Abstract
Neuroinflammation is an important process underlying a wide variety of neurodegenerative diseases. Carvacrol (CAR) is a phenolic monoterpene commonly used as a food additive due to its antibacterial properties, but it has also been shown to exhibit strong antioxidative, anti-inflammatory, and neuroprotective effects. Here, we sought to investigate the effects of CAR on inflammation in the hippocampus and prefrontal cortex, as well as the molecular mechanisms underlying these effects. In our study, lipopolysaccharide was injected into the lateral ventricle of rats to induce memory impairment and neuroinflammation. Daily administration of CAR (25, 50, and 100 mg/kg) for 21 days improved recognition, discrimination, and memory impairments relative to untreated controls. CAR administration significantly attenuated expression of several inflammatory factors in the brain, including interleukin-1β, tumor necrosis factor-α, and cyclooxygenase-2. In addition, CAR significantly increased expression of brain-derived neurotrophic factor (BDNF) mRNA, and decreased expression of Toll-like receptor 4 (TLR4) mRNA. Taken together, these results show that CAR can improve memory impairment caused by neuroinflammation. This cognitive enhancement is due to the anti-inflammatory effects of CAR medicated by its regulation of BDNF and TLR4. Thus, CAR has significant potential as an inhibitor of memory degeneration in neurodegenerative diseases.
Keywords: Brain-derived neurotrophic factor, Cytokines, Inflammation, Lipopolysaccharides, Memory
INTRODUCTION
Neuroinflammation has been linked to a variety of neurological disorders including anxiety, depression, schizophrenia, Alzheimer's disease (AD), ischemic stroke, and Parkinson's disease [1]. As the immune system grows more susceptible to infections with age [2,3], the possibility of severe, long-lasting post-infectious sepsis increases, potentially leading to cognitive dysfunction in elderly patients [4]. Recent studies have identified inflammation as an important factor underlying neurodegenerative diseases, making neuroinflammation an important therapeutic target for the treatment of neurodegenerative diseases [5].
Accordingly, regulation of certain inflammatory factors including interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) have been shown to be critical for the management of neurodegenerative diseases. Resolution of this underlying neuroinflammation has been shown to slow the progression of memory impairment in AD [6,7].
Systemic injection of lipopolysaccharide (LPS), a major component of the external membranes of Gram-negative bacteria and a potent activator of pro-inflammatory cytokines, induces neuroinflammation of the hippocampus and cognitive dysfunction and is a well-established model of neurological pathology [8].
LPS stimulates activation of the innate immune system via Toll-like receptor 4 (TLR4), leading to the expression of several inflammatory cytokines such as IL-6, IL-1β, TNF-α and the nuclear factor-kappa B (NF-κB) system [9,10]. Given the important role of neuroinflammation in disease pathogenesis, resolution of this inflammation has been proposed as a therapeutic option for the treatment of neurodegenerative and neuroinflammatory diseases [11,12].
Non-steroidal anti-inflammatory drugs (NSAIDs) are the most commonly used drugs to treat neuroinflammation [13]. NSAIDs are often prescribed to patients for inflammation control and analgesia; however, long-term use of NSAIDs can lead to gastrointestinal side effects, as well as both liver and kidney toxicity, limiting their use [14]. Given the high risk of adverse effects, there remains a strong desire for new therapies safe enough for long-term treatment [15]. In a recent study, natural products were discussed as an alternative treatment for neuroinflammation and cognitive memory impairment [16].
Carvacrol (CAR, 2-methyl-5-isopropylphenol) is a phenolic monoterpene found in abundance in the essential oils of Lamiaceae plant species. This compound is widely used in the food industry as food additive due to its ability to prevent spoilage [17]. More recently, several studies have proposed the use of CAR as a therapeutic agent in mice and rats due to its widespread antifungal, antioxidant, anti-inflammatory, and anti-apoptotic effects [18,19]. CAR is believed to easily and rapidly cross the bloodbrain barrier [20], greatly enhancing its therapeutic potential in brain-related diseases [21]. These properties likely contribute to the reported anxiolytic and antidepressant effects of CAR in rats [19,22]. At present, the ability of CAR to ameliorate cognitive impairment due to amyloid β or scopolamine-induced cognitive impairments is dependent on its antioxidant and anti-inflammatory effects [23]. In addition, CAR has shown a variety of protective effects in response to 6-hydroxydopamine-induced oxidative stress, including decreases in malondialdehyde and nitrite levels, increased catalase activity, and improved lipid peroxidation profiles [24]. Renal cell injury induced by renal ischemia-reperfusion in rats was reduced by CAR treatment, driven by a combination of antioxidant, cytoprotective, and anti-inflammatory effects [25].
LPS-induced inflammation is a common method used to study the neurobiological mechanisms underlying memory and learning and to develop targeted therapies to treat neuroinflammatory symptoms in animal models. In the present study, the Morris water maze (MWM) test and the object recognition test (ORT) were performed to assess the effects of CAR on cognitive impairment in an LPS-induced neuroinflammation rat model. Additional experiments were performed to better understand the mechanisms underlying the effects of CAR in nerve damage and neuronal inflammation.
METHODS
Animals
Male Sprague-Dawley (SD) rats (weights: 200–220 g, 8 weeks; Samtako Animal Co., Seoul, Korea) were used in this study. The vivarium room was kept on a 12-h light/dark cycle (lights on at 9:00, lights off at 21:00) under a relative humidity of 55 ± 10% and a controlled temperature at 21 ± 2℃. All rats were caged for 7 days to acclimatize before beginning the experimental protocol. All methods and procedures were approved by the Animal Care and Use Committee of Kyung Hee University (KHUASP[SE]-15-115). Experimental procedures were performed according to the Guide for the Care and Use of Laboratory Animals.
LPS administration
Carvacrol, ibuprofen (IBU), and LPS (Escherichia coli; O127:B8) were purchased from Sigma-Aldrich Company (Sigma-Aldrich Co., St. Louis, MO, USA). LPS was administered intracerebroventricularly into the lateral ventricle of the rat brain according to the method of Guo et al. [26] with put under general anesthesia of 50 mg/kg sodium pentobarbital through intraperitoneal injection. Fifty micrograms of LPS dissolved in 10 µl cerebrospinal fluid was microinjected into the lateral ventricle in the rat brains in all lesion groups. Injection of LPS was delivered at a rate of 2 µl/1 min (total 5 min) and injection needles were left in place for an additional 5 min.
Experimental groups
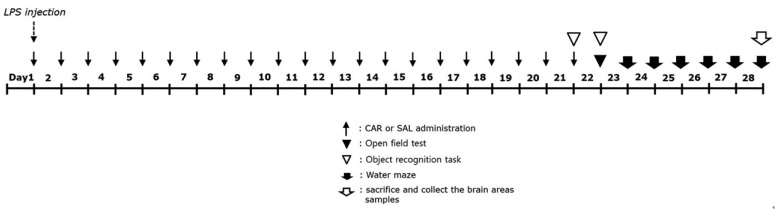
The rats were each divided in an experimental group for treatment and behavioral testing. Rats were separated in 6 groups of 10–12 rats as follows: SAL-injected sham group being regarded as normal (SAL group, n = 10), LPS-injected plus saline-treated group (LPS group as a negative control, n = 10), LPS-injected plus 25 mg/kg CAR-treated group (LPS + CAR25 group, n = 12), LPS-injected plus 50 mg/kg CAR-treated group (LPS + CAR50 group, n = 12), LPS-injected plus 100 mg/kg CAR-treated group (LPS + CAR100 group, n = 12), and LPS-injected plus 40 mg/kg IBU-treated (LPS + IBU group as a positive control, n = 12). CAR and the IBU were administrated intraperitoneally in a volume of 1 ml/kg for 21 day after LPS injection. The standard doses of CAR in the rat and the long-term treatment in the present study was based on a previous study [27]. The CAR and IBU were dissolved in 0.9% physiological saline before use and all drugs were freshly prepared just before every experiment. The whole schedule of behavioral examinations and drug administration are shown in Fig. 1.
Fig. 1. Experimental schedule of lesion generation, CAR administration, and behavioral tests in rats.
CAR, carvacrol; LPS, lipopolysaccharide.
Object recognition task
We used a new ORT to assess cognitive abilities. We used a black-painted acrylic box with a size of 45 × 45 × 45 cm. Rats searched for objects in the acrylic box. The object of discrimination were two similar wooden toys (Familiar objects, A1 and A2) and these objects were large enough that the rats could not move around it. Also, the new wooden toys (B) that were used different in color and shape from A1 and A2. The test consisted of three steps: habituation, training, and testing. The first day was the habituation phase, and the rats were adapted to the test for 10 minutes. Next day 24 h after pre-exposure, the rats were placed in a box containing two similar objects (A1 and A2) and the subject was allowed to explore the objects for 5 min. Two hours later, during the testing phase, the rats were exposed to one new object (B) and one familiar object for 5 min. We measured the sniffing times for the new and familiar objects. Exploration was defined as actively sniffing, pawing at, or whisking with its snout directed toward the object [28].
Morris water maze test
The MWM test was used to measure the time and distance swam to find the submerged platform. The MWM test contained a spatial probe and a place navigation tests. The water tank used as a water maze was a circular cylinder with a diameter of 200 cm and a height of 50 cm. The water temperature used was 22 ± 2℃ and the water height was at 30 cm. The perimeter of the water maze kept the various cues that were hanging around the video camera, the test bench, and the wall around the water tank. The water maze was divided into quadrants and were labeled as NE, NW, SE and SW. The escape zone was located in the center of the NW quadrant, and one of the other quadrants were used as the starting point. The rats underwent three training trials per day for 5 consecutive days, during which they located a hidden platform with the help of visible signals surrounding the evaluation space. Each trial was terminated when the rat found the platform or after 180 s, whichever came first. The spatial probe was performed on day 6, after the removal of the escape platform; each trial was 60 s in duration. All training and probe trails were recorded by a ceiling-mounted video camera and the data were evaluated with the aid of the analyzing program S-MART (PanLab Co., Barcelona, Spain).
Open field test
The open field test (OFT) was based on a previous study [29]. In a dimly lit room, each rat was individually placed in a black-painted square wooden box (60 × 60 × 30 cm) and tracked with a video tracking system for 5 min. The movement of the rats was measured by the distance (locomotor activity) of movements observed by the computerized video-tracking analysis program SMART (PanLab Co.). The number of rearings (exploration) were also manually scored in the black-painted square wooden box for 5 min. The method of the forced swimming test (FST) [30], the elevated plus maze (EPM) test [31], and grip strength test [32] were done according to a previous study.
Inflammatory mediators and NF-κB measurement
Twenty-eight days after LPS injection, IL-6, IL-1β, TNF-α, cyclooxygenase-2 (COX-2), and NF-κB levels were measured in the hippocampus and prefrontal cortex on a previous study [29]. Six rats from each group were severely anesthetized with 1.2 % isoflurane, and were sacrificed. The hippocampus and prefrontal cortex were dissected from each of the rats. The levels of IL-6, IL-1β, TNF-α, COX-2, and NF-κB p65 were assessed through enzyme-linked immunoassay (ELISA) according to the manufacturer's method. IL-6, IL-1β, TNF-α, COX-2, and NF-κB p65 ELISA assay kits were obtained from Abcam (Cambridge, MA, USA).
Total RNA isolation and RT-PCR analysis
The expression of inducible brain-derived neurotrophic factor (BDNF), TLR4, and nitric oxide synthase (iNOS) mRNAs using reverse transcription-polymerase chain reaction (RT-PCR) were analyzed. The total RNA isolation and PT-PCR analysis used in the present study was based on a previous study [33]. Total RNA was extracted from the hippocampus and prefrontal cortex of each rat using TRIzol reagent. cDNA was synthesized from 2 µg total RNA using reverse transcriptase (Takara Bio, Otsu, Japan), and then amplified by PCR at 60℃ for 27 cycles for iNOS, at 58℃ for 28 cycles for TLR4 and at 57℃ for 27 cycles for BDNF using Taq DNA polymerase (Takara, Kyoto, Japan) on a thermal cycler (MJ Research, Watertown, MA, USA). The following sequences were used: for GAPDH (409 bp), (forward) 5′-ATC CCA TCA CCA TCT TCC AG-3′ and (reverse) 5′-CCT GCT TCA CCA CCT TCT TG-3′; for iNOS (170 bp), (forward) 5′-CAC CTT GGA GTT CAC CCA GT-3′ and (reverse) 5′-ACC ACT CGT ACT TGG GAT GC-3′; for TLR4 (219 bp), (forward) 5′-GGT GAG AAA CGA GCT GGT AA-3′ and (reverse) 5′-AGA AAC TGC CAT GTC TGA GC-3′; for BDNF (153 bp), (forward) 5′-CAG GGG CAT AGA CAA AAG-3′ and (reverse) 5′-CTT CCC CTT TTA ATG GTC-3′. The PCR products were electrophoresed on 1.2 % agarose gels and visualized under UV after GelRed (Biotium, Hayward, CA, USA) staining. Quantification of band intensities was performed using Image Master Total Lab (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Data were normalized against glyceraldehyde-3-phosphatede hydrogenase expression in the corresponding sample.
Statistical analysis
All results are expressed as mean ± standard error of mean. The data were analyzed with SPSS 13.0 (Chicago, IL, USA) and were analyzed using multiple way of analysis of variance (ANOVA) and Tukey's post-hoc tests. Statistical significance at p-value < 0.05 has been given symbols in each figure.
RESULTS
Effect of carvacrol on LPS-induced body weight loss
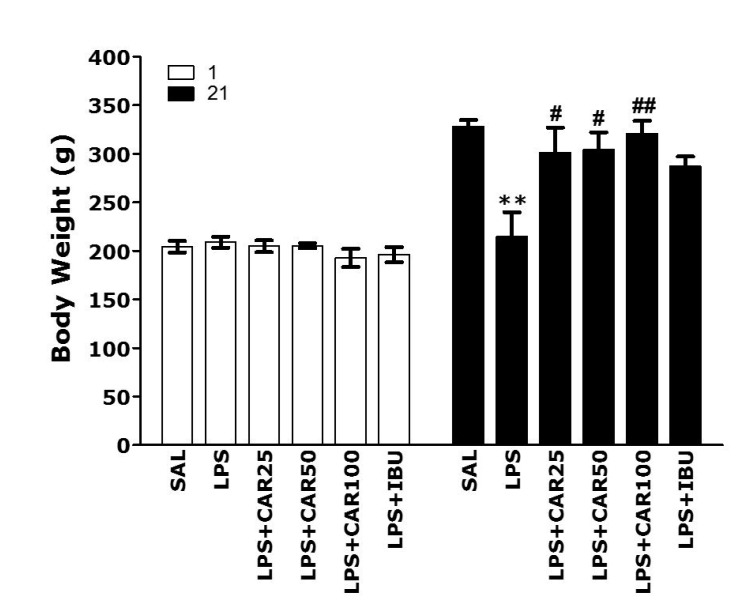
After LPS injection, the body weight of each rat was measured for 21 days and the comparison between day 1 and day 21 are shown in Fig. 2. Normal rats gradually had an increase in body weight over time. On the other hand, LPS-injected rats began to lose body weight on day 1. The body weight loss by LPS usually do not recover to normal levels or increases more slowly than the normal group [34]. On day 1, there was no difference in the body weight of rats in all groups. However, over the curse of 21 days, the rats treated with LPS showed a significant decrease in body weight over 21 days compared with the saline-treated (SAL) group (p < 0.01). However, the group receiving CAR showed a recovery in body weight. The body weight in the LPS + CAR25 group, LPS + CAR50 group and LPS + CAR100 group significantly slowed down body weight loss compared to the LPS group for 21 days (p < 0.05 and p < 0.01). This indicates that rats receiving 25, 50 or 100 mg/kg of CAR were closely associated with the inhibition of body weight loss. As a result, body weight was not correlated with memory deficits recovery, but instead proved that administration of CAR has an inhibitory effect on the loss of body weight, a physiological phenomena and indicator in rats.
Fig. 2. Effects of CAR on body weight on the first day prior to LPS injection and on day 21 after LPS injection (n = 10–12/group).
CAR, carvacrol; LPS, lipopolysaccharide; SAL, saline-treated; IBU, ibuprofen. **p < 0.01 vs. SAL group; #p < 0.05, ##p < 0.01 vs. LPS group.
Effects of carvacrol on LPS-induced memory impairment
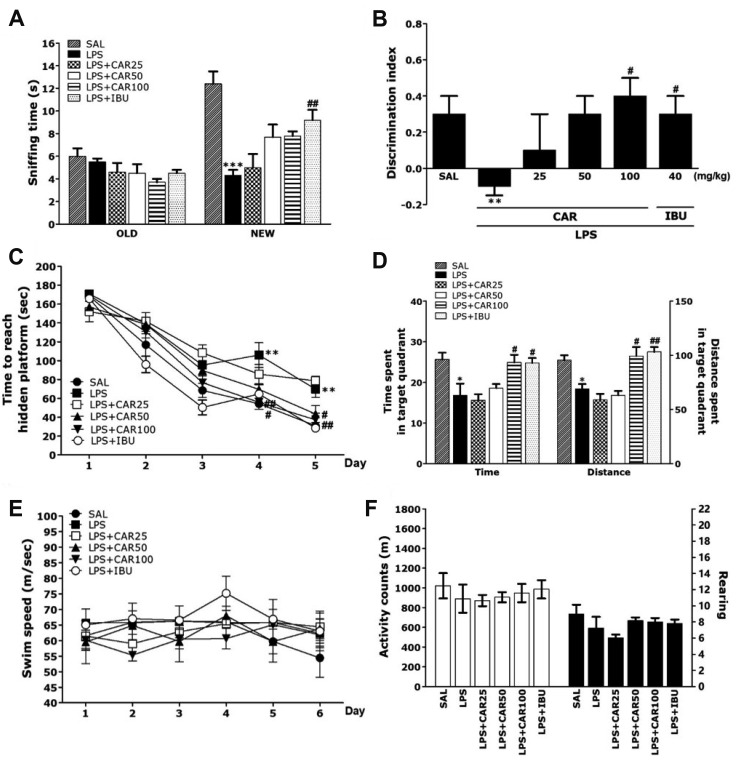
Post-hoc test results showed that the sniffing times were significantly reduced in the LPS-treated group compared to the SAL group (p < 0.001; Fig. 3A). But, the LPS + CAR100 group showed an increase on the new object sniffing time compared with the LPS group. In addition, the discrimination index showed a significant decrease in the LPS-treated group compared to the SAL group (p < 0.01; Fig. 3B). But, the LPS + CAR100 group showed a significantly higher discrimination index than the LPS-treated group (p < 0.05). The LPS + CAR100 group also showed recovery of cognition in LPS-induced memory impairment, similar to that of the LPS + IBU group.
Fig. 3. Effects of CAR on memory impairment in the ORT and MWM test.
The ability to discriminate between familiar objects (A) and new objects (B) in the ORT. The MWM test was used to assess the effect of CAR on spatial learning and memory. The time taken to escape from water (latency) during acquisition trials using a submerged platform (C), the percentages of time spent in the target quadrant and the proportion of the total distance traversed in the target quadrant (D), and swimming speed (E) in the MWM test. Locomotor activity (counts) and total number of rearings (G) in the OFT (n = 10–12/group). CAR, carvacrol; ORT, object recognition test; MWM, Morris water maze; OFT, open field test; LPS, lipopolysaccharide; SAL, saline-treated; IBU, ibuprofen. *p < 0.05, **p < 0.01, ***p < 0.001 vs. SAL group; #p < 0.05, ##p < 0.05 vs. LPS group.
In the MWM test, LPS injection affected performance in the place navigation test. In particular, the LPS group showed poorer performance than did the SAL group (Fig. 3C). ANOVA (6 × 5, treatment × time) disclosed a significant difference among groups (F(4,104) = 233.341, p < 0.01) and an effect of the day of training (F(20,104) = 3.067, p < 0.01); also, a group × day interaction was discovered (F(5,26) = 4.797, p < 0.01). The LPS group indicated worse performance than the SAL group (p < 0.01 on day 4 and 5). The latencies to find the hidden platform by the LPS + CAR100 group was significantly decreased compared to those of the LPS group (p < 0.05 on day 4 and 5). Rats treated with 100 mg/kg of CAR showed a decrease in escape latency during the training period, in comparison to the LPS group in which escape latency markedly increased due to the memory deficits of LPS-induced memory impairment. On the other hand, when the data acquired on day 6 in the retention test were compared, the post-hoc test showed that the LPS + CAR100 group spent more time and distance around the platform than the LPS group (p < 0.05, respectively; Fig. 3D). Also, the escape latency in the LPS + CAR100 group was similar to that in the LPS + IBU group. The average swimming speed showed no difference between the groups, which indicates that the time to reach the platform was simply a result of memory impairment (Fig. 3E).
This study also showed that LPS injection did not affect distance of locomotion and the total number of rearing and evidence that CAR-treatment also did not affect motor functions and exploration (Fig. 3F). This demonstrated that the rats' behavior is caused by memory impairment and not by pathological factors or side effects.
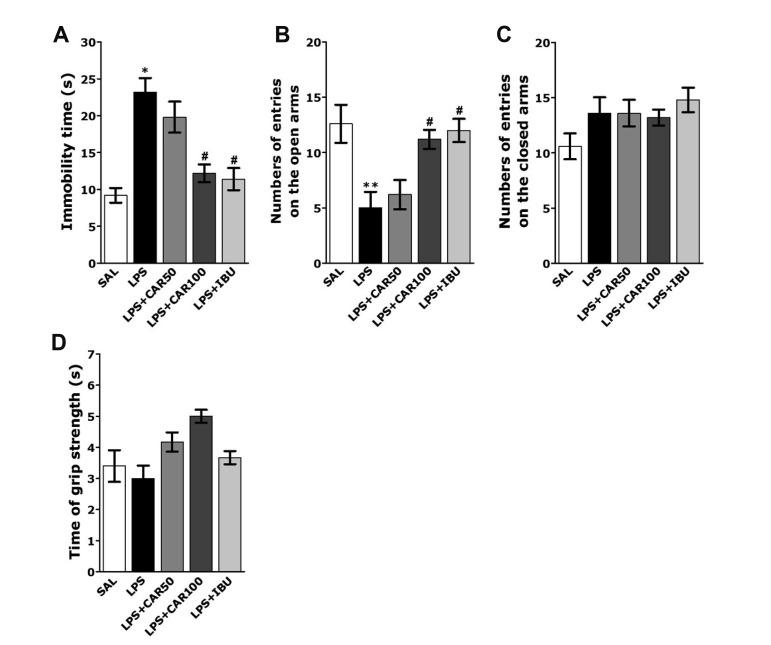
Also, rats subjected to LPS injection exhibited a significant depression phenotype, characterized by increased immobility time during the FST, as compared to SAL group (p < 0.05; Fig. 4A). However, the rats in LPS+CAR100 group showed significant decrease in immobility time during 5 min in the FST, as compared to those in LPS group (p < 0.05), indicating that administration of 100 mg/kg of CAR decreased depression-like behavior. The effects of CAR administration on anxiety-like behaviors, characterized by decreases in open-arm exploration in the EPM test, were also investigated. Post hoc comparisons revealed a significant decrease in the number of entries into the open arms of the maze following the LPS injection compared to the SAL group (p < 0.01; Fig. 4B, C). However, rats in the LPS + CAR100 group also exhibited a significant restoration in the number of entries into the open arms of the maze compared to the LPS group (p < 0.05). The grip strength showed no difference between all groups, which indicates that CAR-treatment also did not affect sensorimotor performance (Fig. 4D).
Fig. 4. Effects of CAR on depression-like and anxiety-like behaviors in the forced swimming test (FST) and elevated plus maze (EPM) test after LPS injection.
Immobility time (A) in the FST, numbers of entries into the open and closed arms in the EPM test (B and C), and grip strength (D). CAR, carvacrol; LPS, lipopolysaccharide; SAL, saline-treated; IBU, ibuprofen. *p < 0.05 and **p < 0.01 vs. SAL group; #p < 0.05 vs. LPS group.
Effects of carvacrol on LPS-induced alternations in inflammatory mediators in the hippocampus and prefrontal cortex
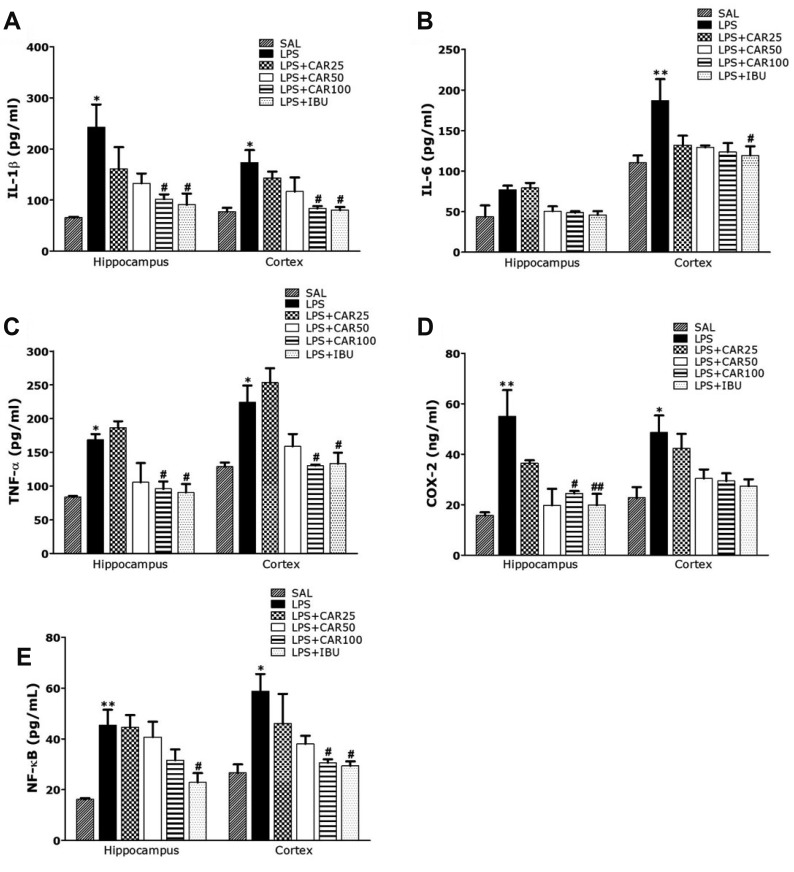
Fig. 5 appeared to investigate the levels of pro-inflammatory markers in the hippocampus and prefrontal cortex activated by LPS-induced neuroinflammation (Fig. 5). The effects of CAR administration on these markers was also investigated. The levels of pro-inflammatory markers, IL-6, IL-1β, TNF-α, COX-2, and NF-κB, were compared in the hippocampus and prefrontal cortex.
Fig. 5. Effects of CAR on inflammatory mediator concentrations in the hippocampus and prefrontral cortex of rats exposed to LPS.
Interleukin (IL)-1β (A), IL-6 (B), tumor necrosis factor (TNF)-α (C), cyclooxygenase-2 (COX-2) (D), and nuclear factor-kappa B (NF-κB) (E) concentrations (n = 6/group). CAR, carvacrol; LPS, lipopolysaccharide; SAL, saline-treated; IBU, ibuprofen. *p < 0.05, **p < 0.01 vs. SAL group; #p < 0.05, ##p < 0.01 vs. LPS group.
Post-hoc test results revealed significantly higher IL-1β level in the hippocampus and prefrontal cortex of the LPS group than in the SAL group (p < 0.05, respectively; Fig. 5A). Daily administration of 100 mg/kg of CAR significantly attenuated LPS-induced increase in IL-1β level in the hippocampus and prefrontal cortex (p < 0.05, respectively). Similar to IL-1β, post-hoc test results revealed higher IL-6 level in the hippocampus of the LPS group than in the SAL group, but it was not statistically significant (Fig. 5B). Daily administration of 100 mg/kg of CAR attenuated LPS-induced increase in IL-6 level in the hippocampus, but it was not statistically significant. However, post-hoc test results revealed significantly higher IL-6 level in the prefrontal cortex of the LPS group than in the SAL group (p < 0.01). Daily administration of 100 mg/kg of CAR attenuated LPS-induced increase in IL-6 level in the prefrontal cortex, but it was not statistically significant. Similar to IL-1β, post-hoc test results revealed significantly higher TNF-α level in the hippocampus and prefrontal cortex of the LPS group than in the SAL group (p < 0.05, respectively; Fig. 5C). Daily administration of 100 mg/kg of CAR significantly attenuated LPS-induced increase in TNF-α level in the hippocampus (p < 0.05, respectively).
Treatment with 100 mg/kg of CAR significantly rescued COX-2 level in the hippocampus and prefrontal cortex by 44.48% and 60.53%, relative to LPS group (p < 0.01 and p = 0.97, respectively; Fig. 5D). Treatment with 100 mg/kg of CAR significantly rescued NF-κB level in the hippocampus and prefrontal cortex by 69.66% and 52.14%, relative to LPS group (p = 0.203 and p < 0.05, respectively; Fig. 5E). Furthermore, COX-2 and NF-κB level in the brain of rats treated with 100 mg/kg of CAR were similar to those of rats treated with 40 mg/kg of IBU.
Effects of carvacrol on LPS-induced expression of iNOS, TLR4, and BDNF mRNAs in the hippocampus and prefrontal cortex
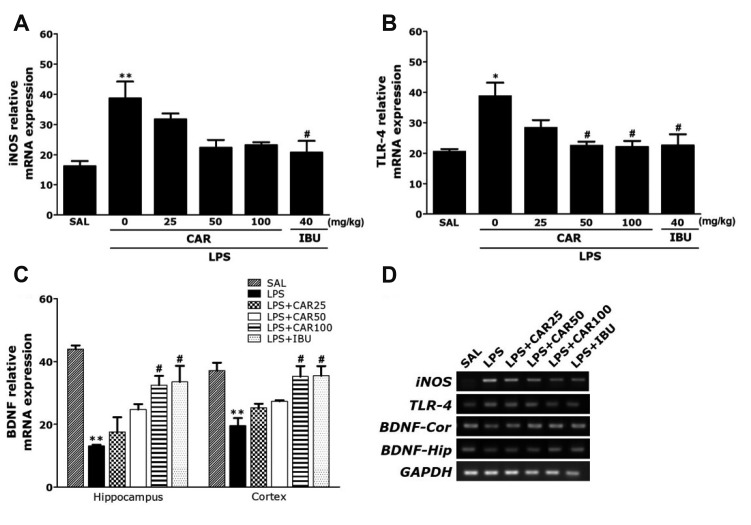
PCR analysis found that LPS injection resulted in significant increases in the iNOS (237.57%) expression in the hippocampus compared to the levels in rats in the SAL group (p < 0.01; Fig. 6A). Administration of 100 mg/kg of CAR inhibited LPS-induced increases in the iNOS levels in the hippocampus, but it was not statistically significant.
Fig. 6. Effects of CAR on immune signaling molecules expression levels in the hippocampus of rats exposed to LPS.
Inducible nitric oxide synthase (iNOS) (A), Toll-like receptor 4 (TLR4) (B), and brain-derived neurotrophic factor (BDNF) (C) mRNAs. PCR bands on agarose gels and relative intensities are shown in (D). The expression levels of iNOS, TLR4, and BDNF mRNAs were normalized to GAPDH mRNA as an internal control (n = 6/group). CAR, carvacrol; LPS, lipopolysaccharide; SAL, saline-treated; IBU, ibuprofen. *p < 0.05 and **p < 0.01 vs. SAL group; #p < 0.05 vs. LPS group.
PCR analysis found that LPS injection resulted in significant increases in the TLR4 (187.78%) expression in the hippocampus compared to the levels in rats in the SAL group (p < 0.05; Fig. 6B). Administration of 100 mg/kg of CAR inhibited LPS-induced increases in the TLR4 levels in the hippocampus (p < 0.05).
Also, we evaluated the effect of chronic CAR treatment on the BDNF expression in rats hippocampus and prefrontal cortex. Chronic treatment of rats with CAR (100 mg/kg) inhibited the decrease of BDNF expression in the hippocampus and prefrontal cortex (p < 0.05, respectively; Fig. 6C).
DISCUSSION
Memory impairment can be caused by the administration of a neuroinflammatory substance. In this study, LPS administration was shown to be sufficient to establish a memory impaired mouse model of neuroinflammation [10]. CAR is an effective anti-inflammatory and potential neuroprotective agent due to its ability to inhibit the production of inflammatory cytokines. LPS injections in rats produce spatial memory and learning disabilities; however, as evidenced by ORT and MWM tests, CAR treatment resulted in significant improvement relative to untreated controls. CAR significantly attenuated increases in IL-1β and TNF-α, two important neuroinflammatory cytokines in the hippocampus and prefrontal cortex. CAR treatment also restored BDNF expression and attenuated TLR-4 levels in the hippocampus. Together, these results show that adequate modulation of TLR4 and BDNF can significantly alleviate LPS-induced cognitive dysfunction. As part of these analyses, we investigated the dose-dependent effects of CAR. Optimal efficacy was achieved at a dose of 100 mg/kg. This dose was well tolerated in this study, consistent with previous findings [27]. In LPS-injected rats, CAR treatment elicited quantifiable recovery of memory impairment at a lower dose (50 mg/kg); however, the best results were observed at higher doses of CAR (> 100 mg/kg). The highest dose of CAR (200 mg/kg) showed positive effects on memory (p < 0.05), though the overall effects were similar to those seen at the 100 mg/kg level (data not shown).
To elucidate the pathophysiological mechanisms of various neurological diseases such as AD, we employed an LPS-induced memory dysfunction model [35]. Neuroinflammation arising after a traumatic brain injury is known to be associated with memory impairment [36]. Accumulating evidence suggests that persistent inflammation can disrupt memory networks, resulting in increased cytokine production and alterations in protein expression, ultimately leading to dementia [5,37]. Inhibition of this inflammation is therefore regarded as a promising option for improving cognitive function [13]. In previous studies, infiltration of LPS into the lateral ventricle led to a significant increase in inflammatory cytokines in the brain of rats, resulting in severe cognitive memory deficits, consistent with our findings [38].
In the present study, administration of CAR after LPS injection restored body weight, demonstrating that CAR can suppress physiological changes due to LPS-induced neuroinflammation [24]. Previous studies have shown that CCl4 injection is associated with fibrosis, decreased body weight, and enhanced liver weight in rats. Consistent with the present findings, CAR treatment helped to prevent body weight loss following CCl4 exposure [39]. In contrast, administration of ibuprofen for 28 days decreased body weight compared to healthy rats, showing a clear divergence from other models. This loss of body weight is thought to be the result of gastrointestinal damage caused by ibuprofen administration [40].
The ORT and MWM tests used in this study were chosen to assess two different memory types, visual recognition and spatial learning, respectively. Our results showed that spatial memory was significantly damaged under LPS-induced impairments, as previously observed [28]. LPS-induced impairment produced a significant increase in exploration time for familiar objects and a marked decrease in memory retention, causing the discrimination index to decrease as the exploration time of new objects decreased significantly. This suggests that, after exposure to a memory impairment event, the brain degenerates dramatically, reducing episodic memory and cognitive abilities [41]. Taken together, these results show that CAR administration significantly increased detection time for new objects and significantly improved memory impairment after LPS injection.
MWM is a hippocampus-dependent learning and memory test commonly used to assess cognitive impairment, spatial learning, and reference memory [41]. During the retention portion of the MWM test, LPS-injected rats spent considerably longer swimming time before finding the platform, indicative of poor spatial learning. In CAR-treated rats, the time needed to find the platform was significantly reduced relative to that of rats that had only received the LPS injection. In addition, the LPS-injected rats without CAR administration demonstrated memory recall and retrieval difficulties, as evidenced by their reduced performance relative to the normal group at in the retention tests at 24 hours after acquisition. CAR improved these behavioral abnormalities and restored spatial learning and memory in LPS-induced rats. Similar results were seen with long-term administration of ibuprofen, a positive control drug [41]. Thus, the results of this study confirmed the hypothesis that CAR improves the memory impairment induced by LPS injection.
No significant differences in OFT results were observed between groups for either locomotion or number of rearings, indicating that CAR does not affect either motor function or sensorimotor performance. Furthermore, this observation indicates that differences in the time needed to reach the platform were due to cognitive impairment in LPS-injected rats rather than differences in locomotion activities. Thus, the change in behavioral performance on the MWM task is the result of memory improvement and not of differences in the active response or psychomotor function.
It has been suggested that infections and other inflammatory processes may be causative factors in emotional disorders, including depression and anxiety. Administration of LPS induced a variety of behavioral responses, collectively known as sickness behavior, some of which could affect the performance on tests used to assess depression and anxiety. In the present study, the administration of CAR significantly decreased immobility during the FST and increased the number of entries into the open arms of the maze in the EPM test. In addition, these results support the possibility that CAR has antidepressant and anxiolytic effects.
Our study showed that LPS infiltration significantly increased expression of IL-1β, TNF-α, and IL-6 in the hippocampus and prefrontal cortex. CAR was found to improve persistent cognitive dysfunction and neuroinflammation by suppressing LPS-induced IL-1β and TNF-α levels [42]. In addition, these results show that the inflammatory reactions to LPS infiltration significantly increased COX-2 expression via activation of the NF-κB pathway in the hippocampus and prefrontal cortex [13]. Previous studies have also shown that injection of LPS activates NF-κB, which primarily regulates inflammatory reactions, confirming the well-established animal model of impaired memory by LPS injection used in our study [43]. In the present study, CAR inhibited LPS-induced memory disturbances and behavioral changes by significantly inhibiting COX-2 levels.
We next analyzed the activation of various immune signaling molecules, including TLR4, the primary immune receptor of LPS: iNOS, an important driver of proinflammatory cytokine production: and NF-κB [44]. Long-term treatment with LPS has been shown to activate the NF-κB pathway via TLR4 and to increases the expression of inflammatory cytokines such as IL-1β and TNF-α in the hippocampus and prefrontal cortex. Our results showed that CAR alleviated the induction of pro-inflammatory cytokines such as IL-1β and TNF-α as well as iNOS expression via the suppression of these pathways. Thus, inhibition of the TLR4 pathway can help to restore cognitive functions and reduce the risk of neurological damage [44].
We also examined the BDNF levels in LPS-treated rats with and without CAR treatment. BDNF is an important neurotrophin associated with cognition and memory, playing an important role in various neurological functions including learning and memory loss [36,45]. As BDNF regulates memory function in the hippocampus, regulation of BDNF signaling may play a major factor in the cognitive function of rats impaired by LPS injection. In the present study, CAR treatment significantly reversed the loss of BDNF mRNA expression by LPS, demonstrating that the beneficial effects of CAR were medicated by an increase in BDNF.
Inflammatory factors such as IL-1β and IL-6 may indirectly influence neuronal functions essential for learning and memory [46]. BDNF has been particularly implicated as a mediator between neuroinflammation and cognitive impairment [47]. Several studies reported increases in BDNF concentrations in the presence of neuroinflammation [48], which could be prevented by administration of an IL-1β receptor antagonist, indicating that IL-1β plays a crucial role in regulating BDNF levels. When considered alongside our results, this suggests that regulation of BDNF levels in the hippocampus and prefrontal cortex is specifically vulnerable to neuroinflammation and cognitive impairment and that CAR treatment was able to reverse the loss of BDNF mRNA expression by suppressing LPS-induced IL-1β and TNF-α levels.
Taken together, the data presented here suggest that CAR administration significantly attenuated LPS-induced defects in cognitive function via the attenuation of neuroinflammation through its effects on TLR4 and BDNF. These findings suggest that CAR may be useful for the treatment of psychologically rooted behaviors and neurochemical alterations seen in memory impairment.
ACKNOWLEDGEMENTS
This research was supported by a Grant from the National Research Foundation of Korea funded by the Korean government (2016R1D1A1A09917012).
Footnotes
Author contributions: B.L. and D.H.H. performed the conception and design. B.L. performed the carried out the experiments. B.L. and D.H.H. performed the acquisition of data. B.L. and D.H.H. performed the analysis and interpretation. B.L. and M.Y. performed the drafting the article. B.L. and D.H.H. performed the statistical analysis. I.S. and H.L. performed the study supervision.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Zipp F, Aktas O. The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci. 2006;29:518–527. doi: 10.1016/j.tins.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Kovaiou RD, Herndler-Brandstetter D, Grubeck-Loebenstein B. Age-related changes in immunity: implications for vaccination in the elderly. Expert Rev Mol Med. 2007;9:1–17. doi: 10.1017/S1462399407000221. [DOI] [PubMed] [Google Scholar]
- 3.Liu WM, van der Zeijst BA, Boog CJ, Soethout EC. Aging and impaired immunity to influenza viruses: implications for vaccine development. Hum Vaccin. 2011;7 Suppl:94–98. doi: 10.4161/hv.7.0.14568. [DOI] [PubMed] [Google Scholar]
- 4.Widmann CN, Heneka MT. Long-term cerebral consequences of sepsis. Lancet Neurol. 2014;13:630–636. doi: 10.1016/S1474-4422(14)70017-1. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham C, Hennessy E. Co-morbidity and systemic inflammation as drivers of cognitive decline: new experimental models adopting a broader paradigm in dementia research. Alzheimers Res Ther. 2015;7:33–40. doi: 10.1186/s13195-015-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mrak RE. Neuropathology and the neuroinflammation idea. J Alzheimers Dis. 2009;18:473–481. doi: 10.3233/JAD-2009-1158. [DOI] [PubMed] [Google Scholar]
- 7.Schwab C, McGeer PL. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J Alzheimers Dis. 2008;13:359–369. doi: 10.3233/jad-2008-13402. [DOI] [PubMed] [Google Scholar]
- 8.Jin Y, Peng J, Wang X, Zhang D, Wang T. Ameliorative effect of ginsenoside Rg1 on lipopolysaccharide-induced cognitive impairment: role of cholinergic system. Neurochem Res. 2017;42:1299–1307. doi: 10.1007/s11064-016-2171-y. [DOI] [PubMed] [Google Scholar]
- 9.Dehkordi NG, Noorbakhshnia M, Ghaedi K, Esmaeili A, Dabaghi M. Omega-3 fatty acids prevent LPS-induced passive avoidance learning and memory and CaMKII-α gene expression impairments in hippocampus of rat. Pharmacol Rep. 2015;67:370–375. doi: 10.1016/j.pharep.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Mirahmadi SM, Shahmohammadi A, Rousta AM, Azadi MR, Fahanik-Babaei J, Baluchnejadmojarad T, Roghani M. Soy isoflavone genistein attenuates lipopolysaccharide-induced cognitive impairments in the rat via exerting anti-oxidative and anti-inflammatory effects. Cytokine. 2018;104:151–159. doi: 10.1016/j.cyto.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Qu Z, Mossine VV, Cui J, Sun GY, Gu Z. Protective effects of AGE and its components on neuroinflammation and neurodegeneration. Neuromolecular Med. 2016;18:474–482. doi: 10.1007/s12017-016-8410-1. [DOI] [PubMed] [Google Scholar]
- 12.Sawikr Y, Yarla NS, Peluso I, Kamal MA, Aliev G, Bishayee A. Neuroinflammation in Alzheimer's disease: the preventive and therapeutic potential of polyphenolic nutraceuticals. Adv Protein Chem Struct Biol. 2017;108:33–57. doi: 10.1016/bs.apcsb.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Gong QH, Pan LL, Liu XH, Wang Q, Huang H, Zhu YZ. S-propargyl-cysteine (ZYZ-802), a sulphur-containing amino acid, attenuates beta-amyloid-induced cognitive deficits and pro-inflammatory response: involvement of ERK1/2 and NF-κB pathway in rats. Amino Acids. 2011;40:601–610. doi: 10.1007/s00726-010-0685-1. [DOI] [PubMed] [Google Scholar]
- 14.Graupera M, García-Pagán JC, Abraldes JG, Peralta C, Bragulat M, Corominola H, Bosch J, Rodés J. Cyclooxygenase-derived products modulate the increased intrahepatic resistance of cirrhotic rat livers. Hepatology. 2003;37:172–181. doi: 10.1053/jhep.2003.50004. [DOI] [PubMed] [Google Scholar]
- 15.Szekely CA, Breitner JC, Fitzpatrick AL, Rea TD, Psaty BM, Kuller LH, Zandi PP. NSAID use and dementia risk in the cardiovascular health study: role of APOE and NSAID type. Neurology. 2008;70:17–24. doi: 10.1212/01.wnl.0000284596.95156.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho YS, So KF, Chang RC. Drug discovery from Chinese medicine against neurodegeneration in Alzheimer's and vascular dementia. Chin Med. 2011;6:15–20. doi: 10.1186/1749-8546-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baser KH. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr Pharm Des. 2008;14:3106–3119. doi: 10.2174/138161208786404227. [DOI] [PubMed] [Google Scholar]
- 18.Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res. 2007;21:308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 19.Melo FH, Moura BA, de Sousa DP, de Vasconcelos SM, Macedo DS, Fonteles MM, Viana GS, de Sousa FC. Antidepressant-like effect of carvacrol (5-Isopropyl-2-methylphenol) in mice: involvement of dopaminergic system. Fundam Clin Pharmacol. 2011;25:362–367. doi: 10.1111/j.1472-8206.2010.00850.x. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Zhang ZL, Chen J, Pei A, Hua F, Qian X, He J, Liu CF, Xu X. Carvacrol, a food-additive, provides neuroprotection on focal cerebral ischemia/reperfusion injury in mice. PLoS One. 2012;7:e33584. doi: 10.1371/journal.pone.0033584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savelev SU, Okello EJ, Perry EK. Butyryl- and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother Res. 2004;18:315–324. doi: 10.1002/ptr.1451. [DOI] [PubMed] [Google Scholar]
- 22.Polli FS, Gomes JN, Ferreira HS, Santana RC, Fregoneze JB. Inhibition of salt appetite in sodium-depleted rats by carvacrol: Involvement of noradrenergic and serotonergic pathways. Eur J Pharmacol. 2019;854:119–127. doi: 10.1016/j.ejphar.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Azizi Z, Ebrahimi S, Saadatfar E, Kamalinejad M, Majlessi N. Cognitive-enhancing activity of thymol and carvacrol in two rat models of dementia. Behav Pharmacol. 2012;23:241–249. doi: 10.1097/FBP.0b013e3283534301. [DOI] [PubMed] [Google Scholar]
- 24.Baluchnejadmojarad T, Hassanshahi J, Roghani M, Mansouri M, Raoufi S. Protective effect of carvacrol in 6-hydroxydopamine hemiparkinsonian rat model. J Basic Clin Physiol Pharmacol. 2014;2:29–34. [Google Scholar]
- 25.Ozturk H, Cetinkaya A, Duzcu SE, Tekce BK, Ozturk H. Carvacrol attenuates histopathogic and functional impairments induced by bilateral renal ischemia/reperfusion in rats. Biomed Pharmacother. 2018;98:656–661. doi: 10.1016/j.biopha.2017.12.060. [DOI] [PubMed] [Google Scholar]
- 26.Guo J, Li F, Wu Q, Gong Q, Lu Y, Shi J. Protective effects of icariin on brain dysfunction induced by lipopolysaccharide in rats. Phytomedicine. 2010;17:950–955. doi: 10.1016/j.phymed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Zhong Z, Wang B, Dai M, Sun Y, Sun Q, Yang G, Bian L. Carvacrol alleviates cerebral edema by modulating AQP4 expression after intracerebral hemorrhage in mice. Neurosci Lett. 2013;555:24–29. doi: 10.1016/j.neulet.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 28.Lee YJ, Choi DY, Yun YP, Han SB, Oh KW, Hong JT. Epigallocatechin-3-gallate prevents systemic inflammation-induced memory deficiency and amyloidogenesis via its anti-neuroinflammatory properties. J Nutr Biochem. 2013;24:298–310. doi: 10.1016/j.jnutbio.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm DH. L-tetrahydropalmatine ameliorates development of anxiety and depression-related symptoms induced by single prolonged stress in rats. Biomol Ther (Seoul) 2014;22:213–222. doi: 10.4062/biomolther.2014.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee B, Sur B, Shim I, Lee H, Hahm DH. Angelica gigas ameliorate depression-like symptoms in rats following chronic corticosterone injection. BMC Complement Altern Med. 2015;15:210. doi: 10.1186/s12906-015-0746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee B, Sur B, Cho SG, Yeom M, Shim I, Lee H, Hahm DH. Ginsenoside Rb1 rescues anxiety-like responses in a rat model of posttraumatic stress disorder. J Nat Med. 2016;70:133–144. doi: 10.1007/s11418-015-0943-3. [DOI] [PubMed] [Google Scholar]
- 32.Tabassum H, Ashafaq M, Parvez S, Raisuddin S. Role of melatonin in mitigating nonylphenol-induced toxicity in frontal cortex and hippocampus of rat brain. Neurochem Int. 2017;104:11–26. doi: 10.1016/j.neuint.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Sur B, Lee B, Yoon YS, Lim P, Hong R, Yeom M, Lee HS, Park H, Shim I, Lee H, Jang YP, Hahm DH. Extract of polygala tenuifolia alleviates stress-exacerbated atopy-like skin dermatitis through the modulation of protein Kinase A and p38 mitogen-activated protein Kinase signaling pathway. Int J Mol Sci. 2017;18:E190. doi: 10.3390/ijms18010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaab J, Rohleder N, Heitz V, Engert V, Schad T, Schürmeyer TH, Ehlert U. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology. 2005;30:188–198. doi: 10.1016/j.psyneuen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Czerniawski J, Miyashita T, Lewandowski G, Guzowski JF. Systemic lipopolysaccharide administration impairs retrieval of context-object discrimination, but not spatial, memory: evidence for selective disruption of specific hippocampus-dependent memory functions during acute neuroinflammation. Brain Behav Immun. 2015;44:159–166. doi: 10.1016/j.bbi.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vasconcelos AR, Yshii LM, Viel TA, Buck HS, Mattson MP, Scavone C, Kawamoto EM. Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment. J Neuroinflammation. 2014;11:85–95. doi: 10.1186/1742-2094-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olsson A, Csajbok L, Ost M, Höglund K, Nylén K, Rosengren L, Nellgård B, Blennow K. Marked increase of beta-amyloid(1-42) and amyloid precursor protein in ventricular cerebrospinal fluid after severe traumatic brain injury. J Neurol. 2004;251:870–876. doi: 10.1007/s00415-004-0451-y. [DOI] [PubMed] [Google Scholar]
- 38.Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, Hong JT. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008;5:37–40. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohseni R, Karimi J, Tavilani H, Khodadadi I, Hashemnia M. Carvacrol ameliorates the progression of liver fibrosis through targeting of Hippo and TGF-β signaling pathways in carbon tetrachloride (CCl4)-induced liver fibrosis in rats. Immunopharmacol Immunotoxicol. 2019;41:163–171. doi: 10.1080/08923973.2019.1566926. [DOI] [PubMed] [Google Scholar]
- 40.Wen C, Zhuang Z, Song H, Tong S, Wang X, Lin Y, Zhan H, Chen Z, Hu L. Metabolism of liver CYP450 and ultrastructural changes after long-term administration of aspirin and ibuprofen. Biomed Pharmacother. 2018;108:208–215. doi: 10.1016/j.biopha.2018.08.162. [DOI] [PubMed] [Google Scholar]
- 41.Lee B, Sur B, Park J, Kim SH, Kwon S, Yeom M, Shim I, Lee H, Hahm DH. Ginsenoside rg3 alleviates lipopolysaccharide-induced learning and memory impairments by anti-inflammatory activity in rats. Biomol Ther (Seoul) 2013;21:381–390. doi: 10.4062/biomolther.2013.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JN, Perry VH. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baluchnejadmojarad T, Zeinali H, Roghani M. Scutellarin alleviates lipopolysaccharide-induced cognitive deficits in the rat: Insights into underlying mechanisms. Int Immunopharmacol. 2018;54:311–319. doi: 10.1016/j.intimp.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 44.Pålsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song X, Zhou B, Zhang P, Lei D, Wang Y, Yao G, Hayashi T, Xia M, Tashiro S, Onodera S, Ikejima T. Protective effect of silibinin on learning and memory impairment in LPS-treated rats via ROS-BDNF-TrkB pathway. Neurochem Res. 2016;41:1662–1672. doi: 10.1007/s11064-016-1881-5. [DOI] [PubMed] [Google Scholar]
- 46.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 47.Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Guan Z, Fang J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav Immun. 2006;20:64–71. doi: 10.1016/j.bbi.2005.04.005. [DOI] [PubMed] [Google Scholar]