Abstract
Chronic pancreatitis (CP) is characterized by progressive, irreversible morphologic and functional changes that are most commonly attributed to environmental insults, particularly when there is a genetic or anatomic predisposition. Heavy alcohol use and cigarette smoking are the most common environmental risk factors, but both may be absent. Antecedent episodes of acute pancreatitis occur in about half of patients, so active interventions are needed to reduce the risk of disease progression. Abdominal pain is the most common symptom, and requires a tailored approach depending on the anatomic changes in the pancreas. Other clinical manifestations include diabetes mellitus, exocrine pancreatic insufficiency, metabolic bone disease, pancreatic cancer, and anatomic complications. Current disease management is centered on risk factor reduction, and screening for and treating disease complications. There are no current therapies to delay or retard disease progression, but there are ongoing efforts to more fully understand the natural history of CP and underlying mechanisms of disease. These studies are expected to provide insights that will transform our approach to disease management and provide further hope to patients.
Keywords: pancreatogenic diabetes, exocrine pancreatic insufficiency, pancreatic cancer, osteoporosis, osteopenia, abdominal pain
Introduction
Chronic pancreatitis (CP) is a disease characterized by a syndrome of symptoms and clinical manifestations related to functional abnormalities that develops as the result of glandular fibrosis and atrophy related to acute and chronic inflammation1. CP most commonly occurs due to environmental insults, with increased risk in patients with genetic or anatomic predisposition. CP may be preceded by antecedent episodes of acute pancreatitis (AP), but is increasingly recognized in patients without a preceding history of AP or abdominal pain. Diagnosis is typically based on the presence of morphologic changes on either CT or MRI, which are frequently associated with functional changes (Figures 1–2)2. Disease-related complications are progressive and generally irreversible3. Unfortunately, there are no medical therapies to interrupt or reverse disease progression, so management primarily consists of screening for and providing early management of complications. Surgery is generally reserved for patients who develop refractory symptoms related to anatomic changes. There are a large number of remaining clinical questions that are being investigated to help clinicians manage this difficult disease.
Figure 1.
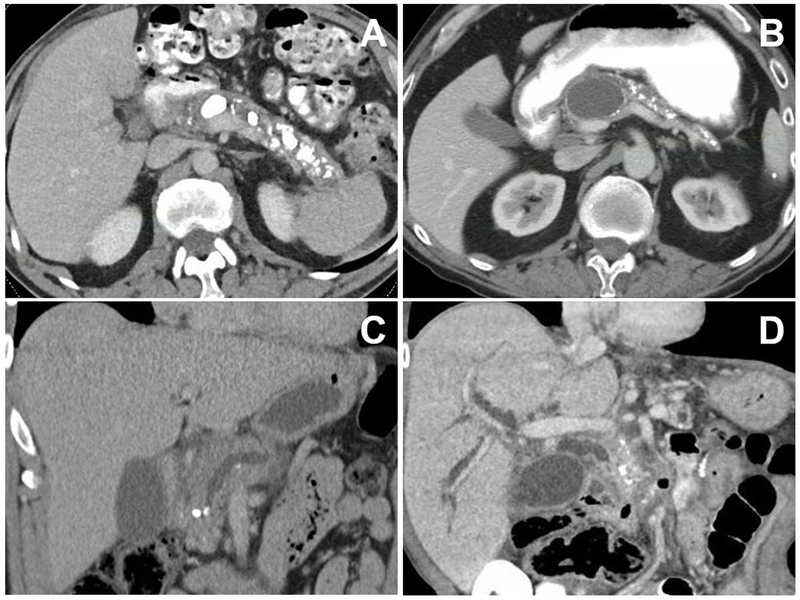
CT imaging features of chronic pancreatitis (from different patients). Panel A: Bulky calcifications in the pancreatic parenchyma and main pancreatic duct. Panel B: Medium-sized pancreatic pseudocyst causing mild pancreatic duct dilation and associated with small pancreatic calcifications and parenchymal atrophy. Panel C: Small pancreatic calcifications, including an intraductal stone causing pancreatic duct obstruction. Panel D: Biliary obstruction secondary to chronic pancreatitis manifest by intra- and extra-hepatic biliary dilation.
Figure 2.
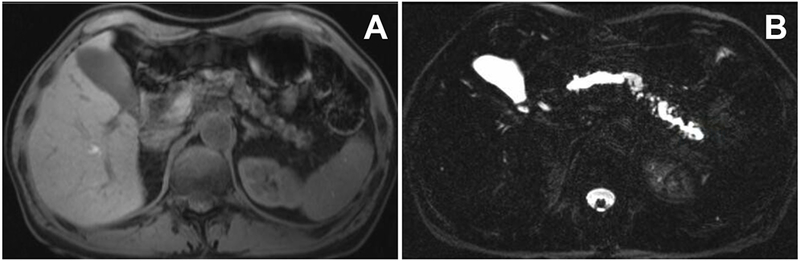
MR imaging features of chronic pancreatitis (in the same patient). Panel A. Decreased T1 signal of the gland and parenchymal atrophy. Panel B. Dilation of the main pancreatic duct and multiple branch ducts on a T2 MRCP image.
Epidemiology
CP is a rare disease (with an estimated prevalence <200,000) in the United States. Although it can develop at an early age of onset in those with a genetic predisposition, this is primarily a disease of adult onset. Despite methodologic variation in studies, prevalence estimates have remained relatively consistent at approximately 35-50 per 100,000 adults, with an incidence rate of 5 per 100,000 patient years4–6. A recent report suggests a two-fold higher prevalence of disease, but this estimate is likely inflated due to the use of diagnostic coding in an administrative database for case ascertainment7. Considering the poor positive predictive value (~50%) for the CP diagnostic code, the true prevalence is likely <50 per 100,000 adults8.
A slight male predominance of disease has been noted for decades, and the recent association with changes at the Claudin (CLDN)2 locus on the X chromosome may partially explain the sex distributions observed in alcohol-induced CP9, 10. Racial differences have been observed in CP, with black patients being more likely to have advanced morphologic changes on imaging and more severe pain and disability compared to whites11. These changes are likely partially explained by a greater frequency of underlying alcohol and/or tobacco use as risk factors. Historically, alcohol was felt to be the primary etiology of CP, but it is now recognized that this is the attributable etiology in <50% of patients10. Thus, patients should not be universally profiled as having an alcohol use disorder, which is often inaccurate and hinders the ability to establish rapport. It has been estimated that patients must consume 4-5 alcoholic drinks per day consistently for over 5 years to be at risk12. There are multiple effects of alcohol exposure to the pancreas, but despite common perceptions it is believed to sensitize the pancreas to injury rather than directly cause CP13.
The deleterious effects of cigarette smoking on the pancreas have recently been established from an epidemiologic standpoint, and there are emerging translational studies potentially explaining the pathogenesis. Previously, the association between CP and cigarette smoking was felt to be confounded by concurrent alcohol consumption, but Yadav et al. convincingly demonstrated an independent, dose dependent response of cigarette smoking on the risk for developing CP14. Importantly, cigarette smoking is a strong risk factor for recurrent acute pancreatitis (RAP), which often progresses to CP. Recent studies using the cerulean-induced model of CP demonstrated the negative effects of cigarette smoking are related to induction of interleukin-22 (IL-22) secondary to aryl hydrocarbons, which promote pancreatic fibrosis15. Collectively, the importance of avoiding heavy alcohol consumption and cigarette smoking cannot be overstated and should be strongly recommended to all patients at risk for the development of CP due to a history of acute pancreatitis, recurrent acute pancreatitis, or with a known genetic predisposition.
While historically CP was categorized as alcoholic or idiopathic an increasing proportion of patients originally considered idiopathic are now recognized to have an underlying genetic factor. A pivotal discovery in the genetics of AP and CP was the observation that an autosomal dominant mutation of the cationic trypsinogen (PRSS1) gene can independently lead to the development of acute and/or chronic pancreatitis16. This so-called hereditary pancreatitis has an autosomal dominant inheritance pattern with a high penetrance of disease (~80%), but it should be noted that patients can also develop the mutation de novo. Other mutations associated with an increased, albeit modest, risk for CP include the genes that encode serine peptidase inhibitor Kazal type 1 (SPINK1), cystic fibrosis transmembrane conductance regulator (CFTR), chymotrypsin C (CTRC), calcium-sensing receptor (CASR), and the aforementioned CLDN2 gene17. Investigations to identify additional genetic mutations remain underway, including carboxypeptidase A1 (CPA1) and carboxyl ester lipase (CEL) genes18–22.
Although uncommon, there are two unique subtypes of CP with clinical features that warrant mention. The first subset is referred to as tropical pancreatitis (previously fibrocalculous pancreatitis) since it is geographically concentrated in those from Southeast Asia, particularly India. Mutations in SPINK1 are commonly observed (40-50%) in tropical pancreatitis, but are not associated with differences in clinical phenotype23. Previously cassava root ingestion was suspected to have an etiologic role; however, this hypothesis hasn’t been supported by more recent studies and the differences in pathogenesis remain unexplained23. These patients tend to have a rapidly progressive disease course. The other unique subtype of CP is autoimmune pancreatitis (AIP), which includes both type 1 AIP (lymphoplasmacytic sclerosing pancreatitis) and type 2 AIP (idiopathic ductcentric pancreatitis)24. AIP is distinct from other types of CP based on specific histopathologic features, which define the two subtypes. There are differences in the clinical course for patients with AIP related to extrapancreatic manifestations (seen only in type 1 AIP) and inflammatory disease relapses. Patients may develop pancreatic calcifications (and subsequent pancreatic insufficiencies) that are indistinguishable from CP, and are more likely in patients with ≥1 disease relapse25. This topic has been recently reviewed in detail, so it is not discussed further26.
Clinical Management of Chronic Pancreatitis
In the absence of disease modifying medical therapies, the clinical management of chronic pancreatitis primarily consists of screening for and managing disease related complications. The contributing pathogenic mechanisms to the development of these complications include damage caused by acute inflammation, low grade chronic inflammation, pancreatic duct obstruction, and/or indirect systemic effects of the disease.
Abdominal pain
The most common symptomatic complaint of patients with CP is chronic abdominal pain, which may affect up to 80% of patients. The pain is classically described as epigastric with radiation to the back, but may present variably. This symptom can be debilitating and is strongly associated with a reduced quality of life, particularly when the pain is constant (as opposed to intermittent)27. Interestingly, the pattern of morphologic changes does not correlate with different pain patterns, illustrating the complex nature of pain in CP28. The origin for abdominal pain in CP is related to parenchymal ischemia caused by either acinar cell injury or pancreatic duct obstruction29. Local ischemia induces inflammation that in turn leads to the generation of nociceptive stimulation by peripancreatic nerves. Even when signals are intermittent, repetitive stimulation can lead to the development of permanent changes in the spinal cord and cerebral cortex (referred to as central sensitization)30, 31. This remodeling explains how some patients have persistent pain despite complete removal of the noxious stimulus (e.g., total pancreatectomy).
The treatment of abdominal pain in patients with CP may consist of combinations of medical, endoscopic, and/or surgical approaches. Unfortunately, these approaches are often not effective or have major consequences, so novel options are needed. Generally, treating pain involves addressing either anatomic or neurologic contributions to pain. Patients with significant anatomic obstruction (evidenced by a dilated main pancreatic duct) are considered candidates for endoscopic or surgical therapy. The aim of an endoscopic approach is to remove obstructing pancreatic duct stones (Figure 1C). When stones are large intraductal or extracorporeal shock wave lithotripsy (ESWL), depending on local expertise, may be required for stone fragmentation. Historically, ERCP was performed following ESWL. However, two studies failed to demonstrate additional benefit, so ERCP is generally reserved for situations in which there is not spontaneous clearance of stones after adequate fragmentation by ESWL32–34. Pancreatic duct strictures can also be treated with serial pancreatic duct stenting, but tend to require prolonged stent placement and often remain refractory34. Surgical options include lateral pancreaticojejunostomy (Puestow), Frey procedure, and pancreaticoduodenectomy (Whipple), which have different anatomic advantages. The improvement in pain is significantly better and more durable in patients undergoing surgery; however, endoscopy is often considered for those who are poor surgical candidates35. Endoscopy is also often considered as a therapeutic trial to identify patients who are most likely to benefit from surgery. Although this approach is intuitive the clinical evidence to support this approach is not robust and further methods to predict pain response are urgently needed to avoid unhelpful interventions36.
Medical therapies are typically recommended for patients without pancreatic duct obstruction, or those with a lower severity of pain. There have been a few recent randomized controlled trials looking at the use of antioxidants and pregabalin. The proposed mechanism for using antioxidant therapy (including vitamins A, C, E, selenium, and methionine) is to decrease nociceptive signaling from the pancreas by reducing systemic oxidative stress and thereby reducing ischemia in the tissue. Two large studies demonstrated mixed results, which may be the consequence of differences in the study populations (age and etiology of CP) and slight variations in the antioxidant formulations37, 38. While this is generally a well-tolerated regimen patients often decline due to the perception of limited efficacy. In another randomized study pregabalin was shown to have improved efficacy in reducing daily pain scores compared to placebo during a 3 week study; however, significant central nervous symptoms were seen in at least one-quarter of subjects on pregabalin, which limits its clinical utility39.
A variety of other therapies have previously been studied and demonstrated either no effects or the sample sizes were too small to confidently assess efficacy including octreotide, pancreatic enzymes, and enteral nutrition. As a result, pain is often managed using the step-wise approach recommended by the World Health Organization, including the use of non-steroidal anti-inflammatory drugs, then low potency opioids, then higher potency and long-acting opioids. Although it is still commonly used, in clinical practice the evidence base supporting the efficacy of celiac plexus block (either endoscopic or percutaneous) in CP remains weak. Importantly, in the absence of sham controlled studies, it is not possible to rule out a placebo effect, which can be inflated in patients receiving an interventional procedure 40. In patients who do respond to injections, the effect is temporary, lasting 3-4 months. Finally, for patients with abdominal pain attributed to CP and no severe ductal obstruction (sometimes referred to as small duct disease) total pancreatectomy may be carefully considered. To reduce the risk of difficult to treat diabetes, islet autotransplantation is concurrently performed (i.e., total pancreatectomy with islet autotransplantation (TPIAT)), if technically feasible41. A proper discussion of the nuances of TPIAT is beyond the scope of this review, but it is worth emphasizing TPIAT is not a panacea and improved methods of patient selection to identify those at risk of persistent opioid dependence (i.e., no pain response) is urgently needed, particularly when recommended for patients without pancreatic calcifications in whom the diagnosis of CP is controversial42.
Chronic pancreatitis-related diabetes mellitus
Diabetes mellitus is a frequent complication of CP with a point prevalence of 40%43, 44. CP-related DM (CP-DM) represents the most common cause of pancreatogenic DM (which has also been referred to as Type 3c DM). DM onset generally develops many years following disease onset, and may ultimately affect up to 80% of patients during their lifetime45. Due to the high prevalence of CP-DM annual screening for DM is recommended46, 47. A recent study of 1,171 subjects with CP demonstrated that DM was more likely to occur in subjects who were older, obese, male sex, black race, or with a family history of DM, while factors independently associated with DM included both obesity and the presence of exocrine pancreatic insufficiency (EPI)43. Other factors that have been associated with CP-DM include a prolonged duration of CP, absence of pain, cigarette smoking, and increased visceral adipose tissue48, 49.
The proposed pathogenic mechanism of CP-DM is primarily insulin deficiency secondary to fibrotic replacement of islets. However, insulin resistance occurs early in the disease course and is explained, in part, by hepatic insulin resistance mediated by pancreatic polypeptide deficiency45. Another contributing factor is an impaired incretin hormone response, but the relative contribution of this abnormality is uncertain, particularly for those without EPI. In the absence of clinical trial evidence to guide treatment decisions, metformin has been recommended as first line therapy based on experience with type 2 DM and translational studies suggesting potential antineoplastic effects46. Insulin is recommended as second line therapy or first line therapy for patients with severe hyperglycemia. In addition to directly addressing the primary pathologic insult, we speculate that insulin may also provide secondary benefits through reversing some of the catabolic processes observed in CP. A detailed characterization of the changes in glucose homeostasis compared to type 2 DM is needed to more clearly understand the pathogenesis, and inform the prevention and treatment of CP-DM50.
Exocrine Pancreatic Insufficiency
Exocrine pancreatic insufficiency (EPI) refers to inadequate production and/or secretion of pancreatic enzymes for nutrient digestion. Since there is a relatively small degree of redundancy to salvage lipids, the predominant symptoms from EPI are related to fat malabsorption. Symptoms of mild EPI include abdominal bloating or discomfort, while severe EPI can lead to overt steatorrhea and weight loss. Many patients deliberately restrict dietary fat intake, so symptoms often underestimate the severity of EPI. Considering the extraordinary reserve of the exocrine pancreas, as well as redundant mechanisms to digest protein and carbohydrates, EPI doesn’t develop until more than a decade after disease onset51. Ultimately, EPI affects over 70% of patients with CP during their lifetime, and is particularly common in those with proximal obstruction of the pancreatic duct or a history of pancreatic resection51. Since quantitative pancreatic function testing (i.e., cholecystokinin stimulated measurement of enzyme output) is no longer used a reduced coefficient of fat absorption (CFA) is generally considered the gold standard for diagnosis of EPI. Even though this is a highly accurate test for fat malabsorption, it is rarely used in clinical practice due to the burden of stool collection for 72 hours. Fecal elastase-1 (FE-1) is an indirect measure of exocrine function that is performed on a random stool sample. False positive test results are commonly encountered if a non-formed specimen is analyzed, because the elastase measurement is a concentration that will appear falsely low if there is increased water content in the stool. Therefore, the FE-1 test should not be used for evaluation of patients with unexplained diarrhea.
The lack of an accurate and convenient test to diagnose and monitor treatment of EPI remains one of the greatest challenges with management of EPI52, 53. In the absence of an ideal testing approach, most clinicians initiate treatment based on the combination of symptoms and pre-test probability. Treatment involves the use of pancreatic enzyme replacement therapy (PERT) at a dose of 25,000 – 50,000 units of lipase with meals, as well as 50% of the meal time dose with snacks3. In the absence of a suitable test to guide titration of the dose, we recommend increasing the dose at least until there is resolution of symptoms (particularly when overt steatorrhea is present). Additionally, patients often benefit from taking a higher dose for meals or snacks with a high fat content. Although weight-based dosing is used in the pediatric population (e.g., cystic fibrosis), this is not recommended for adults since the medication is not systemically absorbed. In patients with EPI with persistent symptoms despite initiation of PERT, additional management options to consider include: escalation of PERT dose, addition of a proton pump inhibitor (if not currently using), consideration of alternative etiologies of symptoms in CP, such as small intestinal bacterial overgrowth or lactose intolerance, and consideration of other causes of fat malabsorption54.
Metabolic Bone Disease
Metabolic bone disease is an increasingly recognized complication of CP and has also been referred to as CP-associated osteopathy. A meta-analysis of 10 studies estimated the pooled prevalence of both osteopenia and osteoporosis is 65%55. Furthermore, patients with CP have increased risk of low trauma fractures56. Although there are no societal guidelines to recommend screening for metabolic bone disease in CP, baseline screening with a DEXA scan is reasonable considering the higher odds of fractures compared to other gastrointestinal conditions, such as celiac disease, cholestatic liver disease, and inflammatory bowel disease, for which this practice has been widely adopted47, 56.
The high observed prevalence of CP-associated osteopathy is partly explained by shared risk factors, including cigarette smoking and heavy alcohol usage. Additionally, chronic inflammation from CP likely contributes to a pro-inflammatory milieu that produces net bone loss57. Lastly, patients with CP are at a high risk for vitamin D deficiency, particularly when EPI is present58. The management of CP-associated osteopathy follows general treatment principles, including supplementation with calcium and vitamin D, weight-bearing exercises, and smoking cessation. When indicated, treatment with oral bisphosphonates should be carefully monitored to ensure patients tolerate therapy, and transitioning to alternate anti-resorptive therapies should be considered if there are problems. Lastly, there are uncontrolled data suggesting that PERT may potentially reduce the risk of fractures in subjects with CP, but further studies are needed before this can be universally recommended59.
Pancreatic Cancer
Epidemiologic studies have consistently shown an increased risk of pancreatic cancer (i.e., pancreatic ductal adenocarcinoma (PDAC)) in patients with CP, which is felt to be a consequence of chronic inflammation leading hyperproliferation of pancreatic stellate cells60. The cumulative life time risk of PDAC is approximately 4-5%; however a history of cigarette smoking and the onset of CP-DM may increase this risk further61, 62. The risk is extraordinarily high in both hereditary and tropical pancreatitis. In hereditary (PRSS1) pancreatitis the cumulative risk for developing PDAC has traditionally be estimated at over 40%, but a recent publication suggests the risk is likely closer to 10%63. The relative risk in patients with tropical pancreatitis may exceed 100; however, contemporary updates are needed to determine if there has been a similar decline over time64. Although screening for PDAC is not endorsed, maintaining a high clinical suspicion in those with unexplained symptoms, such as unexplained weight loss or a change in abdominal pain characteristics, is recommended47. Unfortunately, even when pursued, cross-sectional imaging can be challenging due to the baseline morphologic changes in the pancreas (particularly main pancreatic duct dilation), which can make it difficult to identify a small neoplasm. Similarly, there is a risk for sampling error with EUS-guided fine needle aspiration in patients with multiple calcifications, which can obscure changes in the parenchyma.
Anatomic Complications
A variety of anatomic complications can develop in CP secondary to the local effects of inflammation or glandular fibrosis. One of the common anatomic complications is the development of pancreatic pseudocysts, which may develop in 10–40% of patients during their lifetime (Figure 1B)65. Depending on the anatomic location and size, pseudocysts can lead to gastroduodenal outlet obstruction and/or biliary obstruction. If they become symptomatic, endoscopic intervention using a cystgastrostomy and stent placement is generally preferred over surgical intervention as a first step66. Alternatively, gastroduodenal outlet obstruction and biliary obstruction can simultaneously develop secondary to inflammation and fibrosis involving the pancreatic head, when disease is centered around the pancreaticoduodenal groove (i.e., groove pancreatitis)67. When related to inflammation, management is generally supportive. However, when the obstruction is fixed intervention is required. While endoscopic intervention is typically entertained as the first step in managing a fibrotic biliary stricture (Figure 1D), gastric outlet obstruction has historically been managed with surgery (either a gastrojejunostomy or pancreaticoduodenctomy). Recently groups have reported endoscopic gastrojejunostomy for palliation of malignant disease, but the unknown durability limits the role in patients with CP. Lastly, thrombosis of the splanchnic vasculature (portal, splenic, and superior mesenteric veins) may develop in up to 20% of patients, and is felt to primarily be a consequence of local inflammation near the vascular beds68, 69. The use of anticoagulation has not been rigorously studied, but is generally not recommended, especially if there are chronic vascular changes such as collateralization.
Future Directions
Although our understanding of the pathogenesis and epidemiology of CP have grown over the last couple of decades, major knowledge gaps remain in the natural history and clinical management of CP. There are several factors that make clinical research studies of CP challenging, including the low disease prevalence, lack of standardized study definitions, long time to development of important clinical outcomes, and the lack of validated surrogate measures for those clinical outcomes. Although CP can be accurately diagnosed in the advanced stages of disease, when severe morphologic changes such as pancreatic calcifications are present, the diagnosis of early CP is challenging and controversial. In the absence of a diagnostic biomarker, prospective follow-up of patients with indeterminate clinical and imaging features over many years will be required to determine who truly develops CP or simply has a condition with overlapping symptoms, such as a functional gastrointestinal disorder. Fortunately, the critical nature of this gap has been recognized and is being actively pursued by multi-center consortia44, 70. In addition, the characterization of pediatric pancreatitis and its progression into adulthood is under investigation71. The development of biorepository platforms is included in these studies, and is needed to identify and validate biomarkers associated with the aforementioned disease-related complications. We are hopeful that translational studies will provide further insights into potential mechanisms of these complications that are currently considered irreversible. Identification of markers of disease progression is needed to prioritize pathways that can potentially be targeted by medical therapies. Considering the low disease prevalence, the process of drug development and testing for CP must be carefully considered. It will likely require a combination of evolving animal models72, 73, novel mathematical approaches74, and repurposing existing anti-inflammatory or biologic agents to make clinically measurable improvements.
Summary
CP is a chronic disorder, which is frequently accompanied by multiple disease-related complications, including abdominal pain, DM, EPI, metabolic bone disease, and pancreatic cancer. Current management of CP involves avoidance of cigarette smoking and heavy alcohol usage as well as vigilant clinical follow-up for screening and treatment of complications (Table). Although an early diagnosis would conceptually provide a suitable time window for early intervention, this is not currently feasible. Studies are ongoing to better understand the natural history of CP and its complications, which is needed to close the missing gaps needed to transform the care of our patients with CP.
Table.
Summary of complications associated with chronic pancreatitis.
| Chronic pancreatitis – related diabetes mellitus (CP-DM) | •Lifetime prevalence: up to 180% (point prevalence is ~40%) •Annual screening for DM is recommended. •Most patients will ultimately require insulin therapy, although metformin may be useful for mild hyperglycemia. |
| Exocrine pancreatic insufficiency (EPI) | •Lifetime prevalence: >70% •Accurate and convenient diagnostic testing methods do not exist, so ask about potential symptoms at each visit. •When performed, fecal elastase-1 should be measured in a formed stool specimen. •Initiate pancreatic enzyme replacement therapy at a dose of 25,000 to 50,000 units of lipase with meals, then titrate based on clinical response (including symptoms, body weight, and vitamin and nutritional markers). |
| Metabolic bone disease (CP-associated osteopathy) | •Point prevalence: ~ 66% (includes both osteoporosis and osteopenia) •Consider baseline DXA screening for all patients. •Manage according to general principles. |
| Pancreatic cancer | •Lifetime prevalence: ~ 4-5% •Screening is not universally recommended. •Maintain a high index of clinical suspicion for all patients, and consider periodic imaging for those with additional, strong risk factors. •Changes on imaging can be challenging to interpret, so comparison to baseline scans is needed. |
| Anatomic complications: | •Prevalence: estimates are imprecise. |
| Splanchnic vein thrombosis | •Splenic vein is most commonly involved vessel. •Anticoagulation is unlikely to provide clinical benefit. •Options for management of bleeding gastric varices include endoscopic intervention or splenectomy (for splenic vein thrombosis). |
| Pseudocyst | •Asymptomatic pseduocysts should be observed. •Symptomatic pseudocysts can typically be managed with endoscopic cystgastrostomy, but may require surgical drainage. |
| Duodenal obstruction | •Typically requires surgical bypass. |
| Biliary obstruction | •Can be successfully treated with serial endoscopic stent placement, but refractory cases require surgical biliary bypass. |
Acknowledgements
The authors would like to thank Dr. Mitch Ramsey, MD for his assistance developing a draft of the Table.
Grant Support: Research reported in this publication was supported by the National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under award number U01DK108327 (PH, DC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- AIP
autoimmune pancreatitis
- AP
acute pancreatitis
- CP
chronic pancreatitis
- DM
diabetes mellitus
- EPI
exocrine pancreatic insufficiency
- ESWL
extracorporeal shock wave lithotripsy
- EUS
endoscopic ultrasound
- PDAC
pancreatic ductal adenocarcinoma
Footnotes
Potential conflicts of interest/disclosures:
Dr. Hart has received consulting and honorarium fees from Kangen Pharmaceuticals.
References
- 1.Whitcomb DC, Frulloni L, Garg P, et al. Chronic pancreatitis: An international draft consensus proposal for a new mechanistic definition. Pancreatology 2016;16:218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conwell DL, Lee LS, Yadav D, et al. American Pancreatic Association practice guidelines in chronic pancreatitis: evidence-based report on diagnostic guidelines. Pancreas 2014;43:1143–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsey ML, Conwell DL, Hart PA. Complications of Chronic Pancreatitis. Dig Dis Sci 2017;62:1745–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav D, Timmons L, Benson JT, et al. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol 2011;106:2192–9. [DOI] [PubMed] [Google Scholar]
- 5.Hirota M, Shimosegawa T, Masamune A, et al. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology 2014;14:490–6. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez-Munoz JE, Lucendo A, Carballo LF, et al. A Spanish multicenter study to estimate the prevalence and incidence of chronic pancreatitis and its complications. Rev Esp Enferm Dig 2014;106:239–45. [PubMed] [Google Scholar]
- 7.Sellers ZM, MacIsaac D, Yu H, et al. Nationwide Trends in Acute and Chronic Pancreatitis Among Privately Insured Children and Non-Elderly Adults in the United States, 2007-2014. Gastroenterology 2018;155:469–478 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy NG, Nangia S, DiMagno MJ. The Chronic Pancreatitis International Classification of Diseases, Ninth Revision, Clinical Modification Code 577.1 Is Inaccurate Compared With Criterion-Standard Clinical Diagnostic Scoring Systems. Pancreas 2016;45:1276–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitcomb DC, LaRusch J, Krasinskas AM, et al. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat Genet 2012;44:1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conwell DL, Banks PA, Sandhu BS, et al. Validation of Demographics, Etiology, and Risk Factors for Chronic Pancreatitis in the USA: A Report of the North American Pancreas Study (NAPS) Group. Dig Dis Sci 2017;62:2133–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilcox CM, Sandhu BS, Singh V, et al. Racial Differences in the Clinical Profile, Causes, and Outcome of Chronic Pancreatitis. Am J Gastroenterol 2016;111:1488–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irving HM, Samokhvalov AV, Rehm J. Alcohol as a risk factor for pancreatitis. A systematic review and meta-analysis. JOP 2009;10:387–92. [PMC free article] [PubMed] [Google Scholar]
- 13.Singhvi A, Yadav D. Myths and realities about alcohol and smoking in chronic pancreatitis. Curr Opin Gastroenterol 2018;34:355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav D, Hawes RH, Brand RE, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med 2009;169:1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue J, Zhao Q, Sharma V, et al. Aryl Hydrocarbon Receptor Ligands in Cigarette Smoke Induce Production of Interleukin-22 to Promote Pancreatic Fibrosis in Models of Chronic Pancreatitis. Gastroenterology 2016;151:1206–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfutzer R, Myers E, Applebaum-Shapiro S, et al. Novel cationic trypsinogen (PRSS1) N29T and R122C mutations cause autosomal dominant hereditary pancreatitis. Gut 2002;50:271–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitcomb DC. Genetic risk factors for pancreatic disorders. Gastroenterology 2013;144:1292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witt H, Beer S, Rosendahl J, et al. Variants in CPA1 are strongly associated with early onset chronic pancreatitis. Nat Genet 2013;45:1216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin JH, Boulling A, Masson E, et al. Most unambiguous loss-of-function CPA1 mutations are unlikely to predispose to chronic pancreatitis. Gut 2019. [DOI] [PubMed] [Google Scholar]
- 20.Hegyi E, Sahin-Toth M. Human CPA1 mutation causes digestive enzyme misfolding and chronic pancreatitis in mice. Gut 2019;68:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fjeld K, Weiss FU, Lasher D, et al. A recombined allele of the lipase gene CEL and its pseudogene CELP confers susceptibility to chronic pancreatitis. Nat Genet 2015;47:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou WB, Boulling A, Masamune A, et al. No Association Between CEL-HYB Hybrid Allele and Chronic Pancreatitis in Asian Populations. Gastroenterology 2016;150:1558–1560 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Midha S, Khajuria R, Shastri S, et al. Idiopathic chronic pancreatitis in India: phenotypic characterisation and strong genetic susceptibility due to SPINK1 and CFTR gene mutations. Gut 2010;59:800–7. [DOI] [PubMed] [Google Scholar]
- 24.Hart PA, Zen Y, Chari ST. Recent Advances in Autoimmune Pancreatitis. Gastroenterology 2015;149:39–51. [DOI] [PubMed] [Google Scholar]
- 25.Hart PA, Kamisawa T, Brugge WR, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut 2013;62:1771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagpal SJS, Sharma A, Chari ST. Autoimmune Pancreatitis. Am J Gastroenterol 2018;113:1301. [DOI] [PubMed] [Google Scholar]
- 27.Machicado JD, Amann ST, Anderson MA, et al. Quality of Life in Chronic Pancreatitis is Determined by Constant Pain, Disability/Unemployment, Current Smoking, and Associated Co-Morbidities. Am J Gastroenterol 2017;112:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilcox CM, Yadav D, Ye T, et al. Chronic pancreatitis pain pattern and severity are independent of abdominal imaging findings. Clin Gastroenterol Hepatol 2015;13:552–60; quiz e28-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson MA, Akshintala V, Albers KM, et al. Mechanism, assessment and management of pain in chronic pancreatitis: Recommendations of a multidisciplinary study group. Pancreatology 2016;16:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olesen SS, Frokjaer JB, Lelic D, et al. Pain-associated adaptive cortical reorganisation in chronic pancreatitis. Pancreatology 2010;10:742–51. [DOI] [PubMed] [Google Scholar]
- 31.Bouwense SA, de Vries M, Schreuder LT, et al. Systematic mechanism-orientated approach to chronic pancreatitis pain. World J Gastroenterol 2015;21:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dumonceau JM, Costamagna G, Tringali A, et al. Treatment for painful calcified chronic pancreatitis: extracorporeal shock wave lithotripsy versus endoscopic treatment: a randomised controlled trial. Gut 2007;56:545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaysse T, Boytchev I, Antoni G, et al. Efficacy and safety of extracorporeal shock wave lithotripsy for chronic pancreatitis. Scand J Gastroenterol 2016;51:1380–5. [DOI] [PubMed] [Google Scholar]
- 34.Dumonceau JM, Delhaye M, Tringali A, et al. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Updated August 2018. Endoscopy 2019;51:179–193. [DOI] [PubMed] [Google Scholar]
- 35.Cahen DL, Gouma DJ, Laramee P, et al. Long-term outcomes of endoscopic vs surgical drainage of the pancreatic duct in patients with chronic pancreatitis. Gastroenterology 2011;141:1690–5. [DOI] [PubMed] [Google Scholar]
- 36.Kwon RS, Young BE, Marsteller WF, et al. Narcotic Independence After Pancreatic Duct Stenting Predicts Narcotic Independence After Lateral Pancreaticojejunostomy for Chronic Pancreatitis. Pancreas 2016;45:1126–30. [DOI] [PubMed] [Google Scholar]
- 37.Bhardwaj P, Garg PK, Maulik SK, et al. A randomized controlled trial of antioxidant supplementation for pain relief in patients with chronic pancreatitis. Gastroenterology 2009;136:149–159 e2. [DOI] [PubMed] [Google Scholar]
- 38.Siriwardena AK, Mason JM, Sheen AJ, et al. Antioxidant therapy does not reduce pain in patients with chronic pancreatitis: the ANTICIPATE study. Gastroenterology 2012;143:655–663 e1. [DOI] [PubMed] [Google Scholar]
- 39.Olesen SS, Bouwense SA, Wilder-Smith OH, et al. Pregabalin reduces pain in patients with chronic pancreatitis in a randomized, controlled trial. Gastroenterology 2011;141:536–43. [DOI] [PubMed] [Google Scholar]
- 40.Drewes AM, Kempeneers MA, Andersen DK, et al. Controversies on the endoscopic and surgical management of pain in patients with chronic pancreatitis: pros and cons! Gut 2019;68:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellin MD, Freeman ML, Gelrud A, et al. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology 2014;14:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellin MD, Abu-El-Haija M, Morgan K, et al. A multicenter study of total pancreatectomy with islet autotransplantation (TPIAT): POST (Prospective Observational Study of TPIAT). Pancreatology 2018;18:286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bellin MD, Whitcomb DC, Abberbock J, et al. Patient and disease characteristics associated with the presence of diabetes mellitus in adults with chronic pancreatitis in the United States. Am J Gastroenterol 2017;112:1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmed Ali U, Issa Y, van Goor H, et al. Dutch Chronic Pancreatitis Registry (CARE): design and rationale of a nationwide prospective evaluation and follow-up. Pancreatology 2015;15:46–52. [DOI] [PubMed] [Google Scholar]
- 45.Hart PA, Bellin M, Andersen DK. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet: Gastroenterology and Hepatology 2016;1:226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rickels MR, Bellin M, Toledo FG, et al. Detection, evaluation and treatment of diabetes mellitus in chronic pancreatitis: recommendations from PancreasFest 2012. Pancreatology 2013;13:336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheth SG, Conwell DL, Whitcomb DC, et al. Academic Pancreas Centers of Excellence: Guidance from a multidisciplinary chronic pancreatitis working group at PancreasFest. Pancreatology 2017;17:419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar K, Sharma R, Manrai M, et al. Visceral Adipose Tissue as a Risk Factor for Diabetes Mellitus in Patients with Chronic Pancreatitis: A Cross-sectional, Observational Study. Diabetes Ther 2017;8:1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W, Guo Y, Liao Z, et al. Occurrence of and risk factors for diabetes mellitus in Chinese patients with chronic pancreatitis. Pancreas 2011;40:206–12. [DOI] [PubMed] [Google Scholar]
- 50.Hart PA, Andersen DK, Mather KJ, et al. Evaluation of a Mixed Meal Test for Diagnosis and Characterization of PancrEaTogEniC DiabeTes Secondary to Pancreatic Cancer and Chronic Pancreatitis: Rationale and Methodology for the DETECT Study From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas 2018;47:1239–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Layer P, Yamamoto H, Kalthoff L, et al. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology 1994;107:1481–7. [DOI] [PubMed] [Google Scholar]
- 52.Hart PA, Conwell DL. Challenges and Updates in the Management of Exocrine Pancreatic Insufficiency. Pancreas 2016;45:1–4. [DOI] [PubMed] [Google Scholar]
- 53.Hart PA, Conwell DL. Diagnosis of Exocrine Pancreatic Insufficiency. Curr Treat Options Gastroenterol 2015;13:347–53. [DOI] [PubMed] [Google Scholar]
- 54.Dominguez-Munoz JE. Pancreatic exocrine insufficiency: diagnosis and treatment. J Gastroenterol Hepatol 2011;26 Suppl 2:12–6. [DOI] [PubMed] [Google Scholar]
- 55.Duggan SN, Smyth ND, Murphy A, et al. High prevalence of osteoporosis in patients with chronic pancreatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014;12:219–28. [DOI] [PubMed] [Google Scholar]
- 56.Tignor AS, Wu BU, Whitlock TL, et al. High prevalence of low-trauma fracture in chronic pancreatitis. Am J Gastroenterol 2010;105:2680–6. [DOI] [PubMed] [Google Scholar]
- 57.Duggan SN, Purcell C, Kilbane M, et al. An association between abnormal bone turnover, systemic inflammation, and osteoporosis in patients with chronic pancreatitis: a case-matched study. Am J Gastroenterol 2015;110:336–45. [DOI] [PubMed] [Google Scholar]
- 58.Sikkens EC, Cahen DL, Koch AD, et al. The prevalence of fat-soluble vitamin deficiencies and a decreased bone mass in patients with chronic pancreatitis. Pancreatology 2013;13:238–42. [DOI] [PubMed] [Google Scholar]
- 59.Bang UC, Benfield T, Bendtsen F, et al. The risk of fractures among patients with cirrhosis or chronic pancreatitis. Clin Gastroenterol Hepatol 2014;12:320–6. [DOI] [PubMed] [Google Scholar]
- 60.Algul H, Treiber M, Lesina M, et al. Mechanisms of disease: chronic inflammation and cancer in the pancreas--a potential role for pancreatic stellate cells? Nat Clin Pract Gastroenterol Hepatol 2007;4:454–62. [DOI] [PubMed] [Google Scholar]
- 61.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med 1993;328:1433–7. [DOI] [PubMed] [Google Scholar]
- 62.Liao KF, Lai SW, Li CI, et al. Diabetes mellitus correlates with increased risk of pancreatic cancer: a population-based cohort study in Taiwan. J Gastroenterol Hepatol 2012;27:709–13. [DOI] [PubMed] [Google Scholar]
- 63.Shelton CA, Umapathy C, Stello K, et al. Hereditary Pancreatitis in the United States: Survival and Rates of Pancreatic Cancer. Am J Gastroenterol 2018;113:1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chari ST, Mohan V, Pitchumoni CS, et al. Risk of pancreatic carcinoma in tropical calcifying pancreatitis: an epidemiologic study. Pancreas 1994;9:62–6. [DOI] [PubMed] [Google Scholar]
- 65.Machicado JD, Chari ST, Timmons L, et al. A population-based evaluation of the natural history of chronic pancreatitis. Pancreatology 2018;18:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gurusamy KS, Pallari E, Hawkins N, et al. Management strategies for pancreatic pseudocysts. Cochrane Database Syst Rev 2016;4:CD011392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oza VM, Skeans JM, Muscarella P, et al. Groove Pancreatitis, a Masquerading Yet Distinct Clinicopathological Entity: Analysis of Risk Factors and Differentiation. Pancreas 2015;44:901–8. [DOI] [PubMed] [Google Scholar]
- 68.Agarwal AK, Raj Kumar K, Agarwal S, et al. Significance of splenic vein thrombosis in chronic pancreatitis. Am J Surg 2008;196:149–54. [DOI] [PubMed] [Google Scholar]
- 69.Butler JR, Eckert GJ, Zyromski NJ, et al. Natural history of pancreatitis-induced splenic vein thrombosis: a systematic review and meta-analysis of its incidence and rate of gastrointestinal bleeding. HPB (Oxford) 2011;13:839–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yadav D, Park WG, Fogel EL, et al. PROspective Evaluation of Chronic Pancreatitis for EpidEmiologic and Translational StuDies: Rationale and Study Design for PROCEED From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas 2018;47:1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uc A, Perito ER, Pohl JF, et al. INternational Study Group of Pediatric Pancreatitis: In Search for a CuRE Cohort Study: Design and Rationale for INSPPIRE 2 From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas 2018;47:1222–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sendler M, Beyer G, Mahajan UM, et al. Complement Component 5 Mediates Development of Fibrosis, via Activation of Stellate Cells, in 2 Mouse Models of Chronic Pancreatitis. Gastroenterology 2015;149:765–76 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geisz A, Sahin-Toth M. A preclinical model of chronic pancreatitis driven by trypsinogen autoactivation. Nat Commun 2018;9:5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hao W, Komar HM, Hart PA, et al. Mathematical model of chronic pancreatitis. Proc Natl Acad Sci U S A 2017;114:5011–5016. [DOI] [PMC free article] [PubMed] [Google Scholar]


