Abstract
Background:
Most studies of pulmonary exacerbations (PEx) in cystic fibrosis (CF) focus on intravenous (IV)-treated PEx, though most PEx are treated with oral antibiotics. Our objectives were to describe predictors of antibiotic choice and outcomes for PEx initially identified in clinic.
Methods:
For each patient in the U.S. CF Foundation Patient Registry, we selected the first PEx recorded at a clinic visit in 2013-14 following a clinic visit without a PEx. We used multivariable logistic regression to determine associations between clinical characteristics and antibiotic treatment choice. We determined outcomes in the 90 days after the first PEx.
Results:
Among 14,265 patients with a PEx initially identified in clinic, 21.4% received no antibiotics, 61.5% received new oral and/or inhaled antibiotics, and 17.0% had IV antibiotics within 14 days. Compared to IV antibiotics, patients more likely to receive new oral and/or inhaled antibiotics: were male, <13 years old, had BMI >10th percentile or 18.5 kg/m2, >90 days between clinic visits, FEV1 >70% predicted at the PEx, no prior-year IV-treated PEx, FEV1 decline <10% predicted, and private insurance. Following the PEx, 30.3% of patients had no clinical encounters within 90 days. Treatment with IV antibiotics within 90 days occurred for 23.7% treated without antibiotics, 22.8% of new oral and/or inhaled antibiotics, and 27.1% of IV antibiotics.
Conclusion:
Most PEx identified in clinic are treated with new oral and/or inhaled antibiotics. Markers of disease severity are associated with antibiotic treatment choice. Many patients had no follow-up evaluation within 90 days of treatment.
1. Introduction
Despite significant improvements in morbidity and survival, people with cystic fibrosis (CF) continue to experience periodic worsening signs and symptoms of respiratory health, recognized as pulmonary exacerbations (PEx). Most PEx studies focus on intravenous (IV) antibiotic treatments, yet most PEx are not treated with IV antibiotics. (1) Studies describing use of oral and/or inhaled antibiotics for treatment of PEx report that poor outcomes (e.g., decline in lung function, failure to recover to baseline lung function, and subsequent treatment with IV antibiotics) occur after these events.(1,2) Morgan et al. showed that, among patients with ≥10% decline in FEV1, those treated with IV antibiotics were more likely to recover lung function than patients treated with oral or inhaled antibiotics, or without antibiotics. (3) These observations suggest that some PEx treatments may be superior.
The CF Foundation Patient Registry (CFFPR) contains data on over 30,000 patients in a given year. Since 2003, accredited CF care centers can enter information from clinical encounters at CF Foundation Care Center Network facilities.(4) Beginning midway through 2012, care centers were required to record presence or absence of PEx (as identified by the treating physician) and if antibiotic treatments were prescribed at each outpatient clinic visit. Although many PEx are treated by phone and treatment decisions regarding these events are not recorded in the CFFPR, many events are diagnosed and treated in the clinic setting, allowing for further investigation. We sought to determine how PEx initially identified in clinic were treated and whether outcomes differed according to treatment choice. Our objectives were (1) describe antibiotic types (i.e., IV, oral, inhaled, or none) prescribed for PEx identified at clinic visits; (2) identify predictors of treatment with any antibiotics, IV antibiotics, or without antibiotics; and (3) describe clinical outcomes following PEx identified at clinic visits by type of antibiotic prescribed.
2. Methods
Patients are eligible to be included in the CFFPR if they receive care at one of the CF Foundation’s 121 accredited care centers in the US and they (and/or their legal guardians) provide informed consent. Care centers can be comprised of pediatric, adult, and affiliate care programs. Further details about the CFFPR were previously described.(4) This study was approved by Quorum IRB (IRB # 33029/1).
The current study included patients in the CFFPR with at least one PEx reported during a clinic visit in 2013 or 2014. PEx assessments were made based on the clinician’s assessment and choice of treatment according to data entry guidelines provided by the CF Foundation. Patients from care programs that completed PEx assessments <90% of clinic visits were excluded due to the possibility of bias in PEx reporting at these programs. We included the first PEx recorded at a clinic visit (Index visit) for each patient following a clinic visit in 2013 or 2014 at which a PEx was marked “absent” (Baseline visit). Patients were excluded if they always or never had PEx recorded at clinic visits in 2013 and 2014, received IV antibiotics between Baseline and Index, ever received an organ transplant, or died within 90 days of Index.
Our primary outcome of interest was treatment documented at Index, categorized as no antibiotics, new oral and/or inhaled antibiotics, or IV antibiotics. The CFFPR does not link clinic visits to hospitalizations or episodes of home IV antibiotics, so we included any IV antibiotics begun within 14 days of Index as treatment with IV antibiotics, regardless of whether patients had been prescribed oral or inhaled antibiotics earlier in the 14-day time period.
Demographic and clinical variables were examined to determine if they were associated with PEx treatment type. Demographic variables included sex, age at Index visit (<6, 6 to <13, 13 to <18, 18 to <26, or ≥26 years), mutation class grouping (only class I-III, any class IV/V), race and ethnicity, and insurance status (any public insurance [Medicare, Medicaid, TRICARE, Indian Health Service], private insurance, or no insurance).(1, 5, 6) Clinical variables included best forced expiratory volume in one second (FEV1) percent predicted in the six months prior to Index based on Global Lung Initiative equations, body mass index (BMI) at Index categorized as underweight (<10th percentile according to the CDC growth charts for children and <18.5 kg/m2 for adults), adequate (10th to <85th percentile for children and 18.5 to <25 kg/m2 for adults), and overweight (≥85th percentile for children or ≥25 kg/m2 for adults), CF-related diabetes mellitus, allergic bronchopulmonary dysplasia (ABPA), and having at least one positive culture for methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa (PA), or other respiratory micro-organisms [including non-tuberculous mycobacteria (NTM)] in the twelve months prior to Index, number of days between Baseline and Index, and number of IV-treated PEx in the year prior to Baseline.(7, 8)
We identified next follow-up after Index to determine the proportion of patients who had recorded clinic visits within 30 and 90 days after Index, improved and FEV1 recovery to within 90% of FEV1 baseline within 90 days,(9) and proportion of patients who had IV-treated PEx within 90 days.
2.1. Data Analysis
Multivariable logistic regression was used to test for associations between demographic and clinical variables and Index antibiotic treatment choice. Patients included in the models had to have FEV1 measurements at both Baseline and Index visits, as well as non-missing covariate data. Patients <6 years old were excluded even with FEV1 measurements recorded in the CFFPR due to the possibility that their pulmonary function test performance was unreliable.
Beginning with variables that were significant in bivariate analyses and using backward stepwise logistic regression with a p-value cut-off of 0.05, we developed two logistic regression models to investigate predictors of antibiotic treatment at Index. Our first model compared patients with no antibiotic treatment to patients who received any antibiotic treatment. Our second model compared patients who received oral and/or inhaled antibiotics to patients who received IV antibiotics within 14 days of Index. We analyzed all two-way interaction effects and did not include these in final models as they did not substantially increase model fit [Area Under the Curve (AUC), Hosmer-Lemeshow Test]. SAS 9.4 (SAS Institute, Cary, North Carolina) was used for all data analysis.
We performed additional analyses to test robustness of the regression models. We repeated our analysis, stratified by (1) age (<18 years, ≥18 years), (2) absolute FEV1 decline from the highest recorded FEV1 in the 6 months prior to Index (<10% predicted or ≥10% predicted), and (3) relative FEV1 decline from the highest recorded FEV1 in the 6 months prior to Index (<10% or ≥10%). For the comparison of patients with no antibiotic treatment to those with any antibiotic treatment, we compared (1) patients with increased frequency of airway clearance only, (2) a history of a prior-year PEx identified in clinic with no recorded antibiotic treatment, and (3) a history of prior-year PEx identified in clinic with oral and/or inhaled antibiotics. We also examined the impact of our choice of a 14-day window for categorizing treatment as IV antibiotics by dividing the original cohort into patients treated with IV antibiotics within seven days, and 8-14 days, after Index.
3. Results
3.1. Cohort Characteristics
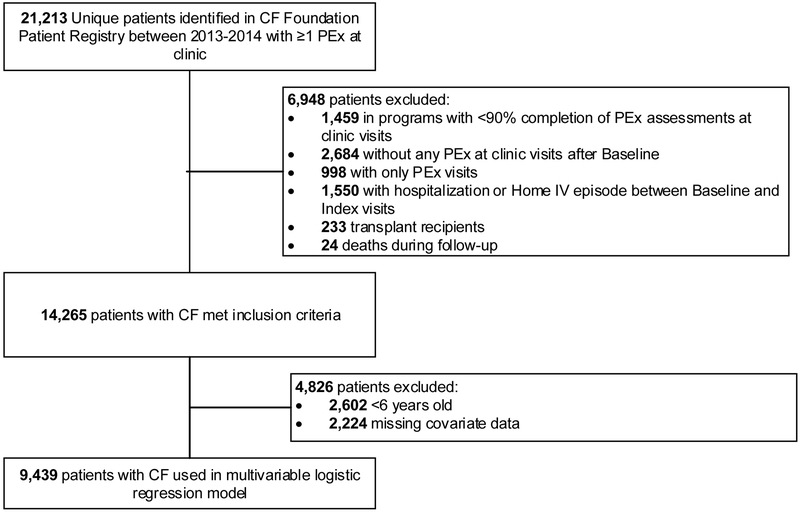
There were 14,265 patients with CF identified in the CFFPR in 2013-14 with at least one PEx diagnosed at a clinic visit (Figure 1). The most common reasons patients were excluded were: receiving IV antibiotics between Baseline and Index visits (1,550 patients); attending one of 11 Adult, 3 Pediatric, or 3 Affiliate CF Centers that completed <90% of PEx assessment fields (1,459 patients), and not having a clinic visit PEx after identifying a baseline visit (2,684 patients).
Figure 1. Study cohort flowchart.
Key demographic and clinical characteristics of patients are shown in Table 1. Median (IQR) age at Index was 14.9 (7.7-24.4) years. Median (IQR) BMI was the 48.8th (26.0-70.7) percentile for pediatric patients and 21.8 (19.8–24.2) kg/m2 for adult patients. The median (IQR) time from Baseline to Index was 83 (47-105) days. The median (IQR) absolute decline in FEV1 % predicted from Baseline to Index was 6.5 (0.9-10.9) % predicted. Change in FEV1 % predicted could not be calculated for 1,819 (12.8%) patients with FEV1 missing at Baseline and/or Index and 2,493 (17.5%) patients who were <6 years old. FEV1 % predicted increased from Baseline to Index in 2,027 (14.2%), decreased <10% predicted in 5,146 (36.1%), and decreased ≥10% predicted in 2,780 (19.5%). In the year prior to Index, 50.7% had at least one respiratory culture positive for PA, 13.8% for MRSA, 32.0% had other organisms (including NTM), and 3.5% had no positive respiratory cultures.
Table 1.
Cohort Characteristics
| Treatment Prescribed at Index | No Antibiotics N (%) |
Oral and/or Inhaled Antibiotics N (%) |
IV AntibioticsaN (%) |
Overall N (%) |
|---|---|---|---|---|
| Number of Patients (%) | 3,059 (21.4%) | 8,774 (61.5%) | 2,432 (17.0%) | 14,265 |
| Age Group (years) | ||||
| <6 | 663 (21.7%) | 1631 (18.6%) | 199 (8.2%) | 2493 (17.5%) |
| 6 to <13 | 592 (19.4%) | 2264 (25.8%) | 395 (16.2%) | 3251 (22.8%) |
| 13 to <18 | 436 (14.3%) | 1512 (17.2%) | 453 (18.6%) | 2401 (16.8%) |
| 18 to <26 | 594 (19.4%) | 1601 (18.2%) | 629 (25.9%) | 2824 (19.8%) |
| ≥26 | 774 (25.3%) | 1766 (20.1%) | 756 (31.1%) | 3296 (23.1%) |
| Female | 1483 (48.5%) | 4352 (49.6%) | 1323 (54.4%) | 7158 (50.2%) |
| Race | ||||
| Caucasian | 2881 (94.2%) | 8221 (93.7%) | 2292 (94.2%) | 13394 (93.9%) |
| African American | 100 (3.3%) | 329 (3.7%) | 75 (3.1%) | 504 (3.5%) |
| Other | 78 (2.5%) | 224 (2.6%) | 65 (2.7%) | 367 (2.6%) |
| Hispanic Ethnicityb | 267 (9.0%) | 692 (8.2%) | 206 (8.7%) | 1165 (8.5%) |
| Insurance Status | ||||
| Private | 1869 (61.1%) | 5251 (59.8%) | 1266 (52.1%) | 8386 (58.8%) |
| Medicare/Medicaid | 1117 (36.5%) | 3342 (38.1%) | 1106 (45.5%) | 5565 (39.0%) |
| Other | 17 (0.6%) | 62 (0.7%) | 26 (1.1%) | 105 (0.7%) |
| Missing | 56 (1.8%) | 119 (1.4%) | 34 (1.4%) | 209 (1.5%) |
| Nutrition Status | ||||
| Underweight | 261 (8.5%) | 720 (8.2%) | 417 (17.1%) | 1398 (9.8%) |
| Adequate | 1962 (64.1%) | 6025 (68.7%) | 1649 (67.8%) | 9636 (67.5%) |
| Overweight | 483 (15.8%) | 1280 (14.6%) | 236 (9.7%) | 1999 (14.0%) |
| Missing | 353 (11.5%) | 749 (8.5%) | 130 (5.3%) | 1232 (8.6%) |
| Time from Baseline to Index Clinic Visit (days) | ||||
| ≤30 | 475 (15.5%) | 964 (11.0%) | 535 (22.0%) | 1974 (13.8%) |
| 31 to 90 | 1256 (41.1%) | 3665 (41.8%) | 1149 (47.2%) | 6070 (42.6%) |
| ≥91 | 1328 (43.4%) | 4145 (47.2%) | 748 (30.8%) | 6221 (43.6%) |
| CF-related Diabetes | 539 (17.6%) | 1407 (16.0%) | 731 (30.1%) | 2677 (18.8%) |
| CFTR Mutation Class | ||||
| Only 1 to 3 | 2136 (69.8%) | 6478 (73.8%) | 1864 (76.6%) | 10478 (73.5%) |
| Any 4 and 5 | 294 (9.6%) | 695 (7.9%) | 157 (6.5%) | 1146 (8.0%) |
| Other | 559 (18.3%) | 1443 (16.4%) | 364 (15.0%) | 2366 (16.6%) |
| Unknown | 70 (2.3%) | 158 (1.8%) | 47 (1.9%) | 275 (1.9%) |
| FEV1 % Predicted at Index | ||||
| Not Applicable <6 years old | 663 (21.7%) | 1631 (18.6%) | 199 (8.2%) | 2493 (17.5%) |
| ≤40 | 279 (9.1%) | 634 (7.2%) | 572 (23.5%) | 1485 (10.4%) |
| 41 to ≤70 | 678 (22.2%) | 2068 (23.6%) | 863 (35.5%) | 3609 (25.3%) |
| 71 to ≤100 | 970 (31.7%) | 3069 (35.0%) | 513 (21.1%) | 4552 (31.9%) |
| >100 | 237 (7.7%) | 765 (8.7%) | 64 (2.6%) | 1066 (7.5%) |
| Missing among ≤6 year olds | 232 (7.6%) | 607 (6.9%) | 221 (9.1%) | 1060 (7.4%) |
| Absolute Change in FEV1 % Predicted Between Baseline and Index | ||||
| Not Applicable <6 years old | 663 (21.7%) | 1631 (18.6%) | 199 (8.2%) | 2493 (17.5%) |
| Increase | 499 (16.3%) | 1260 (14.4%) | 268 (11.0%) | 2027 (14.2%) |
| 0 - <10% decrease | 1100 (36.0%) | 3145 (35.8%) | 901 (37.0%) | 5146 (36.1%) |
| ≥10% decrease | 400 (13.1%) | 1685 (19.2%) | 695 (28.6%) | 2780 (19.5%) |
| Missing among ≥6 year olds | 397 (13.0%) | 1053 (12.0%) | 369 (15.2%) | 1819 (12.8%) |
| Microbiology (≥1 positive culture in year prior to Index) | ||||
| Any culture performed in last year of care | 3,017 (98.6%) | 8,071 (99.2%) | 2,418 (99.4%) | 14,136 (99.1%) |
| Pseudomonas aeruginosa | 1462 (48.5%) | 4129 (47.5%) | 1577 (65.2%) | 7168 (50.7%) |
| Methicillin-resistant Staphylococcus aureus | 341 (11.3%) | 1287 (14.8%) | 316 (13.1%) | 1944 (13.8%) |
| Other bacteria, including NTM | 1058 (35.1%) | 2983 (34.3%) | 489 (20.2%) | 4530 (32.0%) |
| Negative cultures | 156 (5.2%) | 302 (3.5%) | 36 (1.5%) | 494 (3.5%) |
| ABPA | 138 (4.5%) | 318 (3.6%) | 127 (5.2%) | 583 (4.1%) |
| Prior-year IV-treated PEx | ||||
| 0 | 1996 (65.3%) | 5679 (64.7%) | 806 (33.1%) | 8481 (59.5%) |
| 1 | 582 (19.0%) | 1864 (21.2%) | 650 (26.7%) | 3096 (21.7%) |
| 2 | 234 (7.6%) | 697 (7.9%) | 367 (15.1%) | 1298 (9.1%) |
| ≥3 | 247 (8.1%) | 534 (6.1%) | 609 (25.0%) | 1390 (9.7%) |
| PEX within 90 days of baseliine | 524 (17.1%) | 1349 (15.4%) | 753 (31.0%) | 2626 (18.4%) |
Treatment with IV antibiotics defined as any treatment within 14 days of Index clinic visit
Missing = 531
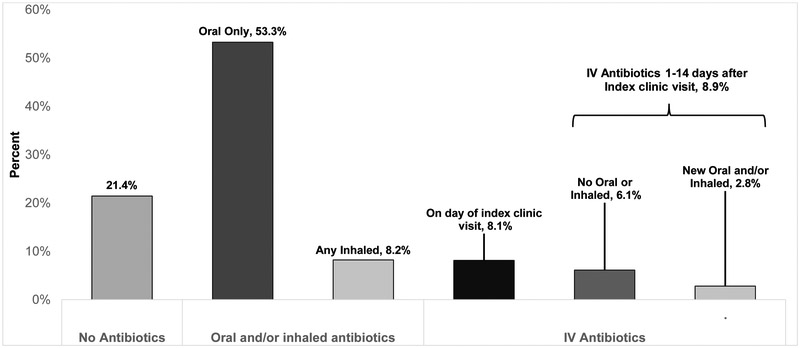
Among eligible patients, 3,059 (21.4%) had no recorded antibiotics prescribed at Index, 8,774 (61.5%) had documented new oral and/or inhaled antibiotics, and 2,432 (17.0%) received IV antibiotics within 14 days after Index (Figure 2). Among patients with no recorded antibiotics, 1,776 (58.1%) were prescribed increased frequency of airway clearance, 698 (22.8%) were prescribed neither antibiotics nor increased frequency of airway clearance, and 585 (19.1%) had a missing entry. Only 344 (2.4%) patients received new inhaled antibiotics alone at Index, so these patients were grouped with patients that received oral antibiotics. Of those receiving IV antibiotics, 1,159 (47.7%) patients began the same day as Index and 1,273 (52.3%) received IV antibiotics within 14 days after Index. Among patients receiving IV antibiotics within 14 days after Index, 433 (17.8%) received new oral and/or inhaled antibiotics at Index and IV antibiotics within 14 days. Mean (SD) decline in FEV1 from Baseline to Index was 4.8 (8.7)% predicted for PEx treated without antibiotics, 6.4 (9.3)% predicted for PEx treated with new oral and/or inhaled antibiotics, and 8.9 (11.7)% predicted for IV-treated PEx (p <0.001).
Figure 2.
Proportion of PEx identified in clinic that were treated with no antibiotics, new oral and/or inhaled antibiotics, and IV antibiotics.
3.2. Predictors of antibiotic treatment choice
There were 9,439 patients included in our multivariate logistic regression models. From our original cohort, 4,826 patients were excluded: 4,312 patients did not have FEV1 values available (2,602 were <6 years old at time of Baseline and 1,710 were ≥6 years with missing FEV1 values at Baseline and/or Index); and 514 had other missing covariate data.
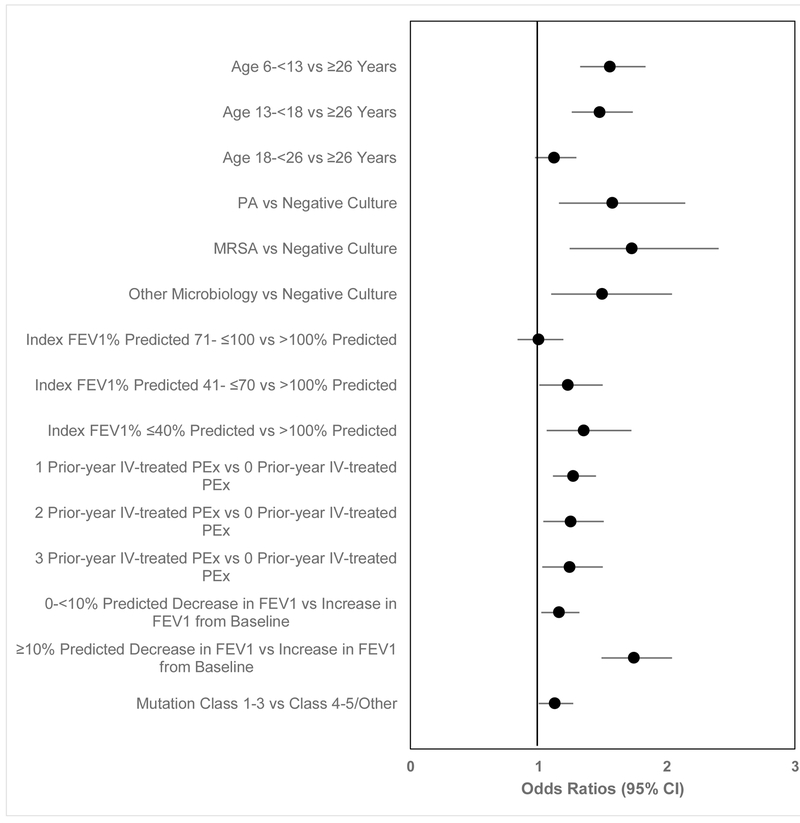
Compared to not receiving antibiotics, the following variables were associated with increased likelihood of being treated with antibiotics (oral, inhaled, or IV) (Figure 3): age <18 years; CFTR mutation class 1-3; positive respiratory culture in the 12 months prior to Index; Index FEV1 ≤70% predicted; having IV-treated PEx in the year prior to Baseline; and decline in FEV1 % predicted from Baseline (p<0.05).
Figure 3. Multivariate logistic regression model comparing odds of treatment with any antibiotics to no antibiotic treatment.
Any antibiotic includes new oral, new inhaled, and IV-treated PEx (see text). Variables were included if they were significant in bivariate analyses and using backward stepwise logistic regression. Odds ratios less than 1 favors no antibiotic treatment, greater than 1 favors treatment with any antibiotics.
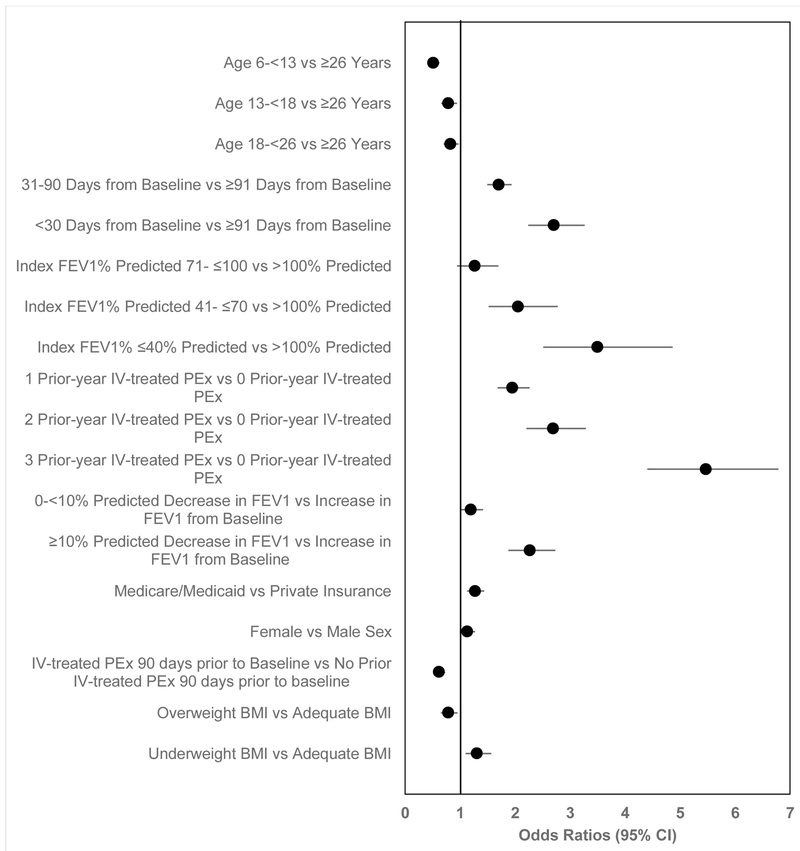
Compared to treatment with oral and/or inhaled antibiotics, characteristics associated with increased likelihood of IV antibiotics were (Figure 4): ages ≥26 years; ≤90 days between Baseline and Index; FEV1 ≤70% predicted at Index; having at least one prior-year IV-treated PEx that was more than 90 days prior to Baseline; decline in FEV1 % predicted from Baseline; female sex; being underweight, and having public insurance (p<0.05 for each).
Figure 4. Multivariable logistic regression model comparing odds of IV-treated PEx to PEx treated with new oral and/or inhaled antibiotic treatment.
Variables were included if they were significant in bivariate analyses and using backward stepwise logistic regression. Odds ratios less than 1 favors oral and/or inhaled antibiotics, greater than 1 favors IV antibiotics.
3.3. Outcomes
Following Index, 3,670 (25.7%) patients had a follow-up clinic visit recorded within 30 days, 4,719 (33.1%) had a visit within 31 to 90 days, and an additional 1,030 (10.9%) were treated with IV antibiotics within 90 days. There were 4,325 (30.3%) patients who did not have any follow-up clinical encounters recorded within 90 days. A PEx was identified at the subsequent clinic visit among 35.0% of patients seen within 30 days and 32.3% among those seen between 31 and 90 days. Among patients seen within 30 days, more patients initially treated with new oral and/or inhaled antibiotics (38.8%) or without antibiotics (35.6%) had a PEx identified at the subsequent clinic visit than those treated with IV antibiotics (26.4%), p<0.001. There were no differences in proportions of patients with a PEx identified at a subsequent clinic visit among patients seen within 31-90 days. Among patients with follow-up within 90 days, treatment with IV antibiotics was reported among 23.7% not initially treated with antibiotics and 22.8% of those initially treated with new oral and/or inhaled antibiotics; re-treatment with IV antibiotics occurred among 27.1% of those initially treated with IV antibiotics.
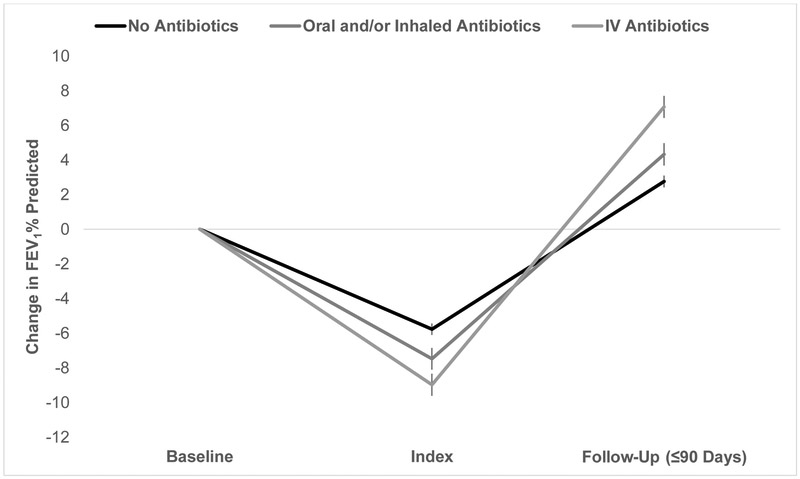
Among 5,337 patients who had FEV1 recorded at Baseline, Index, and subsequent follow-up clinic visit within 90 days, mean (SD) absolute improvement was 4.7 (10.3)% predicted between Index and follow-up. Mean (SD) improvement was significantly higher for patients treated with IV antibiotics [7.1 (11.3)% predicted], compared with oral and/or inhaled antibiotics [4.3 (9.8)% predicted], and no antibiotics [2.8 (9.8)% predicted], (p< 0.001) (Figure 5). Among 1,996 patients with <10% relative decline in FEV1 % predicted at Index, recovery to within 90% of Baseline FEV1 % predicted during 90-day follow-up occurred in 313 (88.7%) patients treated with IV antibiotics, 1,088 (85.0%) treated with oral and/or inhaled antibiotics, and 297 (81.8%) treated without antibiotics, p = 0.03. Among 2,409 patients with ≥10% relative decline in FEV1 % predicted at Index, recovery to within 90% of Baseline FEV1 % predicted during 90-day follow-up occurred in 432 (62.2%) patients treated with IV antibiotics, 845 (60.9%) treated with oral and/or inhaled antibiotics, and 195 (59.6%) treated without antibiotics, p = 0.7.
Figure 5.
Changes in FEV1 % predicted between Baseline, Index, and follow up according to treatment choice at Index. Patients included if data at all 3 visits and follow-up is at clinic within 90 days. Vertical bars represent 95% Cl
3.4. Sensitivity analyses
We repeated our analyses stratified by (1) Index age (<18 years, ≥18 years), (2) absolute FEV1 decline from Baseline (<10% or >10% predicted), and (3) relative FEV1 decline from Baseline to Index (<10% or ≥10%) (Supplemental Table E1-E3). Results were generally similar to the original analysis, although in the model stratified by age, association between treatment with any antibiotics and Index FEV1 was only apparent in patients <18 years old (Supplemental Table E1). In the comparison of patients treated with no recorded antibiotics to those with any antibiotics, the results from our original analysis were not changed substantially when we restricted our analysis to PEx where increased airway clearance was selected (Supplemental Table E4). Having a PEx identified in clinic in the prior year without recorded antibiotics was associated with a decreased odds of being treated with antibiotics at Index (Supplemental Table E4). Similarly, having a PEx identified in clinic in the prior year but no treatment with oral and/or inhaled antibiotics was associated with a reduced odds of being treated with oral and/or inhaled antibiotics at Index. These last two analyses were restricted to patients with a PEx in 2014 since the antibiotic data field was not required until mid-2012. We also examined impact of our choice of a 14-day window for including IV antibiotics by repeating the initial analysis using a 7-day window. Supplemental Table E5 shows IV antibiotic group characteristics using 7- and 14-day windows. Patient characteristics were similar using either window to define IV antibiotic treatment; patients treated within seven days were more likely to have Medicaid insurance, have CF-related diabetes, underweight, and have lower Index FEV1.
4. Discussion
Previous studies of PEx have focused on IV-treated exacerbations.(9-14) However, we confirmed that these exacerbations represent a minority of PEx identified in clinical practice (1, 15, 16): nearly two-thirds of all PEx identified at clinic visits in the CFFPR were treated with oral and/or new inhaled antibiotics, while 17% of PEx were treated with IV antibiotics, fewer than the number of PEx treated without antibiotics. This may still represent an underestimation of PEx treated with oral and/or new inhaled antibiotics, since we are unable to track treatments prescribed by phone, in emergency departments, or by primary care physicians.
As previously reported,(3, 15) >20% of clinically identified PEx did not have any antibiotic treatment recorded, so >80% of PEx that received treatment were treated with oral and/or inhaled antibiotics. As in previous reports,(1) use of inhaled antibiotics alone to treat a PEx was low. Diagnosis of PEx warrants some intervention to avoid negative outcomes associated with their occurrence. Strongest predictors of treatment choice include age, microbiology, severity of baseline lung disease and changes from baseline, and prior IV-treated PEx treatments. The CFFPR does not provide sufficient information to determine why the decision to not use antibiotics is made (e.g., lack of symptoms, patient declined antibiotics), and whether alternate treatments (e.g., increase in airway clearance, oral steroids, addition of chronic medications) are used instead. Characteristics associated with PEx treated without antibiotics suggest this option is used in healthier patients who are less likely to receive antibiotics for a given drop in lung function.(1) Surprisingly, adult patients were also more likely to not receive antibiotics. The reason for this is unclear, but could reflect that adult patients may be more likely to attempt increased airway clearance and/or delay IV antibiotics than pediatric patients. It is also possible that PEx treatment was started prior to clinic visits over the phone and not recorded in the CFFPR; there is no guidance as to whether or not to record these treatments at clinic visits. Nonetheless, 20% of patients with clinically-defined PEx and FEV1 decline >10% predicted had no antibiotic treatment recorded.
Most clinical trials and previous Registry analyses have used IV treatment as part of the definition,(9, 17-20) yet these represent only 17% of PEx initially identified in clinic. Additional IV-treated PEx occur without clinic visits (e.g., via direct hospital admission or via the emergency room), but our focus was on PEx identified in clinic. The frequency with which PEx are treated without antibiotics suggests that including the decision to treat in operational definitions of PEx may limit generalizability in prospective studies. Factors associated with IV-treated PEx are like those reported previously,(1, 3, 15) and generally point to sicker patients. Patients treated with IV antibiotics had a larger decline in FEV1 prior to treatment and had the highest recovery of lung function following treatment. Notably, in a recent prospective study of IV-treated PEx, 50% of patients and 80% of pediatric patients were treated with oral antibiotics prior to starting IV antibiotics, though it is unknown how many of these oral antibiotics were prescribed as a temporizing measure prior to a scheduled course of IV antibiotics (21).
We primarily sought to identify predictors of antibiotic treatment choices associated with the diagnosis of PEx made in clinic; however, another important step in management of PEx should be assessing response to intervention. Even though FEV1 often decreases within two weeks after completion of IV antibiotics, (22) we found that fewer than 25% of patients were seen in clinic or in the hospital within 30 days after PEx was identified in clinic, and 30% were not seen again within 90 days. In contrast to a previous report, we did not demonstrate an improvement in recovery to baseline among patients treated with IV antibiotics compared to no antibiotics or new oral and/or inhaled antibiotics. (3) Furthermore, treatment with IV antibiotics within 90 days did not differ substantially by initial treatment choice. The lack of differences in our study are likely related to indication bias – the sickest patients are more likely to be reevaluated within 90 days. Early follow-up provides an important opportunity to evaluate treatment response and has been proposed as a key component of PEx treatment. (23) We cannot determine whether follow-up visits were scheduled and subsequently cancelled, but these gaps in care represent potential opportunities to improve outcomes, especially as PEx was noted in over 30% of follow-up visits, regardless of timing. We are unable to determine if these follow-up PEx represent the same, or new, PEx. IV-treated PEx resulted in larger improvements in FEV1 and occurred after larger declines from baseline.
There are several limitations to our study. The CFFPR does not capture presumed cause of PEx (e.g, viral infections, non-adherence to chronic therapies, new bacterial infection, etc.), PEx treated over the phone, whether the clinic visit was previously scheduled or an urgent visit, clinicians’ rationale for treatment decisions, or patient and family input into treatment decisions. The CFFPR also does not include information on adherence to prescribed medications or airway clearance frequency, or whether new chronic medications or oral steroids were prescribed in response to a PEx. It is unlikely that flares of ABPA make up a significant portion of the PEx without recorded antibiotics. Only 4.1% of patients had ABPA recorded in the CFFPR (similar to the frequency of ABPA reported in the CFFPR), and the proportion of patients with ABPA who had a PEx treated without antibiotics was similar to our overall study population. Hemoptysis occurs relatively frequently,(24) but it is unlikely that episodes of hemoptysis make up a significant portion of the PEx without recorded antibiotics. In the US, guidelines recommend that antibiotics be part of the treatment regimen in patients with at least mild hemoptysis.(25) PEx assessment in the CFFPR is not standardized, and as with most PEx definitions used, it was determined by the clinician without any additional requirement for or documentation of specific criteria. Audits have been performed on data from the CFFPR (4), and while error rates are generally low, the PEx assessment field was not included in these audits. Clinic visits are not linked directly to IV antibiotics in the CFFPR, and we could not ascertain whether subsequent IV antibiotics were planned or represented initial treatment failure. To account for this, we included any IV antibiotics within 14 days of Index as IV-treated PEx; 58% of IV antibiotic treatments within 90 days after Index occurred within this window. Additionally, the CFFPR does not capture phone follow-up or home spirometry.
PEx are frequent events and published guidelines affirm lack of evidence on best treatment practices.(26) Patient and clinician factors may influence decisions regarding antibiotic treatment choice.(14, 27, 28) Delays in follow-up offer opportunities to improve outcomes if prolonged symptoms and poor PEx recovery can be avoided. Avoiding IV antibiotics is important, but only if recovery from a PEx can be confirmed. A recent proposed algorithm includes a step-wise approach and close follow-up to ensure recovery.(23) Prospective studies are needed to optimize PEx treatment, including when to use antibiotics, by what route, and in what setting, especially in light of the limitations of retrospectively collected data.
Supplementary Material
Highlights.
Over half of pulmonary exacerbations identified in clinic are treated initially with oral antibiotics
Compared to treatment with IV antibiotics, patients treated with oral antibiotics tended to be healthier
Exacerbations treated with IV antibiotics occurred following the largest decline in pulmonary function, but the recovery of pulmonary function was highest following IV antibiotics
Nearly one-third had no clinical encounters within 90 days of exacerbation treatment
ACKNOWLEDGMENTS:
The authors would like to thank the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry (CFFPR) data to conduct this study. Additionally, we would like to thank the patients, care providers, and clinic coordinators at CF Centers throughout the United States for their contributions to the CF Foundation Patient Registry. Portions of the data were presented at the 2016 and 2017 North American Cystic Fibrosis Conference.
P.A.F. is supported by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, through NIH Grant Number UL1 TR000062. The sponsors had no involvement in any aspect of this report.
Footnotes
D.B.S., M.R., M.S.S., G.S.S., P.A.F., and W.J.M. have received honoraria from the CF Foundation for serving as a member of the CF Foundation Patient Registry Committee; no compensation was provided in exchange for the creation of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wagener JS, Rasouliyan L, VanDevanter DR, Pasta DJ, Regelmann WE, Morgan WJ, et al. Oral, inhaled, and intravenous antibiotic choice for treating pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 2013;48(7):666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanojevic S, McDonald A, Waters V, MacDonald S, Horton E, Tullis E, et al. Effect of pulmonary exacerbations treated with oral antibiotics on clinical outcomes in cystic fibrosis. Thorax. 2017;72(4):327–32. [DOI] [PubMed] [Google Scholar]
- 3.Morgan WJ, Wagener JS, Pasta DJ, Millar SJ, VanDevanter DR, Konstan MW, et al. Relationship of Antibiotic Treatment to Recovery after Acute FEV1 Decline in Children with Cystic Fibrosis. Ann Am Thorac Soc. 2017;14(6):937–42. [DOI] [PubMed] [Google Scholar]
- 4.Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The Cystic Fibrosis Foundation Patient Registry. Design and Methods of a National Observational Disease Registry. Ann Am Thorac Soc. 2016;13(7):1173–9. [DOI] [PubMed] [Google Scholar]
- 5.Schechter MS, McColley SA, Regelmann W, Millar SJ, Pasta DJ, Wagener JS, et al. Socioeconomic status and the likelihood of antibiotic treatment for signs and symptoms of pulmonary exacerbation in children with cystic fibrosis. J Pediatr. 2011;159(5):819–24 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konstan M, Morgan W, Butler S, Pasta D, Craib M, Silva S, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151(2):134–9, 9.e1. [DOI] [PubMed] [Google Scholar]
- 7.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.VanDevanter DR, Flume PA, Morris N, Konstan MW. Probability of IV antibiotic retreatment within thirty days is associated with duration and location of IV antibiotic treatment for pulmonary exacerbation in cystic fibrosis. J Cyst Fibros. 2016;15(6):783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders D, Bittner R, Rosenfeld M, Hoffman L, Redding G, Goss C. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010; 182(5):627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schechter M, Shelton B, Margolis P, Fitzsimmons S. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163(6):1331–7. [DOI] [PubMed] [Google Scholar]
- 11.Britto M, Kotagal U, Hornung R, Atherton H, Tsevat J, Wilmott R. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest. 2002;121(1):64–72. [DOI] [PubMed] [Google Scholar]
- 12.Parkins MD, Rendall JC, Elborn JS. Incidence and Risk Factors for Pulmonary Exacerbation Treatment Failures in Patients With Cystic Fibrosis Chronically Infected With Pseudomonas aeruginosa. Chest. 2012;141(2):485–93. [DOI] [PubMed] [Google Scholar]
- 13.Waters V, Stanojevic S, Atenafu EG, Lu A, Yau Y, Tullis E, et al. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J. 2012;40(1):61–6. [DOI] [PubMed] [Google Scholar]
- 14.Schechter MS, VanDevanter DR, Pasta DJ, Short SA, Morgan WJ, Konstan MW, et al. Treatment Setting and Outcomes of Cystic Fibrosis Pulmonary Exacerbations. Ann Am Thorac Soc. 2018;15(2):225–33. [DOI] [PubMed] [Google Scholar]
- 15.Wagener JS, Williams MJ, Millar SJ, Morgan WJ, Pasta DJ, Konstan MW. Pulmonary exacerbations and acute declines in lung function in patients with cystic fibrosis. J Cyst Fibros. 2018;17(4):496–502. [DOI] [PubMed] [Google Scholar]
- 16.Regelmann WE, Schechter MS, Wagener JS, Morgan WJ, Pasta DJ, Elkin EP, et al. Pulmonary exacerbations in cystic fibrosis: young children with characteristic signs and symptoms. Pediatr Pulmonol. 2013;48(7):649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs H, Borowitz D, Christiansen D, Morris E, Nash M, Ramsey B, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331 (10):637–42. [DOI] [PubMed] [Google Scholar]
- 18.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011. ;365(18):1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramsey B, Pepe M, Quan J, Otto K, Montgomery A, Williams-Warren J, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340(1):23–30. [DOI] [PubMed] [Google Scholar]
- 20.VanDevanter DR, Morris NJ, Konstan MW. IV-treated pulmonary exacerbations in the prior year: An important independent risk factor for future pulmonary exacerbation in cystic fibrosis. J Cyst Fibros. 2016;15(3):372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders DB, Solomon GM, Beckett VV, West NE, Daines CL, Heltshe SL, et al. Standardized Treatment of Pulmonary Exacerbations (STOP) study: Observations at the initiation of intravenous antibiotics for cystic fibrosis pulmonary exacerbations. J Cyst Fibros. 2017;16(5):592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West NE, Beckett VV, Jain R, Sanders DB, Nick JA, Heltshe SL, et al. Standardized Treatment of Pulmonary Exacerbations (STOP) study: Physician treatment practices and outcomes for individuals with cystic fibrosis with pulmonary Exacerbations. J Cyst Fibros. 2017;16(5):600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schechter MS, Schmidt HJ, Williams R, Norton R, Taylor D, Molzhon A. Impact of a program ensuring consistent response to acute drops in lung function in children with cystic fibrosis. J Cyst Fibros. 2018;17(6):769–78. [DOI] [PubMed] [Google Scholar]
- 24.Thompson V, Mayer-Hamblett N, Kloster M, Bilton D, Flume PA. Risk of hemoptysis in cystic fibrosis clinical trials: A retrospective cohort study. J Cyst Fibros. 2015;14(5):632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flume PA, Mogayzel PJ Jr., Robinson KA, Rosenblatt RL, Quittell L, Marshall BC, et al. Cystic fibrosis pulmonary guidelines: pulmonary complications: hemoptysis and pneumothorax. Am J Respir Crit Care Med. 2010;182(3):298–306. [DOI] [PubMed] [Google Scholar]
- 26.Flume PA, Mogayzel PJ Jr., Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, et al. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180(9):802–8. [DOI] [PubMed] [Google Scholar]
- 27.Schechter MS, Regelmann WE, Sawicki GS, Rasouliyan L, VanDevanter DR, Rosenfeld M, et al. Antibiotic treatment of signs and symptoms of pulmonary exacerbations: a comparison by care site. Pediatr Pulmonol. 2015;50(5):431–40. [DOI] [PubMed] [Google Scholar]
- 28.Cogen JD, Oron AP, Gibson RL, Hoffman LR, Kronman MP, Ong T, et al. Characterization of Inpatient Cystic Fibrosis Pulmonary Exacerbations. Pediatrics. 2017;139(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.