Abstract
Scope:
Colitis, an inflammatory bowel disease, is associated with aberrant regulation of the colonic mucosal immune system. Resveratrol, a natural plant product, has been found to exert anti-inflammatory properties and attenuate the development of murine colitis. In the current study, we examined the role of microRNA (miR) in the ability of resveratrol to suppress colonic inflammation.
Methods and Results:
BALB/C mice with TNBS-induced colitis, when treated with resveratrol, showed improved clinical outcomes and reduced induction of inflammatory T cells (Th17 and Th1) while increasing CD4+Foxp3+ regulatory T cells (Tregs) and IL-10-producing CD4+ T cells. miR microarray analysis and PCR validation from CD4+ T cells showed treatment with resveratrol decreased the expression of several miRs (miR-31, Let7a, miR-132) that targeted cytokines and transcription factors involved in anti-inflammatory T cell responses (Foxp3 and TGF-β). Transfection studies with miR-31 confirmed that this miR directly regulated the expression of Foxp3. Lastly, analysis of public data from human patients with ulcerative colitis (UC) revealed that miR-31 expression was significantly increased when compared to controls.
Conclusions:
Together, the current study demonstrates that resveratrol-mediated attenuation of colitis may be regulated by miR-31 through induction of Tregs and miR-31 may serve as a therapeutic target for human colitis.
Keywords: colitis, resveratrol, microRNA, miR-31, regulatory T cells
Graphical Abstract
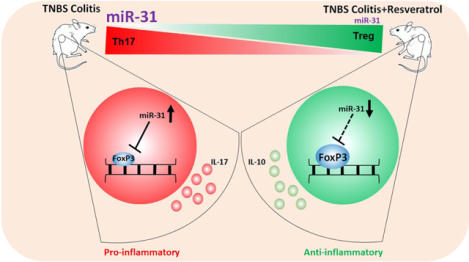
Colitis leads to dyregulation of microRNA (miR) and is characterized by an increase in microRNA-31 (miR-31). During colitis, miR-31 promotes a pro-inflammatory T helper 17 (Th17) phenotype by targeting anti-inflammatory regulatory T cell (Treg) transcription factor FoxP3. Treating colitis with resveratrol, a natural plant product, attenuates the disease by promoting an anti-inflammatory response which includes decreasing miR-31, thus preventing the targeting of Treg-associated FoxP3.
Introduction
Inflammatory bowel diseases (IBDs), such as ulcerative colitis (UC) and Crohn’s disease (CD), are chronic digestive diseases defined by often uncontrollable inflammation along the gastrointestinal tract and colon [1]. The incidence and prevalence of these diseases has risen since 1980 in many parts of the world, particularly in the United States [2], and there is an alarming trend of these diseases increasing in the pediatric population [3, 4]. Even more concerning is the link between colitis and an increased susceptibility to developing colorectal cancer (CRC) in animal models and the human patient population [5–7]. Colitis, as a form of IBD, has a complex etiology often attributed to many interrelated genetic, dietary, and other environmental factors [8, 9]. Current conventional treatment options (e.g. steroids and immunosuppressive drugs) often have adverse side-effects, or in some cases, colitis patients are non-responsive to these conventional therapies [10]. With increasing incidence, a link to development of CRC, and lack of effective treatment options, more studies are focusing on preventative measures to decrease colitis incidences.
Previously, we have shown that treatment with resveratrol, a natural product found in a variety of plant products [11], is able to lessen disease burden in chemical (dextran sodium sulfate, DSS) and genetic (IL-10 knockout) models of colitis [12–14], as well as colitis-associated colon cancers [15, 16]. A current review by Nunes et al. highlights findings from our lab as well as others showing the effectiveness of resveratrol in ameliorating or preventing animal models of colitis [17]. One of the key findings from our previous report in the colitis-associated tumorigenesis model was that resveratrol was able to regulate several microRNAs (miRs) that modulated inflammatory genes and factors [16]. This was a significant finding because miRs, small non-coding RNA molecules that target and regulate gene transcripts [18], were found to be important in both the development and progression of colitis, particularly in terms of regulating inflammation, serving as disease biomarkers, and responding to therapies [19–24]. The importance of miRs in regulating colitis was highlighted in our previous report showing that deficiency in only one miR (miR-155) was able to protect mice from developing severe colitis symptoms by a reduction in the inflammatory T helper (Th) type responses [19]. Our most recent studies in the 2,4,6-Trinitrobenzenesulfonic acid solution (TNBS) model of colitis showed that pro-inflammatory Th17 cells were increased during the disease state and treatment with resveratrol caused reduction in disease severity and alterations in the microbiome, resulting in induction of anti-inflammatory Tregs [14].
In the current report, we expanded our investigation into the effectiveness of resveratrol treatment in the TNBS mouse model of colitis, which is known to activate inflammatory T cells. We found that resveratrol treatment attenuated severe disease development, resulting in a shift from a pro-inflammatory Th17 phenotype to a more anti-inflammatory T cell response characterized by increased regulatory T cells (Tregs) and those producing IL-10. Microarray analysis of miR profiles in cells isolated from the mesenteric lymph node (MLN) revealed several miRs (e.g. miR-31, Let-7a, and miR-132) that target anti-inflammatory T cell factors were downregulated by resveratrol. In this study we further analyzed the role of miR-31, which was found to target Treg transcription factor FoxP3. These findings were even more interesting after analysis of human UC colitis patients revealed this patient population had significantly higher expression of miR-31 in disease-associated tissues. Altogether, the study highlights that miR-31 is a potential target for prevention of colitis and possibly CRC.
Materials and Methods
Animals:
Female mice (BALB/c) aged 8–10 weeks were obtained from Jackson Laboratories (Bar Harbor, ME) and housed at the University of South Carolina, School of Medicine (Columbia, SC) AAALAC-accredited animal facility. All mice were housed in specific pathogen (SPF) free conditions in rooms with controlled temperature, ventilation, and normal light/dark cycles. All procedures performed on mice followed National Institutes of Health (NIH) guidelines under approved animal use protocol AUP2377 by the Institutional Animal Care and Use Committee (IACUC).
Induction of colitis and treatment with resveratrol:
Colitis was induced in experimental mice as previously described [25]. Briefly, TNBS purchased from Sigma-Aldrich (MO, USA) was administered by intrarectal injection using a 38 mm catheter into lightly anesthetized (5% isoflurane) at a concentration of 1 mg TNBS dissolved in 0.1 ml of ethanol (50%). Mice were keep upright for 30 seconds following injection to ensure proper dispersion of the chemical into the colon area. Vehicle control mice were given an injection of 0.1 ml of ethanol (50%) without TNBS to negate any inflammatory or adverse effects caused by the alcohol. Resveratrol, purchased from Sigma-Aldrich, was given 24 hours prior to TNBS injection and given daily until completion of the experiment (5 days) by using a 30 mm oral gavage needle at a dose of 100 mg/kg in 0.1ml of vehicle (1% carboxymethyl cellulose, CMC). In the current study, the following experimental groups were used: Control mice (Vehicle) were given 50% ethanol intrarectal administration and daily oral gavage of vehicle (1% CMC); Naïve mice given treatment (Resveratrol) were given intrarectal administration of 50% ethanol and daily oral gavage of 100 mg/kg of the resveratrol. Disease controls (TNBS+Veh) were given 1mg intrarectal administration of TNBS and daily oral gavage of vehicle (1% CMC). Treatment groups (TNBS+Res) were given 1mg intrarectal administration of TNBS and daily oral gavage of resveratrol (100 mg/kg).
Dosage information:
The human equivalent dose (HED) of resveratrol given to experimental mice daily (100 mg/kg) is approximately 486 mg. The daily HED was calculated using the standard weight ratio Km normalization method previously described [26]. The equation was as follows: HED (mg/kg) = animal dose (mg/kg) × (mouse Km factor/human Km factor); where the mouse Km factor = 3, human Km factor = 37, and the average human weight was estimated at 60 kg (~132 lbs). Resveratrol is available as an over-the-counter dietary supplement in single capsules ranging from 20–500 mg, and pure resveratrol has been shown to be well-tolerated with little to no toxicity at daily doses as high as 2000 mg in human patients [27]. Our laboratory has given mice daily doses of 100 mg/kg resveratrol in previous published reports with no obvious signs of toxicity [14, 28–30].
Assessment of disease parameters:
To assess disease, experimental mice were weighed daily through the entirety of the experiment (5–6 days). Colonoscopies were performed to evaluate extent of damage (ulcerations and bleeding) to the colons prior to the end of the experiment (day 3). At the study endpoint, mice were euthanized by overdose of isoflurane. Excised colons were measured for length and proximal colons sections (1cm) were collected for histology. 10% formalin fixed tissues were stained with hematoxylin and eosin (H&E) to assess colonic damage caused by TNBS administration. To assess T cell response to disease and treatment, flow cytometry was performed on single cell suspensions isolated from MLNs and stained using antibodies for the following T cell and T cell subsets: overall T cells (CD3+), T helper cells (CD4+), cytotoxic T cells (CD8+), Tregs (CD4+FoxP3+), IL-10 producing cells (CD4+IL-10+), Th17 cells (CD4+IL-17+), and Th1 cells (CD4+IFNγ+). All antibodies used in these studies were purchased from BioLegend (CA, USA). For intracellular cytokine staining, MLN cells from experimental groups were cultured overnight (seeded at 1 × 106 cells/ml in complete RPMI media) before being activated for 4 hours with Leukocyte Activation Cocktail with BD GolgiPlug (BD Pharmingen, San Jose, CA). Cells were than collected and stained using the BD Fixation/Permeabilization Solution kit as per instructions from the manufacturer. For transcription factor staining, True-Nuclear Transcription Factor Buffer set from BioLegend was used as per instructions from the manufacturer.
Analysis of miRNA:
MicroRNA arrays were performed on RNA isolated from cells collected from the MLN of experimental mice as previously described [31]. Each sample consisted of a pool of 5 biological replicates. For each miRNA microarray, FlashTag Biotin HSR RNA Labeling kit from Affymetrix (Thermo Fisher Scientific, MA, USA) was used and tagged samples were later hybridized to the Affymetrix miRNA 4.0 chip. Chips were scanned with an Affymetrix GCS 3000 system. For transcriptome microarrays, 100 ng total RNA was used as starting material. RNA was prepared for hybridization by using the Affymetrix GeneChip WT PLUS Reagent Kit according to protocol from the manufacturer. Affymetrix Expression Console Version software was used to evaluate quality control of the samples, as well as initial analysis of the microarray data to include principal component analysis, heatmaps depicting raw signal expression, log2 fold change (FC) calculations, and direct comparisons among the experimental groups. Ingenuity Pathway Analysis (IPA, http://www.ingenuity.com/) was used to generate miRNA-gene target pathways based on differentially regulated miRNA profiles, which was determined to be a greater than ±2 log2 fold change between two different experimental groups. miR validation studies were performed by first preparing complimentary DNA (cDNA) from isolated RNA samples using the miScript II RT kit (Qiagen, MD, USA) followed by quantitative real time PCR (qRT-PCR) using a CFX Connect Real Time System (Bio-Rad, PA, USA). PCR reactions were performed using mouse-specific miR primers purchased from Qiagen. Primers included mmu-miR-31–5p (MI0000579), mmu-Let-7a (MIMAT0004620), and mmu-miR-132–3p (MIMAT0000144). Expression levels were normalized to Snord96a (MS00033733) levels. Fold changes were calculated using the 2−ΔΔCT method.
Transfection experiments with miR-31–5p mimic or inhibitor and target gene quantification:
Transfection experiments were carried out as previously described [29, 32]. Excised MLNs from naïve BALB/c were prepared in a single cell suspension before culturing in complete RPMI 1640 media supplemented with heat inactivated 10% fetal bovine serum, 10mM L-glutamine, 10mM HEPES, 50μM β-mercaptoethanol, and 100μg/ml penicillin/streptomycin. MLN cells were seeded (2 × 105 cells per well) in a 24-well plate and activated with 1 μg/ml of bacterial toxin staphylococcal enterotoxin B (SEB) purchased from Toxin Technologies Inc. (FL, USA). Cells were then transfected with either 20nM of synthetic mmu-miR-31–5p mimic (AGGCAAGAUGCUGGCAUAGCUG) or anti-mmu-miR-31–5p (AGGCAAGAUGCUGGCAUAGCUG) using HiPerfect Transfection Reagent from Qiagen for 24 hours. Expression levels for miR-31 and transcriptional factor FoxP3 (forward: CCCATCCCCAGGAGTCTTG; reverse: ACCATGACTAGGGGCACTGTA) were determined using qRT-PCR. For FoxP3, expression levels were normalized to β actin (forward: GGCTGTATTCCCCTCCG; reverse: CCAGTTGGTAACAATGCCATGT).
Dataset for human colitis patient population:
Data on human miR-31 expression levels was obtained from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) repository. The human dataset used was GEO accession GSE68306 provided by Huang et al. and published elsewhere [33]. The data set consisted of colon tissue biopsies from normal healthy controls (n=16) and ulcerative colitis (UC) patients (n=29). For UC patients, samples consisted of three distinct tissue biopsy types: UC associated with neoplastic tissues (n=11), UC patients without neoplasia (n=9), and non-dysplastic UC mucosa adjacent to a neoplastic lesion (n=9). For the current report, analysis was performed using two different comparisons. First, expression of miR-31 in healthy tissue samples (n=16) was compared to UC patients (n=29). Second, miR-31 expression from healthy controls (n=16) was compared to colonic tissues from UC patients associated with neoplasia (n=11). Raw expression values were obtained from the provided online dataset and based on NanoString nCounter v1.7.0 platform performed on RNA isolated from formalin-fixed paraffin embedded tissue samples.
Statistical Analysis:
GraphPad Prism software (CA, USA) was used for most of the statistical analysis depicted in the current report unless otherwise noted. For in vivo colitis experiments, at least 5 mice were used per experimental group. For in vitro assays, all experiments were performed in triplicate. For statistical differences, significance (p value of ≤ 0.05) was determined using one-way ANOVA followed by Tukey’s post-hoc multiple comparisons test unless otherwise stated.
Results
Treatment with resveratrol reduces severity of TNBS-induced colitis
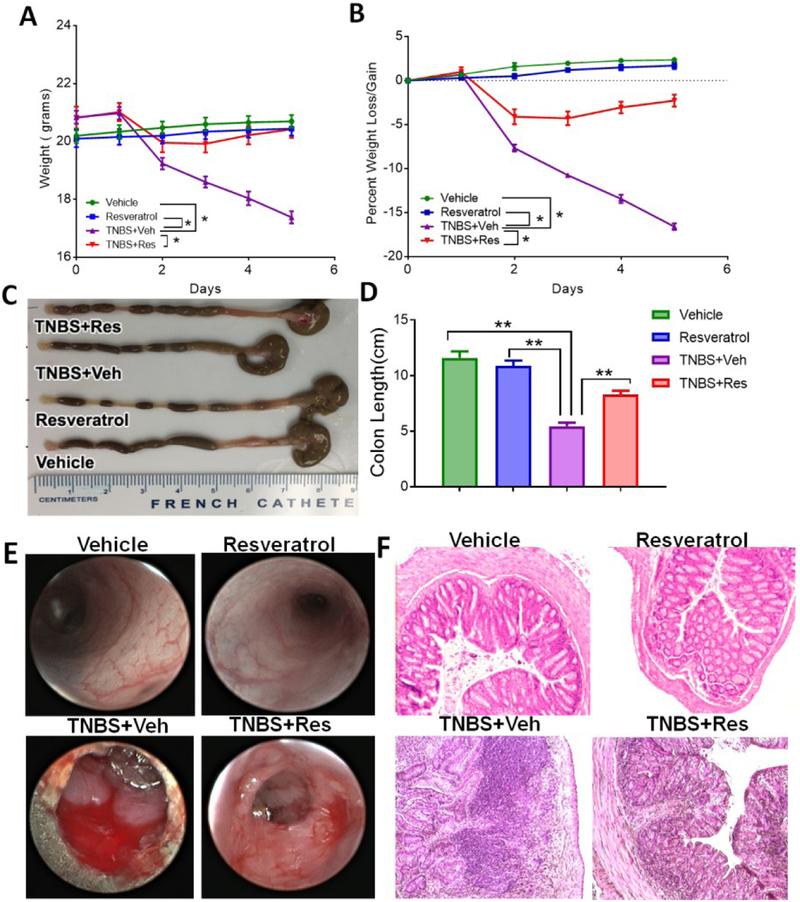
In the current study, we investigated the efficiency of resveratrol to prevent a chemically-induced murine colitis model using TNBS. To investigate prevention of disease by this natural compound, treatment groups were given resveratrol 24 hours prior to induction of disease by TNBS, followed by daily oral administrations of the treatment. As shown, colitis mice (TNBS+Veh) had significant weight loss (~15%) during the course of the study compared to control groups (Vehicle or Resveratrol), but colitis mice pre-treated with resveratrol (TNBS+Res) had significantly decreased incidence of weight loss (Fig. 1A–B). Another hallmark of many colitis models is the shortening of the colon after disease induction. The colons from TNBS mice were significantly shorter while TNBS+Res mice had similar colon lengths when compared to controls (Fig. 1C–D). Colonoscopies performed during the peak of disease (day 3) revealed that while TNBS mice had typical disease-associated features such as ulcerations and bleeding compared to normal colons, colons of TNBS+Res mice had reduced presence of these classical clinical parameters (Fig. 1E). Histological evaluation corroborated with these observations from colonoscopies as there were significant signs of tissue damage (e.g. cellular infiltration, loss of normal colonic tissue architecture) in TNBS mouse colons compared to controls, and this damage was not present or reduced in colons from TNBS+Res mice (Fig. 1F). These observations together demonstrated that resveratrol treatment effectively prevented TNBS-induced colitis.
Figure 1: Treatment with resveratrol reduces clinical parameters in TNBS-induced colitis.
BALB/c mice were injected intrarectally with 1mg of TNBS to induce colitis. Mice treated with resveratrol were given 100mg/kg in vehicle (1% CMC) by oral gavage 24 hours prior to the TNBS injection as well as daily up until the experimental end point (day 4). Experimental groups consisted of: Vehicle (n=5), Resveratrol (n=5), TNBS+Veh (n=5), and TNBS+Res (n=5). Initial clinical parameters consisted of evaluating weight (A), percent weight loss (B) and colon length (C-D). (E) Representative colonoscopies are shown from experimental groups during peak of disease (day 3). (F) Representative colon sections from fixed and paraffin-embedded tissue sections stained with H&E at 20x objective. Significance (p-value: *<0.05, **<0.01, ***<0.005, ****<0.001) was determined by using one-way ANOVA and post-hoc Tukey’s test for bar graphs and Mann-Whitney test for weight data. Data are representative of at least 3 independent experiments.
Treatment of TNBS-induced colitis mice with resveratrol results in a shift from a pro-inflammatory to anti-inflammatory T helper response in MLN
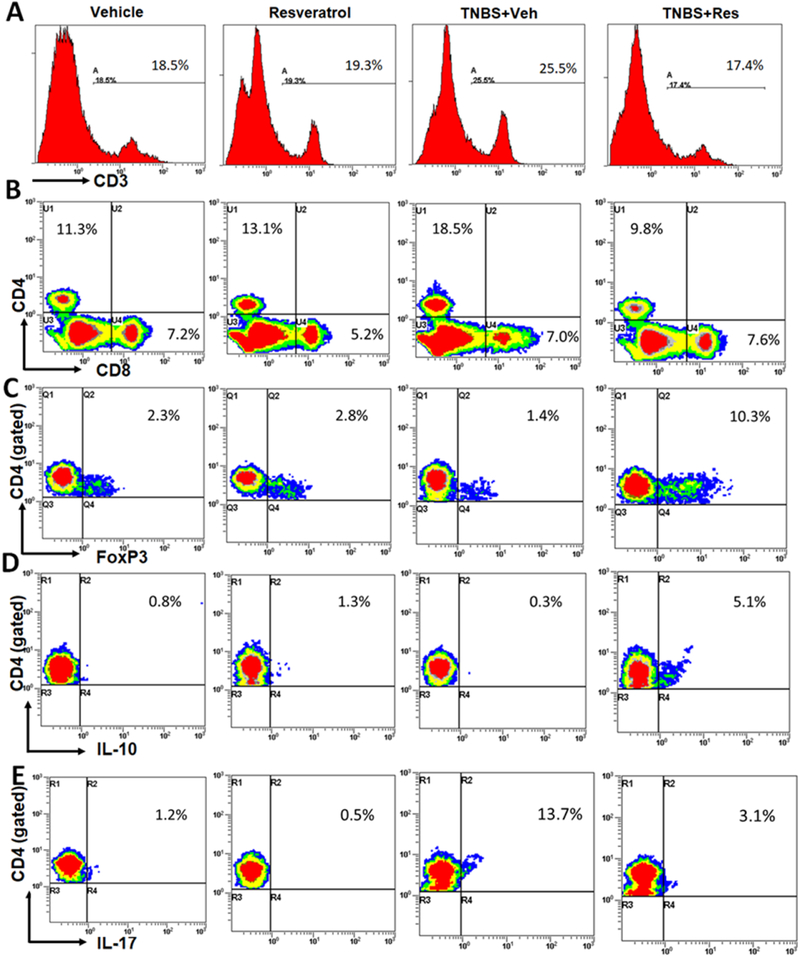
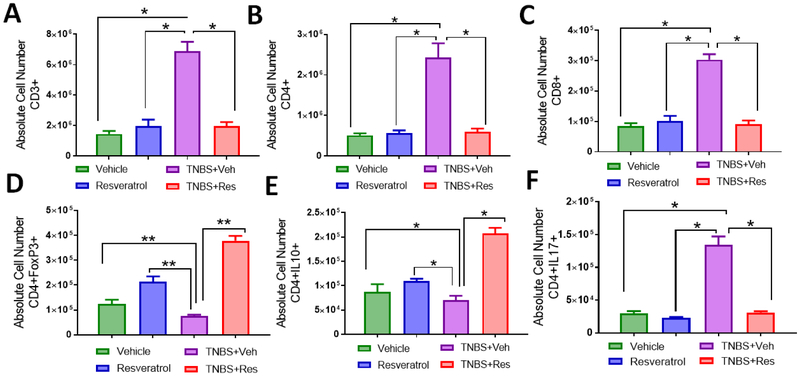
To investigate if this effect of resveratrol was due to reduction in pro-inflammatory T helper responses (IL-17) while inducing anti-inflammatory types (Tregs and IL-10 production), MLNs from the TNBS experimental groups were evaluated to determine T cell distribution during disease and treatment. The T cell subsets evaluated included all T cells (Fig. 2A), T helper and cytotoxic T cells (Fig. 2B), Tregs (Fig. 2C), IL-10 T helper cells (Fig. 2D), and Th17 cells (Fig. 2E). Based on percentages obtained from flow cytometry phenotyping (Figs. 2A–E), absolute cell numbers were assessed in MLNs from all experimental groups. Overall T cells (Fig. 3A), including CD4+ T helper (Fig. 3B) and CD8+ cytotoxic (Fig. 3C), were significantly increased in TNBS+Veh mice compared to the other experimental groups, and TNBS+Res mice reduced these subsets to the levels seen in normal controls. Despite an overall increase in CD4+ T cells, there was a decrease in the anti-inflammatory Treg (Fig. 3D) and IL-10-producing (Fig. 2E) cells during TNBS induction, while colitis mice treated with resveratrol had significantly higher numbers of these anti-inflammatory subsets compared to controls. A significant proportion of the CD4+ T cell subsets in TNBS+Veh mice appeared to be pro-inflammatory Th17 cells (Fig. 3F), and treatment with resveratrol during the disease state showed ablation of the increase in this T cell subset, comparable to control levels. Taken altogether, treatment with resveratrol appeared to prevent colitis-associated increases in pro-inflammatory Th17 cells, likely through the induction of anti-inflammatory subsets such as Tregs and CD4+ IL-10-producing cells.
Figure 2: Treatment with resveratrol alters T cell subsets in the MLN of TNBS-induced mice.
TNBS disease and treatment with resveratrol were performed as described in Figure 1 legend. MLNs were excised from experimental groups (n=5 per experimental group), stained with T cell-specific antibodies, and analyzed by flow cytometry. Representative T cell subset staining by flow cytometry was as follows: (A) CD3+ positive histogram plot; (B) CD4+ and CD8+ dot plot; (C) CD4+-gated FoxP3+ dot plot; (D) CD4+-gated IL-10 dot plot; (E) CD4+-gated IL-17 dot plot.
Figure 3: Treatment with resveratrol increases absolute cell numbers of anti-inflammatory T cell subsets in the MLN of TNBS-induced mice.
TNBS disease and treatment with resveratrol were performed as described in Figure 1 legend. MLNs were excised from experimental groups (n=5 per experimental group), stained with T cell-specific antibodies, and analyzed by flow cytometry (as represented in Figure 2). Bar graphs depict absolute cell numbers in MLN for all T cells (F), T helper cells (G), cytotoxic T cells ( H), Tregs (I), T helper producing IL-10 cells (J), and Th17 cells (K). Significance (p-value: *<0.05, **<0.01, ***<0.005, ****<0.001) was determined by using one-way ANOVA and post-hoc Tukey’s test for bar dot graphs. Data are representative of at least 3 independent experiments.
Resveratrol treatment downregulates miRs that target Treg transcription factor FoxP3 and other anti-inflammatory T cell-associated factors
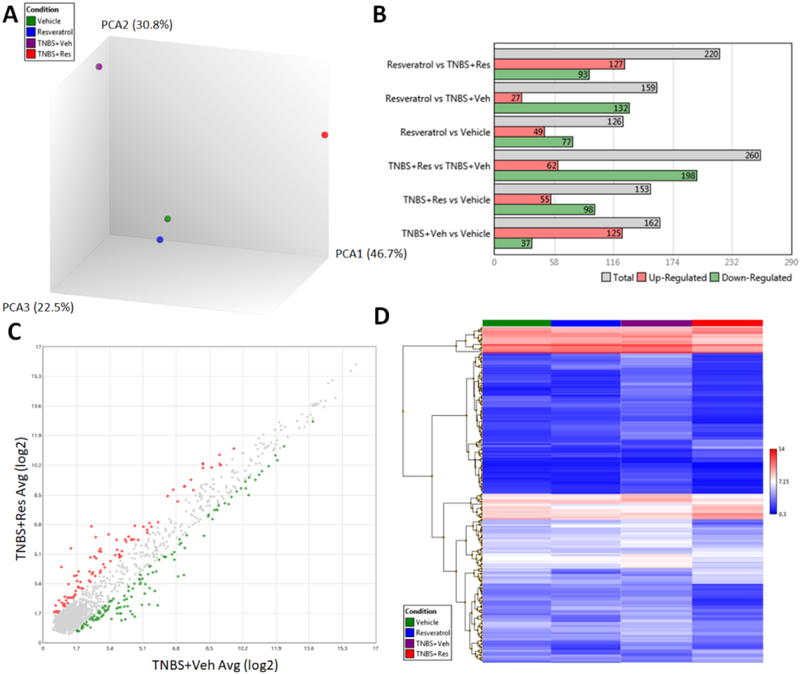
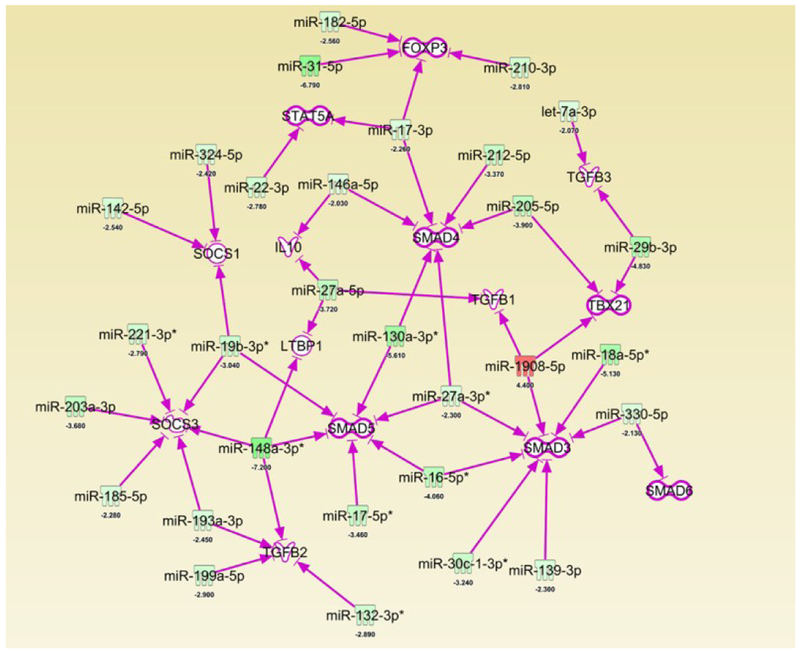
Next, we investigated if miRs regulated the anti-inflammatory properties of resveratrol. To that end, we investigated the miR profile of cells from the MLN. Principal component analysis (PCA) from pooled samples from all experimental groups showed that the miR profiles of controls (Vehicle and Resveratrol) were most similar with opposite deviations occurring when compared to TNBS+Veh and TNBS+Res groups (Fig. 4A). Direct comparisons among two groups at a time, with the significance criteria set to a ±2-fold change, revealed the greatest difference was among the TNBS+Veh vs. TNBS+Res groups (Fig. 4B). Comparison of these two groups showed 260 total miRs (out of 3195 probed) were significantly altered, with TNBS+Res downregulating 198 and upregulating 62 compared to TNBS+Veh (Fig. 4C). A heatmap showing raw expression of these 260 significantly altered miRs for all experimental groups is depicted in Fig. 3D. IPA analysis of these 260 significantly altered miRs revealed several were downregulated in the TNBS+Res group that targeted key anti-inflammatory T cell components such as FoxP3 (miR-31–5p, miR-182–5p, miR-210–3p), IL-10 (miR-146-a-5p and miR-27a-5p), TGFβ2/3 (miR132–3p, miR-1999a-5p, miR193a-3p, miR-148a-3p, let-7a-3p, miR-29b-3p), and several SMAD proteins (miR-330–5p, miR-139–3p, miR-30c-1–3p*, miR-16–5p*, miR-27a-3p) (Fig. 5). Among these miRs targeting key anti-inflammatory T cell factors, miR-31, predicted to target FoxP3, was the most significantly downregulated (−6.790 fold change) when comparing TNBS+Res vs TNBS+Veh groups (Fig. 5). Thus, the microarray data suggested that treatment with resveratrol was able to downregulate miRs that normally targeted anti-inflammatory gene expression and components.
Figure 4: Treatment with resveratrol alters the miR profile in TNBS-induced colitis MLN.
TNBS disease and treatment with resveratrol were performed as described in Figure 1 legend. RNA from MLN of experimental groups was isolated for miR microarray analysis using the murine-specific Affymetrix miRNA 4.0 chip. Experimental groups (Vehicle, Resveratrol, TNBS+Veh, and TNBS+Res) consisted of pools of 5 mice per group. Affymetrix Expression Console Version software was used to generate the following comparisons: (A) 3D PCA plot comparing all experimental groups; (B) multiple bar graph comparisons depicting significantly altered upregulated and downregulated (± 2 fold change) miRs between two experimental groups; (C) scatter plot depicting 260 significantly upregulated (red dots) and downregulated (green dots) miRs out of 3195 total between TNBS+Res and TNBS+Veh; and (D) heat map depicting raw expression values of the 260 aforementioned miRs among the 4 different experimental groups.
Figure 5: Treatment with resveratrol results in downregulation of several miRs that target anti-inflammatory T cell-associated factors.
TNBS disease and treatment with resveratrol were performed as described in Figure 1 legend. 260 significantly altered miRs between TNBS+Res vs. TNBS+Veh noted in Figure 3 legend were subjected to Ingenuity Pathway Analysis (IPA). Depicted is an IPA-generated interaction chart showing significantly altered miRs targeting factors associated with anti-inflammatory T cell responses. Green colors represent downregulated miRs and Red colors represent upregulated miRs. The calculated fold changes between TNBS+Res and TNBS+Veh groups are depicted below each included miR. Purple arrows indicate predicted, highly predicted, and experimentally-proven interactions of the miR with target mRNA shown.
Resveratrol downregulates FoxP3-targeting miR-31 which is highly upregulated in human colitis patients
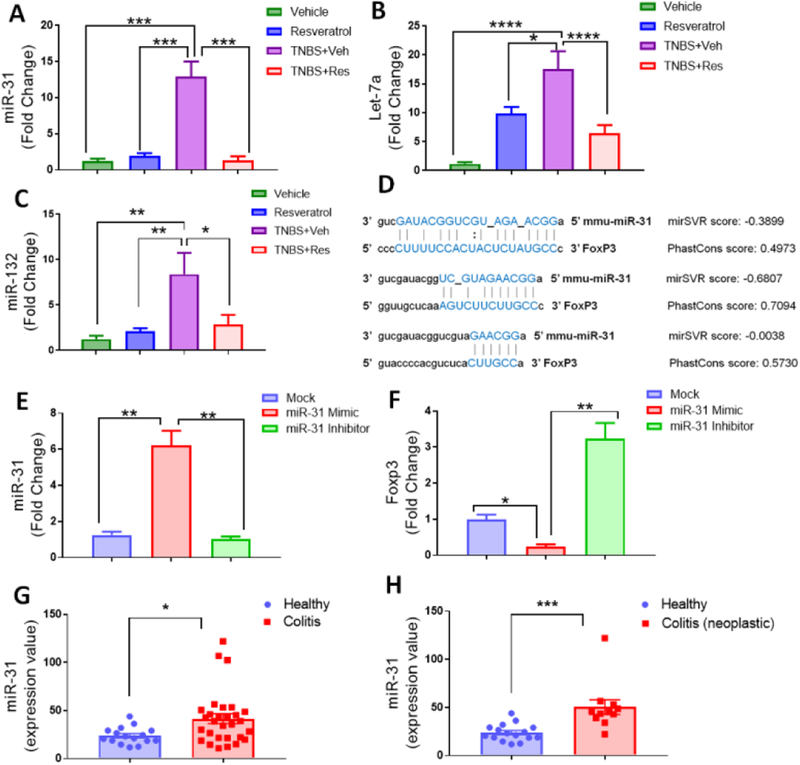
As miR-31 was the most significantly downregulated miR indicated in targeting anti-inflammatory T cell response and factors, PCR was performed to validate the microarray results. Results showed that miR-31 was significantly increased in the MLN of TNBS+Veh mice compared to the control groups, whereas TNBS mice treated with resveratrol had decreased expression of this miRNA (Fig. 6A). Validation was also performed on other miRs highlighted in Fig. 5 to include let-7a (Fig. 6B) and miR-132 (Fig. 6C). As with miR-31, results showed these miRs were increased in the disease state (TNBS+Veh), but treatment with resveratrol (TNBS+Res) prevented their disease-associated upregulation. Next, alignment analysis was performed to determine the potential of FoxP3 being a target. miR-31 was found to have three potential binding sites on the 3’-untranslated region (UTR) of the FoxP3 transcript, two of which had highly-probable miR-target mRNA interactions as predicted by mirSVR and PhastCons scores (Fig. 6D). Transfection experiments were performed to determine if alterations in this miR affected FoxP3 expression by giving SEB-activated T cells from the MLN either mock (just transfection reagent), miR-31-mimic, or miR-31-inhibitor. PCR validation of miR-31 expression showed the transfection was successful (Fig. 6E), and the results indicated that when miR-31 was upregulated (miR-mimic), expression of FoxP3 transcript was downregulated (Fig. 6F). On the contrary, if miR-31 was inhibited, as in the case of treatment with resveratrol, then FoxP3 expression was increased. Collectively, these data showed that FoxP3 expression was altered by miR-31, a diseased-associated miR that resveratrol treatment prevented from becoming upregulated. Lastly, as the TNBS-induced model indicated FoxP3-targeting miR-31 was a potential target in this murine model of colitis, human studies looking at miR expression in colon biopsies of UC patients was investigated to determine any applicable correlation between this animal model and the human patient population. As shown, miR-31 was found to be significantly increased in patients with UC when compared to controls (Fig. 6G). Interestingly enough, this upregulation of miR-31 was found to be even more significant in UC colon biopsies that developed colon cancer-associated neoplastic lesions when compared to healthy controls (Fig. 6H). These data provided a link with observed miR-31 upregulation in the mouse model of colitis and human UC patient population, which resveratrol was able to prevent, effectively inhibiting miR-31 from targeting anti-inflammatory FoxP3-mediated response.
Figure 6: Resveratrol prevents FoxP3-targeting miR-31 upregulation in TNBS-induced colitis which correlates with miR-31 upregulation in human UC patients.
TNBS disease and treatment with resveratrol were performed as described in Figure 1 legend. RNA was isolated from MLN of Vehicle (n=5), Resveratrol (n=5), TNBS+Veh (n=5), and TNBS+Res (n=5) to validate expression levels of miR-31 (A), Let-7a (B), and miR-132 (C). (D) Predicted miR-31 and FoxP3 alignment sites (with mirSVR and Phastcon scores) were obtained from microrna.org. For transfection experiments, single-cell suspensions from normal mouse MLN were seeded (1×105 cells per well) and activated with SEB (1μg/ml) for 24 hours before collecting total RNA from groups. Experimental groups consisted of transfection reagent only mock (n=5), miR-31 mimic (n=5), and miR-31 inhibitor (n=5). Depicted are PCR-generated expression fold changes for miR-31 (E) and FoxP3 (F). For human samples depicted, raw expression values of miR-31 from colonic biopsies were obtained from GEO data GSE68306. Two comparisons are depicted: (G) Normal Healthy controls (n=16) vs. all UC patients (n=29); and (H) Normal Healthy controls (n=16) vs. UC patients associated with neoplasia (n=11). Significance (p-value: *<0.05, **<0.01, ***<0.005, ****<0.001) was determined by using one-way ANOVA and post-hoc Tukey’s test for bar dot graphs when comparing three or more groups. Data are representative of at least 3 independent experiments. For human datasets, significance was determined using an unpaired, two-tailed t test.
Discussion
Previous studies have shown resveratrol is capable of preventing or at least reducing symptoms associated with animal models of colitis by a variety of different mechanisms. For example, in our earliest reports in the DSS-induced model of colitis, we showed resveratrol upregulated silent mating type information regulation-1 (SIRT-1) and downregulated nuclear transcription factor-kappaB (NF-κB) in immune cells [13]. Many of these alterations in signaling pathways in these previous studies correlated with resveratrol-mediated alterations in CD4+ T cells and macrophages, where these effector immune cells were increased upon disease induction, but later reduced upon treatment with resveratrol. In a more recent report by Zhang et al., researchers confirmed our results that resveratrol treatment in the DSS model attenuated colitis which was associated with upregulating colonic tissue levels of SIRT-1 in addition to increasing phosphorylated mechanistic target of rapamycin (pmTOR), while also downregulating other pro-inflammatory factors (e.g. autophagy-related 12, Beclin‑1, and microtubule‑associated protein light chain 3 II) [34]. SIRT1 and mTOR signaling in CD4+ T cells, via TCR stimulation were also found to be affected by low and high doses of resveratrol in studies published by Craveiro et al. [35]. In particular, low dose (20μM) resveratrol was found to increase both SIRT-1 and p-mTOR expression in CD4+ T cells after TCR-mediated stimulation. High dose (100μM) of resveratrol seemed to have the opposite effect, in that it decreased SIRT-1 and mTOR signaling over time. This is interesting given the fact that FoxP3, the key transcription factor of Tregs, can be greatly affected by TCR-mTOR signaling, as it was found that early inhibition of these signaling events can promote expression of FoxP3 in T cells [36]. Such reports highlight how resveratrol can play a dual role depending on the dose and cell types this natural product interacts with. However, the current report shows that in vivo treatment of colitis-induced mice with resveratrol at a larger dose favors Treg induction. These findings were also seen by Yao et al., who showed that resveratrol effectively regulated Treg/Th17 signaling during DSS-induced colitis via modulating hypoxia inducible factor (HIF)-1α/mammalian target of rapamycin (mTOR) signaling pathways [37]. Specifically in these studies, DSS-induced colitis was shown to lead to the upregulation of pro-inflammatory Th17 cells, with decreased Treg numbers in the spleen and colon tissues. Low (50 mg/kg) or high dose (100mg/kg) treatment with resveratrol was able to reverse these colitis-induced observations, effectively reducing Th17 cell numbers while increasing anti-inflammatory Tregs. This is important since even though many mouse models of colitis involve innate immune cells, T Cell Transfers Models, which involve the adoptive transfer of naïve CD4+ T cells into lymphopenic mice, have been instrumental in understanding the contribution of T cells not only in the induction and progression of colitis, but in understanding Treg involvement in disease suppression [38, 39]. For instance, adoptive transfer of CD4+CD45RBhigh expressing T cells can lead to the development of spontaneous colitis [40], whereas, transferring CD4+CD45RBlow or CD4+CD25+ with CD4+CD45RBhigh prevents colitis development since this mixture of T cells leads to increased Treg induction and suppression of effector T cells [41]. In another chemically-induced colitis model in rats using oxazolone, resveratrol treatment was shown to exert anti-inflammatory and pro-apoptotic properties by inhibiting myeloperoxidase (MPO) and sphingosine kinase 1 (SphK1) [42]. In fact, the success of resveratrol treatment in animal models of colitis translates even into the human patient population. A double-blinded, placebo-controlled pilot study in UC patients has shown that supplementation of 500mg/kg of resveratrol for 6 weeks appears to improve quality of life and partially reduce disease severity in this patient population, thought to be due to the ability of this natural product to reduce oxidative stress [43, 44]. In addition to the growing number of studies identifying resveratrol as a potential preventative and therapeutic against colitis and even CRC, there are a number of reports linking dysregulation in miRs possible mechanisms which drive disease development and progression.
As early as a decade ago, reports highlighted the differential expression of certain miRs in patients with colitis (UC and CD) and CRC [45–47]. In one of the earliest animal model reports, Chen et al. identified miR-155 was altered in activated CD4+ cells from TNBS-induced colitis mice [48], which supported our previous report showing miR-155 deficient mice had protection against colitis induction [19]. Since these early reports, the role of miR dysregulation in colitis is becoming more established from both a potential diagnostic tool to areas of therapeutic intervention. For example, a recent published report suggest that serum levels of miR-146–5p are a better diagnostic tool to evaluate UC and CD severity than the standard C-reactive protein levels [49]. miR-449a was suggested to be a possible predictor of colitisassociated CRC progression [24]. Going beyond just diagnostic and biomarkers of disease, miRs are being looked as potential promoters and inhibitors of colitis as well. Increased miR-590–5p, via inhibition of Yes-associated protein 1 (YAP), was shown to reduce intestinal inflammation in both colon cancer cells and mouse models of colitis with significant correlations in intestinal tissues from CD patients [50]. An antagomir for miR-148a was reported to be a potentially effective drug treatment in amelioration of colitis because of its ability to selectively deplete the pro-inflammatory Th1 response without interrupting other protective immunological function during chronic colitis [51]. The current report advances previous studies on miRs by demonstrating that miR-31 is a potential therapeutic target in colitis and CRC prevention.
The current study identifies the miR-31/FoxP3 axis as a means to prevent colitis development, showing this miR was significantly upregulated in the TNBS-induced murine model of colitis as well as documented in a dataset of UC colitis patients. In 2011, researchers reported that miR-31 was found to be highly dysregulated in epithelial cells from chronically inflamed mouse colons and APC(Min/+) tumors [52], and in that same year a report described how miR-31 increase correlated with chronic inflammation in IBD developing into neoplasia [53]. However, while many reports identify miR-31 as being abnormally high in colitis patients and animal models of colitis, the exact role of this miR in disease progression and development is somewhat controversial.
Liu et al. found that colon epithelial-specific deletion of miR-31 resulted in a more severe form of colitis-associated colorectal cancer than wild-type counterparts [54], and another report suggested that overexpression of miR-31 in UC targeted and regulated the pro-inflammatory IL-13 signaling [55]. These reports align with other studies suggesting miR-31 is important in protecting against colitis by way of engaging mucosal healing processes during inflammatory events within the colon [56, 57]. It is important to note that such findings do not necessarily contradict our data suggesting resveratrol-mediated targeting of miR-31 assists in prevention of colitis. Such reports indicated miR-31 seemed to be protective in epithelial cells, whereas our report shows that in CD4+ immune cells, downregulating miR-31 helps initiate a potential anti-inflammatory Treg response. Thus, while upregulation of miR-31 in colonic epithelial cells might serve a protective role, in immune cells it has the potential to promote inflammation by reducing Treg development. This highlights the need to better understand through additional research how regulating miRs in different cell types might have varying consequences. Nevertheless, the current study provides evidence for additional pathways through which resveratrol offers a highly valuable preventative and therapeutic properties against colitis and possibly colitis-associated CRC.
Acknowledgements
The studies were supported in part by NIH grants P01AT003961, R01AI123947, R01AI129788, and P20GM103641. The funding agencies had no role in the experimental design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors would like to acknowledge the Higher Committee for Education Development in Iraq (HCED) and the University of Basrah College of Veterinary Medicine for support provided to HRA. HRA and PBB contributed equally to this manuscript. HRA, PBB MN, and PSN contributed to the overall research design and manuscript preparation. HRA performed the majority of experiments presented in the manuscript, with assistance from PBB. MN and PSN were responsible for obtaining all the funding. PBB assisted in all statistical analysis and interpretation of the data. All authors read and approved the final manuscript.
List of Abbreviations:
- TNBS
2,4,6-trinitrobenzenesulfonic acid solution
- Res
resveratrol
- Veh
vehicle
- MLN
mesenteric lymph node
- miR
microRNA
- Tregs
regulatory T cells
- CRC
colorectal cancer
- UC
ulcerative colitis
- CD
Crohn’s Disease
- DSS
dextran sodium sulfate
- SEB
staphylococcal enterotoxin B
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interests.
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record.
References
- [1].Singh UP, Singh NP, Guan H, Busbee B, Price RL, Taub DD, Mishra MK, Fayad R, Nagarkatti M, Nagarkatti PS, The emerging role of leptin antagonist as potential therapeutic option for inflammatory bowel disease. International reviews of immunology 2014, 33, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG, Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54 e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- [3].Sykora J, Pomahacova R, Kreslova M, Cvalinova D, Stych P, Schwarz J, Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World journal of gastroenterology 2018, 24, 2741–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ong C, Aw MM, Liwanag MJ, Quak SH, Phua KB, Rapid rise in the incidence and clinical characteristics of pediatric inflammatory bowel disease in a South-East Asian cohort in Singapore, 1994–2015. Journal of digestive diseases 2018, 19, 395–403. [DOI] [PubMed] [Google Scholar]
- [5].Foersch S, Waldner MJ, Neurath MF, Colitis and colorectal cancer. Digestive diseases 2012, 30, 469–476. [DOI] [PubMed] [Google Scholar]
- [6].Al Bakir I, Curtius K, Graham TA, From Colitis to Cancer: An Evolutionary Trajectory That Merges Maths and Biology. Frontiers in immunology 2018, 9, 2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang H, Wang W, Romano KA, Gu M, Sanidad KZ, Kim D, Yang J, Schmidt B, Panigrahy D, Pei R, Martin DA, Ozay EI, Wang Y, Song M, Bolling BW, Xiao H, Minter LM, Yang GY, Liu Z, Rey FE, Zhang G, A common antimicrobial additive increases colonic inflammation and colitis-associated colon tumorigenesis in mice. Science translational medicine 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hart A, Diet in the etiology of inflammatory bowel disease. Revista espanola de enfermedades digestivas : organo oficial de la Sociedad Espanola de Patologia Digestiva 2019, 111, 3–4. [DOI] [PubMed] [Google Scholar]
- [9].Mikhailov TA, Furner SE, Breastfeeding and genetic factors in the etiology of inflammatory bowel disease in children. World journal of gastroenterology 2009, 15, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Antonelli E, Villanacci V, Bassotti G, Novel oral-targeted therapies for mucosal healing in ulcerative colitis. World journal of gastroenterology 2018, 24, 5322–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Busbee PB, Rouse M, Nagarkatti M, Nagarkatti PS, Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutrition reviews 2013, 71, 353–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Singh UP, Singh NP, Singh B, Hofseth LJ, Taub DD, Price RL, Nagarkatti M, Nagarkatti PS, Role of resveratrol-induced CD11b(+) Gr-1(+) myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3(+) T cells and amelioration of chronic colitis in IL-10(−/−) mice. Brain, behavior, and immunity 2012, 26, 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M, Nagarkatti PS, Resveratrol (trans-3,5,4’-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. The Journal of pharmacology and experimental therapeutics 2010, 332, 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Alrafas HR, Busbee PB, Nagarkatti M, Nagarkatti PS, Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. Journal of leukocyte biology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cui X, Jin Y, Hofseth AB, Pena E, Habiger J, Chumanevich A, Poudyal D, Nagarkatti M, Nagarkatti PS, Singh UP, Hofseth LJ, Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer prevention research 2010, 3, 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Altamemi I, Murphy EA, Catroppo JF, Zumbrun EE, Zhang J, McClellan JL, Singh UP, Nagarkatti PS, Nagarkatti M, Role of microRNAs in resveratrol-mediated mitigation of colitis-associated tumorigenesis in Apc(Min/+) mice. The Journal of pharmacology and experimental therapeutics 2014, 350, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nunes S, Danesi F, Del Rio D, Silva P, Resveratrol and inflammatory bowel disease: the evidence so far. Nutrition research reviews 2018, 31, 85–97. [DOI] [PubMed] [Google Scholar]
- [18].Guo G, Zhou J, Yang X, Feng J, Shao Y, Jia T, Huang Q, Li Y, Zhong Y, Nagarkatti PS, Nagarkatti M, Role of MicroRNAs Induced by Chinese Herbal Medicines Against Hepatocellular Carcinoma: A Brief Review. Integrative cancer therapies 2018, 17, 1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Singh UP, Murphy AE, Enos RT, Shamran HA, Singh NP, Guan H, Hegde VL, Fan D, Price RL, Taub DD, Mishra MK, Nagarkatti M, Nagarkatti PS, miR-155 deficiency protects mice from experimental colitis by reducing T helper type 1/type 17 responses. Immunology 2014, 143, 478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lopetuso LR, De Salvo C, Pastorelli L, Rana N, Senkfor HN, Petito V, Di Martino L, Scaldaferri F, Gasbarrini A, Cominelli F, Abbott DW, Goodman WA, Pizarro TT, IL-33 promotes recovery from acute colitis by inducing miR-320 to stimulate epithelial restitution and repair. Proceedings of the National Academy of Sciences of the United States of America 2018, 115, E9362–E9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Morilla I, Uzzan M, Laharie D, Cazals-Hatem D, Denost Q, Daniel F, Belleannee G, Bouhnik Y, Wainrib G, Panis Y, Ogier-Denis E, Treton X, Colonic MicroRNA Profiles, Identified by a Deep Learning Algorithm, That Predict Responses to Therapy of Patients With Acute Severe Ulcerative Colitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2018. [DOI] [PubMed] [Google Scholar]
- [22].Minacapelli CD, Bajpai M, Geng X, Van Gurp J, Poplin E, Amenta PS, Brant SR, Das KM, miR-206 as a Biomarker for Response to Mesalamine Treatment in Ulcerative Colitis. Inflammatory bowel diseases 2019, 25, 78–84. [DOI] [PubMed] [Google Scholar]
- [23].Schonauen K, Le N, von Arnim U, Schulz C, Malfertheiner P, Link A, Circulating and Fecal microRNAs as Biomarkers for Inflammatory Bowel Diseases. Inflammatory bowel diseases 2018, 24, 1547–1557. [DOI] [PubMed] [Google Scholar]
- [24].Feng Y, Dong YW, Song YN, Xiao JH, Guo XY, Jiang WL, Lu LG, MicroRNA449a is a potential predictor of colitisassociated colorectal cancer progression. Oncology reports 2018, 40, 1684–1694. [DOI] [PubMed] [Google Scholar]
- [25].Elson CO, Beagley KW, Sharmanov AT, Fujihashi K, Kiyono H, Tennyson GS, Cong Y, Black CA, Ridwan BW, McGhee JR, Hapteninduced model of murine inflammatory bowel disease: mucosa immune responses and protection by tolerance. Journal of immunology 1996, 157, 2174–2185. [PubMed] [Google Scholar]
- [26].Reagan-Shaw S, Nihal M, Ahmad N, Dose translation from animal to human studies revisited. FASEB J 2008, 22, 659–661. [DOI] [PubMed] [Google Scholar]
- [27].Guthrie AR, Chow HS, Martinez JA, Effects of resveratrol on drug- and carcinogen-metabolizing enzymes, implications for cancer prevention. Pharmacol Res Perspect 2017, 5, e00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Alharris E, Alghetaa H, Seth R, Chatterjee S, Singh NP, Nagarkatti M, Nagarkatti P, Resveratrol Attenuates Allergic Asthma and Associated Inflammation in the Lungs Through Regulation of miRNA-34a That Targets FoxP3 in Mice. Frontiers in immunology 2018, 9, 2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Alghetaa H, Mohammed A, Sultan M, Busbee P, Murphy A, Chatterjee S, Nagarkatti M, Nagarkatti P, Resveratrol protects mice against SEB-induced acute lung injury and mortality by miR-193a modulation that targets TGF-beta signalling. Journal of cellular and molecular medicine 2018, 22, 2644–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Singh NP, Singh UP, Hegde VL, Guan H, Hofseth L, Nagarkatti M, Nagarkatti PS, Resveratrol (trans-3,5,4′-trihydroxystilbene) suppresses EL4 tumor growth by induction of apoptosis involving reciprocal regulation of SIRT1 and NF-kappaB. Mol Nutr Food Res 2011, 55, 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Miranda K, Yang X, Bam M, Murphy EA, Nagarkatti PS, Nagarkatti M, MicroRNA-30 modulates metabolic inflammation by regulating Notch signaling in adipose tissue macrophages. International journal of obesity 2018, 42, 1140–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Busbee PB, Nagarkatti M, Nagarkatti PS, Natural indoles, indole-3-carbinol (I3C) and 3,3′-diindolylmethane (DIM), attenuate staphylococcal enterotoxin B-mediated liver injury by downregulating miR-31 expression and promoting caspase-2-mediated apoptosis. PloS one 2015, 10, e0118506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pekow J, Meckel K, Dougherty U, Huang Y, Chen X, Almoghrabi A, Mustafi R, Ayaloglu-Butun F, Deng Z, Haider HI, Hart J, Rubin DT, Kwon JH, Bissonnette M, miR-193a-3p is a Key Tumor Suppressor in Ulcerative Colitis-Associated Colon Cancer and Promotes Carcinogenesis through Upregulation of IL17RD. Clinical cancer research : an official journal of the American Association for Cancer Research 2017, 23, 5281–5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang L, Xue H, Zhao G, Qiao C, Sun X, Pang C, Zhang D, Curcumin and resveratrol suppress dextran sulfate sodiuminduced colitis in mice. Molecular medicine reports 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Craveiro M, Cretenet G, Mongellaz C, Matias MI, Caron O, de Lima MCP, Zimmermann VS, Solary E, Dardalhon V, Dulic V, Taylor N, Resveratrol stimulates the metabolic reprogramming of human CD4(+) T cells to enhance effector function. Science signaling 2017, 10. [DOI] [PubMed] [Google Scholar]
- [36].Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M, T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proceedings of the National Academy of Sciences of the United States of America 2008, 105, 7797–7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yao J, Wei C, Wang JY, Zhang R, Li YX, Wang LS, Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice. World journal of gastroenterology 2015, 21, 6572–6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Song-Zhao GX, Maloy KJ, Experimental mouse models of T cell-dependent inflammatory bowel disease. Methods in molecular biology 2014, 1193, 199–211. [DOI] [PubMed] [Google Scholar]
- [39].Claesson MH, Bregenholt S, Bonhagen K, Thoma S, Moller P, Grusby MJ, Leithauser F, Nissen MH, Reimann J, Colitis-inducing potency of CD4+ T cells in immunodeficient, adoptive hosts depends on their state of activation, IL-12 responsiveness, and CD45RB surface phenotype. Journal of immunology 1999, 162, 3702–3710. [PubMed] [Google Scholar]
- [40].Powrie F, Correa-Oliveira R, Mauze S, Coffman RL, Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. The Journal of experimental medicine 1994, 179, 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A, CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. Journal of immunology 2001, 166, 3008–3018. [DOI] [PubMed] [Google Scholar]
- [42].Abdin AA, Targeting sphingosine kinase 1 (SphK1) and apoptosis by colonspecific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. European journal of pharmacology 2013, 718, 145–153. [DOI] [PubMed] [Google Scholar]
- [43].Samsami-Kor M, Daryani NE, Asl PR, Hekmatdoost A, Anti-Inflammatory Effects of Resveratrol in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Archives of medical research 2015, 46, 280–285. [DOI] [PubMed] [Google Scholar]
- [44].Samsamikor M, Daryani NE, Asl PR, Hekmatdoost A, Resveratrol Supplementation and Oxidative/Anti-Oxidative Status in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Archives of medical research 2016, 47, 304–309. [DOI] [PubMed] [Google Scholar]
- [45].Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Brant SR, Chakravarti S, Kwon JH, MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology 2008, 135, 1624–1635 e1624. [DOI] [PubMed] [Google Scholar]
- [46].Ahmed FE, Jeffries CD, Vos PW, Flake G, Nuovo GJ, Sinar DR, Naziri W, Marcuard SP, Diagnostic microRNA markers for screening sporadic human colon cancer and active ulcerative colitis in stool and tissue. Cancer Genomics Proteomics 2009, 6, 281–295. [PubMed] [Google Scholar]
- [47].Takagi T, Naito Y, Mizushima K, Hirata I, Yagi N, Tomatsuri N, Ando T, Oyamada Y, Isozaki Y, Hongo H, Uchiyama K, Handa O, Kokura S, Ichikawa H, Yoshikawa T, Increased expression of microRNA in the inflamed colonic mucosa of patients with active ulcerative colitis. J Gastroenterol Hepatol 2010, 25 Suppl 1, S129–133. [DOI] [PubMed] [Google Scholar]
- [48].Chen DF, Gong BD, Xie Q, Ben QW, Liu J, Yuan YZ, MicroRNA155 is induced in activated CD4(+) T cells of TNBS-induced colitis in mice. World journal of gastroenterology 2010, 16, 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chen P, Li Y, Li L, Yu Q, Chao K, Zhou G, Qiu Y, Feng R, Huang S, He Y, Chen B, Chen M, Zeng Z, Zhang S, Circulating microRNA146b-5p is superior to C-reactive protein as a novel biomarker for monitoring inflammatory bowel disease. Aliment Pharmacol Ther 2019, 49, 733–743. [DOI] [PubMed] [Google Scholar]
- [50].Yu M, Luo Y, Cong Z, Mu Y, Qiu Y, Zhong M, MicroRNA-590–5p Inhibits Intestinal Inflammation by Targeting YAP. J Crohns Colitis 2018, 12, 993–1004. [DOI] [PubMed] [Google Scholar]
- [51].Maschmeyer P, Petkau G, Siracusa F, Zimmermann J, Zugel F, Kuhl AA, Lehmann K, Schimmelpfennig S, Weber M, Haftmann C, Riedel R, Bardua M, Heinz GA, Tran CL, Hoyer BF, Hiepe F, Herzog S, Wittmann J, Rajewsky N, Melchers FG, Chang HD, Radbruch A, Mashreghi MF, Selective targeting of pro-inflammatory Th1 cells by microRNA-148a-specific antagomirs in vivo. J Autoimmun 2018, 89, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Necela BM, Carr JM, Asmann YW, Thompson EA, Differential expression of microRNAs in tumors from chronically inflamed or genetic (APC(Min/+)) models of colon cancer. PloS one 2011, 6, e18501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Olaru AV, Selaru FM, Mori Y, Vazquez C, David S, Paun B, Cheng Y, Jin Z, Yang J, Agarwal R, Abraham JM, Dassopoulos T, Harris M, Bayless TM, Kwon J, Harpaz N, Livak F, Meltzer SJ, Dynamic changes in the expression of MicroRNA-31 during inflammatory bowel disease-associated neoplastic transformation. Inflammatory bowel diseases 2011, 17, 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Liu Z, Bai J, Zhang L, Lou F, Ke F, Cai W, Wang H, Conditional knockout of microRNA-31 promotes the development of colitis associated cancer. Biochem Biophys Res Commun 2017, 490, 62–68. [DOI] [PubMed] [Google Scholar]
- [55].Gwiggner M, Martinez-Nunez RT, Whiteoak SR, Bondanese VP, Claridge A, Collins JE, Cummings JRF, Sanchez-Elsner T, MicroRNA-31 and MicroRNA-155 Are Overexpressed in Ulcerative Colitis and Regulate IL-13 Signaling by Targeting Interleukin 13 Receptor alpha-1. Genes (Basel) 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tian Y, Xu J, Li Y, Zhao R, Du S, Lv C, Wu W, Liu R, Sheng X, Song Y, Bi X, Li G, Li M, Wu X, Lou P, You H, Cui W, Sun J, Shuai J, Ren F, Zhang B, Guo M, Hou X, Wu K, Xue L, Zhang H, Plikus MV, Cong Y, Lengner CJ, Liu Z, Yu Z, MicroRNA-31 Reduces Inflammatory Signaling and Promotes Regeneration in Colon Epithelium, and Delivery of Mimics in Microspheres Reduces Colitis in Mice. Gastroenterology 2019. [DOI] [PubMed] [Google Scholar]
- [57].Whiteoak SR, Claridge A, Balendran CA, Harris RJ, Gwiggner M, Bondanese VP, Erlandsson F, Hansen MB, Cummings JRF, Sanchez-Elsner T, MicroRNA-31 Targets Thymic Stromal Lymphopoietin in Mucosal Infiltrated CD4+ T Cells: A Role in Achieving Mucosal Healing in Ulcerative Colitis? Inflammatory bowel diseases 2018, 24, 2377–2385. [DOI] [PubMed] [Google Scholar]