Abstract
To better understand the clinical phenotype of acute graft-versus-host disease (GVHD) in children, we examined the GVHD clinical stage, grade, and response to prednisone 60 mg/m2/day PO in a diverse group of 370 pediatric patients with acute GVHD treated from 1990 to 2016 at a single institution. Overall response [complete response (CR) + partial response (PR)] at day 28 occurred in 65%, (CR 52%; PR 13%). Initial GVHD grade did not predict day 28 response. However, the Minnesota GVHD Risk Score predicted response with 68% standard risk (SR)-GVHD patients achieving CR/PR at day 28 versus 48% high risk (HR)-GVHD patients (p < 0.01). Multivariable analysis confirmed that response rates were lower in patients with HR-GVHD [odds ratio (OR), 0.4, p < 0.01] and in recipients of HLA mismatched URD (OR 0.4, p= 0.03). Transplant-related mortality (TRM) at 2 years was greater in HR-GVHD patients, recipients of HLA-partially matched or mismatched unrelated donor (URD) grafts, but not umbilical cord blood (UCB). These data highlight the importance of including children in novel acute GVHD treatment trials. Compared with initial GVHD grade, the Minnesota GVHD Risk Score better demarcates risk of steroid failure and TRM in children and could be used for risk stratification in pediatric acute GVHD studies.
Introduction
Despite many major advances in pediatric hematopoietic cell transplantation (HCT), acute graft-versus-host disease (GVHD) remains a major cause of morbidity and mortality. Most large published GVHD reports focus predominantly on adults with few describing the distinct pediatric aspects of the syndrome [1]. Children with acute GHVD are usually treated with therapies studied predominantly in adults with assumptions as to the expected effectiveness in younger patients. As children have unique underlying diseases, comorbidities, and pharmacokinetics, the characteristics of acute GVHD in children and response to therapy may differ from adults and warrants specific investigation.
At the University of Minnesota, we have the ability to examine the clinical phenotype of acute GVHD and responses to therapy in a large diverse population of children with malignant and nonmalignant diseases undergoing HCT using a variety of graft sources including umbilical cord blood (UCB). We collect GVHD staging and grade, therapy, and response on all patients on a weekly basis until at least day 100 after HCT. We also treat de novo acute GVHD using structured protocol driven therapy, allowing us to examine patient and transplant factors associated with response to uniform GVHD therapy. In order to characterize acute GVHD and response to steroids as primary therapy in a large pediatric cohort treated in a uniform manner, we retrospectively examined the outcomes of 370 children with acute GVHD who underwent HCT at the University of Minnesota.
Patients and methods
Study design
Between January 1990 and December 2016, 370 pediatric (i.e.<18 years of age) University of Minnesota allogeneic HCT patients developed grade I–IV acute GVHD and were treated systemically with prednisone 60 mg/m2/day PO (or methylprednisolone 48 mg/m2/day IV), followed by an 8 week taper [2, 3] as initial therapy. Patients with grade I GVHD not treated with systemic therapy were excluded from this analysis. Clinical and laboratory data were systematically and prospectively collected on all our patients undergoing HCT and entered into the University of Minnesota Blood and Marrow Transplant Database. All HCT protocols were reviewed and approved by the Masonic Cancer Center Protocol Review Committee and the Institutional Review Board (IRB) at the University of Minnesota. All parents/guardians signed IRB approved informed consent for HCT and data collection in accordance with the Declaration of Helsinki.
Patient and transplant characteristics
Patient demographics including year of transplant, recipient age, gender, cytomegalovirus (CMV) serostatus, and underlying diagnosis are shown in Table 1. All patients were recipients of their first allogeneic transplant. For the cohort of 370 patients, the median patient age was 7 years (range, 0.2–17.95) with 67 (18%) <2 years of age. Two thirds of the patients were male. Underlying diseases included malignancies (43%), inborn errors of metabolism (25%), Fanconi anemia (7%), severe aplastic anemia (6%), immune deficiencies (7%), and epidermolysis bullosa (1%). The vast majority of patients (82%) had a Lansky/Karnofsky score ≥90% at time of HCT.
Table 1.
Patient and transplant characteristics
| Factors | N (%) |
|---|---|
| Total | 370 |
| Year of transplant | |
| 1990–1995 | 119 (32%) |
| 1996–2000 | 106 (29%) |
| 2001–2005 | 69 (19%) |
| 2006–2010 | 30 (8%) |
| 2011–2016 | 46 (12%) |
| Age | |
| <2 years | 67 (18%) |
| 2–9 years | 168 (45%) |
| 10–17 years | 135 (36%) |
| Median, years (range) | 7 (0.2–17.95) |
| Gender | |
| Male | 244 (66%) |
| Female | 126 (34%) |
| Disease | |
| Acute lymphoblastic leukemia | 91 (25%) |
| Acute myelogenous leukemia | 60 (16%) |
| CML/JMML | 19 (5%) |
| Myelodsyplastic syndrome | 19 (5%) |
| Hodgkins lymphoma/Non-Hodgkins lymphoma | 8 (2%) |
| Inborn error of metabolism | 94 (25%) |
| Fanconi anemia | 27 (7%) |
| Aplastic anemia | 21 (6%) |
| Immune deficiency | 27 (7%) |
| Epidermolysis bullosa | 4 (1%) |
| Disease risk | |
| Standard risk malignancy | 132 (36%) |
| High risk malignancy | 65 (18%) |
| Nonmalignant disease | 173 (47%) |
| CMV serostatus | |
| Recipient negative, donor negative | 167 (45%) |
| Recipient negative and/or donor positive | 203 (55%) |
| Donor type | |
| HLA-matched sibling | 61 (16%) |
| HLA-mismatched sibling | 8 (2%) |
| Well matched URD | 48 (13%) |
| Partial matched URD | 56 (15%) |
| HLA-mismatched URD | 65 (18%) |
| Single UCB | 97 (26%) |
| Double UCB | 35 (9%) |
| Conditioning | |
| Myeloablative | 351 (95%) |
| Reduced intensity | 19 (5%) |
| GVHD prophylaxis | |
| Cyclosporine or tacrolimus containing | 290 (78%) |
| T-cell depletion | 65 (18%) |
| Methotrexate alone | 14 (4%) |
| Sirolimus and mycophenolate mofetil | 1 (0.3%) |
Standard risk = acute leukemia in CR1 or CR2, CML in first chronic phase, MDS without excess blasts or nonmalignant diseases. High risk malignancy = all others
CML chronic myelogenous leukemia, JMML juvenile myelomonocytic leukemia
Transplant characteristics including donor type, preparative therapy, and GVHD prophylaxis are shown in Table 1. Related donors and recipients were typed at antigen level for HLA-A, -B, and -DRB1 unless adequate family typing was not available to determine haplotypes, which were then confirmed by allele level DNA typing. For unrelated (URD) donors, donors and recipients were initially typed for HLA-A and -B at antigen level and allele level typing at HLA-DRB1 until 2004 when allele typing for HLA-C and other loci was fully implemented. HLA-matching followed the definitions used by the CIBMTR [4]. For UCB transplants, patients and donors were typed for HLA-A and -B at antigen level and for DRB1 at allele level [5, 6]. HLA-DQ and -DP were not considered in URD or UCB donor selection.
Graft sources included HLA identical sibling bone marrow (BM, n = 58) or peripheral blood stem cells (PBSC; n = 3), one antigen mismatched sibling BM (n = 5) or PBSC (n = 1), two antigen mismatched sibling PBSC (n = 1), HLA matched URD BM (n = 46) or PBSC (n = 2), HLA mismatched unrelated BM (n = 55) or PBSC (n = 1), single umbilical cord blood (UCB; n = 97) or double UCB (n = 35).
Details of the preparative therapy, GVHD prophylaxis, and supportive therapies have been previously reported [7–10]. The majority (95%) of patients received a myeloablative conditioning regimen. The preparative therapy was determined by the underlying disease and donor type. Overall 70% patients received a radiation-based regimen and 30% patients receiving a chemotherapy only regimen. The majority (89%) of patients with malignancies received a radiation-based regimen. Most patients (61%) with inborn errors of metabolism received chemotherapy alone and 57% bone marrow failure/immune deficiency patients received a single fraction of irradiation (57%) or chemotherapy alone (43%). GVHD prophylaxis consisted of cyclosporine A (CSA) based therapy in 78% of patients, ex vivo T-cell depletion in 18% of patients, methotrexate (MTX) alone in 4% patients, and sirolimus and mycophenolate mofetil (MMF) in one patient.
Diagnosis, staging, and grading of GVHD
Signs and symptoms of acute GVHD were graded by the Minnesota GVHD grading system [3]. Grade of GVHD refers to clinical (not histologic) grade throughout this report. Initial GVHD grade was calculated using the maximum stage in each organ within a 7 day window preceding initiation of steroid therapy (i.e., days −7 to 0). If no staging was available during this window (n = 64), the maximum stage was determined during the week following initiation of steroid therapy (day +1 to +2, n = 30; day +3 to +7, n = 34). The Minnesota GVHD Risk Score was also used to identify standard risk (SR) vs. high risk (HR) patients by the number of involved organs and severity of GVHD at onset [11]. Prospective real-time organ staging and grading of GVHD was determined weekly by the attending physician, supported by laboratory and clinical information and histologic confirmation when possible. Responses at weekly endpoints were determined by detailed review (MLM, SRH, AR, DJW) of all prospectively recorded staging and grading data.
GVHD therapy
All patients received daily, thrice divided doses of prednisone 60 mg/m2/day PO (or methylprednisolone IV equivalent, 48 mg/m2) for seven consecutive days, followed by daily or twice daily, prednisone for 7 days as initial therapy for acute GVHD. Patients were maintained on therapeutic levels of CSA or tacrolimus. In addition, patients with skin acute GVHD were treated with topical 0.1% triamcinolone cream or 1% hydrocortisone cream (for facial rash) three times daily. If a response to prednisone was observed, patients continued therapy with oral prednisone 60 mg/m2/day through day 14 and then commenced a taper of steroids over 8 weeks [3, 12].
Measurement of GVHD response to prednisone
Response to therapy was evaluated by the attending physician and prospectively recorded weekly in the University of Minnesota BMT Database by determining the GVHD clinical stage for each time point (±3 days) [13]. Response was determined from the maximum GVHD stage and grade in each organ at day 28 (±7 days) after prednisone treatment was initiated. Complete response (CR) was defined as the complete resolution of acute GVHD symptomatology in all organs, without secondary GVHD therapy. Partial response (PR) was defined as improvement in GVHD stage in all initial GVHD target organs without complete resolution, without worsening in any other GVHD target organs and without secondary GVHD therapy. No response was defined as the same severity of GVHD in any organ, or the addition of secondary GVHD therapy or death prior to day 28. Progression was defined as worsening GVHD in ≥1 organ with or without improvement in any organ. Steroid resistant acute GVHD was defined as progression of acute GVHD after 4 days of treatment with prednisone or no improvement after 7 days of treatment. Patients with steroid resistant GVHD were treated with secondary therapy and were considered to have no response. If patients experienced a flare of acute GVHD before day 28 and required therapy with a boost of steroids or the additional GVHD therapy, they were also considered to have no response.
Supportive care
Broad-spectrum prophylactic antibiotics was prescribed in all patients. Patients received acyclovir prophylaxis if they were seropositive for herpes simplex virus and/or cytomegalovirus (CMV). Oral trimethoprim–sulfamethoxazole was given for pneumocystis jiroveci pneumonia prophylaxis. CMV-seronegative recipients received CMV-safe (seronegative or filtered) blood products. Additional intravenous antibacterial and, as indicated antifungal and antiviral antimicrobials were used when patients developed fever.
Statistical analysis
Univariate assessment of various factors on response at day 28 after initiation of steroid therapy was performed by the Chi-square test. Overall survival after treatment was estimated by Kaplan–Meier curves [14]. Treatment-related mortality and the competing risk of relapse or death due to disease were analyzed using cumulative incidence [15]. Comparisons were completed with the Log-Rank or Gray’s test. Assessment of day 28 response on endpoints was performed in a similar manner but with landmark analyses excluding deceased patients (2%) prior to the day 28 assessment [16]. This approach which is conditional on the response status of patients at the landmark time corrects for the bias due to guaranteed survival/observation time among the responders.
Logistic regression was used to examine the independent effect of factors on the endpoint of response. Cox regression was used to assess the independent effect of the indices on 2-year overall survival [17]. Fine and Gray proportional hazards regression was used to assess the independent effect of the indices on TRM [18]. All reported p-values were 2sided. All analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC) and R version 3.3.1.
Results
The median time from HCT to initiation of steroid therapy for acute GVHD was 32 days after HCT (range, 8–156). All patients had at least 6 months follow-up from initiation of acute GVHD treatment (median, 11.8 years; range 0.7–24.3).
The initial GVHD stage in each organ is shown in Table 2. Initial GVHD organ involvement was skin only (n = 250; 68%), upper GI only (n = 23; 6%), lower GI only (n = 26; 7%), upper and lower GI (n = 18; 5%), liver only (n = 3; 1%) or multi-organ (n = 50; 14%). The distribution of initial GVHD organ involvement did not differ by underlying diagnosis, age, or donor type. Skin only GVHD occurred in 100/132 (76%) UCB recipients and 150/ 238 (63%) non-UCB recipients.
Table 2.
Maximum initial GVHD stage at the onset of prednisone therapy
| Organ stage | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Skin | 72 (19%) | 45 (12%) | 95 (26%) | 153 (41%) | 5 (1%) |
| Liver | 361 (97%) | 2 (1%) | 2 (1%) | 2 (1%) | 3 (1%) |
| Lower GI | 286 (77%) | 32 (9%) | 24 (6%) | 19 (5%) | 9 (2%) |
| Upper GI | 312 (84%) | 58 (16%) | |||
Prior to initiation of steroid therapy, initial GVHD grades were grade I in 110 (30%) patients, grade II in 197 (53%) patients, grade III in 49 (13%) patients, and grade IV in 14 (4%) patients. According to the Minnesota acute GVHD Risk Score, 328 (89%) patients had Minnesota SR-GVHD and 42 (11%) patients had Minnesota HR-GVHD. The risk groups had similar clinical characteristics except the time to onset of acute GVHD was earlier in the SR versus the HR patients (median day 29 vs. 36, P < 0.01) as shown in Table 3.
Table 3.
Minnesota GVHD risk score groups
| Standard risk | High risk | P | |
|---|---|---|---|
| N | 328 | 42 | |
| Age at transplant | 0.55 | ||
| Median (range), years | 7 (<1–17) | 8 (<1–17) | |
| Donor type | 0.20 | ||
| HLA-matched sibling donor | 57 (17%) | 4 (10%) | |
| Alternative donor | 271 (83%) | 38 (90%) | |
| Diagnosis | 0.39 | ||
| Malignancy | 172 (52%) | 25 (60%) | |
| Inborn errors of metabolism | 87 (27%) | 7 (17%) | |
| FA/AA/EB/Immune deficiency | 69 (21%) | 10 (24%) | |
| Organ involvement | <0.01 | ||
| Multi-organ | 33 (10%) | 17 (40%) | |
| Skin only | 245 (75%) | 5 (12%) | |
| Lower GI and upper GI | 13 (4%) | 5 (12%) | |
| Liver only | 0 | 3 (7%) | |
| Lower GI only | 14 (4%) | 12 (29%) | |
| Upper GI only | 23 (7%) | 0 | |
| Days to acute GVHD | <0.01 | ||
| Median (range) | 29 (8–100) | 36 (9–100) | |
| Days to chronic GVHD | 0.58 | ||
| Median (range) | 183 (50–1043) | 190 (84–892) | |
FA Fanconi anemia, AA Aplastic anemia, EB Epidermolysis bullosa
At day 14 after steroid initiation, overall response (OR = CR + PR) was observed in 61% patients with CR in 36%, PR in 25% and NR in 39%. At day 28, more patients experienced CR with OR in 65%, CR in 52%, PR in 13% and NR in 35% patients. Similar and thus durable responses were observed at day 56, with OR in 65%, CR in 56%, PR in 9% and NR in 35% patients. Only 13% of patients with CR/PR by day 28 experienced a flare of acute GVHD by day 56.
Patients with multi-organ involvement at onset of acute GVHD were less likely to achieve CR/PR at day 28 (46%) compare with patients with skin only (68%), or those with gut only (70%) GVHD (p < 0.01). Only 1 of 3 patients with isolated liver acute GVHD achieved CR/PR at day 28.
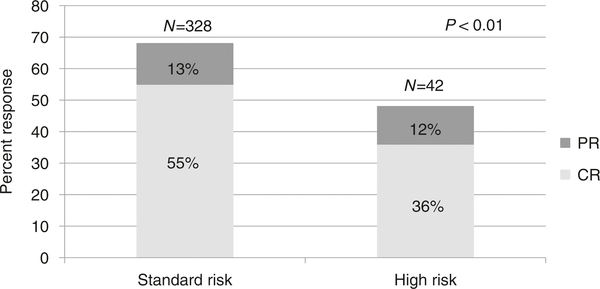
Initial GVHD grade did not predict overall response at day 28 being 68% for grade I, 67% for grade II, 59% for grade III, and 43% for grade IV (p = 0.21). However, as shown in Fig. 1, the Minnesota Risk Score significantly demarcated response with 68% SR patients achieving CR/PR at day 28 compared with only 48% HR patients (p < 0.01).
Fig. 1.
Day 28 response by Minnesota Risk group. CR = complete response. PR = partial response
In multiple regression analysis, adjusted for clinically significant variables in addition to Minnesota Risk group, the probability of day 28 CR/PR was lower in patients with HR-GVHD [Odds ratio (OR) 0.4, 95% CI 0.2–0.7, p < 0.01], and in recipients of HLA-mismatched URD grafts (OR 0.4, 95% CI 0.2–0.9, p= 0.03; Table 4). Organ involvement and GVHD grade were not included in this analysis due to collinearity with Minnesota Risk Score, although initial GVHD grade continued to lack a statistical association with response in the regression analysis when replaced for the Minnesota Risk Score (p = 0.08). Patient age, sex, underlying diagnosis, CMV serostatus, GVHD prophylaxis, time from HCT to GVHD onset showed no statistically significant association with day 28 response.
Table 4.
Factors associated with day 28 CR/PR, 2 year mortality and TRM: Multivariate analysis
| Factors | Odds ratio of CR/PR (95% CI) |
P | HR of mortality (95% CI) |
P | HR of TRM (95% CI) |
P |
|---|---|---|---|---|---|---|
| Minnesota GVHD risk | ||||||
| Standarda | 1.0 | 1.0 | 1.0 | |||
| High | 0.4 (0.2–0.7) | <0.01 | 1.4 (0.9–2.2) | 0.11 | 1.7 (1.0–2.7) | 0.03 |
| Donor type | <0.01 | <0.01 | <0.01 | |||
| Matched/MM siblinga | 1.0 | 1.0 | 1.0 | |||
| URD well matched | 1.5 (0.7–3.4) | 0.31 | 1.5 (0.8–2.6) | 0.22 | 1.8 (0.9–3.6) | 0.10 |
| URD partial matched | 0.9 (0.4–1.9) | 0.78 | 1.9 (1.1–3.4) | 0.02 | 2.1 (1.1–4.0) | 0.03 |
| URD mismatched | 0.4 (0.2–0.9) | 0.02 | 2.4 (1.4–4.0) | <0.01 | 2.9 (1.6–5.2) | <0.01 |
| UCB | 1.7 (0.9–3.3) | 0.10 | 1.2 (0.7–2.0) | 0.41 | 1.0 (0.5–1.8) | 0.92 |
Reference group
Factors tested included all factors from univariate in a backward selection only keeping factors with overall p < 0.10 in final model. Organ involvement and grade of acute GVHD were not included due to collinearity with “new risk”. Lansky/Karnofsky was not included due to too many missing values. Age was treated as a continuous factor. Standard-risk disease includes acute leukemia/NHL in CR1 or CR2, CML in 1st chronic phase, MDS without excess blasts. High risk disease includes all others.
The Minnesota GVHD Risk Score was predictive of response regardless of transplant era
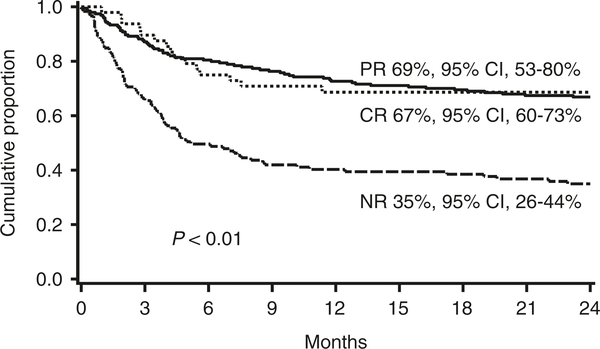
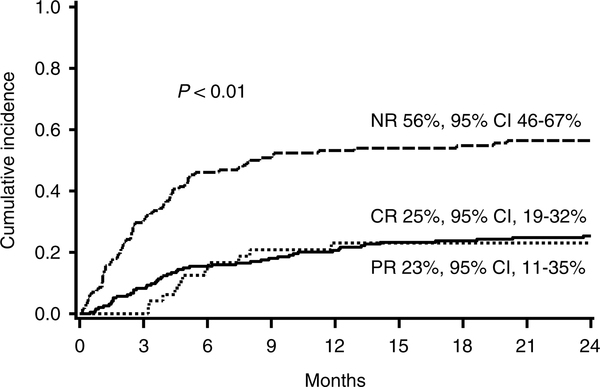
For the entire cohort, the probability of survival at 2 years was 55% [95% confidence interval (CI), 50–60%] and the probability of transplant related mortality (TRM) at 2 years was 32% [95% confidence interval (CI), 27–37%]. In a landmark analysis, patients without CR/PR at day 28 had lower survival at 2 years (35%, 95% CI, 26–44%) than patients with CR (67%, 95% CI, 60–73%) or PR at day 28 (69%, 95% CI, 53–80%; p < 0.01; Fig. 2). Similarly, patients without CR/PR at day 28 had significantly higher TRM at 2 years (52%, 95% CI 42–62%) than patients with either CR (22%, 95% CI, 16–28%) or PR at day 28 (21%, 95% CI, 9–33%; p < 0.01; Fig. 3).
Fig. 2.
Survival at 2 years after onset of steroid therapy by response to steroids at day 28
Fig. 3.
Cumulative incidence of TRM at 2 years after onset of steroid therapy by response to steroids at day 28
Patients with HR-GVHD had a 1.7-fold increased risk of TRM at 2 years [cumulative incidence 47%: HR 1.6, 95% CI, 1.0–2.7] compared with SR-GVHD (cumulative incidence 35% 2 year TRM), p = 0.03; Table 4). Risks of TRM were also significantly higher in recipients of HLA partially matched (HR 2.1, 95% CI, 1.1–4.0, p = 0.03) or HLA-mismatched adult volunteer URD grafts (HR 2.9, 95% CI, 1.6–5.2, p < 0.01), but not in recipients of UCB grafts. When the Minnesota Risk Score was replaced by initial GVHD grade, there was no statistical association between grade and TRM (p = 0.40).
Chronic GVHD
Two years after initiation of steroid therapy, 83 patients had developed chronic GVHD for a cumulative incidence of 22% (95% CI, 18–27%). There was no difference in the incidence (22% vs. 27%, p = 0.39) or time to onset of chronic GVHD from HCT (median 183 vs. 190 days, p = 0.58) between SR-and HR-GVHD patients. Risks of chronic GVHD were significantly lower in UCB recipients (HR 0.4, 95% CI, 0.2–0.8; p = 0.01) and in patients with high risk malignant disease (HR 0.4, 95% CI, 0.2–0.9; p = 0.02). There was no statistically significant interaction between underlying disease and graft source with the development of chronic GVHD. In patients with malignant or nonmalignant disease, the cumulative incidence of chronic GVHD (24% vs. 20%) and its competing risk of 2 year TRM (36% vs. 35%) were similar (p = 0.30).
Within the entire cohort of 370 patients, 165 died by 24 months after onset of acute GVHD. Of those who died, acute GVHD was the cause of death for 45 (51%) patients with malignant diseases, 23 (58%) patients with inborn errors of metabolism and 24 (67%) of patients with other nonmalignant diseases. Of the 197 patients with malignant disease and acute GVHD, 35 (39%) died from relapse. The causes of death did not differ significantly between patients with SR- vs. HR-GVHD.
Discussion
This is the largest pediatric series examining the clinical phenotype of acute GVHD at diagnosis and response to uniform upfront steroid therapy. The strength of these data include weekly GVHD organ staging, structured protocol driven eligibility, and uniform upfront GVHD therapy, facilitating identification of patient and transplant factors associated with outcomes. As well, we were able to examine the clinical phenotype of acute GVHD and responses to therapy in a large diverse population of children with malignant and nonmalignant diseases undergoing HCT using a variety of graft sources including UCB. We observed an overall response at day 28 in 65% children with 2 year survival of 55% and TRM of 32% for the entire cohort. Compared with initial GVHD grade, the Minnesota GVHD Risk Score better demarcates risk of steroid failure and TRM in children and could be used for real time risk stratification in future pediatric GVHD studies, once validated in a unique pediatric multicenter cohort, while awaiting the results of biomarker assays.
In addition to the Minnesota Risk score, donor type was the only other factor associated with response to steroids and later outcomes. Recipients of HLA mismatched URD grafts were less likely to achieve a response to steroids and had at least a 2.4 fold increase risk of mortality and TRM at 2 years than sibling donor recipients. Conversely, the use of unrelated UCB grafts was not associated with worse GVHD outcomes and was associated with lower risks of chronic GVHD, suggesting UCB may be a better alternative donor source for children.
Despite the heterogeneity of diseases in this cohort, the response to steroids for acute GVHD was similar in patients with differing diagnoses and transplant conditioning regimens. We also observed that patients with high risk malignancies had a lower risk of developing chronic GVHD than patients with standard risk patients regardless of stem cell source. These novel findings in pediatric patients with acute GVHD may assist treating physicians with therapeutic decisions.
HLA typing and supportive care measures have substantially improved over the 26 year period of this cohort. Although these practice changes would not play a major role on response to upfront steroid GVHD therapy, some of these changes especially antimicrobials, certainly impact survival and TRM. Interestingly, there was a higher incidence of high risk GVHD in the more recent era.
When compared with our previously published series of mostly adult patients [11], a number of comparative observations can be made. The clinical phenotype of acute GVHD is different in children with a higher incidence of isolated skin involvement, less liver involvement and less multi-organ involvement than adults. However, despite the Minnesota Risk Score being based upon organ staging, a similar proportion of children had HR acute GVHD as in our previous report of predominantly adults (11% vs. 16%). Importantly, children respond to steroids as upfront GVHD therapy to a similar extent as adults, both overall and when assessed by Minnesota GVHD Risk Score.
These results highlight the importance of including children in novel GVHD trials as 1/3 of pediatric patients with acute GVHD are not successfully treated with steroids as primary GVHD therapy. Innovative GVHD trials need to investigate less toxic therapies for standard risk patients and novel approaches for those with high risk GVHD. If established drug treatment regimens from studies in adults are to be repurposed for novel pediatric GVHD trials, careful dose escalation studies may need to be performed in children to establish the optimal dose and schedule of new GVHD agents. As the clinical phenotype of acute GVHD in children is different than adults, pediatric specific GVHD trials may be warranted.
Acknowledgements
The authors thank the nurses, nurse coordinators, pharmacists, and physicians who cared for these patients and their families. In addition, we gratefully acknowledge the nurses and research staff whose dedicated efforts facilitated the prospective collection of the GVHD data.
Funding This study was supported in part by the National Institutes of Health, National Cancer Institute grant P01 CA065493-20.
Footnotes
Compliance with ethical standards
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Carpenter PA, MacMillan ML. Management of acute graft-versu-shost disease in children. Pediatr Clin North Am. 2010;57:273–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weisdorf D, Haake R, Blazar B, Miller W, McGlave P, Ramsay N, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75:1024–30. [PubMed] [Google Scholar]
- 3.MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–94. [DOI] [PubMed] [Google Scholar]
- 4.Weisdorf D, Spellman S, Haagenson M, Horowitz M, Lee S, Anasetti C, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker JN, Davies SM, DeFor T, Ramsay NK, Weisdorf DJ,Wagner JE. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood. 2001;97:2957–61. [DOI] [PubMed] [Google Scholar]
- 6.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–7. [DOI] [PubMed] [Google Scholar]
- 7.MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint foracute GVHD treatment trials. Blood. 2010;115:5412–7. [DOI] [PubMed] [Google Scholar]
- 8.Miller WP, Rothman SM, Nascene D, Kivisto T, DeFor TE, Ziegler RS, et al. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood. 2011;118: 1971–8. [DOI] [PubMed] [Google Scholar]
- 9.Mallhi KK, Smith AR, DeFor TE, Lund TC, Orchard PJ, Miller WP. Allele-Level HLA matching impacts key outcomes following umbilical cord blood transplantation for inherited metabolic disorders. Biol Blood Marrow Transplant. 2017;23:119–25. [DOI] [PubMed] [Google Scholar]
- 10.MacMillan ML, DeFor TE, Young JA, Dusenbery KE, Blazar BR, Slungaard A, et al. Alternative donor hematopoietic cell transplantation for Fanconi anemia. Blood. 2015;125:3798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacMillan ML, Robin M, Harris AC, DeFor TE, Martin PJ, Alousi A, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21:761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hings IM, Filipovich AH, Miller WJ, Blazar BL, McGlave PB,Ramsay NK, et al. Prednisone therapy for acute graft-versus-host disease: short-versus long-term treatment. A prospective randomized trial. Transplantation. 1993;56:577–80. [DOI] [PubMed] [Google Scholar]
- 13.Hings IM, Severson R, Filipovich AH, Blazar BR, Kersey JH, Ramsay NK, et al. Treatment of moderate and severe acute GVHD after allogeneic bone marrow transplantation. Transplantation. 1994;58:437–42. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incompleteobservations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 15.Lin DY. Non-parametric inference for cumulative incidencefunctions in competing risks studies. Stat Med. 1997;16:901–10. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–9. [DOI] [PubMed] [Google Scholar]
- 17.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220 [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]