Abstract
Disruption of usual routines may hinder adherence, increasing the risk of rejection. We aimed to compare weekend versus weekday medication adherence among adolescent and young adult kidney transplant recipients, hypothesizing poorer adherence on weekends. We examined data from the Teen Adherence in Kidney transplant Effectiveness of Intervention Trial (TAKE-IT). We assessed the 3-month run-in period (no intervention) and the 12-month intervention interval, considering a potential interaction between weekend/weekday and treatment group. Adherence was monitored using electronic pillboxes in participants 11–24 years followed in 8 transplant centers in Canada and the United States. We used logistic regression with generalized estimating equations to estimate the association between weekends/weekdays and each of perfect taking (100% of prescribed doses taken) and timing (100% of prescribed doses taken on time) adherence. Taking (OR=0.72 (95%CI 0.65–0.79)) and timing (OR=0.66 (95%CI 0.59–0.74)) adherence were poorer on weekends than weekdays in the run-in (136 participants), and the intervention interval (taking OR=0.74 (0.67–0.81) and timing OR=0.71 (95%CI 0.65–0.77)). There was no interaction by treatment group (64 intervention and 74 control participants). Weekends represent a disruption of regular routines, posing a threat to adherence. Patients and families should be encouraged to develop strategies to maintain adherence when routines are disrupted.
TAKE-IT registration number:
Clinicaltrials.govregistration: NCT01356277
1. Introduction
Adolescent and young adult solid organ transplant recipients have higher rates of graft failure than any other age group.1–5 Poor adherence to immunosuppressive therapy is an important risk factor for acute rejection6,7 and premature graft loss.8,9 Although the degree of deviation from the prescribed treatment regimen needed to compromise graft outcomes is unknown, minor deviations have been associated with adverse outcomes.9,10
Among adolescents and young adults, many factors contribute to the risk for non-adherence including poor family functioning, adverse effects of medications, and adolescents’ desire to be normal.8 However, even in this age group most non-adherence is unintentional; forgetting and poor organization and planning were identified as the most common reasons for non-adherence in studies of adolescent kidney transplant recipients.11,12 In this context, identification of times of high risk for poor adherence may help clinicians and parents to anticipate difficult periods and better support patients’ adherence during these times. For example, weekends may present a disruption in daily routines that are centered around school attendance, leading to poorer adherence. The few prior studies that investigated the association between day of the week and adherence showed inconsistent results. Whereas one study of adult kidney transplant recipients showed a gradual decline in adherence between Monday and Sunday,13 another study of 80 adolescent and adult kidney transplant recipients found poorer adherence on Thursdays and Saturdays compared to other days of the week.14 However, no prior study assessed day of the week as the primary exposure variable, and, to our knowledge, no study focused on adolescents and young adults, in whom the relationship between day of the week and adherence may be different than in older adults.
We aimed to determine whether medication adherence, as measured using electronic monitoring, differs by day of the week among adolescent and young adult kidney transplant recipients. We hypothesized that adherence would be poorer on weekends than on weekdays.
2. Material and Methods
This is a post-hoc analysis of data from the Teen Adherence in Kidney transplant Effectiveness of Intervention Trial (TAKE-IT).15,16 TAKE-IT was a prospective randomized trial testing an adherence-promoting intervention for adolescent and young adult kidney transplant recipients in 8 centers across Canada and USA. The intervention included meetings with a Coach at 3-month intervals, and the option to receive text message, email, and/or visual cue dose reminders. During coaching sessions, intervention participants reviewed electronically-monitored adherence data from the prior 3 months with the Coach, who used ‘Action-Focused Problem-Solving’ to address adherence barriers selected as important by the participant. 16
TAKE-IT was approved by the Research Ethics Boards of all sites. Written informed consent was obtained from all participants and parents (for those <18y).
2.1. Study population
TAKE-IT included prevalent kidney-only transplant recipients with a functioning graft, who were 11 to 24 years old, at least 3 months post-transplant, and who were expected to be followed in one of the 8 participating centers for the 15-month study. Exclusion criteria included: impending graft failure, severe neurocognitive disabilities, exclusive use of liquid immunosuppressive medications, having a sibling participating in the study, participating in another adherence-promoting intervention study, lack of electronic pillbox connectivity, or inability to communicate comfortably in English (or French - Montreal site only).
Electronic adherence assessment:
All participants were given a multi-dose electronic pillbox in which to store all immunosuppressive medications. Participants received a Medminder pillbox (Medminder, Needham, MA) during the first 4–6 months of recruitment. However, some participants encountered technical difficulties with this pillbox. Therefore, subsequent participants received a SimpleMed device (Vaica Medical, Tel Aviv, Israel). The Medminder and SimpleMed were similar, with the same types of adherence-tracking and reminder functions. The date and time of each pillbox compartment opening was recorded automatically in the secure web-based electronic record of the patient who had received this pillbox. Periods of non-use of the pillbox (for example during vacation travel) were reported to study staff by participants, and the dates recorded.
Participants were followed for 3 months (run-in) before randomization to intervention or control groups, and then for 12 months after initiation of the intervention or control condition. During the run-in, group assignment (intervention or control) was unknown to study personnel or participants. The present analysis assesses the run-in period, before initiation of intervention (to reflect baseline behavior patterns), as well as the intervention interval (to determine whether the intervention modified the association between weekend/weekday and adherence).
2.2. Statistical analysis
Primary exposure:
We compared adherence between weekdays and weekends. We defined “weekends” as Friday night, Saturday and Sunday, and “weekdays” as the remaining days (including Friday before 1:00 PM). We initially considered each day of the week separately, and stratified on morning/evening dose; evaluation of plots of the data and comparisons between days of the week led to dichotomization of days into weekends and weekdays (Figure 1).
Figure 1.
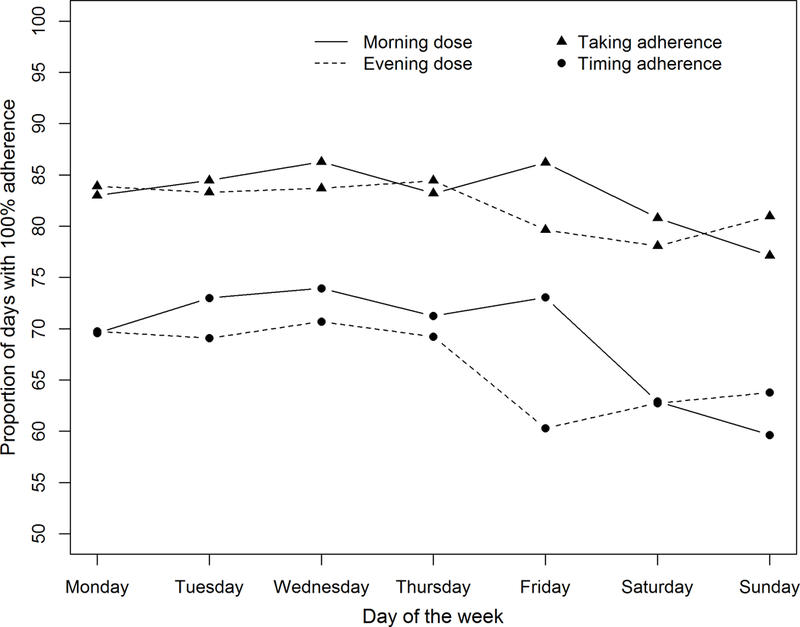
Proportions of days where all patients had 100% taking or 100% timing adherence for each day of the week in the run-in period of the TAKE-IT trial (n=136). The graph indicates the proportion of specific days on which all patients took all prescribed doses of immunosuppressive medications (triangles) or took all prescribed doses on time (circles). For example, all patients took all prescribed doses of medications on ~85% of Mondays; all patients took all prescribed doses on time on ~74% of Tuesdays for morning doses.
Primary outcomes:
“Taking” adherence (defined as the proportion of prescribed doses taken) and “timing” adherence (defined as the proportion of prescribed doses taken within one hour before to two hours after the prescribed dosing time), were the two primary outcomes, both measured using electronic monitoring. For each participant, a taking adherence score (0% if the dose was not taken; 100% if the dose was taken) and a timing adherence score (0% if the dose was not taken on time; 100% if the dose was taken on time) were calculated for each expected dose. Each participant thus had repeated outcome measures. A participant had one score per day if he/she had one dose per day and two scores per day if he/she had two doses per day.
No score was calculated for days that the pillbox was not in use (turned off, not communicating with the server, or participant-reported non-use). The first two weeks of electronic adherence data following enrollment (during the run-in) were deleted to allow adaptation to the pillbox 17.
Association between weekdays/weekends and adherence:
Correlations between weekend and weekday adherences were estimated by Pearson correlation coefficients. Logistic regression with generalized estimating equations was used to estimate the association between weekdays/weekends and each of taking and timing adherence, accounting for correlation of repeated adherence scores within each participant. Because participants had observations on both weekdays and weekends, imbalance of patient characteristics between weekdays and weekends was not possible; therefore, there was no need to adjust for confounders. Separate analyses were conducted using data from the run-in period and from the intervention interval. Analysis of the intervention interval included an interaction between weekend/weekday and treatment group to determine whether the association between day of the week and adherence differed for those in the intervention group compared with those in the control group.
Analyses were conducted using SAS 9.4.
2.3. Sensitivity analyses
First, based on the patterns of adherence by day of the week in the intervention internal (Figure 2), we repeated our analyses considering Sunday night as a weekday for both the run-in and the intervention periods.
Figure 2.
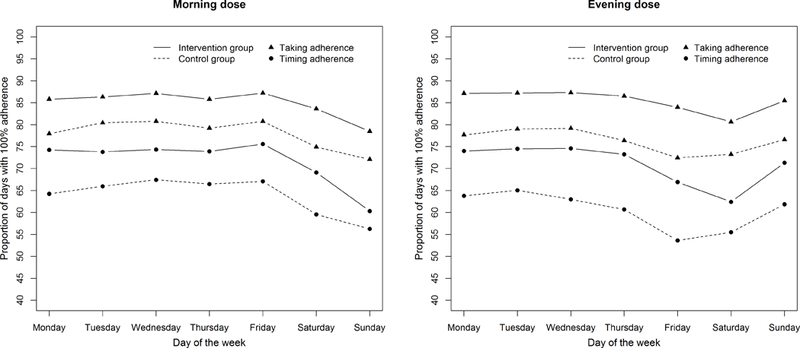
Proportions of days where all patients had 100% taking or 100% timing adherence for each day of the week in the intervention interval of the TAKE-IT trial (n=138). The graph indicates the proportion of specific days on which all patients took all prescribed doses of immunosuppressive medications (triangles) or took all prescribed doses on time (circles). For example, all patients took all prescribed doses of medications on ~85% of Mondays for morning doses in the intervention group; all patients took all prescribed doses on time on ~54% of Friday for evening doses in the control group.
We also repeated our analyses considering holidays as weekend days. For participants at American sites, holidays were Memorial Day, July Fourth, and American Thanksgiving (both Thurs. and Fri.); for Canada, holidays were Good Friday, Easter Monday, Victoria Day, St. Jean Baptiste Day (only for Quebec), Canada Day, and Canadian Thanksgiving (second Mon. in October). Labor Day and the entire period between December 24th and January 2nd were considered holidays for both US and Canada.
Finally, we repeated our analysis using electronic adherence data supplemented with data from logs of doses taken from a source other than the electronic pillbox.
3. Results
3.1. Participant characteristics
The characteristics of the 136 patients with electronic data available in the run-in period and the 138 patients (64 intervention group and 74 control group)16 with electronic data available in the intervention interval are summarized in Table 1. Participants were followed for a median of 2.5 months [interquartile range (IQR) 1.8–2.8] in the run-in (after excluding the first 2 weeks) and 11.7 months [IQR 9.2–12.6] in the intervention interval. Given the large overlap between those contributing data to the run-in and intervention intervals, it was not surprising that participant characteristics were similar in the run-in and intervention intervals. The median time since transplant was about 3 years, the median age at transplant approximately 12 years, and about two-thirds of participants were White. More than half of participants had a living donor. Concerning treatment characteristics, about 65% of participants received 3 immunosuppressive medications per day; 92% took 2 doses of immunosuppressives per day and 8% took a single dose per day.
Table 1.
Baseline characteristics of participants during the run-in and intervention intervals
| Demographics | Run-in interval | Intervention interval |
|---|---|---|
| Number with pillbox data | 136 | 138 |
| Age | 15.8 [13.6–17.4] | 16.0 [13.8–17.6] |
| Male | 83 (61.0) | 84 (60.9) |
| Race | ||
| White | 92 (67.6) | 89 (64.5) |
| Black | 17 (12.5) | 17 (12.3) |
| Other | 27 (19.9) | 32 (23.2) |
| Hispanic or Latino | 12 (8.8) | 12 (8.7) |
| US Study site | 89 (65.5) | 86 (62.3) |
| Healthcare insurer | ||
| U.S. Public | 41 (30.1) | 36 (26.1) |
| U.S. Private | 48 (35.3) | 50 (36.2) |
| Canadian provincial | 47 (34.6) | 52 (37.7) |
| Medication insurer | ||
| U.S. Public | 44 (32.3) | 40 (29.0) |
| Private | 62 (45.6) | 66 (47.8) |
| Canadian provincial | 16 (11.8) | 17 (12.3) |
| Other | 14 (10.3) | 15 (10.9) |
| Household income per year | ||
| Less than $50,000 | 57 (41.9) | 55 (39.9) |
| Greater than $50,000 | 62 (45.6) | 67 (48.6) |
| Unknown/Prefer not to answer | 17 (12.5) | 16 (11.6) |
| Disease characteristics | ||
| Years post-transplant | 2.9 [0.8–7.1] | 3.3 [1.0–7.5] |
| Number of prior transplants (including current one) | ||
| 1 | 123 (90.4) | 125 (90.6) |
| 2 | 13 (9.6) | 13 (9.4) |
| Donor source | ||
| Living | 71 (52.2) | 71 (51.4) |
| Deceased | 65 (47.8) | 67 (48.6) |
| Duration of dialysis before current transplant | ||
| 0 month | 39 (28.7) | 39 (28.3) |
| > 0 month | 97 (71.3) | 99 (71.7) |
| Total lifetime duration of dialysis | ||
| 0 month | 37 (27.2) | 37 (26.8) |
| > 0 month | 99 (72.8) | 101 (73.2) |
| Age at transplant (in years) | 12.0 [8.3–14.9] | 11.9 [8.0–14.9] |
| Primary disease | ||
| CAKUT | 55 (40.4) | 57 (41.3) |
| Glomerulonephritis | 13 (9.6) | 14 (10.1) |
| FSGS | 14 (10.3) | 14 (10.1) |
| Other | 54 (39.7) | 53 (38.4) |
| Number of past acute rejections | ||
| 0 | 109 (80.1) | 110 (79.7) |
| ≥ 1 | 27 (19.9) | 28 (20.3) |
| Comorbidities | ||
| None | 70 (51.5) | 72 (52.2) |
| ≥ 1 | 66 (48.5) | 66 (47.8) |
| Treatment characteristics | ||
| Number of immunosuppressive medications | ||
| 1 | 4 (2.9) | 3 (2.3) |
| 2 | 43 (31.6) | 44 (33.1) |
| 3 | 89 (65.5) | 86 (64.7) |
| Number of doses of immunosuppressive per day | ||
| 1 | 11 (8.1) | 11 (8.3) |
| 2 | 125 (91.9) | 122 (91.7) |
| Total number of medications | 7.0 [5.0-9.0] | 7.0 [5.0-9.0] |
| Total number of doses per day | ||
| 1 | 4 (2.9) | 5 (3.8) |
| 2 | 120 (88.2) | 115 (86.5) |
| 3 or 4 | 12 (8.9) | 13 (9.8) |
In columns, data given as median [interquartile range] or number (%)
3.2. Association between weekdays/weekends and adherence
Figure 1 shows, for each day of the week during the run-in period, by dose (morning or evening), the proportion of days for which all participants had 100% taking or 100% timing adherence. Figure 2 shows, for each day of the week during the intervention interval, the proportion of days for which all participants had 100% taking or 100% timing adherence, stratified by treatment group. Both taking and timing adherence were significantly better in the intervention than control group16. However, the association between weekend/weekday and adherence did not differ significantly by treatment group (interaction p-value = 0.39).
Summarizing each participant’s adherence across each observation interval, the median [IQR] taking adherence for weekdays was 89.2% [73.1–95.5] in the run-in period and 87.4% [75.1–94.2] in the intervention interval; median [IQR] timing adherence for weekdays was 72.9% [50.3–87.6] in the run-in period and 74.6% [49.1–84.6] in the intervention interval. Focusing on weekend adherence, the median [IQR] taking adherence was 84.9% [63.1–95.1] in the run-in period and 82.5% [64.6–92.4] in the intervention interval; median [IQR] timing adherence was 60.0% [38.1–83.2] in the run-in period and 64.3% [37.3–78.3] in the intervention interval. In both the run-in and the intervention interval, weekend adherence was highly correlated with weekday adherence (Figures 3 and 4). In the run-in period, 65.9% of patients had weekend taking adherence lower than their weekday taking adherence, and 71.9% had weekend timing adherence lower than their weekday timing adherence. In the intervention interval, 69.3% of patients had weekend taking adherence lower than their weekday taking adherence and 78.8% had weekend timing adherence lower than their weekday timing adherence.
Figure 3.
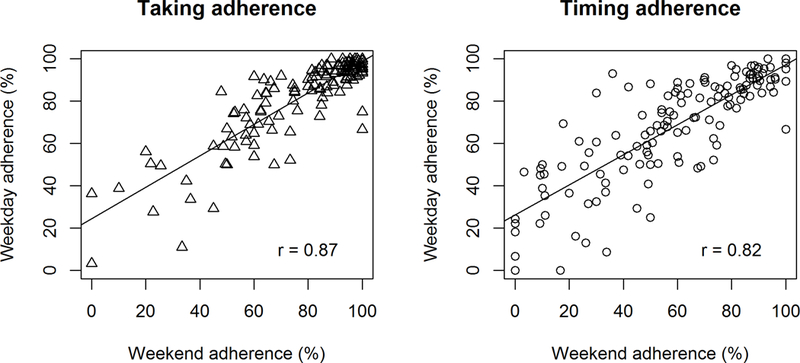
Correlation between weekend and weekday adherence in the run-in period of the TAKE-IT trial.
Figure 4.
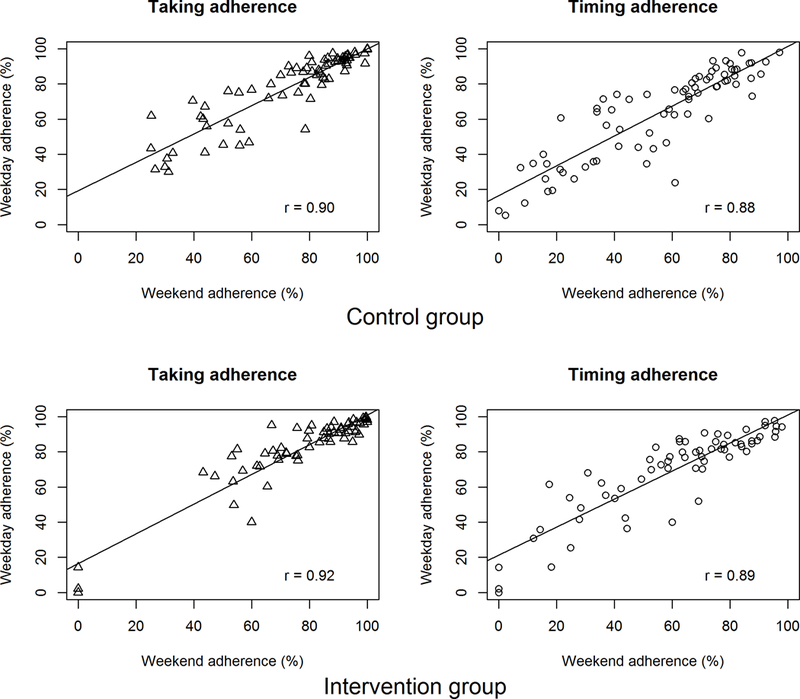
Correlation between weekend and weekday adherence in the intervention interval of the TAKE-IT trial.
Table 2 shows the results of the logistic regression models used to estimate the association between weekdays/weekends and each of taking and timing adherence, both in the run-in period and the intervention interval. In the run-in period, perfect taking adherence was significantly less likely on weekends than weekdays (OR=0.71 (95%CI 0.65–0.79)). Participants also had a significantly lower likelihood of on-time dosing on weekends than weekdays (OR=0.66 (95%CI 0.59–0.74)). Results were similar in the intervention interval. Associations between other patient characteristics and adherence were described in a previous paper.18
Table 2.
Results of logistic regressions with taking and timing adherence scores as outcomes and day of the week as exposure (weekdays as the reference, weekends as the compared group)
| Run-in period | Intervention interval | |||
|---|---|---|---|---|
| Taking adherence Odds ratio (95%CI) |
Timing adherence Odds ratio (95%CI) |
Taking adherence Odds ratio (95%CI) |
Timing adherence Odds ratio (95%CI) |
|
| Main analysis | 0.71 (0.65-0.79) | 0.66 (0.59-0.74) | 0.74 (0.67-0.81) | 0.71 (0.65-0.77) |
| Sunday night as weekday | 0.71 (0.65-0.79) | 0.67 (0.60-0.75) | 0.72 (0.65-0.79) | 0.68 (0.62-0.75) |
| Weekends combined with holidays | 0.70 (0.63-0.78) | 0.65 (0.58-0.73) | 0.74 (0.67-0.81) | 0.70 (0.64-0.77) |
| Electronic adherence data supplemented with log data | 0.73 (0.66-0.81) | 0.69 (0.61-0.77) | 0.75 (0.69-0.82) | 0.73 (0.67-0.80) |
An odds ratio < 1 means that odds of higher taking or timing adherence scores are lower for weekends compared to weekdays.
3.3. Sensitivity analysis
Results of all sensitivity analyses were similar to those obtained in our main analyses (Table 2). Results were essentially identical when holidays were included as weekend days, and when electronic adherence data were supplemented with data from logs of doses taken from a source other than the electronic pillbox.
4. Discussion
Among adolescent and young adult kidney transplant recipients, both taking and timing adherence were significantly poorer on weekends compared to weekdays. These findings indicate that even a change in routine as regular and recurrent as weekday to weekend may have an impact on adherence. A break in usual routines has been repeatedly identified as a risk factor for poor adherence.19 Our results support the results of prior studies suggesting that difficulties in organization and planning are a common cause of non-adherence.11,12
However, it is also important to recognize that non-adherence did not occur exclusively on weekends. There was a strong correlation between weekend and weekday adherence. This study cannot determine whether poor adherence during weekdays was also related to disruptions in usual routines, but this is possible. For example, poor adherence on a weekday may be related to outings or activities that are limited to one or two days per week. Weekends represent a time when routines are fairly consistently disrupted for all patients, even among the most adherent, so allow some assessment of the impact of disruption of routines on adherence.
It is notable that not only was there poorer on-time dosing on weekends, but more doses were missed as well. Late dosing may be explained by participants sleeping later on weekends11 and therefore delaying taking their medication. A higher rate of missing doses on weekends has several possible explanations. Patients may be unsure as to what to do if their dose is late, and simply skip the dose. The difference in usual routine between weekends and weekdays may disrupt patients’ organizational systems resulting in forgotten doses, even though these patients has electronic pillboxes to help their organizational skills. Adolescents may be particularly sensitive to changes in routine, especially if their weekday schedule is highly structured around the school day, and medication dosing is planned to fit in this schedule. Awareness of the high risk of poor adherence on weekends and holidays may encourage healthcare professionals and parents to engage young people in contingency planning whereby a plan is made in advance to ensure that medications are taken on time even outside their weekday routines.
This study has limitations. First, this was a post-hoc analysis of data collected during a randomized trial; the study was not specifically designed to compare weekday with weekend adherence. Second, participants of a trial, and particularly those for whom electronic adherence data are available, may have better adherence than the general population of patients. We cannot exclude the possibility that selection bias affected the results. However, this bias would most likely underestimate the impact of weekends on adherence. It seems unlikely that the most non-adherent patients would have more consistent adherence across all days of the week, or better adherence on weekends than weekdays. In an unselected sample of patients in the ‘real world’, the differences between weekend and weekdays may be even more striking.
A third limitation is that the sample is relatively small. It is possible that we lacked the power to detect an interaction between day of the week and treatment group. A difference between the intervention and control groups in the association between adherence and day of the week may exist. However, the plots shown in Figure 2 suggest that, while adherence was better in the intervention than the control group, the intervention did not result in more consistent adherence across all days of the week.
Fourth, we cannot exclude the possibility that participants did not use the electronic pillbox in the same way on weekends as on weekdays. For example, if participants were not at home at dosing times more often on weekends, then adherence would appear poorer on weekends than weekdays, even if the medications were actually taken from a source other than the pillbox. Although imperfect, electronic monitoring is the only possible method to measure adherence on each day separately. Patients were also asked to log doses taken away from the pillbox, but did not consistently do so. Analyses that included logged doses taken away from the pillbox showed the same results as the primary analysis (Table 2).
Finally, it is unknown whether poorer adherence on weekends than weekdays has any impact on graft outcomes. Given the strong correlation between weekend and weekday adherence, it would be difficult to decide how to compare graft outcomes based on weekend vs. weekday adherence. Adherence on weekdays, which represents a greater proportion of time than weekends, is clearly also important to graft outcomes. We hypothesize that poor adherence on weekends may reflect poor contingency planning and problem-solving; our findings highlight the importance of discussing with patients strategies to maintain good adherence even when usual routines are disrupted. Existing evidence suggests that even minor deviations from the prescribed immunosuppression schedule may influence graft outcomes. 9,10 This study had neither adequate power nor adequate duration of follow-up to assess associations between adherence and graft function, rejection rates, or graft failure rates. 15,16
Based on electronic monitoring, both taking adherence and on-time dosing are poorer on weekends compared with weekdays among adolescent and young adult kidney transplant recipients. These findings highlight the importance of working with patients and their caregivers to identify adherence barriers and develop targeted strategies to prevent poor adherence when usual routines are disrupted, particularly during weekends.
Acknowledgments
The authors would like to acknowledge the devotion of the study coaches and the generous participation of patients and families without whom this study would not have been possible.
The study was funded by the American National Institutes of Health, National Institutes of Diabetes, Digestive and Kidney diseases (NIDDK; R01DK092977).
The funder had no role in study design, data collection, analysis, interpretation of data, writing the report, or and the decision to submit the report for publication. J.B., who was a postdoctoral fellow at the Research Institute of the McGill University Health Centre when this study was done, was supported by an RI MUHC-Desjardins Studentship in Child Health Research. B.J.F., a member of the Research Institute of the McGill University Health Centre, was supported by a Fonds de recherche du Quebec Santé Chercheur-Boursier Clinicien award.
The authors would like to acknowledge the devotion of the study coaches and the generous participation of the patients and their families without whom this study would not have been possible.
Abbreviations
- (CI)
Confidence interval
- (IQR)
Interquartile range
- (OR)
Odds ratio
- (TAKE-IT)
Teen Adherence in Kidney transplant Effectiveness of Intervention Trial
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose. Dr. Foster is a co-investigator on 2 investigator-initiated studies funded by Astellas Canada. The other authors have no conflicts of interest to disclose.
References
- 1.Kabore R, Couchoud C, Macher MA, et al. Age-Dependent Risk of Graft Failure in Young Kidney Transplant Recipients. Transplantation. 2017;101(6):1327–1335. [DOI] [PubMed] [Google Scholar]
- 2.Lepeytre F, Dahhou M, Zhang X, et al. Association of Sex with Risk of Kidney Graft Failure Differs by Age. J Am Soc Nephrol. 2017;28(10):3014–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster BJ, Dahhou M, Zhang X, Dharnidharka V, Ng V, Conway J. High Risk of Graft Failure in Emerging Adult Heart Transplant Recipients. Am J Transplant. 2015;15(12):3185–3193. [DOI] [PubMed] [Google Scholar]
- 4.Foster BJ, Dahhou M, Zhang X, Dharnidharka VR, Conway J, Ng VL. High Risk of Liver Allograft Failure During Late Adolescence and Young Adulthood. Transplantation. 2016;100(3):577–584. [DOI] [PubMed] [Google Scholar]
- 5.Foster BJ, Dahhou M, Zhang X, Platt RW, Samuel SM, Hanley JA. Association between age and graft failure rates in young kidney transplant recipients. Transplantation. 2011;92(11):1237–1243. [DOI] [PubMed] [Google Scholar]
- 6.Takemoto SK, Pinsky BW, Schnitzler MA, et al. A retrospective analysis of immunosuppression compliance, dose reduction and discontinuation in kidney transplant recipients. Am J Transplant. 2007;7(12):2704–2711. [DOI] [PubMed] [Google Scholar]
- 7.Nevins TE, Thomas W. Quantitative patterns of azathioprine adherence after renal transplantation. Transplantation. 2009;87(5):711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dew MA, Dabbs AD, Myaskovsky L, et al. Meta-analysis of medical regimen adherence outcomes in pediatric solid organ transplantation. Transplantation. 2009;88(5):736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN. Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant. 2010;14(5):603–613. [DOI] [PubMed] [Google Scholar]
- 10.De Geest S, Abraham I, Moons P, et al. Late acute rejection and subclinical noncompliance with cyclosporine therapy in heart transplant recipients. J Heart Lung Transplant. 1998;17(9):854–863. [PubMed] [Google Scholar]
- 11.Simons LE, McCormick ML, Mee LL, Blount RL. Parent and patient perspectives on barriers to medication adherence in adolescent transplant recipients. Pediatr Transplant. 2009;13(3):338–347. [DOI] [PubMed] [Google Scholar]
- 12.Zelikovsky N, Schast AP, Palmer J, Meyers KE. Perceived barriers to adherence among adolescent renal transplant candidates. Pediatr Transplant. 2008;12(3):300–308. [DOI] [PubMed] [Google Scholar]
- 13.Denhaerynck K, Steiger J, Bock A, et al. Prevalence and risk factors of non-adherence with immunosuppressive medication in kidney transplant patients. Am J Transplant. 2007;7(1):108–116. [DOI] [PubMed] [Google Scholar]
- 14.Henriksson J, Tyden G, Hoijer J, Wadstrom J. A Prospective Randomized Trial on the Effect of Using an Electronic Monitoring Drug Dispensing Device to Improve Adherence and Compliance. Transplantation. 2016;100(1):203–209. [DOI] [PubMed] [Google Scholar]
- 15.Foster BJ, Pai A, Zhao H, Furth S, TAKE-IT Study Group. The TAKE-IT study: aims, design, and methods. BMC Nephrol. 2014;15:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster BJ, Pai ALH, Zelikovsky N, et al. A Randomized Trial of a Multicomponent Intervention to Promote Medication Adherence: The Teen Adherence in Kidney Transplant Effectiveness of Intervention Trial (TAKE-IT). Am J Kidney Dis. 2018;72(1):30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denhaerynck K, Schafer-Keller P, Young J, Steiger J, Bock A, De Geest S. Examining assumptions regarding valid electronic monitoring of medication therapy: development of a validation framework and its application on a European sample of kidney transplant patients. BMC Med Res Methodol. 2008;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boucquemont J, Pai AL, Dharnidharka VR, Hebert D, Furth SL, Foster BJ. Gender differences in medication adherence among adolescent and young adult kidney transplant recipients. Transplantation. 2018;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasylyeva TL, Singh R, Sheehan C, Chennasamudram SP, Hernandez AP. Self-reported adherence to medications in a pediatric renal clinic: psychological aspects. PLoS One. 2013;8(7):e69060. [DOI] [PMC free article] [PubMed] [Google Scholar]


