Abstract
Objective:
To investigate associations of lifetime traumatic brain injury (LT-TBI) prior to an index deployment, and/or deployment-acquired TBI (DA-TBI), with post-deployment binge and heavy drinking.
Setting:
Soldiers from 3 Brigade Combat Teams deployed to Afghanistan in 2012.
Participants:
4,645 Soldiers who participated in the Army STARRS Pre/Post Deployment Study and completed 4 assessments: T0 (1–2 months pre-deployment), T1 (upon return to U.S.), T2 (3-months post-deployment), and T3 (9-months post-deployment).
Design:
Prospective, longitudinal study controlling for baseline binge drinking.
Main Measures:
Self-reported past month binge drinking (5+ alcoholic beverages on the same day) and past month heavy drinking (binge drinking at least weekly) at T2 and T3.
Results:
34.3% screened positive for LT-TBI, and 19.2% screened positive for DA-TBI. At T2 only, LT-TBI, but not DA-TBI, was associated with increased odds of binge drinking [adjusted odds ratio (AOR) = 1.39, 95% CI 1.20–1.60, p < .001] and heavy drinking [AOR = 1.28, 95% CI 1.09–1.49, p = .007]. Among the subgroup with LT-TBI, also having DA-TBI was associated with increased risk of heavy drinking at T3 [AOR = 1.42. 95% CI 1.03–1.95, p = 0.047].
Conclusion:
Routine screening for LT-TBI may help target efforts to prevent alcohol misuse among military members.
Keywords: traumatic brain injury, military, post-deployment, binge drinking, heavy drinking
INTRODUCTION
There is a strong neurobehavioral basis for traumatic brain injury (TBI) leading to alcohol misuse. Wherever else there may be damage to the brain, there is frequently damage in the frontal areas, which are critical to the executive functions that regulate thinking, behavior, and emotional expression.1,2 Executive function weaknesses may increase vulnerability to alcohol misuse due to multiple factors, including diminished social connectedness, poor insight into consequences, and impaired impulse control.3,4 Moreover, there are neurochemical abnormalities that may contribute to this predisposition to alcohol misuse after TBI. Risk for alcohol use disorders also arises from dysfunction of dopaminergic pathways,5 particularly those sub-serving the prefrontal cortex, a common consequence of TBI in animal studies,6,7 although the evidence in humans is less extensive.8 Thus, whether due to neurobehavioral or neurochemical influences, TBI may predispose to later alcohol misuse.
Over the past decade, studies of military members and veterans who had deployed to Afghanistan or Iraq have found that alcohol misuse is prevalent after experiencing a deployment-acquired TBI (DA-TBI).9–12 Experiencing a DA-TBI has been associated with increased risk for post-deployment alcohol misuse,11 binge and heavy drinking (i.e., binge drinking at least weekly),9,10 and receiving an alcohol or drug-related diagnosis in the Veterans Health Administration.12 Furthermore, even though one study found that the association between DA-TBI and post-deployment problem alcohol use became insignificant after controlling for posttraumatic stress disorder (PTSD),13 other studies have found that the relationship between DA-TBI and increased post-deployment binge and heavy drinking was independent of post-deployment mental health problems such as PTSD and depression.10,14 Yet, previous studies have not explored the role of pre-deployment risk factors for post-deployment alcohol misuse (e.g., lifetime TBI (LT-TBI), pre-deployment binge drinking, or pre-deployment mental disorders).
There is growing evidence that TBI in childhood may be particularly associated with substance use in adolescents and adults. McKinlay and colleagues found that children under 6 who had been hospitalized with a mild TBI were more likely to develop substance misuse problems in early adolescence and adulthood than children without a TBI.15 Ilie and colleagues reported a significantly increased risk of alcohol and other drug misuse among adolescents who reported at least one TBI, although temporal onset could not be determined.16 A birth cohort study conducted in Southwest England found that by age 17, children with a history of TBI were more likely to have an alcohol use disorder than either the general population or those with an orthopedic injury. These comparisons were adjusted for pre-birth socio-demographic factors, family environment, parenting style and history of criminal activity.17 Together, these epidemiological studies suggest a relationship between TBI experienced in childhood or adolescence with future alcohol misuse. Animal studies have also found associations between juvenile TBI and adult preference for alcohol,18 which may have multiple pathways of action.3 The association of childhood TBI with adult alcohol misuse has not been studied among military samples.
The purpose of this study was to increase knowledge about the association of DA-TBI with post-deployment alcohol misuse by examining the role of pre-deployment risk factors. Using data from the Army STARRS Pre/Post Deployment Study (PPDS),19 we examined the associations of LT-TBI and DA-TBI (and their interaction) with binge and heavy drinking at 3 and 9 months post-deployment, controlling for pre-deployment binge drinking, pre-deployment lifetime mental disorder, deployment stressors, and socio-demographic characteristics. Additionally, we investigated whether the associations identified between LT-TBI and post-deployment binge and heavy drinking varied by age of first TBI.
METHODS
Participants and Procedures
The PPDS is a multi-wave panel survey of Regular Army soldiers from three US Army Brigade Combat Teams (BCTs).19,20 Baseline assessment was conducted 1–2 months before the BCTs deployed to Afghanistan in 2012 (T0), referred to as the index deployment. Post-deployment assessment occurred approximately 1 month (T1), 3 months (T2), and 9 months (T3) after return from the index deployment. Respondents provided written, informed consent to participate in the surveys. Procedures were approved by the Human Subjects Committees of Army STARRS collaborating organizations.
Among soldiers who were present for duty at T0 (N=9949), 95.3% consented to participate in the baseline PPDS survey (n=9488) and 86.0% completed core sections of the survey and consented to Army and Department of Defense (DoD) record linkage (n=8558). Within the latter group, 7742 deployed with their BCTs and were eligible for inclusion in the current study. The sample was restricted to the 60.0% of eligible soldiers who completed all 3 follow-up surveys (n=4645). Of those with complete follow-up data, 153 were missing data on one or more predictor variables, resulting in a sample size of 4,492 for the main analysis (refer to Supplemental Digital Content for a diagram illustrating the selection of the analysis sample).
Combined weights were applied in the analysis, which incorporated (1) a propensity-based adjustment for baseline attrition due to incomplete surveys and inability to link to Army/DoD administrative data; (2) post-stratification of the propensity weights to socio-demographic and military career characteristics of all soldiers in the three combined BCTs that deployed to Afghanistan after T0; and (3) a propensity-based attrition adjustment to account for loss of participants due to incomplete data in one or more of the follow-up waves.20
Measures
More detail on study measures described below is available by viewing the Army STARRS PPDS questionnaires at http://starrs-ls.org.21
Drinking Outcome Measures
The dependent variables were past-month binge drinking (BD) and past-month heavy drinking (HD) at T2 and T3. The assessment of alcohol use in the PPDS surveys was based on items adapted from the Composite International Diagnostic Interview Screening Scales (CIDI-SC).22. Soldiers were asked “How often in the past 30 days did you drink 5 or more drinks of alcohol on the same day?” Response options were: never, less than one day a week, 1–2 days a week, 3–4 days a week, and every or nearly every day, and were coded 0–4 for analyses. The binary BD and HD variables were based on respondents’ ratings of this binge frequency item. The frequency thresholds for BD (“less than one day a week) and HD (“1–2 days a week” or greater) were selected to maximize consistency with the Substance Abuse and Mental Health Services Administration (SAMHSA) definitions of BD and HD.23,24 Thus, binge drinking was defined as 5 or more alcoholic beverages on the same day at least once in the past 30 days, and heavy drinking was defined as binge drinking at least 1 day a week during the preceding 30 days.
Key Independent Variables – TBI
Pre-deployment lifetime TBI (LT-TBI) was assessed at T0. A restricted definition of pre-deployment LT-TBI, which required endorsement of loss of consciousness (LOC), was used to mitigate concerns about the validity of retrospective self-report of details pertaining to older injuries.25 Regarding history of TBI with LOC, respondents were asked how many times in their lives (including childhood and adulthood) they had experienced a head, neck, or blast injury that led to either: a LOC (knocked you out) for less than 30 minutes; between 30 minutes and 24 hours; or more than 24 hours. LT-TBI was defined as endorsement (yes/no) of 1 or more head, neck, or blast injuries that led to LOC of any duration. Soldiers were also asked how old they were at the time of their first LT-TBI. Age of first LT-TBI was defined as the age at which the earliest TBI with LOC occurred and categorized as <13, 13–17, 18+, or never.
Deployment-acquired TBI (DA-TBI) was assessed at T1 to screen for TBI that occurred during the index deployment, to be consistent with the Assistant Secretary of Defense’s severity definitions for TBI which ensured consistency with guidelines from other medical groups (e.g., Centers for Disease Control and Prevention, World Health Organization, National Institutes of Health).26,27 Respondents indicated how many times they had experienced head, neck, or blast injuries that led to an: (1) alteration of consciousness (AOC; didn’t knock you out but caused you to be dazed or “see stars”); (2) LOC (knocked you out) for <30 minutes; (3) LOC for 30 minutes or more; (4) posttraumatic amnesia (PTA; caused you to have a lapse in memory of events before, during, or after the injury) for less than 30 minutes; and (5) PTA for 30 minutes or more. DA-TBI was defined as any endorsement (yes/no) of 1 or more head, neck, or blast injuries during the index deployment that led to AOC, LOC, or PTA of any duration. This definition encompasses TBI of any severity, including all injuries that would be recognized as mild TBI using the American Congress of Rehabilitation Medicine’s definition.28 Preliminary analyses yielded no evidence of an association between probable severity of DA-TBI with post-deployment BD or HD; thus, DA-TBI was dichotomized (yes/no) in all analyses.
Other Independent Variables Assessed at T0
Past-month pre-deployment binge drinking was measured at T0 (yes/no) and was included in all models to account for the effects of baseline alcohol misuse. Pre-deployment lifetime mental disorders (LT-MD) were assessed with the self-administered CIDI-SC22 and a 6-item screening version of the PTSD Checklist (PCL).29 CIDI-SC/PCL diagnoses were validated against structured clinical interviews in the Army STARRS clinical reappraisal study.30 Respondents who met criteria for 1 or more of these diagnoses were considered to have a LT-MD: major depressive disorder, mania/hypomania, generalized anxiety disorder, panic disorder, PTSD, intermittent explosive disorder, conduct disorder, oppositional defiant disorder, alcohol or other substance use disorder, and adult attention deficit-hyperactivity disorder (which was required to be symptomatic during the preceding 6 months).
Other Independent Variables Assessed at T1
Posttraumatic stress symptoms experienced during the index deployment were assessed using 5 items that assessed repetitive, disturbing memories of stressful experiences; physical reactions to reminders of stressful experiences; feeling as if one’s future would be cut short; difficulty concentrating; and feeling jumpy or easily startled. Respondents’ ratings of the 5 items on a 5-point Likert scale (not at all to extremely) were summed to create a total PTSD symptom severity score (theoretical range=0–20; Cronbach’s α=.84).23 Combat/deployment stress was quantified using a Deployment Stress Scale (theoretical range=0–16) that reflects overall exposure to stressful or traumatic events during the index deployment (e.g., firing at the enemy/taking enemy fire; having members of one’s unit seriously wounded or killed).23 Personal life stress was assessed with the Personal Life Stress scale, based on 5 items which evaluated severity of stress arising from financial matters, romantic relationships, legal problems, family relationships, and problems experienced by loved ones during the index deployment.23 Respondents’ ratings of each of these items on a 5-point Likert scale (none to very severe) were summed for a total score (theoretical range=0–20; Cronbach’s α=.76). To facilitate interpretation of results, raw scores from the PTSD symptom severity, combat/deployment stress, and personal life stress scales were standardized prior to regression analysis.
Socio-demographic and Military Service Covariates – Assessed at T0
Socio-demographic variables included sex, age, race, ethnicity, marital status, and educational attainment. Military service characteristics included BCT and number of prior deployments (0, 1, or 2 or more).
Data Analysis
Weights-adjusted multivariable logistic regression models were fit to estimate associations of LT-TBI, DA-TBI, and the interaction of LT-TBI x DA-TBI with BD and HD at T2 and at T3. Models adjusted for pre-deployment BD, pre-deployment LT-MD, combat/deployment stress severity, personal life stress during deployment, PTSD symptoms during deployment, and socio-demographic and military service characteristics. When the interaction term was non-significant, it was dropped from the model and main effects results were reported. For sensitivity analyses, models were re-run using categorical age groupings of when the first TBI with LOC occurred.
PPDS data are clustered (by BCT and administration session) and weighted; thus, the design-based Taylor series linearization method was used to estimate standard errors. Multivariable significance was examined using design-based Wald Χ2 tests. Two-tailed p<.05 was considered significant. Analyses were conducted using R Version 3.1.3.31
RESULTS
Study Population
Weights-adjusted characteristics of the sample have been published previously,26 indicating that the sample was predominantly male (94.7%), and white (71.8%), with approximately 16.1% of soldiers identifying as Hispanic. The majority of the sample was married (57.6%), had a high school degree without a college degree (68.9%), and a mean age of 26.9. Almost half of the sample (44.8%) indicated that the index deployment was their first.
Descriptive Findings
Previous analyses revealed that self-reported BD held constant from pre-deployment to 3-months postdeployment at 52.5%, and decreased to 41.3% at 9-months postdeployment.23 A little over 23% of PPDS soldiers reported HD pre-deployment, which increased slightly at 3-months post-deployment (26.1%) and decreased back to pre-deployment levels at 9-months post-deployment (22.3%).23
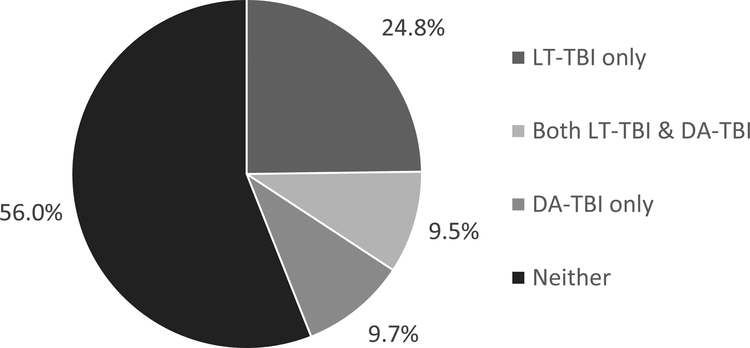
Among the PPDS sample, 1551 soldiers (34.3%) reported pre-deployment LT-TBI with LOC. Most only reported LT-TBI with LOC < 30 minutes (29.1%); prevalence of LT-TBI with LOC ≥ 30 minutes was substantially lower at 5.2%. 898 soldiers (19.2%) reported DA-TBI that occurred during the index deployment (Table 1). A previous report indicated that prevalence of probable very mild (AOC only), mild, and moderate-to-severe DA-TBI was 13.2%, 4.8%, and 1.2%, respectively.26 Over half of the sample (56.0%) had neither LT-TBI nor DA-TBI, 24.8% reported LT-TBI only, 9.7% had DA-TBI only, and 9.5% had both LT-TBI and DA-TBI (Figure 1).
Table 1:
Bivariate associations of pre-deployment factors and deployment-acquired TBI with binge drinking and heavy drinking at T2 and T3 among Army STARRS Pre/Post Deployment Survey respondents (n=4,645) weighted percentagesa
| Size of sub-group n (weighted percent) |
Drinking Outcomes at T2 | Drinking Outcomes at T3 | |||
|---|---|---|---|---|---|
| Binge Drinking n = 2438 (52.5%) |
Heavy Drinking n = 1230 (26.1%) |
Binge Drinking n = 1924 (41.3%) |
Heavy Drinking n = 1052 (22.3%) |
||
| Lifetime TBIb at T0 | 1551 (34.3%) | 958 (62.0%)*** | 507 (32.3%)*** | 741 (47.9%)*** | 397 (25.3%)*** |
| Lifetime Mental Health Diagnosis at T0 | 1982 (43.4%) | 1201 (61.0%)*** | 677 (34.0%)*** | 962 (48.5%)*** | 572 (28.6%)*** |
| Past-month Binge Drinking at T0 | 2418 (52.5%) | 1776 (73.4%)*** | 987 (40.5%)*** | 1389 (57.7%)*** | 783 (32.2%)*** |
| Deployment-acquired TBIc at T1 | 898 (19.2%) | 494 (55.4%) | 271 (30.0%)** | 423 (46.6%)*** | 246 (27.2%)*** |
Weighted percentages are shown to display policy relevant findings. The table shows unweighted numbers. Chi-square test significance are displayed for each bivariate association.
Lifetime TBI was defined as at least 1 TBI with a loss of consciousness prior to the index deployment.
DA-TBI was defined as any endorsement (yes/no) of 1 or more head, neck, or blast injuries during the index deployment that led to alteration of consciousness, loss of consciousness, or posttraumatic amnesia of any duration.
p≤.05;
p≤.01;
p≤.001
Note. TBI = traumatic brain injury. The analysis sample is comprised of baseline Pre/Post Deployment Survey respondents who deployed to Afghanistan and completed all follow-up surveys. Lifetime TBI, lifetime mental health diagnosis (includes alcohol or substance use disorder), and past-month binge drinking were assessed 1–2 months prior to the index deployment (T0). Deployment-acquired TBI was assessed within one month of return from deployment (T1). Drinking outcomes were assessed 3 months (T2) and 9 months (T3) after return from deployment.
Figure 1.
Weighted Prevalence of Lifetime-TBI and Deployment-Acquired TBI in the PPDS sample
Abbreviations and definitions:
LT-TBI = Lifetime traumatic brain injury (TBI) was defined as at least 1 TBI with a loss of consciousness prior to the index deployment (reported at T0).
DA-TBI = Deployment-acquired TBI was defined as any endorsement of 1 or more head, neck, or blast injuries during the index deployment that led to alteration of consciousness, loss of consciousness, or posttraumatic amnesia of any duration (reported at T1).
Table 1 displays the weighted bivariate associations of LT-TBI, DA-TBI, and pre-deployment factors with past-month BD and HD at T2 and T3. Soldiers with LT-TBI with LOC exhibited significantly higher rates of both binge and heavy drinking at T2 and T3 compared to soldiers with no LT-TBI. Soldiers who reported DA-TBI exhibited significantly higher rates of BD at T3, and HD at T2 and T3, compared to soldiers without DA-TBI. The highest rates of BD and HD at T2 and T3 were among soldiers who reported pre-deployment BD, compared to soldiers who did not report pre-deployment BD.
Multivariable Models of Binge Drinking and Heavy Drinking at T2 and T3
LT-TBI with LOC was associated with increased odds of BD and increased odds of HD at T2, compared to no LT-TBI (Table 2). LT-TBI was not associated with BD or HD at T3. Pre-deployment BD had the largest associations in the multivariable models for BD and HD at T2 and T3. For instance, pre-deployment BD was associated with more than six times the odds of BD at T2 and more than five times the odds of HD at T2, relative to no pre-deployment BD. DA-TBI was not significantly associated with drinking outcomes at T2 or T3. The interaction of LT-TBI and DA-TBI was not significantly associated with the outcomes, yet did approach significance in the model of HD at T3 (p = .07).
Table 2:
Adjusted odds ratios of binge drinking and heavy drinking at 3 months (T2) and 9 months (T3) post-deployment among Army STARRS Pre/Post Deployment Survey respondents (n=4,492)
| Adjusted Odds-Ratio (95% CI) | ||||
|---|---|---|---|---|
| Drinking Outcomes at T2 | Drinking Outcomes at T3 | |||
| Binge Drinking | Heavy Drinking | Binge Drinking | Heavy Drinking | |
| Lifetime TBIa at T0 | 1.39 (1.20–1.60)*** | 1.28 (1.09–1.49)** | 1.16 (0.98–1.38) | 0.91 (0.74–1.10) |
| Lifetime Mental Health Diagnosisb at T0 | 1.28 (1.07–1.55)* | 1.49 (1.26–1.76)*** | 1.32 (1.10–1.59)** | 1.55 (1.29–1.85)*** |
| Past-month Binge Drinking at T0 | 6.01 (4.95–7.28)*** | 5.37 (4.38–6.58)*** | 4.07 (3.46–4.79)*** | 3.20 (2.64–3.87)*** |
| Deployment-acquired TBIc at T1 | 0.83 (0.67–1.02) | 0.90 (0.74–1.10) | 0.99 (0.83–1.18) | 0.92 (0.71–1.20) |
|
Interaction Term: Lifetime TBI & Deployment-acquired TBI |
N/Ad | N/A | N/A | 1.45 (1.00–2.10)^ |
Note. CI=confidence interval; TBI=traumatic brain injury. Each column displays the results for a separate weights-adjusted logistic regression model. Models also adjusted for Brigade Combat Team, number of prior deployments, combat/deployment stress at T1, personal life stress at T1, posttraumatic stress severity at T1, age, gender, race, ethnicity, education, and marital status. T0 assessment occurred 1–2 months before deployment to Afghanistan. T1 assessment occurred within 1 month of return from deployment. Due to missing demographic and/or T1 data, 153 eligible subjects were excluded from the models.
p≤.001;
p≤.01;
p≤.05;
p=.07
Lifetime TBI was defined as at least 1 TBI with a loss of consciousness prior to the index deployment.
Includes alcohol or substance use disorder.
DA-TBI was defined as any endorsement (yes/no) of 1 or more head, neck, or blast injuries during the index deployment that led to alteration of consciousness, loss of consciousness, or posttraumatic amnesia of any duration.
N/A = not applicable because the interaction term was insignificant, therefore the results from the main effects models are presented.
Although the LT-TBI x DA-TBI effect on heavy drinking at T3 only approached significance, we pursued the subset analysis stratified by LT-TBI status to explore possible reasons for discrepancies between the current results and those of prior studies that found associations of DA-TBI with post-deployment drinking outcomes (see Supplemental Digital Content).9,10 We found that, among soldiers with LT-TBI with LOC, those with DA-TBI had significantly increased odds of HD at T3, compared to those without DA-TBI (AOR, 1.42; 95% CI, 1.03–1.95, p = .047). Among soldiers without LT-TBI, DA-TBI was not associated with HD at T3 (AOR, 0.88; 95% CI, 0.67–1.16, p = 0.38).
Sensitivity Analyses
We also examined the relationship of age of first LT-TBI with LOC with the drinking outcomes at T2 and T3 (Table 3). All of the TBI age-of-onset categories were associated with increased odds of BD at T2, compared to no LT-TBI. Even though the odds of BD at T2 were highest among soldiers with a first LT-TBI at age 12 or younger, the AOR was not statistically different from the other age-of-onset groups. With respect to HD at T2, only soldiers with a first LT-TBI at age 12 or younger had significantly increased odds compared to those with no LT-TBI. None of the TBI age-of-onset categories was significantly associated with drinking outcomes at T3, and DA-TBI remained non-significant in all models.
Table 3:
Adjusted odds ratios of binge and heavy drinking at 3 months (T2) and 9 months (T3) post-deployment examining the association of age of first lifetime TBI among Army STARRS Pre/Post Deployment Survey respondents (n=4,330)
| Adjusted Odds-Ratio (95% CI) | ||||
|---|---|---|---|---|
| Drinking Outcomes at T2 | Drinking Outcomes at T3 | |||
| Binge Drinking | Heavy Drinking | Binge Drinking | Heavy Drinking | |
| Lifetime TBIa at T0 (ref = no) | ||||
| First LT TBI age 12 or younger | 1.58 (1.22–2.05)** | 1.35 (1.10–1.65)* | 1.14 (0.88–1.48) | 1.08 (0.87–1.33) |
| First LT TBI age 13–17 | 1.28 (1.07–1.53)* | 1.27 (1.01–1.61) | 1.15 (0.95–1.40) | 1.01 (0.79–1.30) |
| First LT TBI age 18 or older | 1.43 (1.11–1.85)* | 1.17 (0.91–1.51) | 1.23 (0.90–1.67) | 0.78 (0.57–1.08) |
| Lifetime Mental Health Diagnosis at T0 | 1.28 (1.06–1.55)* | 1.50 (1.26–1.78)*** | 1.32 (1.10–1.60)* | 1.54 (1.27–1.87)*** |
| Past-month Binge Drinking at T0 | 5.99 (4.92–7.28)*** | 5.27 (4.30–6.46)*** | 4.08 (3.45–4.82)*** | 3.25 (2.64–4.01)*** |
| Deployment-acquired TBIb at T1 | 0.85 (0.68–1.05) | 0.89 (0.72–1.10) | 1.04 (0.87–1.25) | 1.11 (0.91–1.36) |
Note. CI=confidence interval; TBI=traumatic brain injury. Each column displays the results for a separate weights-adjusted logistic regression model. Models also adjusted for Brigade Combat Team, number of prior deployments, combat/deployment stress at T1, personal life stress at T1, posttraumatic stress severity at T1, age, gender, race, ethnicity, education, and marital status. T0 assessment occurred 1–2 months before deployment to Afghanistan. T1 assessment occurred within 1 month of return from deployment. A total of 315 eligible subjects were excluded from the models due to missing demographic and/or T1 data (n=153) or missing age-of-first TBI data (n=162).
p≤.05;
p≤.01;
p≤.001
Lifetime TBI was defined as at least 1 TBI with a loss of consciousness prior to the index deployment.
DA-TBI was defined as any endorsement (yes/no) of 1 or more head, neck, or blast injuries during the index deployment that led to alteration of consciousness, loss of consciousness, or posttraumatic amnesia of any duration.
DISCUSSION
Binge and heavy drinking can impede reintegration post-deployment and often lead to negative consequences, including impaired health and reduced force readiness.32,33 This prospective, longitudinal study is the first to describe the associations of both LT-TBI and DA-TBI with post-deployment binge and heavy drinking. This study improves upon previous studies9,10 by including controls for pre-deployment binge drinking and lifetime mental disorder (including substance use disorders).
Among the PPDS sample, 34.3% screened positive for LT-TBI with LOC prior to the index deployment. This estimate is higher than among non-military samples, in which approximately 20% of non-institutionalized adults have experienced at least one TBI with LOC during their lifetime.34,35 We note that more than half of the PPDS sample had been deployed prior to the index deployment, so this elevated estimate of LT-TBI may reflect previous DA-TBIs among this sample. Yet, evidence suggests that the majority of TBIs among military members occur during non-deployment periods.36 Because the PPDS instrument did not assess cause of injury, we do not know to what extent the LT-TBI estimate reflects TBIs that occurred during previous deployments, during military service while not deployed, or prior to joining the military. Previous estimates of LT-TBI among military members have been limited by small, convenience samples and inconsistent screening for complete lifetime history of TBI inclusive of non-deployment periods and prior to joining the military.37–40 We further note that these previous studies had samples with military members from other Services or multiple Services, while the PPDS sample was Army only. Similar to estimates of DA-TBI, we might expect prevalence of LT-TBI to vary by Service, yet this remains unknown.
Upon return from Afghanistan, 19.2% of soldiers in the PPDS sample screened positive for DA-TBI during the index deployment, which is similar to other studies that reported estimates of DA-TBI from the most recent deployment among soldiers.9,41,42 The fact that a significant proportion of military members preparing to deploy have already experienced at least one TBI with LOC raises the possibility that this history of TBI may interact with new head injuries and exacerbate or prolong symptoms.43
Multivariable models revealed that LT-TBI with LOC prior to the index deployment was significantly associated with increased odds of both BD and HD at 3-months post-deployment, yet was not associated with drinking outcomes at 9-months post-deployment. Unlike previous studies,9,10 DA-TBI was not associated with binge or heavy drinking at either post-deployment assessment. Further, DA-TBI remained non-significant even when we excluded LT-TBI from the models (data not shown), conflicting with results of previous studies.9,10 These discrepancies may be attributable to the inclusion of controls for pre-deployment BD and LT-MD in the current analysis, which were not applied in previous studies. Pre-deployment BD had the highest AORs in all models, and having a pre-deployment lifetime mental disorder was significantly associated with BD and HD in all models.
The interaction term for LT-TBI x DA-TBI was not significantly associated with the drinking outcomes, other than approaching significance when examining HD at T3 (p = .07). However, to reconcile the current results with those of prior studies9,10 that found associations between DA-TBI and post-deployment alcohol misuse,9,10 we ran parallel analyses stratifying the T3 HD model by LT-TBI status. These analyses revealed that soldiers with LT-TBI with LOC and recent DA-TBI had significantly increased odds of HD at T3, compared to soldiers without DA-TBI. This finding is in the same direction as two large, population-based studies that found an association between experiencing a DA-TBI on a recent deployment and increased odds of HD during the post-deployment year, controlling for comorbid post-deployment mental health problems.9,10 Results of our subset analysis raise the possibility that the association found in those prior studies was driven by DA-TBI that occurred against a backdrop of (unmeasured) LT-TBI. However, those previous studies also were limited by a lack of other pre-deployment measures; most notably, pre-deployment BD. They also assessed military members during earlier years of the conflicts (2007–2011), and one included military members from all Service branches (with only 37% Army) rather than Army only, which may reflect differences in exposure to TBIs and differences in types of combat exposures.9
Military operational tempo has shifted during the decade-long conflicts, and soldiers in the PPDS sample who deployed to Afghanistan in 2012 may have had different deployment experiences which influenced cumulative TBI burden or other factors influencing post-deployment drinking behaviors compared to prior studies. Furthermore, the PPDS study occurred after the implementation of a DoD policy44 in 2012 regarding management of mild TBI in deployed settings that rolled out incident-based screening for potentially concussive events and mandated rest in theater. Raised awareness in the U.S. Armed Forces about the potential deleterious effects of TBI, even when injuries were mild, along with the implementation of the DoD screening and early rest protocols, may have had effects that in turn influenced post-deployment drinking behaviors.
Sensitivity analyses were conducted to expand upon what is known from civilian studies,15–17,45 to examine if the age of first LT-TBI provides additional information about the relationships found in the main models. We did not find strong evidence of differential effects of LT-TBI on the drinking outcomes related to the age of first LT-TBI. However, the sensitivity analyses did suggest that the association of HD at T2 may have been largely attributable to those with a first LT-TBI prior to age 13. These results may be consistent with both animal and human data suggesting that a first TBI in childhood or adolescence may predispose to adult consequences, especially behaviors mediated by reward networks.3,6,18,46,47 The association of prior TBI with processing of rewards and consequences may also contribute to the significantly increased odds of suicide following a history of TBI,48–50 which would be further exacerbated by alcohol misuse. More research is needed to explore the impact of age of first LT-TBI with adult alcohol misuse.
There are several limitations to the current study. Similar to most other military studies, positive screens for both LT-TBI and DA-TBI were limited by reliance on retrospective self-report and were not verified with a clinically confirmed diagnosis nor captured via interview where recall could have been enhanced through systematic probes.51,52 Similarly, retrospective self-report of both BD and HD may have been vulnerable to recall or social desirability biases. Yet, social desirability biases for both TBI assessment and post-deployment drinking were likely reduced due to the confidential nature of the PPDS study. The pre-deployment LT-MD measure included a broad range of internalizing and externalizing disorders (including substance use disorders); and we did not examine to what extent those diagnostic entities may have been driving the significant AORs for LT-MD in the models. Despite use of weights to enhance generalizability to the larger population of deployed soldiers and to mitigate impacts of attrition,23 the sample may have been biased if soldiers separated from the military early or were unable to complete a follow-up assessment due to problems related to a TBI, post-deployment drinking, or other reasons impacting study outcomes. Lastly, study results may not be generalizable to military members from other Services, female military members, or civilians.
In conclusion, results from this study suggest that experiencing lifetime TBI with LOC prior to deployment is associated with increased odds of binge and heavy drinking at 3-months post-deployment among soldiers returning from an Afghanistan deployment. This relationship appeared more pronounced among soldiers with a first LT-TBI that occurred prior to age 13. Furthermore, among those with LT-TBI, also experiencing recent DA-TBI was associated with increased odds of heavy drinking at 9-months post-deployment. Taken together, these findings suggest that the DoD could reduce future harms associated with binge and heavy drinking by implementing evidence-based methods (i.e., screening and brief intervention, motivational interviewing) to identify and intervene early for alcohol misuse, particularly among military members with a history of TBI, whether or not it was incurred during deployment.53,54 Targeted alcohol screening for military members with a history of TBI could be improved if the DoD implemented universal screening for history of TBI when military members first join the military to gather baseline information about an individual’s lifetime history of TBI (e.g., number of injuries, age of first injury, severity). This baseline lifetime history of TBI could be integrated into electronic health databases to be accessible to clinicians, which would allow them to implement targeted alcohol screening, and to improve upon treatment of future head injuries by understanding a person’s lifetime history of TBI. Implementing this type of screening policy change would need to be evaluated in relation to possible barriers to obtaining a valid self-report of lifetime TBI (e.g., concerns of prospective military members regarding possible negative consequences of disclosing TBI history; limitations of retrospective self-report). Given the high prevalence of LT-TBI and DA-TBI among military members, future research is needed to explore how LT-TBI and DA-TBI, as well as cumulative burden of TBI, are associated with post-deployment unhealthy drinking behaviors, ideally using validated, structured clinical interviews to assess for history of TBI.51,55
Supplementary Material
Acknowledgments:
The Army STARRS Team consists of Co-Principal Investigators: Robert J. Ursano, MD (Uniformed Services University of the Health Sciences) and Murray B. Stein, MD, MPH (University of California San Diego and VA San Diego Healthcare System)
Site Principal Investigators: Steven Heeringa, PhD (University of Michigan), James Wagner, PhD (University of Michigan) and Ronald C. Kessler, PhD (Harvard Medical School)
Army liaison/consultant: Kenneth Cox, MD, MPH (US Army Public Health Center)
Other team members: Pablo A. Aliaga, MA (Uniformed Services University of the Health Sciences); COL David M. Benedek, MD (Uniformed Services University of the Health Sciences); Laura Campbell-Sills, PhD (University of California San Diego); Carol S. Fullerton, PhD (Uniformed Services University of the Health Sciences); Nancy Gebler, MA (University of Michigan); Robert K. Gifford, PhD (Uniformed Services University of the Health Sciences); Meredith House, BA (University of Michigan); Paul E. Hurwitz, MPH (Uniformed Services University of the Health Sciences); Sonia Jain, PhD (University of California San Diego); Tzu-Cheg Kao, PhD (Uniformed Services University of the Health Sciences); Lisa Lewandowski-Romps, PhD (University of Michigan); Holly Herberman Mash, PhD (Uniformed Services University of the Health Sciences); James E. McCarroll, PhD, MPH (Uniformed Services University of the Health Sciences); James A. Naifeh, PhD (Uniformed Services University of the Health Sciences); Tsz Hin Hinz Ng, MPH (Uniformed Services University of the Health Sciences); Matthew K. Nock, PhD (Harvard University); Nancy A. Sampson, BA (Harvard Medical School); CDR Patcho Santiago, MD, MPH (Uniformed Services University of the Health Sciences); LTC Gary H. Wynn, MD (Uniformed Services University of the Health Sciences); and Alan M. Zaslavsky, PhD (Harvard Medical School).
Conflicts of Interest and Source of Funding: Army STARRS was sponsored by the Department of the Army and funded under cooperative agreement number U01MH087981 with the U.S. Department of Health and Human Services, National Institutes of Health, and National Institute of Mental Health (NIH/NIMH). Subsequently, STARRS-LS was sponsored and funded by the Department of Defense (USUHS grant number HU0001–15-2-0004). Drs. Adams and Larson were supported in part by the National Center for Complementary and Integrative Health (NCCIH; R01 AT008404). Contents are solely the responsibility of the authors and do not necessarily represent the views of the Department of Health and Human Services, NIMH, NCCIH, NIH, the Veterans Administration, the Department of the Army, or the Department of Defense.
Disclosures: Dr. Stein has in the past three years been a consultant for Actelion, Aptinyx, Bionomics, Janssen, Jazz Pharmaceuticals, Neurocrine, Oxeia Biopharmaceuticals, Pfizer, and Resilience Therapeutics. In the past 3 years, Dr. Kessler received support for his epidemiological studies from Sanofi Aventis; was a consultant for Johnson & Johnson Wellness and Prevention, Sage Pharmaceuticals, Shire, Takeda; and served on an advisory board for the Johnson & Johnson Services Inc. Lake Nona Life Project. Kessler is a co-owner of DataStat, Inc., a market research firm that carries out healthcare research. The remaining authors have no disclosures.
Contributor Information
Rachel Sayko Adams, Institute for Behavioral Health, Heller School for Social Policy and Management, Brandeis University, Waltham, MA; VHA Rocky Mountain Mental Illness Research Education and Clinical Center, Aurora, CO.
Laura Campbell-Sills, Department of Psychiatry, University of California, San Diego, La Jolla, CA.
Murray B. Stein, Department of Psychiatry and Department of Family Medicine and Public Health, University of California, San Diego, La Jolla, CA; VA San Diego Healthcare System, San Diego, CA.
Xiaoying Sun, Department of Family Medicine and Public Health, University of California, San Diego, La Jolla, CA.
Mary Jo Larson, Institute for Behavioral Health, Heller School for Social Policy and Management, Brandeis University, Waltham, MA.
Ronald C. Kessler, Department of Health Care Policy, Harvard Medical School, Boston, MA.
Robert J. Ursano, Department of Psychiatry, Uniformed Services University of the Health Sciences, Bethesda, MD.
Sonia Jain, Department of Family Medicine and Public Health, University of California, San Diego, La Jolla, CA.
John D. Corrigan, Department of Physical Medicine & Rehabilitation, Wexner Medical Center, The Ohio State University, Columbus, OH.
REFERENCES
- 1.Bigler ED, Maxwell WL. Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging Behav. 2012;6(2):108–136. [DOI] [PubMed] [Google Scholar]
- 2.Stuss DT. Traumatic brain injury: Relation to executive dysfunction and the frontal lobes. Curr Opin Neurol. 2011;24(6):584–589. [DOI] [PubMed] [Google Scholar]
- 3.Weil ZM, Corrigan JD, Karelina K. Alcohol abuse after traumatic brain injury: Experimental and clinical evidence. Neuroscience and biobehavioral reviews. 2016;62:89–99. [DOI] [PubMed] [Google Scholar]
- 4.Corrigan JD, Mysiw WJ. Substance Misuse among Persons with TBI In: Nathan Zasler, Ross Zafonte, Douglas Katz, eds. Brain Injury Medicine. Second Edition ed2012. [Google Scholar]
- 5.Martinez D, Gil R, Slifstein M, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58(10):779–786. [DOI] [PubMed] [Google Scholar]
- 6.Wagner AK, Sokoloski JE, Ren D, et al. Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J Neurochem. 2005;95(2):457–465. [DOI] [PubMed] [Google Scholar]
- 7.Hutson CB, Lazo CR, Mortazavi F, Giza CC, Hovda D, Chesselet MF. Traumatic brain injury in adult rats causes progressive nigrostriatal dopaminergic cell loss and enhanced vulnerability to the pesticide paraquat. Journal of neurotrauma. 2011;28(9):1783–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnemiller E, Brenneis C, Wissel J, et al. Impaired dopaminergic neurotransmission in patients with traumatic brain injury: a SPECT study using 123I-beta-CIT and 123I-IBZM. Eur J Nucl Med. 2000;27(9):1410–1414. [DOI] [PubMed] [Google Scholar]
- 9.Adams RS, Larson MJ, Corrigan JD, Horgan CM, Williams TV. Frequent binge drinking after combat-acquired traumatic brain injury among active duty military personnel with a past year combat deployment. The Journal of Head Trauma Rehabilitation,. 2012;27(5):349–360, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams RS, Corrigan JD, Mohr BA, Williams TV, Larson MJ. Traumatic brain injury and post-deployment binge drinking among male and female Army active duty service members returning from Operation Enduring Freedom/Operation Iraqi Freedom. Journal of neurotrauma. 2017;34(7):1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rona RJ, Jones M, Fear NT, et al. Mild traumatic brain injury in UK military personnel returning from Afghanistan and Iraq: cohort and cross-sectional analyses. The Journal of Head Trauma Rehabilitation,. 2012;27(1):33–44. [DOI] [PubMed] [Google Scholar]
- 12.Carlson K, Nelson D, Orazem R, Nugent S, Cifu D, Sayer N. Psychiatric Diagnoses Among Iraq and Afghanistan War Veterans Screened for Deployment-Related Traumatic Brain Injury. Journal of traumatic stress. 2010;23(1):17–24. [DOI] [PubMed] [Google Scholar]
- 13.Polusny MA, Kehle SM, Nelson NW, Erbes CR, Arbisi PA, Thuras P. Longitudinal effects of mild traumatic brain injury and posttraumatic stress disorder comorbidity on postdeployment outcomes in national guard soldiers deployed to Iraq. Archives of General Psychiatry. 2011;68(1):79–89. [DOI] [PubMed] [Google Scholar]
- 14.Adams RS, Larson MJ, Corrigan JD, et al. Combat-acquired traumatic brain injury, posttraumatic stress disorder, and their relative associations with postdeployment binge drinking. Journal of Head Trauma Rehabilitation,. 2016;31(1):13–22, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKinlay A, Dalrymple-Alford JC, Horwood LJ, Fergusson DM. Long term psychosocial outcomes after mild head injury in early childhood. Journal of neurology, neurosurgery, and psychiatry. 2002;73(3):281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilie G, Adlaf EM, Mann RE, et al. Associations between a History of Traumatic Brain Injuries and Current Cigarette Smoking, Substance Use, and Elevated Psychological Distress in a Population Sample of Canadian Adults. Journal of neurotrauma. 2015;32(14):1130–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy E, Cohen M, Munafo M. Childhood Traumatic Brain Injury and the Associations With Risk Behavior in Adolescence and Young Adulthood: A Systematic Review. The Journal of head trauma rehabilitation. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weil ZM, Karelina K, Gaier KR, Corrigan TE, Corrigan JD. Juvenile Traumatic Brain Injury Increases Alcohol Consumption and Reward in Female Mice. Journal of neurotrauma. 2016;33(9):895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ursano RJ, Colpe LJ, Heeringa SG, et al. The Army study to assess risk and resilience in servicemembers (Army STARRS). Psychiatry. 2014;77(2):107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kessler RC, Heeringa SG, Colpe LJ, et al. Response bias, weighting adjustments, and design effects in the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). Int J Methods Psychiatr Res. 2013;22(4):288–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.STARRS-LS: Study to Assess Risk & Resilience in Servicemembers - Longitudinal Study. 2019; http://starrs-ls.org/#/. Accessed March 15, 2019.
- 22.Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int J Methods Psychiatr Res. 2004;13(2):93–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell-Sills L, Ursano RJ, Kessler RC, et al. Prospective risk factors for post-deployment heavy drinking and alcohol or substance use disorder among US Army soldiers. Psychological medicine. 2018;48(10):1624–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Center for Behavioral Health Statistics and Quality. 2016 National Survey on Drug Use and Health: Methodological summary and definitions. Rockville, MD: Substance Abuse and Mental Health Services Administration;2017. [Google Scholar]
- 25.McKinlay A, Corrigan JD, Bogner JA, Horwood LJ. Obtaining a History of Childhood Traumatic Brain Injury Using the Ohio State University TBI Identification Method to Elicit Adult Recall. The Journal of head trauma rehabilitation. 2017;32(6):E24–E28. [DOI] [PubMed] [Google Scholar]
- 26.Stein MB, Kessler RC, Heeringa SG, et al. Prospective longitudinal evaluation of the effect of deployment-acquired traumatic brain injury on posttraumatic stress and related disorders: results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). The American journal of psychiatry. 2015;172(11):1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assistant Secretary of Defense. Traumatic Brain Injury: Updated Definition and Reporting. In. Washington, DC: Department of Defense,; 2015. [Google Scholar]
- 28.Kay T, Harrington DE, Adams R, et al. Definition of Mild Traumatic Brain Injury. J Head Trauma Rehabil. 1993;8(3):86–87. [Google Scholar]
- 29.Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility International Society for Traumatic Stress Studies; 1993; San Antonio, TX. [Google Scholar]
- 30.Kessler RC, Santiago PN, Colpe LJ, et al. Clinical reappraisal of the Composite International Diagnostic Interview Screening Scales (CIDI-SC) in the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). Int J Methods Psychiatr Res. 2013;22(4):303–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R: A language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 32.Adams RS, Larson MJ, Corrigan JD, Ritter GA, Williams TV. Traumatic brain injury among US active duty military personnel and negative drinking-related consequences. Substance Use & Misuse,. 2013;48:821–836, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santiago PN, Wilk JE, Milliken CS, Castro CA, Engel CC, Hoge CW. Screening for Alcohol Misuse and Alcohol-Related Behaviors Among Combat Veterans. Psychiatric services. 2010;61(6):575–581. [DOI] [PubMed] [Google Scholar]
- 34.Corrigan JD, Yang J, Singichetti B, Manchester K, Bogner J. Lifetime prevalence of traumatic brain injury with loss of consciousness. Inj Prev. 2017. [DOI] [PubMed] [Google Scholar]
- 35.Whiteneck GG, Cuthbert JP, Corrigan JD, Bogner JA. Risk of negative outcomes after traumatic brain injury: a statewide population-based survey. The Journal of head trauma rehabilitation. 2016;31(1):E43–E54. [DOI] [PubMed] [Google Scholar]
- 36.Helmick KM, Spells CA, Malik SZ, Davies CA, Marion DW, Hinds SR. Traumatic brain injury in the US military: epidemiology and key clinical and research programs. Brain imaging and behavior. 2015;9(3):358–366. [DOI] [PubMed] [Google Scholar]
- 37.Spira JL, Lathan CE, Bleiberg J, Tsao JW. The impact of multiple concussions on emotional distress, post-concussive symptoms, and neurocognitive functioning in active duty United States marines independent of combat exposure or emotional distress. Journal of neurotrauma. 2014;31(22):1823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bryan CJ, Clemans TA. Repetitive traumatic brain injury, psychological symptoms, and suicide risk in a clinical sample of deployed military personnel. JAMA psychiatry. 2013;70(7):686–691. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy CH, Porter Evans J, Chee S, Moore JL, Barth JT, Stuessi KA. Return to Combat Duty after Concussive Blast Injury. Archives of Clinical Neuropsychology. 2012;27(8):817–827. [DOI] [PubMed] [Google Scholar]
- 40.Dretsch MN, Silverberg ND, Iverson GL. Multiple Past Concussions Are Associated with Ongoing Post-Concussive Symptoms but Not Cognitive Impairment in Active-Duty Army Soldiers. Journal of neurotrauma. 2015;32(17):1301–1306. [DOI] [PubMed] [Google Scholar]
- 41.Terrio H, Brenner LA, Ivins BJ, et al. Traumatic brain injury screening: Preliminary findings in a US Army Brigade Combat Team. The Journal of Head Trauma Rehabilitation,. 2009;24(1):14–23. [DOI] [PubMed] [Google Scholar]
- 42.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain Injury in U.S. soldiers returning from Iraq. New England Journal of Medicine. 2008;358(5):453–463. [DOI] [PubMed] [Google Scholar]
- 43.Yumul JN, McKinlay A. Do Multiple Concussions Lead to Cumulative Cognitive Deficits? A Literature Review. PM R. 2016;8(11):1097–1103. [DOI] [PubMed] [Google Scholar]
- 44.Department of Defense. DoD Policy Guidance for Management of Mild Traumatic Brain Injury/Concussion in the Deployed Setting. Number 6490.11. In. Washington D.C: 2012. [Google Scholar]
- 45.McKinlay A, Corrigan J, Horwood LJ, Fergusson DM. Substance abuse and criminal activities following traumatic brain injury in childhood, adolescence, and early adulthood. The Journal of head trauma rehabilitation. 2014;29(6):498–506. [DOI] [PubMed] [Google Scholar]
- 46.Yan HQ, Kline AE, Ma X, Li Y, Dixon CE. Traumatic brain injury reduces dopamine transporter protein expression in the rat frontal cortex. Neuroreport. 2002;13(15):1899–1901. [DOI] [PubMed] [Google Scholar]
- 47.Kennedy E, Heron J, Munafó M. Substance use, criminal behaviour and psychiatric symptoms following childhood traumatic brain injury: findings from the ALSPAC cohort. European child & adolescent psychiatry. 2017;26(10):1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmedani BK, Peterson EL, Hu Y, et al. Major Physical Health Conditions and Risk of Suicide. American journal of preventive medicine. 2017;53(3):308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madsen T, Erlangsen A, Orlovska S, Mofaddy R, Nordentoft M, Benros ME. Association Between Traumatic Brain Injury and Risk of Suicide. Jama. 2018;320(6):580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boggs JM, Beck A, Hubley S, et al. General Medical, Mental Health, and Demographic Risk Factors Associated With Suicide by Firearm Compared With Other Means. Psychiatric services. 2018:appips201700237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corrigan JD, Bogner J. Initial reliability and validity of the Ohio State University TBI Identification Method. The Journal of head trauma rehabilitation. 2007;22(6):318–329. [DOI] [PubMed] [Google Scholar]
- 52.Vanderploeg RD, Groer S, Belanger HG. Initial developmental process of a VA semistructured clinical interview for TBI identification. Journal of Rehabilitation Research & Development. 2012;49(4). [DOI] [PubMed] [Google Scholar]
- 53.Walker DD, Walton TO, Neighbors C, et al. Randomized trial of motivational interviewing plus feedback for soldiers with untreated alcohol abuse. J Consult Clin Psychol. 2017;85(2):99–110. [DOI] [PubMed] [Google Scholar]
- 54.Saitz R Unhealthy Alcohol Use. New England Journal of Medicine. 2005;352(6):596–607. [DOI] [PubMed] [Google Scholar]
- 55.Bogner J, Corrigan JD. Reliability and predictive validity of the Ohio State University TBI identification method with prisoners. The Journal of head trauma rehabilitation. 2009;24(4):279–291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.