Abstract
Siplizumab, a humanized anti-CD2 monoclonal antibody, has been used in conditioning regimens for hematopoietic cell transplantation and tolerance induction with combined kidney-bone marrow transplantation. Siplizumab-based tolerance induction regimens deplete T cells globally while enriching regulatory T cells (Tregs) early post-transplantation. Siplizumab inhibits allogeneic mixed-lymphocyte reactions (MLRs) in vitro. We compared the impact of siplizumab on Tregs versus other T-cell subsets in HLA-mismatched allogeneic MLRs using PBMCs. Siplizumab predominantely reduced the percentage of CD4+ and CD8+ effector memory T cells, which express higher CD2 levels than naïve T cells or resting Tregs. Conversely, siplizumab enriched proliferating CD45RA− FoxP3HI cells in MLRs. FoxP3 expression was stable over time in siplizumab-containing cultures, consistent with enrichment for bona fide Tregs. Consistently, high-throughput TCRβ CDR3 sequencing of sorted unstimulated and proliferating T cells in MLRs revealed selective expansion of donor-reactive Tregs along with depletion of donor-reactive CD4+ effector/memory T cells in siplizumab-containing MLRs. These results indicate that siplizumab may have immunomodulatory functions that may contribute to its success in tolerance-inducing regimens. Our studies also confirm that naïve in addition to effector/memory T cells contribute to the allogeneic MLR and mandate further investigation of the impact of siplizumab on alloreactive naïve T cells.
Introduction
Siplizumab is a humanized IgG1κ monoclonal antibody directed against the human CD2 glycoprotein, a receptor expressed on virtually all mature human T cells and on the vast majority of thymocytes1. CD2 promotes the adhesion of T cells to antigen-presenting cells through its interaction with the ligand LFA-3 (CD58)2. Binding of CD2 to LFA-3 also leads to a cascade of intracellular signals necessary for T-cell activation, conferring an important costimulatory function to this molecule3–5, and blockade of CD2 has been shown to promote islet allograft tolerance induction in a murine model6,7.
Ligation of either siplizumab or its parent antibody (BTI-322/Lo-CD2a) to human CD2 promotes T-cell depletion through antibody-dependent cell-mediated cytotoxicity (ADCC), an effect that is notably more pronounced on activated T cells8,9. In addition, these drugs efficiently inhibit the in vitro allogeneic mixed-lymphocyte reaction (MLR), with subsequent hyporesponsiveness upon allogeneic restimulation, while preserving reactivity to non-specific stimulation. This effect was found to be mediated, at least in part, by specific activation-associated T-cell depletion10,11.
We have previously used siplizumab as part of the conditioning regimen for HLA-mismatched, haploidentical hematopoietic cell transplantation alone12 or for combined HLA-mismatched kidney-bone marrow transplantation (CKBMT), a protocol that achieved allograft tolerance in association with transient mixed chimerism induction13,14. In recipients of hematopoietic cell transplantation to treat malignancies15 and CKBMT recipients16, a marked early enrichment in regulatory T cells (Tregs) was observed during the T-cell reconstitution phase. Phenotypic analysis suggested that the increase in Tregs was due to a combination of de novo generation in the thymus and lymphopenia-driven expansion of residual T cells, occurring in the presence of antigenic pressure from the graft17. These observations were consistent with previous evidence indicating a suppressive/regulatory mechanism of tolerance in the first year after transplantation in CKBMT patients16. Consistently, we have used a TCR sequencing approach to demonstrate preferentially expanded donor-specific Tregs at 6 months post-transplant in the circulation of tolerant patients but not in a patient who failed to achieve tolerance18.
Taken together, these data point towards a possible novel, additional effect of siplizumab as a selective modulator of alloimmunity towards the graft, i.e. the selective expansion of donor-specific suppressive Tregs. For these reasons, we studied CD2 expression on different T-cell subsets and tested the hypothesis that siplizumab may selectively spare Tregs from depletion in vitro in HLA-mismatched allogeneic MLRs. We also analyzed TCRβ CDR3 repertoires to determine whether: (1) donor-specific Treg clones would be selectively expanded by allostimulation in the presence of siplizumab; and (2) donor-specific effector/memory T cells would be selectively depleted. Our data demonstrate these potential tolerance-promoting effects of siplizumab.
Materials and Methods
Detailed methods, including cell isolation, mixed lymphocyte reactions, flow cytometry analyses, cell sorting, genomic DNA isolation and sequencing, clonal and statistical analysis may be found in Supplementary Material.
Allogeneic CFSE-Violet Dye MLRs
All experiments were performed with the approval of the local institutional review board (protocols IRB-AAAF0548 and IRB-AAAR0324). Human PBMCs from ten HLA-typed healthy donors were isolated from fresh whole blood. PBMCs from responder-stimulator pairs, selected to have the greatest number of HLA-mismatches available, were labelled with CFSE and Violet-Dye respectively, as described17, and combined in a 1:1 ratio. Siplizumab (produced by Medimmune Oncology Inc, Gaithersburg, MD USA – Lot#: 01AZ19B-2 for ITB-Med AB, kindly provided by Dr. David H.Sachs) was added to cell suspensions at several concentrations (Supplementary Figure 1). Cells were cocultured at 37°C for 6 days. For prolonged MLR cultures, 500 μl of fresh MLR medium was added to each well at day 6 and day 9. In some experiments, modified MLRs were used, as described in Supplementary Methods.
Clonal Analysis
Analysis of TCRβ CDR3 sequencing data was performed with R programming language, as detailed in the Supplementary Methods. CD4+ and CD8+ clones were defined as alloreactive based on the comparison between unstimulated and post-MLR CD4+/CD8+ CFSELO samples, as previously described19. Alloreactive CD4+ clone sequences were compared with unstimulated CD4 Treg and CD4 Non-Treg sequences to determine to which subset these alloreactive clones belonged at the time of input, thus defining the alloreactive Treg and alloreactive Non-Treg subsets. A similar approach was applied to the analysis of alloreactive CD8+ T cells (Supplementary Figure 3).
Statistical Analysis
Continuous variables from experimental data were reported as mean ± SD or median [IQR], as appropriate. Parametric and non-parametric tests were employed to analyze unpaired and matched data, as fully detailed in the supplementary material. P-values for ANOVA and non-parametric equivalents are reported in the text, while significant differences in the follow-up comparisons are represented in figures. The relative change of the mean or median (range across drug concentrations) is provided for significant differences. Statistically significant differences were assumed at 5% level of probability. All analyses were performed with GraphPad Prism version 7 (GraphPad Software Inc, La Jolla, CA USA).
Results
CD2 expression in unstimulated T-cell subsets
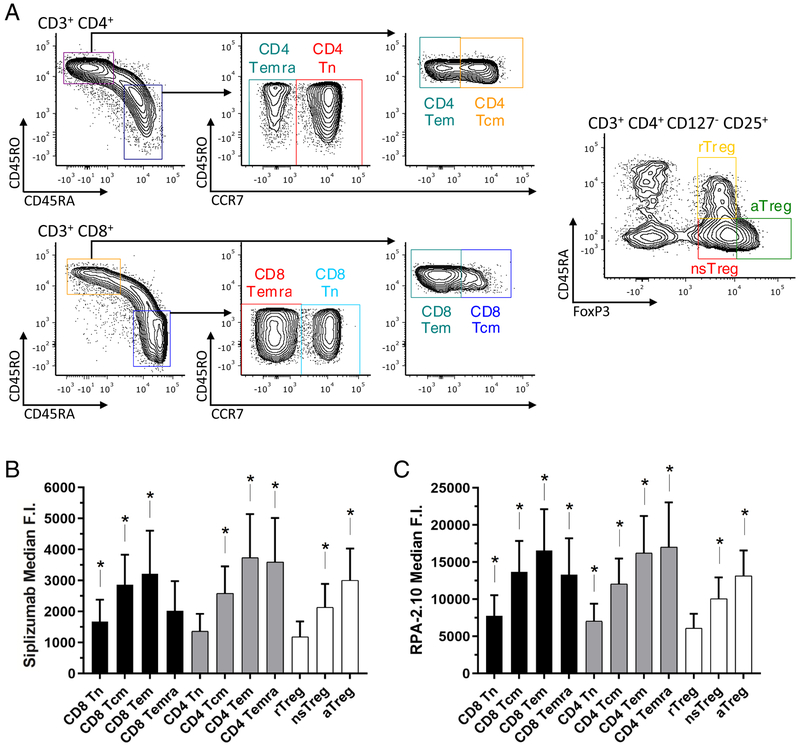
Using PBMCs, we analyzed the relative surface expression of CD2 on unstimulated CD4+ and CD8+ Naive, Central Memory, Effector Memory and TEMRA subsets and on Treg subsets defined as resting (rTreg), non-suppressive (nsTreg) and activated (aTreg) according to Miyara et al20 (Figure 1A). CD2 expression levels were significantly different among the T-cell subsets analyzed (p < 0.0001 with both siplizumab and RPA-2.10 mAbs). Both of the anti-CD2 antibodies employed (Figures 1B and 1C) returned similar results in terms of relative CD2 density, with memory T cells showing the highest expression of the molecule.
Figure 1. CD2 expression among unstimulated T-cell subsets.
(A) Gating strategy for the subsets of interest on which CD2 expression was assessed. (B,C) Median fluorescence intensity of CD2 (using Siplizumab and the commercially available clone RPA-2.10) among T-cell subsets (mean ± SD); * p < 0.05 compared to rTreg, n = 10 unique donors (repeated-measures ANOVA and Tukey’s multiple comparisons test). Significant differences in selected additional comparisons:
- [Siplizumab] CD4+ Naïve vs Central Memory: p < 0.0001 – CD4+ Naïve vs Effectory Memory p = 0.0004 – CD8+ Naïve vs Central Memory: p < 0.0001 – CD8+ Naïve vs Effectory Memory p = 0.0047 – nsTregs vs CD4+ Central Memory: p = 0.0116 – nsTregs vs CD4+ Effector Memory: p = 0.0016 – nsTregs vs CD8+ Central Memory: p = 0.0003 – nsTregs vs CD8+ Effector Memory: p = 0.0459 – aTregs vs CD4+ Effector Memory: p = 0.0249.
- [RPA-2.10] CD4+ Naïve vs Central Memory: p < 0.0001 – CD4+ Naïve vs Effectory Memory p < 0.0001 – CD8+ Naïve vs Central Memory: p < 0.0001 – CD8+ Naïve vs Effectory Memory p = 0.0003 – nsTregs vs CD4+ Central Memory: p = 0.0025 – nsTregs vs CD4+ Effector Memory: p = 0.0006 – nsTregs vs CD8+ Central Memory: p = 0.0019 – nsTregs vs CD8+ Effector Memory: p = 0.0092 – aTregs vs CD4+ Effector Memory: p = 0.0154.
Our studies with both mAbs confirmed previous studies21 indicating that naïve T cells express lower levels of CD2 compared to central and effector memory T cells. This observation applied to both CD4+ and CD8+ subsets. Interestingly, Tregs appeared to have lower CD2 expression than their effector counterparts: the rTreg subset consistently showed the lowest CD2 expression among the subsets investigated, with statistically significant differences compared to most of the other subsets (Figure 1B,C). The level of CD2 on nsTregs was lower than that on the memory subsets and the level on aTreg was lower than that on CD4+effector memory T cells.
Effect of Siplizumab on T-cell proliferation and CD2 expression in the allogeneic MLR
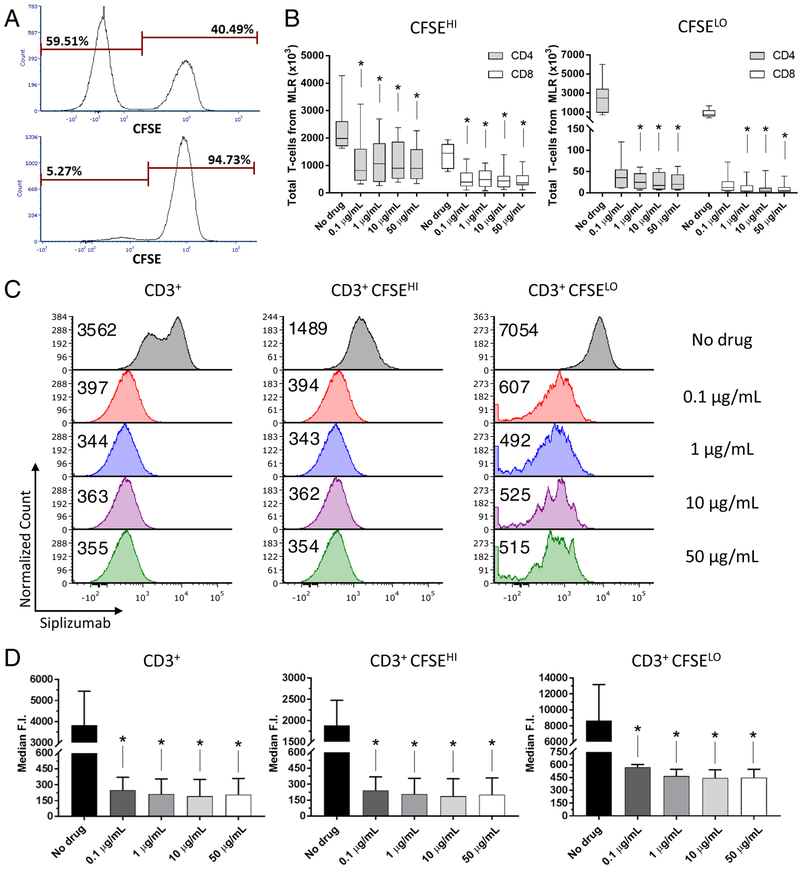
Addition of siplizumab at all concentrations tested (0.1 – 50 μg/mL) to the allogeneic HLA-mismatched MLR resulted in a significant reduction of total CD4+ and CD8+ T-cell absolute numbers (Supplementary Table 1). This reduction was much more pronounced for proliferating T cells (CFSELO CD4+: [−98.5%;−99.3%], p < 0.0001; CFSELO CD8+: [−98.3%;−99.3%], p < 0.0001), but was significant even for non-proliferating T cells (CFSEHI CD4+: [−46.5%;−58.8%], p = 0.0008; CFSEHI CD8+: [−66.3%;−74.6%], p = 0.0004) (Figure 2A and B, Supplementary Figure 4). These findings validate the previous suggestion that the depleting effect of siplizumab is preferentially directed toward activated T cells9. Additionally, a dose effect was evident when the ratio of CFSEHI to CFSELO T cells was considered, showing that increasing drug concentrations had a greater effect on proliferating than non-proliferating cells in both the CD4+ and CD8+ subsets (p < 0.0001 for both) (Supplementary Figure 5).
Figure 2. Cell proliferation and CD2 expression in both proliferating and non-proliferating T cells are reduced in MLRs containing siplizumab at day 6.
(A) Proliferation of CD3+ T cells in MLRs containing Siplizumab (bottom panel, 1 μg/mL) compared to control MLRs (top panel). (B) Numbers of non-proliferating (CFSEHI) and proliferating (CFSELO) T cells recovered from allogeneic MLRs at increasing drug concentrations (median [IQR]); n = 10 unique donor pairs (Friedman’s test and Dunn’s multiple comparisons tests). (C) Siplizumab median fluorescence intensity (with PE-Dazzle594 anti-human IgG Fc) in a representative sample of total, non-proliferating and proliferating T cells at increasing drug concentration, with (D) summary of the analysis (mean ± SD); n = 5 unique donor pairs. * p < 0.05 compared to No drug MLR (repeated-measures ANOVA and Dunnet’s multiple comparisons test).
No difference in CD69 or HLA-DR surface expression was observed between control and siplizumab-containing cultures on days 1, 2 or 4 of MLR, whereas at day 7 the expression of both markers was significantly lower in siplizumab-treated samples compared to control (Supplementary Figure 6). Of note, CD69 and HLA-DR expression post-MLR was directly correlated to the frequency of proliferating T cells, which expressed higher levels of both markers compared to resting T cells (data not shown). No dose-response relationship could be observed in the tested concentration range.
Analysis of CD2 expression by indirect staining (Siplizumab + anti-human IgG Fc) on CD3+ cells revealed significant surface CD2 down-regulation post-MLR in cultures containing siplizumab compared to controls ([−93.5%;−95.0%], p = 0.0089) (Figure 2C and D). This effect was apparent in both CFSEHI and CFSELO CD3+ cells ([−87.2%;−90.1%], p = 0.0052 and [−93.4%;−94.8%], p = 0.0162, respectively). Proliferating cells showed higher CD2 expression than non-proliferating cells in control MLRs (+358.9%, p = 0.0184) and to a lesser extent in cultures containing siplizumab ([+123–3%;+138.6%], p < 0.05 at every drug concentration). Levels of surface-bound siplizumab over time during the MLR were also tracked with a secondary antibody (Supplementary Figure 7).
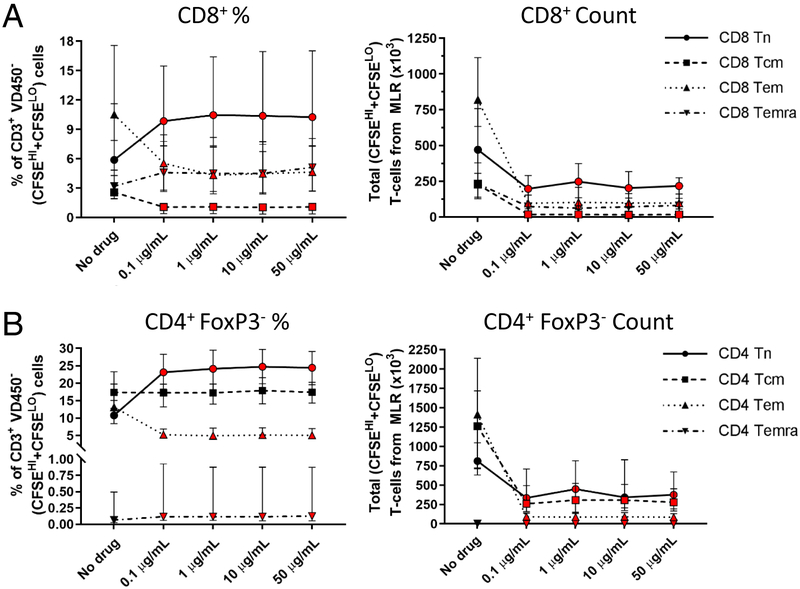
Effect of siplizumab on CD8+ and CD4+ FoxP3− subsets in the allogeneic MLR
Siplizumab reduced the absolute number of cells in all subsets recovered from the allogeneic MLR at day 6 of culture (Supplementary Table 1), and also consistently modified the relative abundance of specific T-cell subsets compared to the control MLR (Figure 3 and Supplementary Figure 8). Overall, the percentage of both CD4+ and CD8+ naive T cells increased in siplizumab-containing cultures compared to the control MLR ([+105.9%;+118.5%], p < 0.0001 and [+52.4%;+60.3%], p = 0.0002, respectively). In addition, the TEMRA fraction of CD8+ T-cells was significantly increased with siplizumab ([+40.4%;+59.6%], p < 0.0001). On the other hand, T-cell memory subsets were reduced by siplizumab addition to the culture, with a significant reduction of CD8+ central memory ([−73.4%;−74.6%], p = 0.0018) and CD4+ and CD8+ effector memory T cells ([−63.3%;−65.1%], p < 0.0004 and [−52.7%;−59.3%], p = 0.0013, respectively). Most of these changes were already evident with the lowest concentration of the drug.
Figure 3. Siplizumab lymphodepleting effect varies among different CD8+ and CD4+ FoxP3− T-cell subsets in allogeneic HLA-mismatched MLRs.
CD8+ (A) and CD4+ FoxP3− (B) T-cell subsets recovered from the HLA-mismatched MLRs expressed both as percentage of total CD3+ responders and as absolute cell numbers (median [IQR]); n = 10 unique donor pairs. Red-colored symbol: p < 0.05 compared to No drug MLR (repeated-measures ANOVA and Dunnet’s multiple comparisons test, or Friedman’s test and Dunn’s multiple comparisons tests, as appropriate).
Separate analysis of non-proliferating (CFSEHI) subsets (Supplementary Figure 8) revealed a significant albeit marginal reduction of CD8+ central memory ([−37.4%;−42–4%], p = 0.0005), as well as a small decline in CD4+ and CD8+ effector memory T cells ([−23.1%;−26.5%], p = 0.0034 and [−29.1%;−36.1%], p = 0.0007, respectively) in siplizumab-containing cultures. Proliferating (CFSELO) subsets (Supplementary Figure 8) showed significant reductions in CD4+ and CD8+ effector memory T cells ([−57.5%;−66.8%], p = 0.0019 and [−34.9%;−52.0], p < 0.0001, respectively) in the presence of siplizumab.
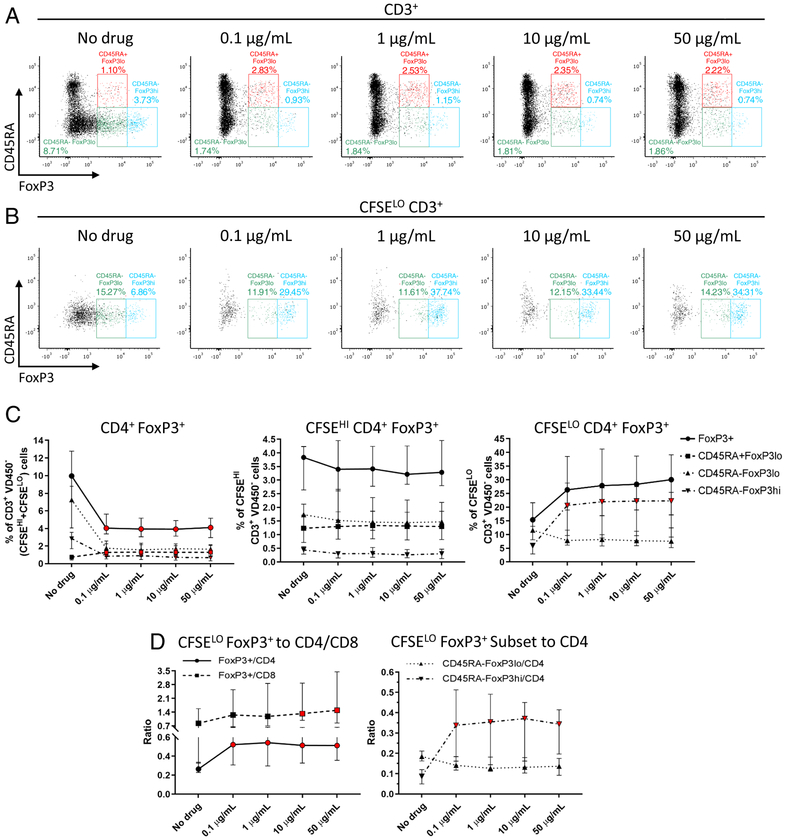
Effect of siplizumab on CD4+FoxP3+ T cells in the allogeneic MLR
The absolute numbers and frequencies of CD4+ CD25+ CD127− FoxP3+cells among total (CFSEHI plus CFSELO) CD3+ T cells (Figure 4A and 4C, Supplementary Figure 4) decreased significantly ([−55.0%;−59.4%], p < 0.0001) in siplizumab-containing cultures compared to controls, as did the percentages of CD45RA− FoxP3LO (nsTregs) and CD45RA− FoxP3HI (aTregs) cells ([−75.7%;−78.4%], p < 0.0001 and [−63.2%;−72.0%], p = 0.0002, respectively). Consistent with their lower CD2 expression, however, CD45RA+ FoxP3LO cells (resting phenotype) showed a significant frequency increase ([+98.9%;+109.2%], p = 0.0067) in siplizumab-containing MLR compared with control. No significant differences in these FoxP3+ subsets were detected among the non-proliferating cells (CFSEHI; Figure 4C). However, when proliferating cells (CFSELO) were analyzed separately (Figure 4B and 4C), we observed a significant increase in the percentage of CD4+FoxP3+ cells (p = 0.0163), with borderline significance at high drug concentrations on the post-hoc test (10 μg/mL +70.0%, p = 0.05 and 50 μg/mL +72.3%, p = 0.06). This difference was clearly due to a marked enrichment in CD45RA− FoxP3HI Tregs (i.e. those with an activated phenotype) compared to control ([+250.9%;+262.6%], p = 0.0002), while CD45RA− FoxP3LO Tregs (i.e. those with a non-suppressive phenotype) showed a non-significant tendency to decline (p = 0.10) in siplizumab-containing cultures. Overall, ratios of CFSELO CD4+ FoxP3+ cells to CFSELO CD4+ or CFSELO CD8+ T cells (Figure 4D) were significantly increased by siplizumab ([+70.5%;+75.0%], p = 0.0034 and [+42.2%;+79.3%], p = 0.0073, respectively), with a significant increase also observed in ratios of CFSELO CD45RA− FoxP3HI to both CFSELO CD4+ and CFSELO CD8+ cells ([+235.2%;+247.5%], p < 0.0001 and [+201.9%;+356.7%], p < 0.0001, respectively).
Figure 4. Siplizumab reduces the percentage of total FoxP3+ cells, but expands the fraction of proliferating FoxP3HI cells and the ratio of proliferating FoxP3+ to CD4+ and CD8+ cells in allogeneic HLA-mismatched MLRs.
Total (A) and proliferating (B) CD4+ CD25+ CD127− FoxP3+ subsets post-MLR at different drug concentrations, back-gated on responder total and proliferating CD3+ T cells respectively. FoxP3+ cells were further characterized on the basis of CD45RA (+/−) and FoxP3 (hi/lo) expression. (C) Total, non-proliferating (CFSEHI) and proliferating (CFSELO) CD4+ CD25+ CD127− FoxP3+ cells represented as percentage of total, non-proliferating and proliferating CD3+ T cells respectively (median [IQR]); n = 10 unique donor pairs. (D) Ratios of proliferating (CFSELO) CD4+ CD25+ CD127− FoxP3+ cells to either CFSELO CD4+ or CFSELO CD8+ cells, and ratio of CFSELO CD45RA− FoxP3HI/LO subsets to CFSELO CD4+ cells (median [IQR]); n = 10 unique donor pairs. Red-colored symbol: p < 0.05 compared to No drug MLR (repeated-measures ANOVA and Dunnet’s multiple comparisons test, or Friedman’s test and Dunn’s multiple comparisons tests, as appropriate).
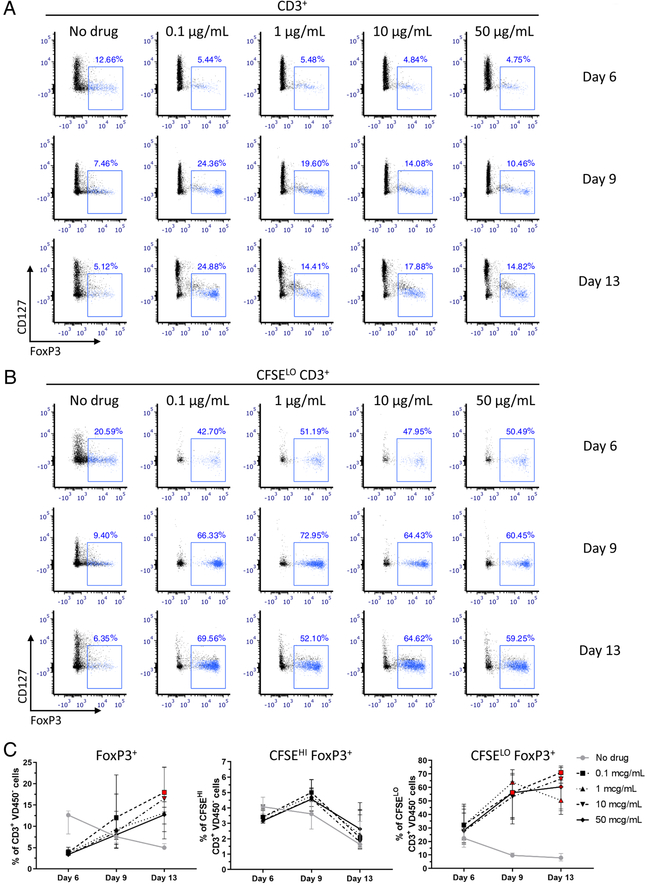
Stability of FoxP3 expression over time in Siplizumab-containing and control cultures
FoxP3 not only identifies Tregs, but is also expressed by activated human conventional T cells that lack suppressive function. However, FoxP3 expression in these cells tends to be lower and more transient compared to that of Tregs22–24. In order to differentiate between transient activation-dependent and stable Treg-associated FoxP3 expression, we extended four allogeneic HLA-mismatched MLRs until day 13. Phenotypic data were obtained by applying the same gating to the analysis at every time point in a given MLR, and results from days 6, 9 and 13 were compared (Figure 5, Supplementary Figures 9–10). We identified Tregs according to expression of CD4, FoxP3, CD25 and CD127 and assessed their frequency in total T cells as well as in proliferating (CFSELO) and in non-proliferating (CFSEHI) T cells separately. As expected, the frequency of FoxP3+ cells among total T cells showed a progressive decrease from day 6 to day 13 in the control MLR, likely due to progressive loss of FoxP3 expression by activated conventional T cells. On the other hand, the percentage of FoxP3+ cells in siplizumab-containing cultures showed an opposite trend over time, increasing by approximately 2- to 4-fold on day 13 compared to day 6. When linear regression of FoxP3 expression over time was performed, a statistically significant difference between the slopes was observed between siplizumab-containing and control MLRs (p < 0.0001) (Supplementary Table 2), which was most pronounced at the lowest drug concentration. At day 13 post-culture, the total CD4+ FoxP3+ CD25+ CD127− Treg percentage was significantly greater in siplizumab-containing compared to control MLRs ([+120.6%;+255.9%], p = 0.0233) (Figure 5C). Similar trends were observed when the same analysis was performed on proliferating T-cells (Figure 5C), for which linear regression analysis of FoxP3 expression over time also revealed a statistically significant difference between siplizumab-containing and control MLRs (p = 0.0042). The borderline significant difference in percentage of FoxP3+ cells observed at day 6 between siplizumab-containing and control MLRs progressively increased over time, reaching statistical significance at both day 9 ([+411.6%;+474.8%], p = 0.0177) and day 13 ([+537.3%;+770.4%], p = 0.0103). Conversely, the percentage of FoxP3+ cells among non-proliferating T cells in siplizumab-containing MLRs did not differ significantly from that in control MLRs at days 9 and 13 (Figure 5C).
Figure 5. FoxP3 expression in total and proliferating (CFSELO) T cells declines over time in control allogeneic HLA-mismatched MLRs, but shows an opposite trend in allogeneic HLA-mismatched MLRs containing siplizumab.
Total (A) and proliferating (B) CD4+ CD25+ CD127− FoxP3+ cells post-MLR at different drug concentrations and at different timepoints, back-gated on responder total and proliferating CD3+ T cells respectively. (C) Total, non-proliferating (CFSEHI) and proliferating (CFSELO) CD4+ CD25+ CD127− FoxP3+ cells over time represented as percentage of responder total, non-proliferating and proliferating CD3+ T-cells respectively (median [IQR]); n = 4 unique donor pairs. Red-colored symbol: p < 0.05 compared to No drug MLR (repeated-measures ANOVA and Dunnet’s multiple comparisons test).
To gain further insight into the commitment of post-MLR FoxP3+ T cells, we assessed the FoxP3 promoter methylation status of CD4+CD25+FoxP3+ cells sorted after a 14 days MLR, cultured with and without the addition of siplizumab, and compared it with that of sorted unstimulated Tregs isolated from the same donors. The FoxP3 promoter region, known as the Treg-specific demethylated region (TSDR) is heavily methylated in activated human conventional T cells, whereas suppressive Tregs display complete FoxP3 promoter demethylation25. The TSDR methylation status did not vary significantly over time in cultures to which siplizumab was added (−13.7%, p = 0.58), whereas the TSDR in FoxP3+ cells had a more methylated phenotype compared to unstimulated Tregs in control MLRs (+92.2%, p = 0.0096) (Supplementary Figure 11).
Clonal Analysis
The relative and sustained enrichment of FoxP3+ cells among proliferating T-cells in siplizumab-containing MLRs strongly supported the hypothesis that these were alloreactive Tregs. This enrichment could theoretically be due to a combination of three different mechanisms, including selective depletion of alloreactive non-Treg cells, expansion of pre-existing alloreactive Tregs and/or the induction of alloreactive Tregs from CD4+ effector T cells during the culture period. In order to obtain additional insight into this phenomenon, we performed high-throughput TCRβ CDR3 sequencing on genomic DNA extracted from unstimulated CD4+ and post-MLR CFSELO CD4+ sorted T cells. Using this method, alloreactive CD4+ T cell sequences were identified in eight pairs of Siplizumab-containing (1 μg/mL) and control MLRs (Supplementary Table 3). By additionally interrogating FACS-sorted, unstimulated CD4+ Treg and CD4+ Non-Treg sequences for these alloreactive CD4+ sequences, we were able to map their origin to the Treg or Non-Treg populations in the unstimulated sample.
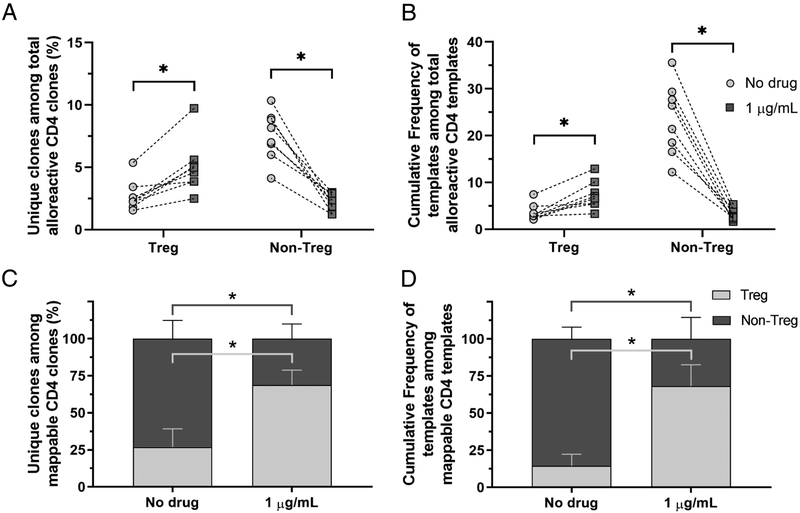
The fraction of unique alloreactive CD4+ sequences that mapped to the Treg fraction of the unstimulated population was significantly higher in siplizumab-containing MLRs compared to control cultures (+110.9%, p = 0.0078) (Figure 6A). Consistently, the cumulative frequency of sequences identified as alloreactive Treg in the alloreactive CD4+ subset increased significantly with siplizumab compared to control (+132.7%, p = 0.0078) (Figure 6B). On the other hand, both the clone fraction and the cumulative frequency of identifiable Non-Treg among alloreactive CD4+ cells was significantly reduced in siplizumab-containing cultures compared to control (−71.2%, p = 0.0002 and −86.2%, p < 0.0001, respectively).
Figure 6. Siplizumab promotes a relative expansion of alloreactive Treg clones over CD4+ Non-Treg clones.
(A) Percentage of unique clones identified either as alloreactive Treg or alloreactive Non-Treg, based on detection in CFSELO populations and sorted unstimulated Tregs or non-Tregs, among total CD4+ clones identified as alloreactive in the CFSELO population (median [IQR]). (B) Cumulative frequency of sequences identified either as alloreactive Treg or alloreactive Non-Treg among total CD4+ sequences identified as alloreactive in the CFSELO population (median [IQR]). (C) Fraction of unique clones identified as either alloreactive Treg or alloreactive Non-Treg among total CD4+ mappable clones (i.e. those identifiable as alloreactive and detectable in either unstimulated Treg or untimulated Non-Treg PBMCs) (mean ± SD). (D) Cumulative frequency of sequences identified either as alloreactive Treg or alloreactive Non-Treg among mappable CD4+ sequences (i.e. those identifiable as alloreactive and detectable in either unstimulated Treg or untimulated Non-Treg PBMCs) (mean ± SD). n = 8 unique donor pairs for all experiments. * p < 0.01 compared to No drug MLR (paired t-test or Wilcoxon’s matched-pairs signed rank test, as appropriate).
Because Adaptive’s deep sequencing platform does not readily capture sequences present at frequencies below 10−5 and most alloreactive T cells detected after expansion in an MLR are present in the circulation at very low frequencies and are therefore not detected in the unstimulated T cell pool26, only a minor fraction of alloreactive CD4+ clones could be mapped to the sorted unstimulated Treg or Non-Treg population. Consequently, the data in Figure 6A and B underestimates the clone fraction and the cumulative frequency of each subset in the CFSELO population, as most sequences could not be assigned to (i.e. was not “mappable” to) either subset. To overcome this limitation, we also analyzed the clone fractions and cumulative frequencies of each subset in the alloreactive population using only the mappable clones (to either Treg or Non-Treg) as the denominator (Figures 6C and 6D, Supplementary Figure 12). The Treg clone fraction in siplizumab-containing MLRs again increased significantly, with an average of 68.8% of sequences identifiable as Tregs compared to 26.9% of sequences in control cultures (p < 0.0001). Conversely, the mappable Non-Treg clone fraction decreased significantly to a mean of 31.2% in siplizumab-containing cultures compared to 73.1% in control cultures (p < 0.0001). Likewise, the cumulative frequency of mappable Tregs in siplizumab-containing MLRs was markedly increased to an average of 68.2% compared to 14.4% in control cultures (p < 0.0001), while the cumulative frequency of Non-Tregs decreased in the siplizumab-containing cultures (31.9% vs 85.6 in control cultures, p < 0.0001).
In three pairs of samples the analysis was extended to allow identification of alloreactive clones that mapped to the CD4+ Naive, CD4+ Memory, CD8+ Naive, CD8+ Effector Memory and CD8+ TEMRA populations in the unstimulated circulating PBMCs (Supplementary Figure 13). The cumulative frequency of alloreactive sequences identifiable as CD4+ Memory in the unstimulated repertoire decreased significantly in siplizumab-containing MLRs compared to controls (−68.7%, p = 0.0169), whereas the alloreactive clone fraction identifiable as CD4+ Memory did not change significantly (p = 0.11), though each individual showed a downward shift in the siplizumab cultures. In contrast, the contribution of CD8+ Effector Memory and TEMRA subsets to the alloreactive population did not change consistently in either direction in the presence of siplizumab (Supplementary Figure 13).
The above study provided an opportunity to examine the relative contribution of naïve and memory T cells to the human MLR. While only small numbers of clones identifiable as naïve CD4+ or CD8+ T-cells contributed to the alloreactive cell populations in this assay, when only mappable (to naïve or memory subsets in unstimulated populations) clones were used as the denominator in the analysis, the contribution of naïve CD4+ and CD8+ cells to the response was readily apparent (Supplementary Figure 14A–D). The greater diversity of naïve compared to memory T cells in unstimulated human PBMCs (Supplementary Figure 14E and F) is associated with a lower frequency of each naïve clone in a given sample, resulting in a bias against their detection in the unstimulated repertoire and hence against the identification of a naïve T cell contribution to the CFSELO population. The inability to assign a naïve or memory phenotype to about 80–95% of CFSELO CD4+ or CD8+ clones in the MLR (Supplementary Figure 13) is consistent with this interpretation.
To further corroborate this point, we assessed the alloreactivity of sorted naïve and memory T cells in a modified MLR, in which the two subsets were stained with different proliferation dyes: our experiments confirmed that both naïve and memory T cells participate in the alloresponse, both when responder cells are cultured separately or are recombined in the same MLR (Supplementary Figure 15).
Discussion
Siplizumab is a T cell-depleting agent that binds to a specific region of the human CD2 molecule, which differs from the classic CD2 epitopes described in earlier work and that is not shared with most non-human primates27. Even though the exact role of the CD2 molecule in human T-cell immunology is still incompletely understood, perturbation of specific regions of this receptor can inhibit T-cell proliferation28. In addition to this inhibitory effect, siplizumab also mediates a robust T-cell depletion through ADCC, which is in turn dependent on the activity of monocytes and NK-cells9. Interestingly, this action is more pronounced against activated T lymphocytes, likely because these cells express CD2 at higher density compared to resting T cells, and because NK-cell activity is enhanced by the high IL-2 concentrations that follow T-cell activation29.
Our in vitro experiments showed that siplizumab may have additional features of potential interest for solid organ transplantation: when added to the allogeneic MLR, siplizumab predominantly reduced the frequency of CD4+ and CD8+ effector memory T cells compared to other subsets. This effect likely reflects both reduced T-cell proliferation and selective depletion of effector memory T cells. Indeed, our data show that both CD4+ and CD8+ effector memory T cells express CD2 at high density, which likely renders them a preferential target for the drug. Similar results have been described for alefacept, a CD2-specific LFA-3-Ig fusion protein that was shown to selectively deplete memory T cells30,31. In contrast, T cell-depleting agents currently available, such as polyclonal anti-thymocyte globulin (ATG) and alemtuzumab, are predominantly active against naïve T cells and do not efficiently deplete alloantigen-primed T cells32–34. Considering the relevance of alloreactive effector memory T cells in graft rejection35, siplizumab may confer additional protection compared to other T cell-depleting agents, especially in recipients at high immunological risk.
Our data also demonstrate that siplizumab selectively increases the percentage of proliferating Tregs in the allogeneic MLR compared to control. Since we found that resting Tregs express the lowest surface CD2 levels among T-cell subsets, they may be selectively spared from siplizumab-mediated T-cell depletion and inhibition of proliferation, leading in turn to a relative expansion of regulatory T cells during the alloresponse. This effect differs from that originally described for ATG, which featured FoxP3 induction from conventional T cells that was not associated with suppressive activity36,37. Notably, in the published experiments, FoxP3 upregulation was completely lost after ten days of culture, in contrast to our observations of siplizumab-containing MLRs, in which FoxP3 expression tended to increase over time.
Despite efficiently inhibiting T-cell proliferation, siplizumab did not affect early T-cell activation as assessed by CD69 and HLA-DR expression. This lack of downregulation is probably important in permitting expansion of alloreactive Tregs, since the expression of these molecules has been shown to play a central role in the development and suppressive function of this regulatory subset38,39.
While we did not functionally confirm the suppressive ability of CFSELO Tregs in siplizumab cultures, the stability and increasing enrichment of FoxP3 expression over time in siplizumab-containing compared to control MLRs is inconsistent with the transient upregulation of FoxP3 that is typical of activated conventional T cells. Moreover, FoxP3+ T cells harvested from siplizumab-containing cultures retained a high degree of FoxP3 promoter demethylation, which was comparable to that assessed in unstimulated Tregs. Most importantly, the TCR repertoire analysis we performed demonstrated that the enrichment in alloreactive FoxP3+ T cells observed post-MLR derives from the expansion of Tregs pre-existing in the unstimulated T-cell pool. Indeed, the striking predominance of Treg-derived sequences among total alloreactive sequences in siplizumab MLRs that could be mapped to the unstimulated pool suggests that siplizumab MLRs may provide a means of expanding donor-specific Tregs in vitro. Expansion of donor-specific Tregs has been reported to occur in MLRs to which belatacept has been added40. However, in our hands, this method also expands large numbers of non-donor-specific Tregs18 and it would be of interest to compare these two methodologies side by side. Since siplizumab greatly reduces the number of proliferating T-cells, as shown by the steep decrease in absolute T-cell counts observed in siplizumab MLRs, additional expansion of this population would be needed to obtain sufficient quantities for infusion.
Our mapping of MLR-expanded T cells to sorted unstimulated T cell subpopulations also allowed us to compare the contributions of naïve and memory T cells to the allogeneic HLA-mismatched MLR, a type of analysis that has not been previously reported. We were able to map markedly greater numbers of clones to the memory T-cell pool than to the naïve subset for both CD4+ and CD8+ cells. While one interpretation of this result would be that most of the T-cells responding to alloantigens during alloreaction are cross-reactive memory T-cells, we believe the result is biased to detection of memory cells due to sampling effect. Similar to previous studies41, we observed that unstimulated circulating memory T cells have a lower diversity (higher clonality and Simpson’s index) compared to their naïve counterparts, resulting in a low frequency of each naïve T cell in the circulation and a reduced chance of detecting it in a pool of 105 TCR templates, which is the order of magnitude used for Adpative’s deep sequencing method. Consistent with this interpretation, we have shown that the vast majority of alloreactive T-cell clones detected with this MLR method are undetectable in the unstimulated population due to their low frequency in the circulation26. Thus, we interpret the ability to map even a small fraction of alloreactive T cells in the MLR to the naïve population in a relatively small sample as an indication that naïve T cells make a major contribution to the alloreactive pool. These conclusions are consistent with our experiments and previous data obtained from both CFSE-based proliferation and limiting dilution assays, indicating that the alloreactive T-cell repertoire is not restricted to a specific subset, and that both naïve and memory T cells contribute to the MLR42,43.
In view of the relatively low CD2 expression we detected on naïve CD4+ and CD8+ T-cell populations, it is possible that siplizumab may be relatively ineffective at inhibiting this component of the allograft response if used as induction therapy. Since naïve human T-cells convert to the memory phenotype in a T-cell-deficient environment44, however, further studies are needed to assess CD2 expression on naïve T cells undergoing this lymphopenia-driven activation.
The experiments described herein were designed to evaluate within-subject differences. Hypothesis testing with a matched-pair design increases the power to detect significant differences. Therefore, particular caution should be paid when interpreting results from small changes. The major effects of siplizumab on Tregs and effector memory cells, however, were large.
In conclusion, we have shown that the T-cell depleting effect of siplizumab targets mainly the effector memory subset and that the drug promotes a relative expansion of alloreactive regulatory T-cells in vitro. These activities help to explain the marked enrichment of Tregs observed in hematopoietic stem cell transplant recipients and tolerant CKBMT recipients conditioned with a siplizumab-based regimen, which recent studies have shown to reflect an expansion of donor-specific Tregs18. Further studies will be needed to assess the mechanisms by which these effects occur in vivo.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Ying Kuen Cheung for his assistance with statistical analysis, Thomas M. Savage and Ben Sprangers for helpful review of the manuscript and Nicole Casio for assistance with its submission. This work was supported by ITB-Med AB. Some of the experiments reported in this publication used the resources of the CCTI Flow Cytometry Core, supported in part by the Office of the Director, National Institutes of Health under awards S10RR027050 and S10OD020056, the Herbert Irving Comprehensive Cancer Center Flow Cytometry Shared Resources, funded in part through Center Grant P30CA013696, and the Diabetes and Endocrinology Research Center Flow Core Facility, funded in part through Center Grant 5P30DK063608. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- ADCC
antibody-dependent cell-mediated cytotoxicity
- CKBMT
combined kidney-bone marrow transplantation
- MLR
mixed-lymphocyte reaction
- Tregs
regulatory T-cells
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr. Sykes is the head of the Scientific Advisory Board of ITB-Med AB. Dr. Berglund is the Chief Scientific Officer of ITB-Med AB. Dr. Sellberg and C. Binder are both employees of ITB-Med AB. The other authors have no conflicts of interest to disclose.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Bierer BE, Burakoff SJ. T-lymphocyte activation: the biology and function of CD2 and CD4. Immunol Rev. 1989;111:267–294. [DOI] [PubMed] [Google Scholar]
- 2.van der Merwe PA, Barclay AN, Mason DW, et al. Human cell-adhesion molecule CD2 binds CD58 (LFA-3) with a very low affinity and an extremely fast dissociation rate but does not bind CD48 or CD59. Biochemistry. 1994;33(33):10149–10160. [DOI] [PubMed] [Google Scholar]
- 3.Suthanthiran M T-cell differentiation antigen cluster 2 (CD2) is a receptor for accessory cells and can generate and/or transduce accessory signals. Cell Immunol. 1988;112(1):112–122. [DOI] [PubMed] [Google Scholar]
- 4.Bockenstedt LK, Goldsmith MA, Dustin M, Olive D, Springer TA, Weiss A. The CD2 ligand LFA-3 activates T cells but depends on the expression and function of the antigen receptor. J Immunol. 1988;141(6):1904–1911. [PubMed] [Google Scholar]
- 5.Suthanthiran M Transmembrane signalling via the T cell antigen receptor heterodimer and the CD2 antigen. A synergistic pathway for activation of T cells. Transplantation. 1989;47(2):348–351. [DOI] [PubMed] [Google Scholar]
- 6.Kapur S, Sharma V, Khanna A, Li B, Sukol K, Suthanthiran M. Regulation of the anti-allograft response by targeting the CD2 antigen: a potential strategy for the creation of transplant tolerance. Surg Technol Int. 1996;5:233–240. [PubMed] [Google Scholar]
- 7.Kapur S, Khanna A, Sharma VK, Li B, Suthanthiran M. CD2 antigen targeting reduces intragraft expression of mRNA-encoding granzyme B and IL-10 and induces tolerance. Transplantation. 1996;62(2):249–255. [DOI] [PubMed] [Google Scholar]
- 8.Branco L, Barren P, Mao SY, et al. Selective deletion of antigen-specific, activated T cells by a humanized MAB to CD2 (MEDI-507) is mediated by NK cells. Transplantation. 1999;68(10):1588–1596. [DOI] [PubMed] [Google Scholar]
- 9.Nizet Y, Chentoufi AA, de la Parra B, et al. The experimental (in vitro) and clinical (in vivo) immunosuppressive effects of a rat IgG2b anti-human CD2 mAb, LO-CD2a/BTI-322. Transplantation. 2000;69(7):1420–1428. [DOI] [PubMed] [Google Scholar]
- 10.Latinne D, De La Parra B, Nizet Y, et al. An anti-CD2 mAb induces immunosuppression and hyporesponsiveness of CD2+ human T cells in vitro. Int Immunol. 1996;8(7):1113–1119. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Kolber-Simonds D, Hope JA, et al. The anti-CD2 monoclonal antibody BTI-322 generates unresponsiveness by activation-associated T cell depletion. Clin Exp Immunol. 2004;138(3):476–483. doi: 10.1111/j.1365-2249.2004.02650.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spitzer TR, McAfee SL, Dey BR, et al. Nonmyeloablative haploidentical stem-cell transplantation using anti-CD2 monoclonal antibody (MEDI-507)-based conditioning for refractory hematologic malignancies. Transplantation. 2003;75(10):1748–1751. doi: 10.1097/01.TP.0000064211.23536.AD [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4):353–361. doi: 10.1056/NEJMoa071074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai T, Sachs DH, Sprangers B, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14(7):1599–1611. doi: 10.1111/ajt.12731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaffer J, Villard J, Means TK, et al. Regulatory T-cell recovery in recipients of haploidentical nonmyeloablative hematopoietic cell transplantation with a humanized anti-CD2 mAb, MEDI-507, with or without fludarabine. Exp Hematol. 2007;35(7):1140–1152. doi: 10.1016/j.exphem.2007.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreola G, Chittenden M, Shaffer J, et al. Mechanisms of donor-specific tolerance in recipients of haploidentical combined bone marrow/kidney transplantation. Am J Transplant. 2011;11(6):1236–1247. doi: 10.1111/j.1600-6143.2011.03566.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sprangers B, DeWolf S, Savage TM, et al. Origin of Enriched Regulatory T Cells in Patients Receiving Combined Kidney-Bone Marrow Transplantation to Induce Transplantation Tolerance. Am J Transplant. 2017;17(8):2020–2032. doi: 10.1111/ajt.14251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savage TM, Shonts BA, Obradovic A, et al. Early expansion of donor-specific Tregs in tolerant kidney transplant recipients. JCI Insight. 2018;3(22):e124086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris H, DeWolf S, Robins H, et al. Tracking donor-reactive T cells: Evidence for clonal deletion in tolerant kidney transplant patients. Sci Transl Med. 2015;7(272):272ra10. doi: 10.1126/scitranslmed.3010760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019 [DOI] [PubMed] [Google Scholar]
- 21.Lo DJ, Weaver TA, Stempora L, et al. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. Am J Transplant. 2011;11(1):22–33. doi: 10.1111/j.1600-6143.2010.03317.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kmieciak M, Gowda M, Graham L, et al. Human T cells express CD25 and Foxp3 upon activation and exhibit effector/memory phenotypes without any regulatory/suppressor function. J Transl Med. 2009;7:89. doi: 10.1186/1479-5876-7-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziegler SF. FOXP3: not just for regulatory T cells anymore. Eur J Immunol. 2007;37(1):21–23. doi: 10.1002/eji.200636929 [DOI] [PubMed] [Google Scholar]
- 24.Allan SE, Crome SQ, Crellin NK, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19(4):345–354. doi: 10.1093/intimm/dxm014 [DOI] [PubMed] [Google Scholar]
- 25.Janson PCJ, Winerdal ME, Marits P, Thörn M, Ohlsson R, Winqvist O. FOXP3 Promoter Demethylation Reveals the Committed Treg Population in Humans. PLOS ONE. 2008;3(2):e1612. doi: 10.1371/journal.pone.0001612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeWolf S, Grinshpun B, Savage T, et al. Quantifying size and diversity of the human T cell alloresponse. JCI Insight. 2018;3(15). doi: 10.1172/jci.insight.121256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damschroder MM, Kozhich AA, Woods RM, et al. Analysis of human and primate CD2 molecules by protein sequence and epitope mapping with anti-human CD2 antibodies. Mol Immunol. 2004;41(10):985–1000. doi: 10.1016/j.molimm.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 28.Bell GM, Imboden JB. CD2 and the regulation of T cell anergy. J Immunol. 1995;155(6):2805–2807. [PubMed] [Google Scholar]
- 29.Domzig W, Stadler BM, Herberman RB. Interleukin 2 dependence of human natural killer (NK) cell activity. J Immunol. 1983;130(4):1970–1973. [PubMed] [Google Scholar]
- 30.Weaver TA, Charafeddine AH, Agarwal A, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat Med. 2009;15(7):746–749. doi: 10.1038/nm.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S, Yamada Y, Tonsho M, et al. Alefacept promotes immunosuppression-free renal allograft survival in nonhuman primates via depletion of recipient memory T cells. Am J Transplant. 2013;13(12):3223–3229. doi: 10.1111/ajt.12500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levitsky J, Leventhal JR, Miller J, et al. Favorable effects of alemtuzumab on allospecific regulatory T-cell generation. Hum Immunol. 2012;73(2):141–149. doi: 10.1016/j.humimm.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia C-Q, Chernatynskaya AV, Wasserfall CH, et al. Anti-thymocyte globulin (ATG) differentially depletes naïve and memory T cells and permits memory-type regulatory T cells in nonobese diabetic mice. BMC Immunol. 2012;13:70. doi: 10.1186/1471-2172-13-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearl JP, Parris J, Hale DA, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5(3):465–474. doi: 10.1111/j.1600-6143.2005.00759.x [DOI] [PubMed] [Google Scholar]
- 35.Benichou G, Gonzalez B, Marino J, Ayasoufi K, Valujskikh A. Role of Memory T Cells in Allograft Rejection and Tolerance. Front Immunol. 2017;8:170. doi: 10.3389/fimmu.2017.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol JASN. 2006;17(10):2844–2853. doi: 10.1681/ASN.2006050422 [DOI] [PubMed] [Google Scholar]
- 37.Broady R, Yu J, Levings MK. ATG-induced expression of FOXP3 in human CD4(+) T cells in vitro is associated with T-cell activation and not the induction of FOXP3(+) T regulatory cells. Blood. 2009;114(24):5003–5006. doi: 10.1182/blood-2009-04-214437 [DOI] [PubMed] [Google Scholar]
- 38.Cibrián D, Sánchez-Madrid F. CD69: from activation marker to metabolic gatekeeper. Eur J Immunol. 2017;47(6):946–953. doi: 10.1002/eji.201646837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176(8):4622–4631. [DOI] [PubMed] [Google Scholar]
- 40.Guinan EC, Cole GA, Wylie WH, et al. Ex Vivo Costimulatory Blockade to Generate Regulatory T Cells From Patients Awaiting Kidney Transplantation. Am J Transplant. 2016;16(7):2187–2195. doi: 10.1111/ajt.13725 [DOI] [PubMed] [Google Scholar]
- 41.Oakes T, Heather JM, Best K, et al. Quantitative Characterization of the T Cell Receptor Repertoire of Naïve and Memory Subsets Using an Integrated Experimental and Computational Pipeline Which Is Robust, Economical, and Versatile. Front Immunol. 2017;8. doi: 10.3389/fimmu.2017.01267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macedo C, Orkis EA, Popescu I, et al. Contribution of naïve and memory T-cell populations to the human alloimmune response. Am J Transplant. 2009;9(9):2057–2066. doi: 10.1111/j.1600-6143.2009.02742.x [DOI] [PubMed] [Google Scholar]
- 43.Lombardi G, Sidhu S, Daly M, Batchelor JR, Makgoba W, Lechler RI. Are primary alloresponses truly primary? Int Immunol. 1990;2(1):9–13. [DOI] [PubMed] [Google Scholar]
- 44.Onoe T, Kalscheuer H, Chittenden M, Zhao G, Yang Y-G, Sykes M. Homeostatic expansion and phenotypic conversion of human T cells depend on peripheral interactions with APCs. J Immunol. 2010;184(12):6756–6765. doi: 10.4049/jimmunol.0901711 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.