Abstract
Purpose
To investigate the feasibility of using Brillouin microscopy for assessment of corneal edema in patients with Fuchs’ Endothelial Corneal Dystrophy (FECD). Brillouin microscopy analyzes the frequency shift of light inelastically scattered by naturally occurring acoustic waves in a small volume of tissue. The resulting frequency shift is a measure of the local hydromechanical properties of the tissue.
Methods
Participants were scanned using a clinical Brillouin imaging system (780 nm laser, 5 mW) and a color-coded map of the mean Brillouin shift laterally across the corneal stroma was created.
Results
Brillouin maps of normal subjects (n = 8) were relatively homogeneous, while FECD patients’ (n = 7) maps exhibited significantly reduced Brillouin shifts (unpaired t test, P < .001) centrally. The mean difference of 83 MHz corresponds to approximately 3.9% higher water content (percentage difference in volume fraction) in central corneas of FECD group relative to normal subjects. The Brillouin scan of a FECD patient 1-month after Descemet membrane endothelial keratoplasty (DMEK) measured a 62 MHz increase in Brillouin shift relative to pre-operative level, indicating normalization of corneal hydration.
Conclusions
All FECD patients scanned exhibited a centralized reduction in Brillouin shift, distinct from the normal subjects measured, and consistent with centralized edema characterized by pachymetry. Brillouin scans revealed substantially reduced water content after DMEK. These results suggest that Brillouin microscopy could aid treatment planning and assessment of FECD. Moreover, corneal hydration mapping may be useful in understanding fluid pump function dynamics of the cornea and developing early interventions for FECD.
Keywords: Cornea, Edema, Fuchs, Endothelial Disease, Imaging
Introduction
Fuchs’ Endothelial Corneal Dystrophy (FECD) is a progressive condition of the cornea caused by endothelial dysfunction and inability to maintain fluid pump function, resulting in significant visual impairment in advanced stages. The characteristic clinical feature is the formation of focal projections, termed “guttae”1, excreted from the thickened posterior band of Descemet’s membrane, followed by a progressive increase in corneal stromal edema2. The current standard of care treats FECD during the later stages of the disease with a complete or partial corneal transplantation. However, its progression rates vary, and quantitative methods to predict progression rates are not currently available.
FECD and other eye diseases alter the water concentration in the corneal stroma3, as do procedures such as photorefractive keratectomy or corneal graft surgery4,5. Current clinical methods to assess water content are based on monitoring corneal thickness, typically using ultrasound- or optical coherence tomography-based pachymetry or Scheimpflug-camera topography, or identifying concomitant structural features using slit-lamp biomicroscopy. These are indirect measurements of water concentration and cannot take into account factors such as the natural variation in corneal thickness between people6.
Brillouin microscopy is an emerging technique developed to characterize the biomechanical properties of tissues, and has shown potential in corneal disease diagnosis7. In Brillouin microscopy, laser light is focused to a small volume of tissue, and the Brillouin-scattered light from this region is collected at 180° and measured with a spectrometer. The technique is based on spontaneous Brillouin scattering, which is the inelastic scattering of light by naturally occurring acoustic waves in material. The resulting frequency shift, called the Brillouin frequency shift, depends on the local hydromechanical properties of the tissue. Recent studies have established that Brillouin frequency shifts are modulated by water concentration8,9. Here, we investigate whether Brillouin microscopy has sufficient sensitivity to detect corneal edema in patients with FECD and to visualize quantitative, spatial patterns of water concentration in FECD patients.
Materials and Methods
This pilot study recruited 8 subjects with normal corneas (mean ± SD age, 35 ± 9 years) and 7 subjects diagnosed with FECD (53 ± 10 years) from the Massachusetts Eye and Ear Cornea Service in Boston, Massachusetts, and the Woolfson Eye Institute in Atlanta, Georgia, between May 2018 and December 2018. It is noted that previous studies found no significant age-dependence in corneal Brillouin shift values10. All subjects underwent a comprehensive ophthalmic examination by a cornea specialist including pachymetry, slit-lamp biomicroscopy, and ancillary Pentacam (Oculus, Wetzlar, Germany) Scheimpflug imaging in order to quantify the central corneal thickness. Exclusion criteria for participating in the study included prior refractive or intraocular surgery, dry eye syndrome, glaucoma, and diagnosis of any corneal dystrophies other than FECD. Institutional Review Board (IRB)/Ethics Committee approval was obtained from the Partners Human Research Committee (Partners Healthcare Institutional Review Board) and Woolfson Eye Institute Ethics Committee. Written informed consent was obtained from all subjects prior to Brillouin imaging. All experiments were performed in accordance with the principles of the Declaration of Helsinki.
Participants were scanned using two clinical Brillouin imaging systems (780 nm laser, 5 mW) with identical specifications, which have been described previously10. Axial scans were taken at ~30 different transverse locations within a radius of about 4 mm from the corneal apex. From each axial scan, a mean Brillouin shift was computed by averaging the Brillouin shift values measured within the span of the corneal stroma. A color-coded Brillouin map was obtained by 2-dimensional interpolation of the mean Brillouin shift laterally across the corneal stroma.
Results
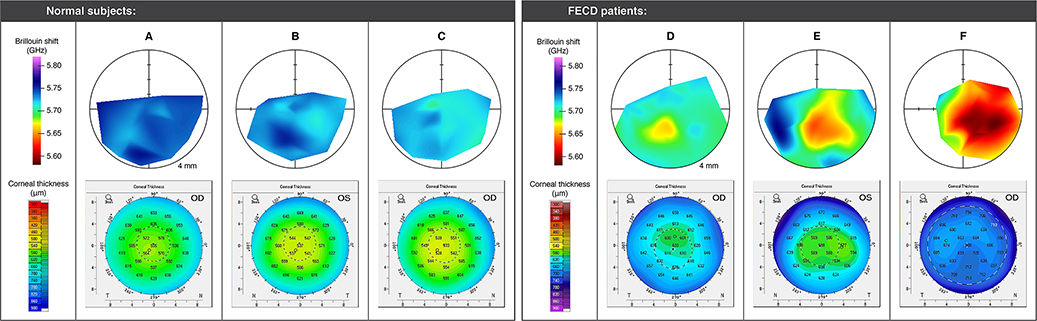
Brillouin maps of the 7 FECD patients exhibit greater heterogeneity than those of the 8 normal subjects (see representative examples in Figure 1). A pattern of centralized reduction in Brillouin shift, corresponding to increased water content in the central region of the corneal stroma is apparent for all FECD patients. This pattern is more pronounced in patients with advanced-stage FECD (see Figure 1), consistent with progressive edema that is characteristic of the condition.
Figure 1:
Representative examples of Brillouin maps (top row; radius of outer circle is 4 mm) and corneal topographies (bottom row) for normal subjects (A, B, C) and FECD patients (D, E, F) scanned in this study. Normal subjects A and B both had a central-to-peripheral thickness ratio (CPTR) of 0.86. Subject C had a CPTR of 0.89. FECD subject D had the lowest CPTR of the FECD patients measured (0.89) and presented with central guttae with areas of pigmented confluence. Subject E had a CPTR of 0.94 and presented with central guttae with patchy confluence. Subject F had the largest CPTR of the FECD patients measured (0.99) and presented with centrally confluent guttae and haziness.
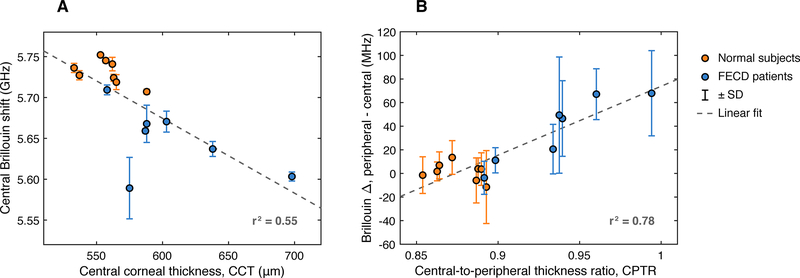
The mean Brillouin shift in the central region — defined here as the zone < 1 mm from the corneal apex — is 5731 ± 15 MHz (mean ± SD) for normal subjects, and 5648 ± 42 MHz for Fuchs’ patients, a statistically significant difference (unpaired t test, P < .001). The average CCT for the FECD group is 607 ± 47 μm (mean ± SD), expectedly higher than 557 ± 17 μm for the normal group (unpaired t test, P < .05). Figure 2–A plots the central Brillouin shift versus CCT for all study subjects. A trend towards decreasing Brillouin value with increasing CCT is evident, with a fitted slope [95% confidence interval] of −0.92 [−1.42, −0.42] MHz/μm. This result is consistent with previous measurements of individual diurnal changes in healthy volunteers9, which reported a slope of −1.06 ± 0.2 MHz/μm.
Figure 2:
(A) Plot of Brillouin shift in the central region (< 1 mm from the corneal apex) vs. central corneal thickness (CCT) for normal subjects (orange) and FECD patients (blue). Dotted line shows a linear fit to the data with a fitted slope [95% confidence interval] of −0.92 [−1.42, −0.42] MHz/μm. (B) Plot of Brillouin regional difference (peripheral minus central) vs. central-to-peripheral thickness ratio (CPTR) for normal subjects (orange) and FECD patients (blue). Dotted line shows a linear fit to the data with a fitted slope [95% confidence interval] of 580 [400, 770] MHz/CPTR.
Regional difference in Brillouin shift is less affected by interpersonal variation10. Similarly, we found that the ratio of corneal thickness in the peripheral region to central corneal thickness, denoted CPTR (“central-to-peripheral thickness ratio”) could be a useful measure of FECD severity11. We found that the CPTR is higher on average for the FECD group, 0.94 ± 0.04, versus 0.88 ± 0.01 for the normal group, and that the difference is statistically significant (unpaired t test, P < .005). We computed the difference between the mean Brillouin shift in the periphery of the cornea (defined here as the region > 3 mm from the corneal apex) and the Brillouin shift in the central area (again defined as the region < 1 mm from the corneal apex) for each subject. Figure 2–B shows Brillouin regional difference versus CPTR for all study subjects. A trend towards increasing Brillouin regional difference with decreasing CPTR is apparent, with a fitted slope [95% confidence interval] of 580 [400, 770] MHz/CPTR.
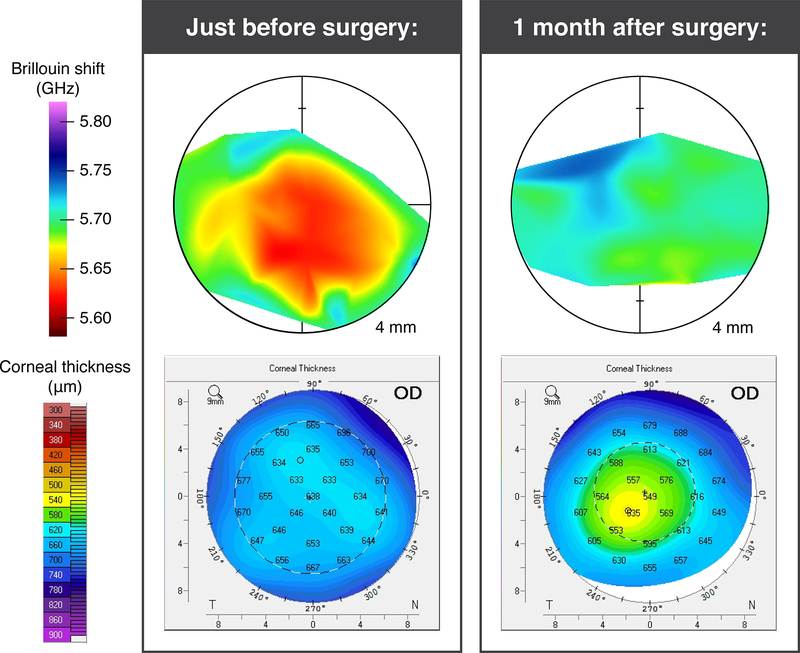
One FECD patient was scanned just prior to a partial-thickness cornea transplant (Descemet membrane endothelial keratoplasty, DMEK), and then scanned again 1 month post-operatively. This case study can be seen in Figure 3. The mean Brillouin shift in the patient’s central corneal region increased significantly from 5637 ± 9 MHz (mean ± SD) just before DMEK, to 5699 ± 8 MHz one month after DMEK (unpaired t test, P < .001), consistent with the expected reduction in corneal edema following treatment. The Brillouin value after DMEK is slightly less than the lowest value measured in the normal group, 5702 ± 21 MHz. The CTPR improved from 0.96 to 0.87, close to 0.88 for the normal group.
Figure 3:
Case study of an FECD patient scanned just prior to DMEK surgery and again one month post-operatively. Top row shows Brillouin shift map and bottom row shows corneal thickness. Before surgery, the patient had a CPTR of 0.96 and presented with confluent guttae. One month after surgery their CPTR was 0.87 (with the graft).
Discussion
Previous work has established a relationship between Brillouin shift and corneal hydration9. Assuming there is a typical ~ 0.78 volume-fraction of water in the healthy cornea, we estimate that there is 3.9% higher water content on average in the central corneas of the FECD group compared to normal (percentage difference in volume fraction). The Brillouin spatial maps of the normal subjects are relatively uniform, with negligible difference (1.3 ± 7.7 MHz) on average between peripheral and central corneal regions. By contrast, FECD patients exhibit a centralized reduction in Brillouin shift, with an average peripheral-to-central difference of 37 ± 28 MHz. This corresponds to an average of 1.5% higher water content (percentage difference in volume fraction) in the central regions relative to the peripheral regions of the FECD patients.
Brillouin microscopy allows for non-contact mapping of corneal water content (volume fraction) with a sensitivity of approximately ±0.2%.9 The results of this pilot study of FECD patients suggest that this technique may be useful for assessment of FECD or other dystrophies that cause fluid accretion or reduction in the cornea stroma. Further study is warranted to investigate what correlation exists between Brillouin shifts and guttata in early-stage FECD, and to determine whether the patterns of edema identified by Brillouin measurements can be applied to predict the rate of FECD progression and aid in surgical planning. Because of its sensitivity to water content, Brillouin microscopy may also prove beneficial for understanding the role that fluid dynamics play in different corneal disorders, as well as possibly for monitoring corneal grafts after transplantation.
Acknowledgements
We thank Dr. R. Doyle Stulting for assistance with the study taking place at the Woolfson Eye Institute in Atlanta, Georgia. This study was supported by funding from the National Institutes of Health (Bethesda, MD), grants R01-EY025454, P41-EB015903, and R41-EY028820.
Financial Support: This study was supported by funding from the National Institutes of Health (Bethesda, MD), grants R01-EY025454, P41-EB015903, and R41-EY028820.
Footnotes
Conflict of Interest: Drs. Eltony, Shao and Yun hold patents related to the technology. Dr. Yun is the scientific founder of Intelon Optics, Inc., which licensed the patents.
References
- 1.Eghrari AO, Riazuddin SA, Gottsch JD. Fuchs Corneal Dystrophy. Prog Mol Biol Transl Sci. 2015;134:79–97. [DOI] [PubMed] [Google Scholar]
- 2.Wilson SE, Bourne WM. Fuchs’ Dystrophy. Cornea. 1988;7:2–18. [PubMed] [Google Scholar]
- 3.Sherwin JC, Kokavec J, Thornton SN. Hydration, fluid regulation and the eye: in health and disease. Clin Experiment Ophthalmol. 2015;43:749–764. [DOI] [PubMed] [Google Scholar]
- 4.Fields CR, Taylor SM, Barker FM. Effect of Corneal Edema Upon the Smoothness of Excimer Laser Ablation. Optom Vis Sci. 1994;71:109–114. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty PJ, Wellish KL, Maloney RK. Excimer Laser Ablation Rate and Corneal Hydration. Am J Ophthalmol. 1994;118:169–176. [DOI] [PubMed] [Google Scholar]
- 6.Doughty MJ, Zaman ML. Human Corneal Thickness and Its Impact on Intraocular Pressure Measures: A Review and Meta-analysis Approach. Surv Ophthalmol. 2000;44:367–408. [DOI] [PubMed] [Google Scholar]
- 7.Yun SH, Chernyak D. Brillouin microscopy: Assessing ocular tissue biomechanics. Curr Opin Ophthalmol. 2018;29:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu P-J, Kabakova IV., Ruberti JW, et al. Water content, not stiffness, dominates Brillouin spectroscopy measurements in hydrated materials. Nat Methods. 2018;15:561–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao P, Seiler TG, Eltony AM, et al. Effects of Corneal Hydration on Brillouin Microscopy In Vivo. Investig Opthalmology Vis Sci. 2018;59:3020–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shao P, Eltony AM, Seiler TG, et al. Spatially-resolved Brillouin spectroscopy reveals biomechanical abnormalities in mild to advanced keratoconus in vivo. Scientific Reports 2019;9:7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Repp DJ, Hodge DO, Baratz KH et al. Fuchs’ Endothelial Corneal Dystrophy: Subjective Grading versus Objective Grading Based on the Central-to-Peripheral Thickness Ratio. Ophthalmology. 2013;120:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]