Abstract
Solid organ transplant recipients are at risk for potentially life-threatening infections due to life-long immunosuppression. Vaccine-preventable infections result in graft injury, morbidity, mortality, and significantly increased medical costs. Unfortunately, the majority of transplant recipients continue to be under-immunized at the time of transplant and thereafter. Given the rising rates of vaccine hesitancy and refusal in the general population, transplant recipients can no longer rely on herd immunity to protect them from vaccine preventable infections. Novel tools are desperately needed to overcome transplant-specific immunization barriers to improve immunization rates in this high-risk population. Digital health technologies may offer a solution by addressing transplant specific barriers; specifically, providing accurate information about vaccine safety, efficacy, and timing in the pre- and post-transplant periods; making a complete immunization record universally available and easily accessible; enabling communication between patients and multiple providers; and providing automated vaccine reminders to both patients and providers when vaccines are due using transplant-specific immunization guidelines. Digital health has transformed health care by empowering patients with their own health information and connecting patients, their providers, and public health officials. In doing so, it offers a potential platform to address and overcome the problem of under-immunization in the transplant population.
Vaccine-Preventable Infections in the Solid Organ Transplant Population
Vaccine preventable infections (VPIs) including influenza, pneumococcus, herpes zoster (HZ), human papillomavirus (HPV), meningococcus, Hepatitis A, Hepatitis B, Haemophilus influenzae, pertussis, rotavirus, measles, mumps, varicella and rubella) are a common and significant problem across all solid organ transplant (SOT) recipients. In the adult SOT population, the traditional emphasis has been on prevention of influenza and invasive pneumococcal disease (IPD). The significant impact of influenza has been demonstrated across multiple studies. In a multicenter 5-year prospective study of 477 primarily adult SOT recipients with medically-attended influenza infection, 69% required hospitalization, 11% required admission to the intensive care unit (ICU), 8% required mechanical ventilation, and there was a 6-month mortality rate of 4.6%.(1) Likewise, in a multicenter cohort study of 237 SOT recipients with microbiologic confirmation of influenza during the 2009 H1N1 pandemic, 71% were hospitalized, 32% had pneumonia, 16% required admission to the ICU, and 4% died.(2) In comparison, across the general population in the United States, 1–2% of influenza cases require hospitalization and there is a 0.1–0.2% mortality rate.(3) Streptococcus pneumoniae is responsible for pneumonia, meningitis, bacteremia and other invasive manifestations in SOT recipients. In a pooled meta-analysis performed in 2017, the incidence of IPD in the SOT population was 465 per 100,000 (range 170–1272 per 100,000) compared to 10 per 100,000 (range 8–14 per 100,000) in a healthy control cohort.(4)
More recently, there has been a new emphasis on prevention of HZ and HPV. The incidence of HZ post-transplant ranges from 17–40 per 1000 person-years (depending on the type of transplant), compared to 9–11 per 1000 person-years in immunocompetent persons >50 years of age.(5–8) In general, the rates of HZ are highest in thoracic transplant recipients.(6) A study of 239 lung transplant recipients showed that 20% of patients had an episode of HZ by 5-years post-transplant.(9) HPV also occurs more frequently in the SOT population than in the general population. In a cohort study of 187,649 transplant recipients, 890 HPV related cancers were observed including 500 in situ and 390 invasive cancers.(10) Compared to the general population, transplant recipients had a 3–20 fold increased risk for in situ cancers and a 2–7 fold increased risk for invasive cancers excluding cervical cancer.(10)
SOT recipients are also at increased risk for meningococcus, Hepatitis A and Hepatitis B. In a study of 11,000 solid organ transplant recipients in the Netherlands, the annual incidence of bacterial meningitis was 7‐fold higher (95% confidence interval [CI] 2.94–17.02, P < 0.001) for renal transplant recipients as compared with the general population (9.56 [95% CI 3.98–22.96] vs. 1.35 [95% CI 1.28–1.43] per 100,000 patients per year).(11) Hepatitis A and B are a concern not only for those SOT recipients travelling to endemic areas, but are also locally significant in certain settings. Outbreaks of hepatitis A have been widespread in the United States since 2016, and persons with chronic liver disease are at greater risk of complications.(12) Although the overall U.S. incidence of hepatitis B has decreased since the 1980s likely due to universal childhood immunization; hepatitis B infections are increasing in certain groups such as injection drug users and immigrants from HBV-endemic countries.(13) Hemodialysis was a significant risk factor for de novo Hepatitis B infections in the 1970s however, this risk has been significantly reduced by vaccination and infection control practices such that the rate is now <1%.(14)
VPIs are also a common occurrence in pediatric SOT recipients. Pediatric recipients are likely at increased risk for VPIs compared to adult recipients as children may lack previous immunity from natural exposure and may not have had time to finish their primary immunization series by the time of transplant. In a recent study of nearly 7,000 pediatric SOT recipients from 45 tertiary care centers in the United States, 1 in 6 pediatric SOT recipients was hospitalized with a VPI in the first five years post-transplant.(15) Children who underwent transplant before two years of age and recipients of a lung, heart, intestinal or multi-visceral organ transplant were at increased risk for a hospitalization with a VPI. Compared to the annual rates of hospitalization in the general pediatric population, the rates of hospitalization in the pediatric SOT population were significantly higher; the rate was 50 times higher for influenza, 6 times higher for respiratory syncytial virus (RSV), 87 times greater for rotavirus, and 2 times higher for pneumococcus. These concerning rates do not even take into account the many transplant recipients who have VPIs that are managed in the outpatient setting.
VPIs result in significant morbidity, mortality and increased medical costs for pediatric SOT recipients. Initial transplant hospitalizations complicated by RSV and VPI had longer lengths of stay (55 days vs 16 days; P<.001) and increased hospitalization costs ($268,626 vs $148,128, p<.001) than those transplant hospitalizations not complicated by VPIs.(15) Hospitalizations for RSV and VPIs resulted in case-fatality rates greatly exceeding those rates in the general pediatric population; the death rate for RSV was 53 times greater than in the general pediatric population, for pneumococcus was 17 times greater, for rotavirus was 23 times greater and for influenza was 4 times greater.(15)
Under-Immunization in the Solid Organ Transplant Population
Despite a clear increased risk for vaccine-preventable infections, and multiple published recommendations from the Infectious Diseases Society of America and the American Society of Transplantation for “all solid organ transplant candidates to receive age-appropriate vaccines based on the Centers for Disease Control and Prevention Schedule”,(16, 17) and even in the face of frequent medical visits in the pre-transplant time period, the majority of transplant recipients are under-immunized.(18)
Immunization has generally been under-prioritized in the adult SOT population. A review of influenza vaccination coverage amongst 1800 adult kidney, liver and heart transplant recipients from 1995–2005 showed that only 45% of the study population was vaccinated against influenza during the full vaccination season preceding transplant and 52% received an influenza vaccination in the first full vaccination season post-transplant.(19) Although one would expect that kidney transplant candidates would be up-to-date on immunizations given their frequent pre-transplant exposure to healthcare providers during dialysis, in a cross-sectional point-prevalence study of 362 adult kidney transplant candidates, 75% of whom were receiving dialysis, immunization rates were as low as 36% for pneumococcus, 55% for influenza, 7% for zoster and 2.5% for tetanus.(20) The same study showed that African-American transplant candidates were less likely than whites to be vaccinated (OR 0.24, 95% CI 0.12–0.47) suggesting potential racial disparities in vaccination.
Preventative care including immunizations is generally a priority in pediatric medicine. However, studies have shown that the majority of pediatric transplant recipients are under-immunized. In a 12-year review of Swiss pediatric liver transplant recipients from 1990 to 2002, only 43% of children were up to date at the time of transplant for diphtheria, tetanus, acellular pertussis, and polio vaccines, 44% of children older than 12 months had received their measles‐mumps‐rubella (MMR) vaccines, 14% of children had received at least one dose of Hepatitis B vaccine, and less than 5% had received at least one dose of Hepatitis A vaccine.(21) Similarly, in a recent study of over 300 pediatric liver transplant recipients form 39 centers across North America, less than thirty percent were up-to-date on age-appropriate immunizations at the time of transplant.(18)
In the past, transplant recipients were conferred some degree of protection from VPIs secondary to “herd immunity” in which immunization of a significant portion of the healthy population provides some degree of protection for those members of society who have not or cannot develop immunity. However, with the rising rates of vaccine hesitancy and refusal amongst the general population,(22) herd immunity may no longer protect non-immune individuals such as unvaccinated transplant recipients. Sadly, we are in the midst of a national and international measles epidemic (23), therefore, it is critical that we ensure protection pre-transplant and examine pathways to select patients for live vaccination post-transplant.(24)
Successful immunization should not only be measured by the number of vaccines given, but also by the ability of the vaccine to protect against the intended infection. Clearly, immune responses to vaccines post-transplant and during end-stage organ disease are suboptimal and new strategies are required to enhance vaccine response. Further research is needed to better understand 1) the best method for monitoring immune response to vaccines, particularly in people on immunosuppressive medications, 2) what antibody levels equate with disease protection, 3) what is the best time post-transplant to administer needed immunizations, and 4) when to recheck immune response after administering vaccines post-transplant. Maintenance of vaccine protection becomes especially important when a transplant candidate is offered an organ from a PHS-high-risk donor.(25)
Barriers to Immunization in the Transplant Population
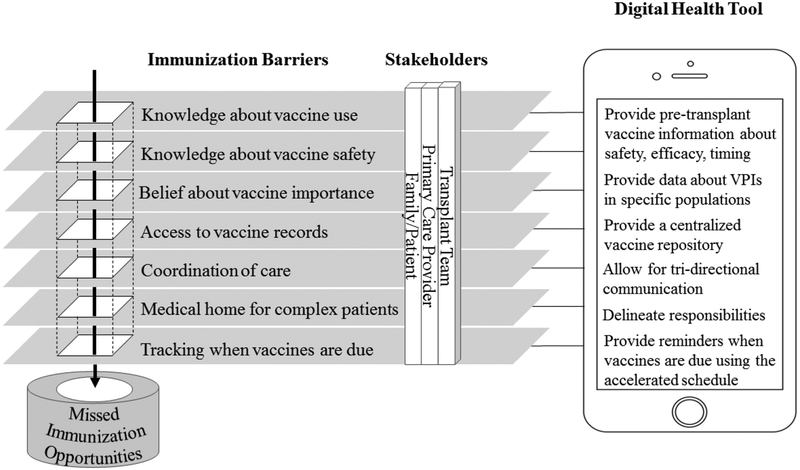
In addition to encountering common immunization barriers faced by healthy individuals (concern for vaccine pain, adverse events and side effects, moral or religious hesitations and lack of access to health care); transplant candidates also face additional immunization barriers secondary to their medical complexity, acutely ill-status, unique vaccine requirements and care by multiple subspecialists that may result in a medical home which exists outside of the primary care provider’s (PCP’s) office. Providers may choose to forego certain live immunizations (varicella and MMR) due to concern that their patient is too sick to wait the recommended four weeks between live vaccination and transplant. In a study of 53 pediatric liver transplant stakeholders including parents of liver transplant recipients, PCPs, transplant nurse coordinators, transplant infectious diseases physicians and pediatric transplant hepatologists, five major themes were identified as transplant-specific barriers to immunization: 1) inaccurate factual knowledge about safety, efficacy and timing of vaccines pre-transplant, 2) lack of coordination and communication between team members , 3) non-centralized location of immunization records, 4) difficulty tracking when immunizations are due and 5) immunization not being a priority.(26)
Despite the fact that multiple studies have shown that immunizations do not cause graft rejection and actually are associated with a lower risk of allograft loss and death following transplant,(27–29) in a 2009 survey of 181 kidney transplant providers, 10/181 (5%) still did not routinely recommend influenza vaccine secondary to beliefs that the vaccine lacked efficacy and continued safety concerns including concern for graft rejection.(30) In a separate study of 82 SOT recipients, the main refusal cited for influenza immunization refusal was fear of adverse events from the vaccine, specifically graft rejection.(31) Clearly, further education of patients and providers around vaccine safety and efficacy in the transplant population is needed.
Finally, if the number of vaccines is a barrier to immunization, perhaps certain vaccines need to be prioritized based on the individual transplant recipient. For example, meningococcal vaccine is a priority in patients who are starting eculizumab, and influenza vaccine is a priority during periods of high influenza circulation. Certain vaccines require multiple doses for optimal protection; therefore, programmatic initiatives are needed to ensure follow-up for future doses of a vaccine series or vaccines that need to be administerd annually.
Potential Solutions to Address Under-Immunization
Several techniques are used to increase immunization rates in the general population including education, advertising, immunization registries and electronic reminders. However, in addition to these, specific solutions for SOT are required. One potential solution to under-immunization of SOT candidates and recipients that has been trialed at certain institutions is supplying immunizations in subspecialty/transplant clinic to increase access to immunizations and decrease missed vaccine opportunities(32). However, in a study of 73 North American pediatric hepatologists representing 32 centers, the majority (94%) stated that they were not able to administer all childhood vaccines in liver/transplant clinic.(32) Reasons cited for not being able to administer vaccines in liver/transplant clinic included vaccines not available in clinic, perception that vaccine administration was the job of the PCP, nursing time to administer vaccines and vaccine cost and reimbursement. Another potential solution that some centers have utilized is a pre-transplant infectious disease consultation to help review immunization records and make personal recommendations for immunization catch-up or acceleration when appropriate. Although this has shown to improve vaccination uptake in some transplant populations;(33) such interventions require significant resources and may not be feasible at all centers.
Vaccination of patients attending dialysis units would be opportune and is recommended by the National Kidney Foundation(34); however, this is made complicated by the limited availability of certain vaccines in dialysis units eg, PCV13, and by reimbursement rates for vaccines administered in dialysis units. Standing orders policies in dialysis units are helpful and may improve vaccination rates; however, the percentage of patients actually receiving vaccines still remains low.(20, 35, 36) Incentivized programs are necessary to encourage and reward those dialysis centers with a high percentage of immunized individuals.
Digital health tools may be a more universally-available solution to help facilitate immunization delivery. Digital health tools (mobile-phone, electronic medical record and web-based) have shown initial success in creating population-based immunization registries, implementing vaccine reminder/recall systems, providing education about vaccines, providing automated clinical decision support alerts and increasing immunization rates.(37, 38) Apps can help create a secure, mobile accessible repository of vaccination information, accessible by everyone in the circle of care. In addition, mobile apps can be leveraged to offer other features such as real-time, remote reporting of VPIs and vaccine adverse events, and vaccine product bar code scanning.
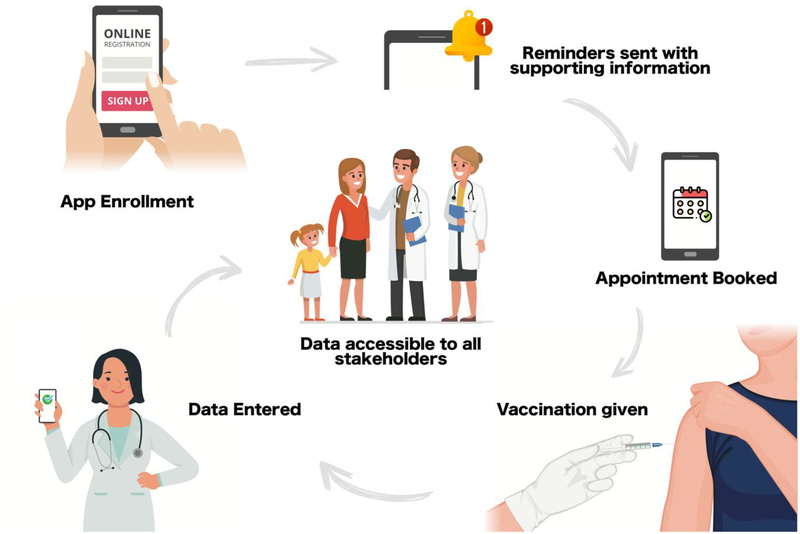
Future cloud-based tools could be developed that are tailored to the needs of the transplant population including education about pre- and post-transplant immunizations, communication portals for multiple providers, and individualized vaccine reminders. Transplant programs wishing to operationalize a tool would first need to identify the relevant vaccination schedule for the patients they care for (such as an accelerated schedule for pediatric transplant candidates). This would be entered by the software provider, or through an administrator portal to create the basis for the platform. The schedule will allow patients and providers to compare a patient vaccination history to the schedule and identify which vaccines are outstanding, known as “forecasting”. Second, the transplant program would determine the platform’s reminder logic. It could be as simple as “send a notification to all users one month prior to a vaccination’s due date”. Alternatively, it be customized such that patients/caregivers receive different content, at different times compared to providers. For example, the platform could send a reminder to the parent 1 month prior to a vaccination telling them to make an appointment with their PCP and a reminder to the PCP 2 weeks prior to the visit asking them to confirm that an appointment has been made. Once the vaccination schedule and reminder logic is inputted to the platform and tested, patient teams could enroll (Figure 2). At enrollment, vaccination history and patient specific requirements such as transplant type and date would be entered into the client side. The platform’s forecaster would generate a vaccination and notification schedule, delivering the reminders at the set timepoints incorporating entered data. When a new vaccination is administered (by the PCP for example), it would be entered into the platform and shared with all users. The forecaster would update and future messages would account for that new vaccine.
Figure 2:
A transplant digital health tool could be created to help increase immunization rates. At enrollment, vaccination history and patient specific requirements such as transplant type and date would be entered into the client side. The platform’s forecaster would generate a vaccination and notification schedule, delivering the reminders at the set timepoints incorporating entered data. When a new vaccination is administered (by the PCP for example), it would be entered into the platform and shared with all users. The forecaster would update and future messages would account for that new vaccine.
Through an agile development cycle these solutions can be developed, tested, iterated and then further evaluated. Mobile applications have the additional benefits of being flexible to incorporate new information and providing technology that can be designed to “speak” to several different EMR systems. Ideally, interoperability would be established between EMR systems and the platform to reduce the need for manual entry of data by all parties.
Conclusion
VPIs continue to negatively affect SOT recipients across all ages and graft types. Immunizations are a relatively inexpensive, safe way to provide some degree of protection against these infections. As a transplant community, we must begin to prioritize immunizations as an essential part of pre- and post-transplant care. We must take primary ownership for ensuring that our patients receive their needed immunizations. In order to do this, focused national vaccine-centric educational modules and novel tools are needed to inform all transplant providers about current immunization recommendations in the transplant population and to help providers appreciate the value of vaccines in prevention of disease post-transplant.(39) New tools are needed to help support transplant programs in keeping track of immunizations that have been given and immunizations that are due. National policies through the United Network of Organ Sharing are necessary to enforce that non-emergent transplant candidates are fully up-to-date for age on immunizations. Economic incentives should be provided to ensure “first-dollar coverage” (insurance coverage of vaccines without copayments or coinsurance costs for all ages) and to reward those transplant centers who are successfully immunizing all of their patients.(39)
Digital health tools could provide enhanced education and support for families and front-line providers in understanding safety, efficacy, and transplant-specific timing of immunizations. As new vaccines against bacterial and viral VPIs are currently in the pipeline for development (for example against Clostridium difficile, methicillin resistant staph aureus, cytomegalovirus and RSV), it will be essential for the transplant community to have mechanisms in place to disseminate and implement new immunizations.
Figure 1:
A digital health tool could help overcome barriers to pre-transplant immunization by providing education about immunizations in the setting of transplant, enhancing communication between providers and families, sending out computerized reminder when immunizations are due, and providing a central easily accessible repository for vaccines.
Acknowledgements/Funding:
AF is funded by a National Institutes of Health and National Center for Advancing Translational Sciences Clinical and Translation Science Award (KL2 TR002534), an Agency of Healthcare Research and Quality Award (K08 HS026510-01A1), and a Children’s Hospital Colorado Research Scholar Award. KA is supported through a Canadian Institute for Health Research (CIHR) Doctoral Research Award (CIHR-IRSC:0092002293). The authors wish to acknowledge Cameron Bell for his help in preparing Figure 2.
Disclosures: The authors have conflicts of interest to disclose as defined by the American Journal of Transplantation. DK has received clinical trials grant from Roche and GSK as well as honoraria from GSK and Sanofi. KA and KW are creators of the CANImmunize platform, a digital immunization tracking tool. CANImmunize was developed with financial contribution from the Public Health Agency of Canada.
Abbreviations
- HZ
Herpes zoster
- HPV
Human papillomavirus
- IIS
Immunization information system
- ICU
Intensive care unit
- IPV
Invasive pneumococcal disease
- MMR
Measles, mumps and rubella
- PCP
Primary care provider
- RSV
Respiratory syncytial virus
- SOT
Solid organ transplantation
- VPI
Vaccine preventable infection
Contributor Information
Amy G. Feldman, Section of Gastroenterology, Hepatology and Nutrition and the Digestive Health Institute, Adult and Child Consortium for Health Outcomes Research and Delivery Science (ACCORDS), Children’s Hospital Colorado and the University of Colorado School of Medicine, Aurora, CO.
Katherine Atkinson, Department of Public Health Sciences, Karolinska Institutet, Stockholm, Sweden 17177.
Kumanan Wilson, Department of Medicine, University of Ottawa, Ottawa Hospital Research Institute, The Ottawa Hospital, Civic Campus, Ottawa, ON.
Deepali Kumar, Transplant Infectious Diseases and Multi Organ Transplant Program, University Health Network, Toronto ON..
References
- 1.Kumar D, Ferreira VH, Blumberg E, Silveira F, Cordero E, Perez-Romero P et al. A 5-Year Prospective Multicenter Evaluation of Influenza Infection in Transplant Recipients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2018;67(9):1322–1329. [DOI] [PubMed] [Google Scholar]
- 2.Kumar D, Michaels MG, Morris MI, Green M, Avery RK, Liu C et al. Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: a multicentre cohort study. Lancet Infect Dis 2010;10(8):521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Disease Burden of Influenza. 2019; Available from: https://www.cdc.gov/flu/about/burden/index.html
- 4.van Aalst M, Lotsch F, Spijker R, van der Meer JTM, Langendam MW, Goorhuis A et al. Incidence of invasive pneumococcal disease in immunocompromised patients: A systematic review and meta-analysis. Travel Med Infect Dis 2018;24:89–100. [DOI] [PubMed] [Google Scholar]
- 5.Hamaguchi Y, Mori A, Uemura T, Ogawa K, Fujimoto Y, Okajima H et al. Incidence and risk factors for herpes zoster in patients undergoing liver transplantation. Transpl Infect Dis 2015;17(5):671–678. [DOI] [PubMed] [Google Scholar]
- 6.Pergam SA, Forsberg CW, Boeckh MJ, Maynard C, Limaye AP, Wald A et al. Herpes zoster incidence in a multicenter cohort of solid organ transplant recipients. Transpl Infect Dis 2011;13(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015;372(22):2087–2096. [DOI] [PubMed] [Google Scholar]
- 8.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005;352(22):2271–2284. [DOI] [PubMed] [Google Scholar]
- 9.Manuel O, Kumar D, Singer LG, Cobos I, Humar A. Incidence and clinical characteristics of herpes zoster after lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2008;27(1):11–16. [DOI] [PubMed] [Google Scholar]
- 10.Madeleine MM, Finch JL, Lynch CF, Goodman MT, Engels EA. HPV-related cancers after solid organ transplantation in the United States. Am J Transplant 2013;13(12):3202–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Veen KE, Brouwer MC, van der Ende A, van de Beek D. Bacterial meningitis in solid organ transplant recipients: a population-based prospective study. Transpl Infect Dis 2016;18(5):674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Widespread outbreaks of Hepatitis A Across the United States. August 9, 2019]; Available from: https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm
- 13.Hyun S, Lee S, Ventura WR, McMenamin J. Knowledge, Awareness, and Prevention of Hepatitis B Virus Infection Among Korean American Parents. J Immigr Minor Health 2018;20(4):943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onuigbo MA, Onuigbo NT. De novo HBV infection in a Mayo Clinic hemodialysis population: economic impact of reduced HBV testing and a call for changes in current US CDC guidelines on HBV testing protocols. Hemodial Int 2012;16 Suppl 1:S32–38. [DOI] [PubMed] [Google Scholar]
- 15.Feldman AG, Beaty BL, Curtis D, Juarez-Colunga E, Kempe A. Incidence of Hospitalization for Vaccine-Preventable Infections in Children Following Solid Organ Transplant and Associated Morbidity, Mortality, and Costs. JAMA Pediatr 2019;173(3):260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2014;58(3):e44–100. [DOI] [PubMed] [Google Scholar]
- 17.Danziger-Isakov L, Kumar D. Vaccination of solid organ transplant candidates and recipients: Guidelines from the American society of transplantation infectious diseases community of practice. Clinical transplantation 2019:e13563. [DOI] [PubMed] [Google Scholar]
- 18.Feldman AG, Sundaram SS, Beaty BL, Curtis DJ, Torres R, Kempe A. Immunization Rates at the Time of Pediatric Liver Transplant: A Prospective Multicenter Study through the Study of Liver Diseases. Hepatology 2018;68(Supplement 1):149A. [Google Scholar]
- 19.Harris K, Baggs J, Davis RL, Black S, Jackson LA, Mullooly JP et al. Influenza vaccination coverage among adult solid organ transplant recipients at three health maintenance organizations, 1995–2005. Vaccine 2009;27(17):2335–2341. [DOI] [PubMed] [Google Scholar]
- 20.Lee DH, Boyle SM, Malat G, Sharma A, Bias T, Doyle AM. Low rates of vaccination in listed kidney transplant candidates. Transpl Infect Dis 2015. [DOI] [PubMed] [Google Scholar]
- 21.Diana A, Posfay-Barbe KM, Belli DC, Siegrist CA. Vaccine-induced immunity in children after orthotopic liver transplantation: a 12-yr review of the Swiss national reference center. Pediatric transplantation 2007;11(1):31–37. [DOI] [PubMed] [Google Scholar]
- 22.Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kang Y. Vaccination Coverage Among Children Aged 19–35 Months — United States, 2017. MMWR Morb Mortal Wkly Rep 2018;67:1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Measles cases in 2018. Available from: https://www.cdc.gov/measles/cases-outbreaks.html
- 24.Pittet LF, Verolet CM, McLin VA, Wildhaber BE, Rodriguez M, Cherpillod P et al. Multimodal safety assessment of measles-mumps-rubella vaccination after pediatric liver transplantation. Am J Transplant 2019;19(3):844–854. [DOI] [PubMed] [Google Scholar]
- 25.Volk ML, Wilk AR, Wolfe C, Kaul DR. The “PHS Increased Risk” Label Is Associated With Nonutilization of Hundreds of Organs per Year. Transplantation 2017;101(7):1666–1669. [DOI] [PubMed] [Google Scholar]
- 26.Feldman A, Marsh R, Kempe A, Morris M. Barriers to Pre-transplant Immunization: A Qualitative Study of Pediatric Liver-transplant Stakeholders In: American Transplant Congress; 2019; Boston, MA; 2019. [Google Scholar]
- 27.Kumar D, Blumberg EA, Danziger-Isakov L, Kotton CN, Halasa NB, Ison MG et al. Influenza vaccination in the organ transplant recipient: review and summary recommendations. Am J Transplant 2011;11(10):2020–2030. [DOI] [PubMed] [Google Scholar]
- 28.White-Williams C, Brown R, Kirklin J, St Clair K, Keck S, O’Donnell J et al. Improving clinical practice: should we give influenza vaccinations to heart transplant patients? The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2006;25(3):320–323. [DOI] [PubMed] [Google Scholar]
- 29.Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011;6(5):1192–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chon WJ, Kadambi PV, Harland RC, Thistlethwaite JR, West BL, Udani S et al. Changing attitudes toward influenza vaccination in U.S. Kidney transplant programs over the past decade. Clin J Am Soc Nephrol 2010;5(9):1637–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Restivo V, Vizzini G, Mularoni A, Di Benedetto C, Gioe SM, Vitale F. Determinants of influenza vaccination among solid organ transplant recipients attending Sicilian reference center. Hum Vaccin Immunother 2017;13(2):346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feldman AG, Kempe A, Beaty BL, Sundaram SS, Studies of Pediatric Liver Transplantation Research G. Immunization practices among pediatric transplant hepatologists. Pediatric transplantation 2016;20(8):1038–1044. [DOI] [PubMed] [Google Scholar]
- 33.Kasper AK, Pallotta AM, Kovacs CS, Spinner ML. Infectious diseases consult improves vaccination adherence in kidney transplant candidates. Vaccine 2018;36(34):5112–5115. [DOI] [PubMed] [Google Scholar]
- 34.Vaccines and Dialysis: What You Need to Know. Available from: https://www.kidney.org/sites/default/files/PatBro_Vaccines_and_%20Dialysis.pdf
- 35.Bond TC, Patel PR, Krisher J, Sauls L, Deane J, Strott K et al. Association of standing-order policies with vaccination rates in dialysis clinics: a US-based cross-sectional study. Am J Kidney Dis 2009;54(1):86–94. [DOI] [PubMed] [Google Scholar]
- 36.Duval L, George C, Hedrick N, Woodruff S, Kleinpeter MA. Network 13 partnership to improve the influenza, pneumococcal pneumonia, and hepatitis B vaccination rates among dialysis patients. Adv Perit Dial 2011;27:106–111. [PubMed] [Google Scholar]
- 37.Wilson K, Atkinson KM, Westeinde J. Apps for immunization: Leveraging mobile devices to place the individual at the center of care. Hum Vaccin Immunother 2015;11(10):2395–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szilagyi PG, Bordley C, Vann JC, Chelminski A, Kraus RM, Margolis PA et al. Effect of patient reminder/recall interventions on immunization rates: A review. Jama 2000;284(14):1820–1827. [DOI] [PubMed] [Google Scholar]
- 39.Recommendations for Incentivizing the Development of Vaccines, Diagnostics, and Therapeutics to Combat Antibiotic-Resistance. In: Bacteria PACoCA-R, (ed). 2017. [Google Scholar]