Abstract
Older patients are at increased risk for complications and death following allogeneic hematopoietic cell transplantation (allo-HCT). Traditional transplant-specific prognostic indices such as hematopoietic cell transplant comorbidity index (HCT-CI) may not capture all underlying geriatric vulnerabilities, and in-depth evaluation by a geriatrician prior to transplant may not always be available. We hypothesize that routine pre-transplant interdisciplinary clinical assessment may uncover prognostically important geriatric deficits. Using an institutional database of 457 adults aged 60 years and older who underwent first allo-HCT for hematological malignancies from 2010 to 2017, we examined the prognostic impact of pre-transplant deficits in geriatric domains of function, mobility, mood, medication, nutrition, and relevant biochemical markers. We found that impairment in instrumental activities of daily living (IADL) was associated with reduced survival through increased non-relapse mortality (NRM, HR = 1.82; 95% CI, 1.04-3.19). The combination of IADL impairment with either HCT-CI/age index or disease risk index readily stratified NRM and overall survival, respectively. In addition, we found that even mild renal dysfunction adversely impacted survival in older transplant patients. Our findings establish important geriatric vulnerabilities in older patients prior to allo-HCT and may provide an entry point for prospective, interventional trials to improve their outcomes.
Introduction
Advanced hematologic malignancies in older patients are unmet medical needs due to increased incidence in the aging population, heightened sensitivity to therapeutic toxicities, and the lack of effective, curative treatment strategies[1,2]. Allogeneic hematopoietic cell transplantation (allo-HCT) represents the only potential curative option for most of hematologic malignancies, yet it remains unavailable to many older patients or results in poor outcomes due to regimen-related toxicities, higher risk of relapse, and access barriers [1,3].Therefore, it is crucial to develop effective strategies to assess risks in older allo-HCT patients.
Hematopoietic cell transplant comorbidity index (HCT-CI) and its subsequent modification to include age (HCT-CI/age) is the most widely used tool to stratify mortality risk after allo-HCT[4,5]. Recent efforts to improve its prognostic accuracy have included various combinations with modified disease risk index (DRI) and serum biomarkers such as albumin, ferritin, and c-reactive protein [6-8]. The improved prognostication and the development of reduced intensity conditioning (RIC) thus has allowed increasing number of older patients to proceed to allo-HCT in recent years. However, non-relapse mortality (NRM) rates have remained approximately 25-35% at two years across studies [1,9-11].
Comprehensive geriatric assessment (GA), defined as a multidisciplinary process to assess medical, psychosocial, and functional limitations of an older adult, has emerged as an important risk stratification tool for survival and treatment toxicities in advanced hematologic malignancies, including allo-HCT [12-14]. Specific domains of GA, such as instrumental activities of daily living (IADL), cognition, and gait speed, indicate functional reserve and vulnerabilities, and may predict survival beyond chronologic age and performance status. Of these, IADL impairment has been consistently shown to associate with inferior overall survival (OS) and progression-free survival (PFS) in HCT patients [15-17].
GA should be obtained ideally through an official geriatrics consultation or through prospectively collected, patient-reported outcomes supplemented by clinician evaluation, although these require adequate resource support and considerable physician expertise. We previously reported a small cohort of older patients who had received an official geriatrics evaluation prior to HCT and found a high prevalence of geriatric deficits [18]. We hypothesize that routine pre-transplant assessment from interdisciplinary clinical providers may help uncover geriatric deficits in older patients who have not received official geriatrics evaluation, and that these assessments may be utilized as screening tools for additional evaluation.
Materials and Methods
Patients and Transplant Care
This analysis included patients aged 60 years or older who underwent first allo-HCT for a hematologic malignancy between 2010 and 2017 at our institution. We chose 60 years or older as it is the common cutoff used in many studies of older transplant patients including the CIBMTR summary [9]. A waiver of authorization for this retrospective review was obtained from the Institutional Review and Privacy Board at the Memorial Sloan Kettering Cancer Center. Routine pre-transplant assessment, transplant and post-transplant care, and disease monitoring followed standard institutional guidelines. Demographic data, disease and transplant characteristics, NRM, relapse/disease progression, cause of death, and survival data were retrieved from our institutional transplant database. All data were anonymized to protect the identity of subjects involved. The HCT-CI and revised DRI were assigned according to published criteria [19,20].
Geriatric and Laboratory Characteristics
We systematically reviewed clinical notes from physicians, advanced practice providers, nursing staff, and ancillary clinical staff such as physical therapists, occupational therapists, and dietitians. Pre-transplant assessments of all geriatric variables such as basic activities of daily living (Katz’s ADL), IADL (Lawton’s IADL), cognition, mood, nutrition, prior falls, weight loss, and medication use were defined and documented as described in Supplemental Table 1. Potentially inappropriate medication (PIM) use was defined by the American Geriatrics Society 2015 Updated Beers Criteria [21]. Pre-conditioning laboratory biomarkers were either from most recent clinic visit prior to admission or on the day of transplant admission as described in Supplemental Table 1.
Statistical Methods
OS and PFS were estimated using the Kaplan-Meier method, while NRM was estimated using the cumulative incidence method for competing risks. Relapse/progression of disease was considered a competing risk for NRM. All comparisons across groups were based on the log-rank test for OS and PFS, and the Gray’s test for NRM. The association of clinical, transplant, geriatric, and laboratory characteristics with survival outcomes was evaluated using the Cox proportional hazards model, with cause-specific model used for NRM. Variables included: age, gender, Karnofsky Performance Scale (KPS), diagnosis, HCT-CI/age index, revised DRI, donor type, conditioning intensity, and recipient cytomegalovirus (CMV) serostatus; geriatric variables of impairments in ADL, IADL, nutrition, mood, mobility, and PIM use; and biochemical markers. Cognitive impairment was excluded due to high percentage of missing data. Multivariate models were built with all univariate covariates of p < 0.05. All statistical analyses were performed with R 3.5.0 (R development core team, Vienna, Austria).
Results
Patients Characteristics
Baseline characteristics of the study cohort were listed in Table 1. The median age was 66 years (range 60-78.7 years) with 17% aged 70 years or older. Thirty-nine percent of patients were female. Approximately two-third of patients had myeloid malignancies. Twenty-eight percent of patients had high/very high DRI. One-third of patients were in the high HCT-CI/age risk group (≥5). KPS was below 90 in 47% of patients. Sixty percent of patients received a myeloablative conditioning regimen, including 43% on the CD34+ selection platform. Most patients had fully matched donors and used peripheral blood stem cells.
Table 1.
Demographic, Clinical, and Transplant Characteristics
| N (%) | |
|---|---|
| Number of patients | 457 |
| Patient age, years (median, range) | 65.9 (60-78.7) |
| Patient age, ≥70 | 77 (17) |
| Female Gender | 177 (39) |
| Disease Indication | |
| Myeloid malignancies | 315 (69) |
| Lymphoid malignancies | 142 (31) |
| Karnofsky Performance Score (KPS) | |
| ≥90 | 242 (53) |
| <90 | 211 (47) |
| Missing information | 4 |
| Hematopoietic Cell Transplant-Comorbidity/Age Index (HCT-CI/age) | |
| <5 | 307 (67) |
| ≥5 | 150 (33) |
| Disease Risk Index (DRI) | |
| Low/Intermediate | 327 (72) |
| High/Very high | 130 (28) |
| Donor HLA-type | |
| Match related donor | 127 (28) |
| Match unrelated donor | 247 (54) |
| Mismatch (≤7/8) unrelated donor | 58 (13) |
| Haploidentical donor | 25 (5) |
| Conditioning Intensity | |
| Myeloablative | 274 (60) |
| NMA/RIC | 183 (40) |
| Patient CMV Status | |
| Negative | 190 (42) |
| Positive | 267 (58) |
| Graft Source | |
| Bone marrow | 55 (12) |
| Peripheral blood | 402 (88) |
| Follow-up of survivors, months (median, range) | 37 (2 – 102) |
Abbreviations: HLA, Human Leukocyte Antigen; NMA/RIC, Non-myeloablative/Reduced intensity conditioning; CMV, Cytomegalovirus.
As illustrated in Table 2, baseline geriatric impairments were common in domains of function, mobility, cognition, mood, nutrition, and medications, including 11% with impaired IADL, 18% with prior fall within last year, 16% with significant weight loss, 18% with depressive symptoms, and 44% with PIM use. Only 340 patients (74%) had pre-transplant Montreal Cognitive Assessment (MoCA) performed, with 44% scoring abnormal (score <26). Nine percent of patients had abnormal calculated creatinine clearance (<60 mL/min/1.73m2), 7% had serum albumin <3.5 g/dL, and 15% had absolute lymphocyte count below normal (0.5 K/μl).
Table 2.
Geriatric Deficits and Laboratory Markers
| N (%) | |
|---|---|
| Number of patients | 457 |
| Activities of Daily Living (ADL) | |
| Not impaired | 438 (96) |
| Impaired | 19 (4) |
| Instrumental Activities of Daily Living (IADL) | |
| Not impaired | 364 (89) |
| Impaired | 44 (11) |
| Missing information | 49 |
| Cognition (MOCA) | |
| Not impaired | 190 (56) |
| Impaired | 150 (44) |
| Missing information | 117 |
| Polypharmacy (median, range) | 5 (0 - 22) |
| Potentially Inappropriate Medication Use | |
| No | 257 (56) |
| Yes | 200 (44) |
| Prior fall within last year | |
| No | 376 (82) |
| Yes | 81 (18) |
| Weight loss 10lbs or more in 3-month | |
| No | 382 (84) |
| Yes | 75 (16) |
| Depressive symptoms | |
| No | 376 (82) |
| Yes | 81 (18) |
| Pre-transplant serum albumin | |
| ≥3.5 | 426 (93) |
| <3.5 | 31 (7) |
| Pre-transplant creatinine clearance (CrCl) | |
| ≥60 | 415 (91) |
| <60 | 42 (9) |
| Pre-transplant absolute lymphocyte count | |
| ≥500 | 388 (85) |
| <500 | 69 (15) |
Abbreviations: MOCA, Montreal Cognitive Assessment.
Transplant Outcomes
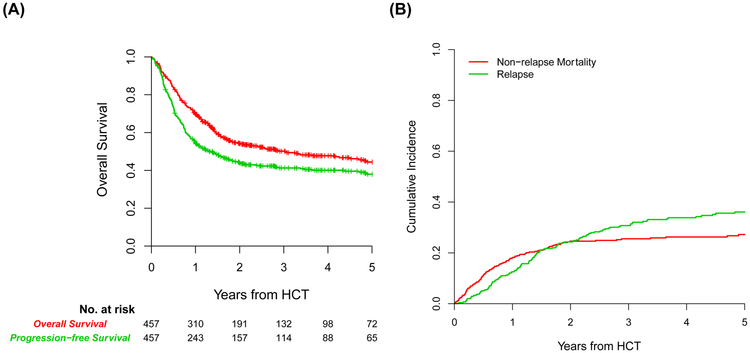
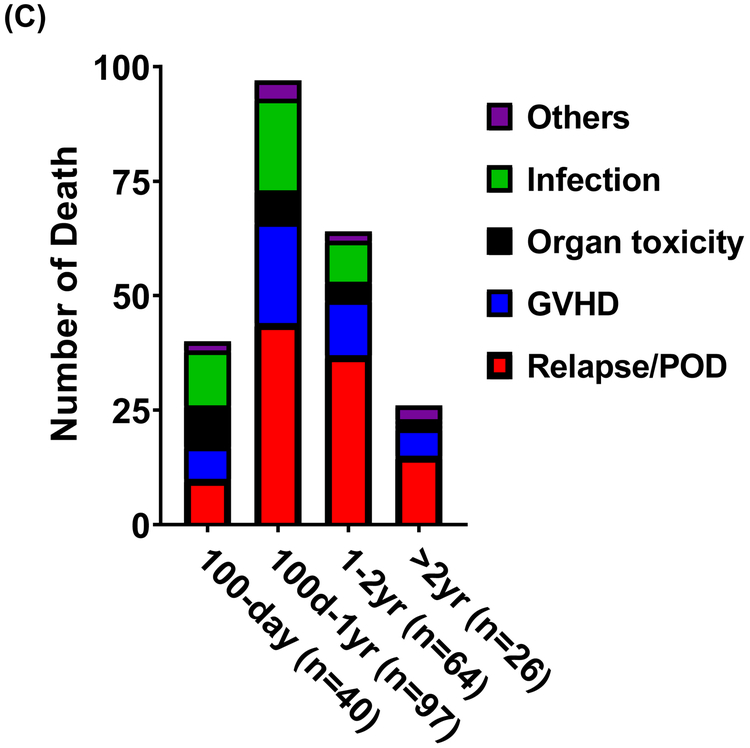
With a median follow-up of 37 months for survivors, the 1- and 3-year probability of OS was 70% (95% confidence interval (CI), 65-74) and 50% (95% CI, 45-55), respectively (Figure 1A). The 1- and 3-year probability of PFS was 54% (95% CI, 50-59) and 41% (95% CI, 36-46), respectively (Figure 1A). The 2-year cumulative incidence of NRM was 25% (95% CI, 21-29) and relapse/disease progression 26% (95% CI, 22-30) (Figure 1B). By the data cutoff date, 227 patients had died, the majority of whom during the 100-day to 1-year period post-transplant. The causes of death from common etiologies were illustrated in Figure 1C.
FIGURE 1.
(A) Overall survival (red line) and progression-free survival (green line) of the cohort. (B) Non-relapse mortality (red line) and relapse/progression of disease (green line) of the cohort. (C) Causes of death in specified periods.
Geriatric Vulnerabilities Impact on NRM and OS
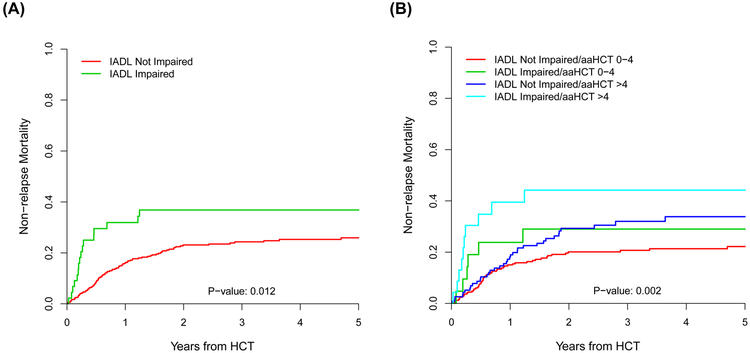
In univariate analyses, the only geriatric or laboratory variable associated with NRM was IADL impairment (Hazard ratio (HR) = 1.98; 95% CI, 1.16-3.38, p = 0.012) (Supplemental Table 2). In addition, reduced KPS, high HCT-CI/age risk, mismatched unrelated donor, and recipient CMV seropositivity were also associated with NRM (Supplemental Table 2). As illustrated in Figure 2, IADL impairment readily stratified NRM either alone (grey’s p = 0.012), or in combination with HCT-CI/age index (grey’s p = 0.002). Patients with high HCT-CI/age risk and IADL impairment had a very high estimated 2-year NRM of 45% (95% CI, 36-53) (Figure 2B).
FIGURE 2.
(A) Impact of IADL impairment (green line) on NRM (p=0.012). (B) Combining IADL impairment with HCT-CI/age group stratifies NRM (p=0.002).
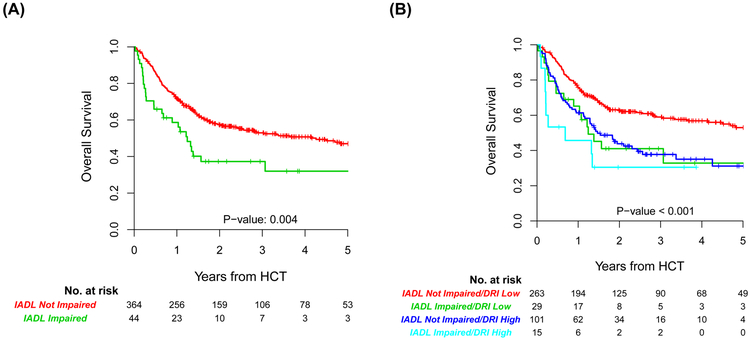
Similarly, IADL impairment was associated with inferior OS (HR = 1.84; 95% CI, 1.21-2.73, p = 0.004) and PFS (HR = 1.76; 95% CI, 1.2-2.58, p = 0.004) in univariate analysis (Supplemental Table 2). Reduced KPS, high HCT-CI/age risk, high/very high DRI, mismatched unrelated donor, recipient CMV seropositivity, and mild renal dysfunction were also associated with inferior OS and PFS in univariate analysis (Supplemental Table 2). As illustrated in Figure 3, IADL impairment readily stratified OS either alone (log-rank p = 0.004), or in combination with modified DRI (log-rank p < 0.001). Patients with low/intermediate DRI and normal IADL had a superior estimated 3-year OS of 60% (95% CI, 56-64) (Figure 3B).
FIGURE 3.
(A) Impact of IADL impairment (green line) on OS (p=0.004). (B) Combining IADL impairment with DRI group stratifies OS (p<0.001).
Multivariate Analyses of NRM, PFS, and OS
We summarized multivariate analyses of OS, PFS, and NRM in Table 3. IADL impairment remained significantly associated with increased NRM (HR = 1.82; 95% CI, 1.04-3.19, p = 0.036), OS (HR = 1.58; 95% CI, 1.01-2.45, p = 0.043), and PFS (HR = 1.54; 95% CI, 1.02-2.33, p = 0.039). HCT-CI/age was significantly associated with NRM (HR = 1.49; 95% CI, 1.01-2.21, p = 0.045), while revised DRI was significantly associated with inferior OS (HR = 1.87; 95% CI, 1.36-2.57, p < 0.001), and PFS (HR = 1.53; 95% CI, 1.14-2.07, p = 0.005). Additionally, recipient CMV seropositivity and reduced KPS were significantly associated with inferior OS and PFS (Table 3). Finally, mild renal dysfunction prior to transplant remained significantly associated with inferior OS (HR = 1.76; 95% CI, 1.15-2.67, p = 0.009) and PFS (HR = 1.52; 95% CI, 1.01-2.28, p = 0.044) in multivariate analysis.
Table 3.
Multivariate Analyses of OS, PFS, and NRM
| OS | PFS | NRM | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| KPS | 0.039 | 0.044 | 0.081 | |||
| <90 | Reference | Reference | Reference | |||
| ≥90 | 0.74 (0.55-0.98) | 0.76 (0.58-0.99) | 0.70 (0.47-1.05) | |||
| HCT-CI/age | 0.991 | 0.984 | 0.045 | |||
| <5 | Reference | Reference | Reference | |||
| ≥5 | 1.00 (0.73-1.36) | 1.00 (0.75-1.34) | 1.49 (1.01-2.21) | |||
| DRI | <0.001 | 0.005 | ||||
| Low/Interm. | Reference | Reference | ||||
| High/very high | 1.87 (1.36-2.57) | 1.53 (1.14-2.07) | ||||
| Donor type | ||||||
| MRD | Reference | Reference | Reference | |||
| MUD | 0.90 (0.63-1.27) | 0.538 | 0.87 (0.64-1.2) | 0.403 | 1.16 (0.71-1.91) | 0.548 |
| MMUD | 1.52 (0.97-2.39) | 0.067 | 1.28 (0.84-1.95) | 0.255 | 2.23 (1.21-4.1) | 0.01 |
| Haploidentical | 1.18 (0.61-2.31) | 0.622 | 1.18 (0.65-2.13) | 0.594 | 1.4 (0.56-3.53) | 0.474 |
| Patient CMV | 0.016 | 0.026 | 0.103 | |||
| Negative | Reference | Reference | Reference | |||
| Positive | 1.45 (1.07-1.96) | 1.37 (1.04-1.81) | 1.41 (0.93-2.14) | |||
| IADL | 0.043 | 0.039 | 0.036 | |||
| Not impaired | Reference | Reference | Reference | |||
| Impaired | 1.58 (1.01-2.45) | 1.54 (1.02-2.33) | 1.82 (1.04-3.19) | |||
| CrCl | 0.009 | 0.044 | ||||
| ≥60 | Reference | Reference | ||||
| <60 | 1.76 (1.15-2.67) | 1.52 (1.01-2.28) | ||||
Abbreviations: HR, Hazard Ratio; CI, Confidence Interval; KPS, Karnofsky Performance Scale; HCT-CI, Hematopoietic cell transplantation-comorbidity index; DRI, Disease Risk Index; Interm., Intermediate; MRD, Matched-related donor; MUD, Matched-unrelated donor; MMUD, Mismatched-unrelated donor; CMV, Cytomegalovirus; IADL, Instrumental Activities of Daily Living; CrCl, Creatinine Clearance in milliliter/minute/1.73m2.
Discussion
Here we systemically examined pre-transplant assessment of individual geriatric domains from interdisciplinary clinical providers and found that geriatric vulnerabilities such as functional, mobility, mood, medications, cognition, and nutrition could be readily captured. We demonstrated the adverse impact of certain geriatric deficits, such as IADL impairment and mild renal dysfunction, on transplant outcomes. While this approach appeared feasible and identified significant number of deficits, we noted that the overall incidence of individual impairment appeared lower compared to previous studies [15-17]. This could be due to inadequate documentation or provider assessment versus utilizing patients’ self-report. In addition, it is possible that our patient population was a fitter cohort. Nevertheless, we demonstrated a similar association of IADL impairment with increased NRM and reduced survival. The prognostic importance of IADL impairment in geriatric oncology and hematology has been shown in many cancer types and settings, presumably due to its sensitivity in detecting early reduction of functional reserve (22). While no other GA variable was found to associate with adverse outcomes in this study, we lacked information on several important domains such as cognition, mobility, and social support, which could provide additional prognostic value [15-17]. We anticipate significant improvement in geriatric assessment and documentation with our increased effort to deliver geriatrics education to interdisciplinary clinical providers.
In our cohort of older patients with heterogeneous diagnosis, disease risk, conditioning regimen, donors, and post-transplant maintenance, we observed 3-year OS and PFS of 50% and 41%, respectively, as well as 2-year NRM of 25%. Our results compared favorably with several studies in this population of patients [3,11,23]. This is likely due to the inherent selection of a contemporary cohort of patients with improved supportive care, infection management, and modern transplant care. Even under these conditions, we showed here that geriatric vulnerabilities such as IADL impairment further risk stratified survival and supplemented traditional HCT-CI/age and DRI. These findings could provide an easily obtained, rapid risk assessment screening tool for older patients. Patients with significant impairment could then be referred to geriatrics to institute targeted interventions.
We identified several other factors including KPS and CMV serostatus which were associated with adverse outcomes. Importantly, we demonstrated that mild renal dysfunction was associated with inferior survival, even though none of these patients had creatinine above 2 as defined by the original HCT-CI [19,24]. These results, along with two recent reports [25,26], suggest that even mild renal dysfunction is poorly tolerated in the older patient population, thus defining a biochemical geriatric vulnerability.
Our study is not without limitations. First, given its retrospective design, the true incidence of individual geriatric deficit may be underestimated. second, the heterogeneity in diagnosis, donor type especially a small percentage of haploidentical donor, transplant platforms, and post-transplant care may introduce selection biases. Third, in addition to pre-transplant risk factors, allo-HCT itself may introduce additional risk factors for survival such as graft-versus-host disease and geriatric syndromes [27].Finally, this is a single institutional study which may not be applicable to other settings. We expect that the upcoming Blood and Marrow Transplant Clinical Trials Network (BMT CTN 1704) CHARM study will help address many of these issues ().
Nevertheless, our results have important implications for the selection of older patients for potential curative allo-HCT, and for management interventions in patients with geriatric deficits. It is conceivable that the true impact of GA on transplant outcomes should be evaluated by a prospectively designed, GA-adapted, interventional trial, where the transplant strategy is modified by GA-defined vulnerabilities, or non-transplant options are chosen for overly frail patients. It is also equally important to develop management strategies based on GA findings to improve outcomes. Since geriatric comorbidities are difficult to modify in the short-term, potential GA-directed intervention could include specific management assistance programs, subspecialty consultations, intensified rehabilitation, medication management, and psychosocial support to target other deficits [28].
In conclusion, we demonstrate the feasibility of collecting GA data retrospectively from pre-transplant interdisciplinary clinical provider assessments and illustrate the prognostic value of IADL impairment and mild renal dysfunction in older allo-HCT recipients. Our results add to the growing literature on the impact of geriatric vulnerabilities on HCT outcomes and raise the possibility of rapid geriatric screening by interdisciplinary clinical providers. Most importantly, our results provide an entry point for prospective, geriatric vulnerability-directed, interventional trials for older allo-HCT patients to improve their outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported in part by National Institutes of Health award numbers P01 CA23766 and NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Lin received support from the New York State Empire Clinical Research Investigator Program (ECRIP) and the Elsa U. Pardee Foundation for Cancer Research. This work was presented in part at the 60th American Society of Hematology Annual Meeting & Exposition, December 1-4, 2018, San Diego, CA; and at the 2019 American Society of Clinical Oncology (ASCO) Annual Meeting, May 31-June 4, 2019, Chicago, IL.
Footnotes
COMPETING INTERESTS:
Dr. Giralt reports research funding from Amgen, Actinium, Celgene, Johnson & Johnson, Miltenyi Biotec, and Takeda and serves as a consultant for Amgen, Actinium, Celgene, Johnson & Johnson, Jazz Pharmaceuticals, Miltenyi Biotec, Takeda, Novartis, Kite Pharma, and Spectrum Pharmaceuticals.
Dr. Perales reports honoraria from Abbvie, Bellicum, Bristol-Myers Squibb, Incyte, Merck, Novartis, Nektar Therapeutics, and Takeda. He serves on DSMBs for Servier and Medigene, and the scientific advisory boards of MolMed and NexImmune. He has also received research support for clinical trials from Incyte and Miltenyi Biotec.
Dr. Sauter has served as a paid consultant on advisory boards for: Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite, a Gilead Company and GSK. He has received research funds for investigator-initiated trials from: Juno Therapeutics and Sanofi-Genzyme.
All other authors declare no competing financial interests in relation to the work described.
REFERENCES:
- 1.Giralt SA. Hematopoietic cell transplantation for older adults, in Korc-Grodzicki B and Tew WP (ed): Handbooks of Geriatrics Oncology – Practical Guide to Caring for the Older Cancer Patient. New York, NY, Demos Medical Publishing, 2017, pp 241–254. [Google Scholar]
- 2.Pulte D, Jansen L, Castro FA, Brenner H. Changes in the survival of older patients with hematologic malignancies in the early 21st century. Cancer. 2016; 122: 2031–2040. [DOI] [PubMed] [Google Scholar]
- 3.McClune BL, Weisdorf DJ, Pedersen TL, da Silva G, Tallman MS, Sierra J et al. Effect of Age on Outcome of Reduced-Intensity Hematopoietic Cell Transplantation for Older Patients With Acute Myeloid Leukemia in First Complete Remission or With Myelodysplastic Syndrome. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2010; 28: 1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elsawy M, Sorror M. Up-to-date tools for risk assessment before allogeneic hematopoietic cell transplantation. Bone Marrow Transplantation 2016; 51: 1283–1300. [DOI] [PubMed] [Google Scholar]
- 5.Sorror ML, Storb RF, Sandmaier B, Maziarz RT, Pulsipher MA, Maris MB et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2014; 32: 3249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bejanyan N, Brunstein CG, Cao Q, Lazaryan A, Ustun C, Warlick ED et al. Predictive value of disease risk comorbidity index for overall survival after allogeneic hematopoietic transplantation. Blood Adv 2019; 3: 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaughn JE, Storer BE, Armand P, Raimondi R, Gibson C, Rambaldi A et al. Design and Validation of an Augmented Hematopoietic Cell Transplantation-Comorbidity Index Comprising Pretransplant Ferritin, Albumin, and Platelet Count for Prediction of Outcomes after Allogeneic Transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 2015; 21: 1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Artz AS, Logan B, Zhu X, Akpek G, Bufarull RM, Gupta V et al. The prognostic value of serum C-reactive protein, ferritin, and albumin prior to allogeneic transplantation for acute myeloid leukemia and myelodysplastic syndromes. Haematologica 2016; 101: 1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Souza A, Fretham C. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides, 2017. http://www.cibmtr.org. Accessed on April 15, 2019
- 10.Hahn T, McCarthy PL Jr, Hassebroek A, et al. Significant Improvement in Survival After Allogeneic Hematopoietic Cell Transplantation During a Period of Significantly Increased Use, Older Recipient Age, and Use of Unrelated Donors. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology 2013; 31: 2437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorror ML, Sandmaier B, Storer BE, Franke GN, Laport GG, Chauncey TR et al. Long-term Outcomes Among Older Patients Following Nonmyeloablative Conditioning and Allogeneic Hematopoietic Cell Transplantation for Advanced Hematologic Malignancies. JAMA 2011; 306: 1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy VE, Muffly LS. Assessment of older adult candidates for allogeneic hematopoietic cell transplantation: updates and remaining questions. Expert Rev Hematol 2019; 12: 99–106. [DOI] [PubMed] [Google Scholar]
- 13.Rosko A, Artz A. Aging: Treating the Older Patient. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 2017; 23: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Artz A Biologic vs physiologic age in the transplant candidate. Hematology 2016; 2016: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muffly LS, Kocherginsky M, Stock W, Chu Q, Bishop MR, Godley LA et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica 2014; 99: 1373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deschler B, Ihorst G, Schnitzler S, Bertz H, Finke J. Geriatric assessment and quality of life in older patients considered for allogeneic hematopoietic cell transplantation: a prospective risk factor and serial assessment analysis. Bone Marrow Transplantation 2018; 53: 565–575. [DOI] [PubMed] [Google Scholar]
- 17.Nawas MT, Andreadis C, Martin TG, Wolf JL, Ai WZ, Kaplan LD et al. Limitation in patient-reported function is associated with inferior survival in older adults undergoing autologous hematopoietic cell transplantation. Biology Blood Marrow Transplant J Am Soc Blood Marrow Transplant 2019. doi: 10.1016/j.bbmt.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 18.Lin RJ, Shahrokni A, Dahi PB, Jakubowski AA, Devlin S, Maloy MA et al. Pretransplant comprehensive geriatric assessment in hematopoietic cell transplantation: a single center experience. Bone Marrow Transplantation 2018; 53: 1184–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood 2013; 121: 2854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 2014; 123: 3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.the Panel B. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society 2015; 63: 2227–46. [DOI] [PubMed] [Google Scholar]
- 22.DuMontier C, Liu MA, Murillo A, Hshieh T, Houman J, Soiffer R, et al. Functional, survival, and care utilization among older adults with hematologic malignancies. Journal of the American Geriatrics Society 2019; 67: 889–897. [DOI] [PubMed] [Google Scholar]
- 23.Rashidi A, Ebadi M, Colditz GA, DiPersio JF. Outcomes of Allogeneic Stem Cell Transplantation in Elderly Patients with Acute Myeloid Leukemia: A Systematic Review and Meta-analysis. Biology of Blood and Marrow Transplantation 2016; 22: 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106: 2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fein JA, Shimoni A, Labopin M, Shem-Tov N, Yerushalmi R, Magen H et al. The impact of individual comorbidities on non-relapse mortality following allogeneic hematopoietic stem cell transplantation. Leukemia 2018; 32: 1787–1794. [DOI] [PubMed] [Google Scholar]
- 26.Shouval R, de Jong CN, Fein J, Broers A, Danylesko I, Shimoni A et al. Baseline Renal Function and Albumin are Powerful Predictors for Allogeneic Transplantation-Related Mortality. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 2018; 24: 1685–1691. [DOI] [PubMed] [Google Scholar]
- 27.Lin RJ, Hilden PD, Elko TA, Dahi PB, Shahrokni A, Jakubowski AA et al. Burden and impact of multifactorial geriatric syndromes in allogeneic hematopoietic cell transplantation for older adults. Blood Advances 2019; 3: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology 2018; 36: 2326–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.