Abstract
Although neutropenia is a common complication following lung transplantation, its relationship to recipient outcomes remains understudied. We evaluated a retrospective cohort of 228 adult lung transplant recipients between 2008 and 2013 to assess the association of neutropenia and Granulocyte Colony Stimulating Factor (GCSF) treatment with outcomes. Neutropenia was categorized as mild (ANC 1000–1499), moderate (500–999) or severe (<500) and also as a time-varying continuous variable. Association with survival, acute rejection (ACR) and chronic lung allograft dysfunction (CLAD) were assessed by Cox proportional hazards regression. GCSF therapy impact on survival, CLAD and ACR development was analyzed by propensity score matching. 101 of 228 patients (42.1%) developed neutropenia. Recipients with severe neutropenia had higher mortality when compared to recipients with no (adjusted hazard ratio (aHR) 2.97, 95% CI 1.05–8.41, p=0.040), mild (aHR 14.508, 95% CI 1.58–13.34, p=0.018), or moderate (aHR 3.27, 95% CI 0.89–12.01, p=0.074) neutropenia. Surprisingly, GCSF treatment was associated with a higher risk for CLAD in mildly neutropenic patients (aHR 3.49, 95% CI 0.93–13.04, p=0.063), although it did decrease death risk in severely neutropenic patients (aHR 0.24, 95% CI 0.07–0.88, p=0.031). Taken together, our data point to an important relationship between neutropenia severity and GCSF treatment in lung transplant outcomes.
INTRODUCTION
Neutropenia after solid organ transplant is a known risk factor for adverse events.1 Neutropenia after renal transplant is associated with death and allograft loss.2 It is also a risk factor for death after liver transplant.3 Although neutropenia is common after lung transplant, its effects on outcomes in this population has not been as thoroughly investigated and no formal guidelines for management exist. Most neutropenia is predominantly thought to result from treatment with anti-proliferative agents such as azathioprine or mycophenolic acid (MPA) or (cytomegalovirus) CMV prophylaxis or treatment with antiviral drugs such as valganciclovir or ganciclovir.1,4 Currently, MPA is the anti-proliferative agent of choice with almost 80% of lung transplant recipients maintained on it through the first year post-transplant according to ISHLT registry data.5 Neutropenia is usually managed primarily by a dose reduction and/or discontinuation of immunosuppression therapy, which itself is a risk factor for allograft rejection.6 In addition to limiting or discontinuing use of MPA, physicians often manage neutropenia in lung recipients with granulocyte colony stimulating factor (GCSF), but its effects on lung allograft survival and rejection remain unknown.
Studies examining the effectiveness of GCSF in solid organ recipient survival have been primarily limited to its effects on acute cellular rejection (ACR). In kidney and liver transplant recipients, GCSF therapy did not change ACR incidence, but there is conflicting evidence among heart and lung transplant recipients.7–9 It also remains unclear whether GCSF treatment improves long-term survival, or if severity of neutropenia plays a role in GCSF-associated outcomes in solid organ transplant recipients. In lung recipients this is especially important to determine given their higher dependence on immunosuppression10, more frequent acute rejection11, and poorer overall survival5, when compared to other solid organ recipients. The goals of this study were to (1) evaluate the association between severity of neutropenia and clinical outcomes, such as allograft rejection and survival, and (2) determine how GCSF administration might attenuate that relationship.
MATERIALS AND METHODS
Study Design and Patient Selection
We conducted a retrospective cohort study of all adult (≥18 years) patients who underwent a lung transplantation at Barnes-Jewish Hospital between 2008 and 2013 who were previously enrolled in a lung transplant recipient database at our institution.12 Patients were excluded if they were transplanted before the age of 18 or transplanted at another institution. The primary outcome was overall survival. Secondary outcomes included ACR, the development of chronic lung allograft dysfunction (CLAD) and infection.
Diagnosis of Neutropenia.
All blood counts obtained after transplantation were analyzed for each patient. Onset of neutropenia was defined as the first instance of absolute neutrophil count (ANC) less than 1500 and was categorized as mild (ANC≤1500), moderate (ANC≤1000) or severe (ANC≤500) based on the nadir of the ANC during the index episode. Duration was defined as time to next ANC>1500 and dichotomized with prolonged being more than 1 week.13
Diagnosis of ACR and CLAD.
During the study period, all patients at our institution underwent protocolized bronchoscopy with bronchoalveolar lavage and transbronchial biopsy to evaluate for infection and rejection at 1-, 3-, 6- and 12-months as part of standard post-lung transplant care. Additional bronchoscopic evaluations were based on changes in a patient’s clinical status at the discretion of the treating transplant pulmonologist. All transbronchial biopsies were evaluated by a trained pathologist and scored for ACR according to guidelines outlined by the International Society for Heart and Lung Transplantation (ISHLT).14 Severity and timing of each episode of ACR was recorded for all participating patients through the end of the follow up period. CLAD was diagnosed as a persistent drop in forced expiratory volume in one second (FEV1) or forced vital capacity (FVC) of greater than 10% in the absence of infection or other reversible cause as previously described.15 Although this does not represent the latest guidelines released in early 201916, this definition is reflective of clinical practice during the study period17.
Diagnosis of Infection.
Evaluation for infection was performed at each protocolized and clinically-indicated bronchoscopic exam. Only respiratory infections were considered. Bacterial (gram-positive and gram-negative) and aspergillus infections were diagnosed with positive respiratory cultures from bronchoscopic evaluation, irrespective of clinical symptoms. For CMV, infection was diagnosed if both respiratory and blood CMV PCR were positive and the patient underwent treatment for the positive cultures.
All data were collected through December 31, 2017. Data were maintained in a secure REDCap database with access limited to essential study personnel.
Immunosuppression Regimen.
All patients at our institution are maintained on a standard 3-drug immunosuppression regimen: a calcineurin inhibitor (tacrolimus or cyclosporine), an anti-proliferative agent (MPA) and corticosteroids. Corticosteroids were initiated on postop day 0 at 1 gram of methylprednisolone daily for 3 days followed by 1mg/kg of prednisone (max of 40mg) with a predetermined taper down to 5mg by 3 months. Induction therapy was given per protocol with the preferred agent changing during the study time frame from (anti-thymocyte globulin) ATGAM to basiliximab. Alterations to immunosuppression were made on an individual basis at the discretion of the treating transplant pulmonologist.
This study was approved by the Institutional Review Board at Washington University (IRB#:201105421).
Statistical Analyses
Baseline demographic and clinical factors were evaluated using the Wilcoxon rank-sum test for continuous variables and the chi-square test for categorical variables.
Construction of Cox Models.
Survival, ACR and CLAD were evaluated as time-to-event outcomes utilizing Cox proportional hazards (PH) regression. Separate Cox models were constructed using neutropenia as a categorical variable of none, mild, moderate, and severe as previously defined (basic Cox model) and using ANC as a continuous time-varying covariate with both a natural log and a quadratic transformation (extended Cox models). For time to ACR, the first episode of ACR after the diagnosis of neutropenia was utilized. For multivariable modelling in both the basic and extended Cox models, covariates of interest were chosen based on prior known or suspected confounding of the outcomes of interest to improve causal inference in this retrospective observational study as previously described.18 Covariates included in the final Cox model for Survival, ACR and CLAD included age, donor gender, transplant indication, PGD grade at 72 hours and CMV mismatch status. For Survival, CLAD status, CMV infection and gram-negative bacterial infection were included in the final model.
Predictors of Neutropenia.
Predictors of neutropenia were evaluated utilizing Cox regression. For all analyses, univariable analyses were conducted for relevant clinical and biological covariates. Variables with p≤0.20 or with prior literature intimating potential confounding in the outcome of interest were offered into the multivariable Cox regression models.
Propensity Score Matching.
Propensity score matching (PSM) for GCSF use was carried out for the entire cohort. Patients were matched on age, gender, neutropenia severity, CMV mismatch status (high risk vs. not high risk where high risk was defined as recipient CMV negative and donor CMV positive) and whether or not they had ACR prior to the first neutropenic episode. Logistic regression was performed to generate propensity scores for GCSF use and patients were matched by score with a match tolerance of 0.1. 51 patients (27 in GCSF and 24 in non-GCSF groups) were included in the PSM analysis. Cox PH regression analyses for survival, time to CLAD and time to ACR were conducted for the matched cohort by severity of neutropenia as outlined above (basic Cox Models).
For all analyses, we considered a two-sided p < 0.05 statistically significant. We performed regression diagnostics to ensure proportional hazards assumption was met for Cox regression analyses. We conducted all analyses using SPSS 24 (IBM Corp., Armonk, NY) with the SPSS extension for R work environment (R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/) and SAS v9.4 (copyright ©2013 SAS Institute Inc., Cary, NC, USA).
RESULTS
Patient Population
A total of 228 patients were included in the study. 101 of 228 patients (44.3%) developed neutropenia: 42 Mild, 34 Moderate and 25 Severe. Table 1 depicts the demographics for these patients according to degree of neutropenia. Patients with moderate and severe neutropenia were more likely to have received basiliximab and less likely to receive ATGAM for induction. Additionally, patients with both donor and recipient negative CMV serostatus were more likely to have no neutropenia while patients with donor positive and recipient negative CMV serostatus were more likely to develop neutropenia. 71 of the 101 (70.3%) patients developed neutropenia within the first year after transplant while the other 30 (29.7%) had their first episode after 1 year. Table 2 outlines factors related to neutropenic episodes. The top panel reports differences between neutropenia severity group. The bottom panel reports differences in GCSF therapy within neutropenia severity group. Overall, the median length of neutropenia was 8 days (IQR 5–28). GCSF administration in response to neutropenia was significantly different between the mild, moderate and severe groups with patients more likely to have received GCSF with increasing degree of severity. GCSF was not associated with any difference in the duration of neutropenia for patients with moderate or severe disease. However, patients with mild neutropenia who received GCSF had a significantly shorter duration of neutropenia (9.5 days vs. 5 days, p=0.023). Additionally, there was a significant difference among all patients who received GCSF with mild patients having a significantly shorter duration than moderate or severe patients (Table 2, bottom panel, row 1 p=0.026). Patients who developed neutropenia late (after the first-year post-transplant) were neutropenic for a longer time than patients who developed it early (within the first-year post-transplant) [median 23 days (5–69) vs. 7.5 (5–20.5) days, p=0.035]. Patients with moderate and severe neutropenia were more likely to have at least one dose of MPA held in response to neutropenia than patients with only mild neutropenia. Overall there were no differences in MPA dose between the groups.
Table 1:
Baseline Characteristics by Degree of Neutropenia
| None (n=125) |
Mild (n=42) |
Moderate (n=34) |
Severe (n=25) |
p-value | |
|---|---|---|---|---|---|
| Recipient Age, years, mean (SD) | 54.3 (13.0) | 53.7 (12.3) | 49.9 (13.4) | 49.2 (15.8) | 0.287 |
| Recipient Gender | 0.135 | ||||
| Female | 44 (35.2) | 18 (42.9) | 13 (38.2) | 15 (60) | |
| Male | 81 (64.8) | 24 (57.1) | 21 (61.8) | 10 (40) | |
| Recipient Race | 0.528 | ||||
| White | 118 (94.4) | 38 (90.5) | 32 (94.1) | 25 (100) | |
| African-American | 5 (4) | 4 (9.5) | 2 (5.9) | 0 (0) | |
| Asian | 2 (1.6) | 0 (0) | 0 (0) | 0 (0) | |
| Transplant Diagnosis | 0.570 | ||||
| ILD | 62 (49.6) | 21 (50) | 13 (38.2) | 11 (44) | |
| COPD | 30 (24) | 12 (28.6) | 6 (17.6) | 4 (16) | |
| CF | 16 (12.8) | 7 (16.7) | 9 (26.5) | 8 (32) | |
| A1AT | 5 (4) | 1 (2.4) | 2 (5.9) | 0 (0) | |
| PH | 2 (1.6) | 0 (0) | 1 (2.9) | 0 (0) | |
| Other | 10 (8) | 1 (2.4) | 4 (8.8) | 2 (8) | |
| Recipient CMV Positive | 75 (60) | 23 (54.8) | 14 (41.2) | 15 (60) | 0.258 |
| Donor Age, years, mean (SD) | 35.9 (14.9) | 34.6 (13.3) | 38.9 (14.9) | 39.8 (16) | 0.771 |
| Donor Male Gender | 85 (68) | 27 (64.3) | 20 (58.8) | 14 (56.0) | 0.585 |
| Donor Race | 0.184 | ||||
| White | 101 (80.8) | 33 (78.6) | 20 (58.8) | 20 (80) | |
| African-American | 23 (18.4) | 9 (21.4) | 14 (41.2) | 5 (20) | |
| Asian | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | |
| CMV Mismatch Status | |||||
| R−/D− | 28 (22.4) | 8 (19) | 3 (8.8) | 2 (8) | |
| R+/D+ | 51 (40.8) | 21 (50) | 8 (23.5) | 11 (44) | |
| R+/D− | 24 (19.2) | 2 (4.8) | 6 (17.6) | 4 (16) | 0.005 |
| R−/D+ | 22 (17.6) | 11 (26.2) | 17 (50) | 8 (32) | |
| Transplant Type | 0.622 | ||||
| Single | 6 (4) | 0 (0) | 1 (2.9) | 1 (4) | |
| Bilateral | 119 (96) | 42 (100) | 33 (97.1) | 24 (96) | |
| Ischemic Time, minutes, mean (SD) | 278.2 (67.6) | 264.3 (73.6) | 289 (65.8) | 288.1 (80.3) | 0.322 |
| Induction Therapy | 0.007 | ||||
| Basiliximab | 81 (65.3) | 27 (64.3) | 26 (76.5) | 18 (72) | |
| ATGAM | 42 (33.9) | 9 (21.4) | 6 (17.6) | 5 (20) | |
| Thymoglobulin | 0 (0) | 2 (4.8) | 0 (0) | 0 (0) | |
| Other | 1 (0.8) | 3 (7.1) | 1 (2.9) | 1 (4) | |
| None | 0 (0) | 1 (2.4) | 1 (2.9) | 1 (4) | |
| PGD @ 72hours | 0.602 | ||||
| 0 | 54 (43.2) | 11 (26.2) | 10 (29.4) | 10 (40) | |
| 1 | 60 (48) | 26 (61.9) | 20 (58.8) | 11 (44) | |
| 2 | 8 (6.4) | 4 (9.5) | 2 (5.9) | 3 (12) | |
| 3 | 3 (2.4) | 1 (2.4) | 2 (5.9) | 1 (4) |
Table 2:
Patient Characteristics by Degree of Neutropenia
| Mild (n=42) |
Moderate (n=34) |
Severe (n=25) |
p-value* | |
|---|---|---|---|---|
| Median time to neutropenia, months (IQR) | 4.9 (2.4–13.2) | 6.2 (3.4–27.2) | 3.8 (2.1–6.8) | 0.342 |
| Valcyte at time of Neutropenia, n(%) | 19 (45.2) | 16 (47.1) | 14 (56) | 0.680 |
| Calcineurin Inhibitor at Neutropenia | 0.181 | |||
| Tacrolimus | 41 (97.6) | 31 (91.2) | 25 (100) | |
| Cyclosporine | 1 (2.4) | 3 (.8) | 0 | |
| Prednisone Dose at time of Neutropenia, mg, median (IQR) | 7.5 (5–60) | 10 (5–35) | 10 (5–50) | 0.296 |
| Mycophenolate Dose at time of Neutropenia, g, Median (IQR) | 1 (0.5–2) | 1 (0.5–2) | 1 (0.5–2) | 0.826 |
| Mycophenolate Response to Neutropenia, n (%) | 0.141 | |||
| No Change | 12 (28.6) | 5 (14.7) | 5 (20) | |
| Dose Decrease | 6 (14.3) | 3 (8.8) | 2 (8) | |
| Dose Held | 24 (57.1) | 26 (76.5) | 16 (64) | |
| Medication Change | 0 | 0 | 2 (8) | |
| G-CSF Administered for Neutropenia, n(%) | 6 (14.3) | 9 (26.5) | 12 (48) | 0.011 |
| Duration of Neutropenia, days, median (IQR) | ||||
| GCSF Administered | 5 (1–7) | 7 (6–37) | 8 (4–11) | 0.026 |
| GCSF Not Administered | 9.5 (6–34.5) | 8 (5–28) | 8 (3–32) | 0.656 |
| p-value for Duration of Neutropenia^ | 0.023 | 0.274 | 0.956 |
Reported p-value is for comparison between neutropenia severity groups.
reported p-value is for comparison within each neutropenia severity group based on whether or not they received GCSF.
Neutropenia and Survival
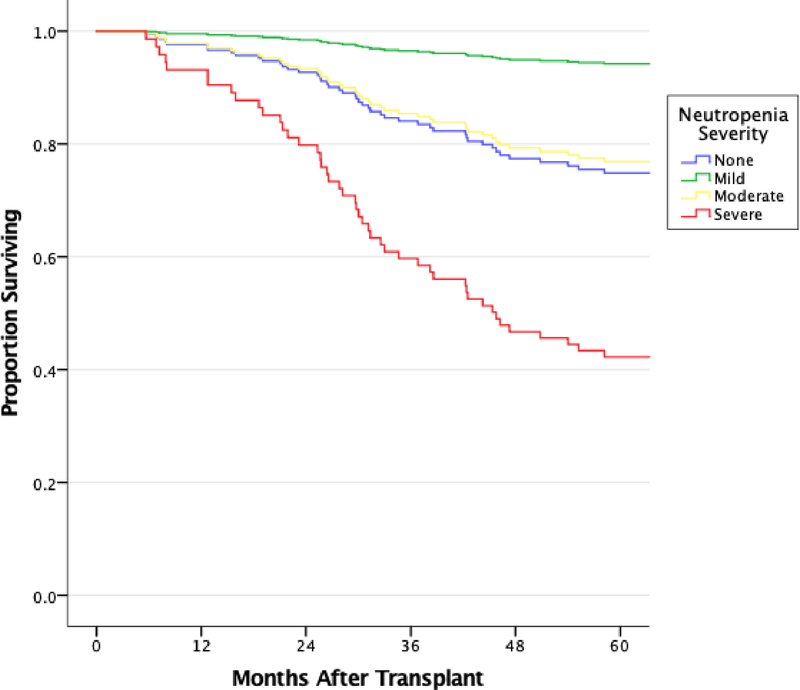
Overall median survival (IQR) was 86.92 months (79.07–94.77) for patients with no neutropenia, 78.03 months (61.03–95.04) for severe neutropenia, 90.76 months (77.05–104.48) for moderate neutropenia and 93.96 months (80.86–107.07) for mild neutropenia. In the basic Cox model, multivariable modelling revealed severe neutropenia to be an independent risk factor for death when compared to patients with no neutropenia [aHR 2.97 (1.05–8.41), p=0.040]. Additionally, severe neutropenia patients were also at a significantly higher risk than patients with mild or moderate neutropenia (Figure 1, Table 3). In the extended Cox model, increasing ANC was significantly associated with improved survival (aHR 3.304, 95% CI 1.982–5.508, p<0.001, Supplemental Table 1). Additionally, in an analysis of ANC-squared, an ANC of 3329 (95% CI 1677 to 10,072) appeared to maximize survival (Supplemental Figure 1).
Figure 1: Basic Multivariable Cox Regression Modelling of Survival after Lung Transplantation.
All patients who developed neutropenia at any time point after lung transplant were evaluated. Model was adjusted for age, gender, CMV infection, CLAD diagnosis, and gram-negative bacterial infection. Severe neutropenia was associated with poorer survival than no, mild or moderate neutropenia. Severe vs. no neutropenia: aHR=2.97 (1.05–8.41), p=0.04; Severe vs. Mild Neutropenia: aHR=14.51 (1.58–33.41), p=0.018; Severe vs. Moderate: aHR=3.27 (0.89–12.01), p=0.07.
Table 3:
Basic Cox Regression Model of Risk of Death after Lung Transplantation
| UNIVARIABLE ANALYSIS | MULTIVARIABLE ANALYSIS | |||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) |
p-value | Hazard Ratio (95% CI) |
p-value |
| Degree of Neutropenia (ref: None) | 0.020 | 0.075 | ||
| Mild | 0.16 (0.02–1.18) | 0.072 | 0.20 (0.03–1.49) | 0.117 |
| Moderate | 0.99 (0.42–2.30) | 0.975 | 0.91 (0.39–2.12) | 0.823 |
| Severe | 3.26 (1.29–8.21) | 0.012 | 2.97 (1.05–8.41) | 0.040 |
| Recipient Age | 0.99 (0.98–1.01) | 0.524 | ||
| Recipient Male Gender | 1.24 (0.80–1.90) | 0.335 | ||
| Recipient Race | 1.41 (0.57–3.48) | 0.457 | ||
| Transplant Diagnosis (ref: COPD) | 0.045 | |||
| Donor Age | 0.99 (0.98–1.01) | 0.933 | ||
| Donor Male Gender | 1.51 (0.95–2.40) | 0.079 | 1.94 (1.02–3.67) | 0.043 |
| Donor Race | 1.37 (0.86–2.18) | 0.178 | ||
| CMV Mismatch Status (ref: R−/D−) | 0.588 | |||
| Transplant Ischemic Time, min | 1.00 (0.99–1.01) | 0.612 | ||
| Transplant Type | 1.56 (0.38–6.33) | 0.537 | ||
| ACR | 0.75 (0.48–1.17) | 0.206 | ||
| CLAD | 2.98 (1.82–4.86) | <0.001 | 2.68 (1.42–5.04) | 0.002 |
| CMV Infection | 1.62 (1.01–2.58) | 0.044 | 1.73 (0.96–3.10) | 0.066 |
| Aspergillus Infection | 1.72 (1.13–2.63) | 0.011 | ||
| Gram Positive Bacterial Infection | 1.43 (0.93–2.19) | 0.102 | ||
| Gram Negative Bacterial Infection | 1.70 (1.10–2.64) | 0.018 | 1.87 (1.08–3.24) | 0.026 |
Neutropenia and Rejection
There were no significant differences in time to ACR in either the basic or extended Cox models (data not shown). There was no significant association between severity of neutropenia and development of CLAD in the basic Cox models (data not shown). However, in extended Cox modelling, increasing ANC was associated with a significantly increased risk for CLAD (aHR 2.64, 95% CI 1.71–4.06, p<0.001, Supplemental Table 2).
Neutropenia and Infection
Table 4 shows the distribution of infection among patients with no, mild, moderate and severe neutropenia. There were no significant differences in rates of CMV infection. For gram positive infections, patients with severe neutropenia had significantly higher rates than patients with no neutropenia (p=0.004) or only mild neutropenia (p=0.026). Moderately and severely neutropenic patients had significantly higher rates of gram-negative infections than mildly neutropenic patients (Moderate vs. Mild, p=0.040; Severe vs. Mild, p=0.033). Mildly neutropenic patients had a significantly lower rate of aspergillus infection than patients without neutropenia (p=0.045). In extended Cox modelling, there was no significant association between ANC and any of the infectious outcomes.
Table 4:
Rate of Infection by Overall Degree of Neutropenia
| None | Mild | Moderate | Severe | |
|---|---|---|---|---|
| Aspergillus*, n(%) | 66 (53.6) | 15 (35.7) | 18 (52.9) | 13 (52) |
| CMV*, n (%) | 80 (64) | 26 (61.9) | 25 (73.5) | 18 (72) |
| Gram Negative**, n(%) | 71 (56.8) | 19 (45.2) | 23 (67.6) | 18 (72) |
| Gram Positive^, n(%) | 33 (26.4) | 12 (28.6) | 12 (35.3) | 14 (56) |
No Overall significant comparisons for aspergillus or CMV infection
Severe vs Mild (OR 3.11, 95% CI 1.07–9.02, p=0.033) and Moderate vs Mild (OR 2.78, 95% CI 1.07–7.27, p=0.034) had significantly higher rate of gram-negative infection.
Severe patients had significantly higher rate of gram-positive infection than none (OR 2.89, 95% CI 1.25–6.69, p=0.011) and mild (OR 3.18, 95% CI 1.13–8.96, p=0.026) neutropenia patients.
Association between GCSF and Neutropenia Outcomes
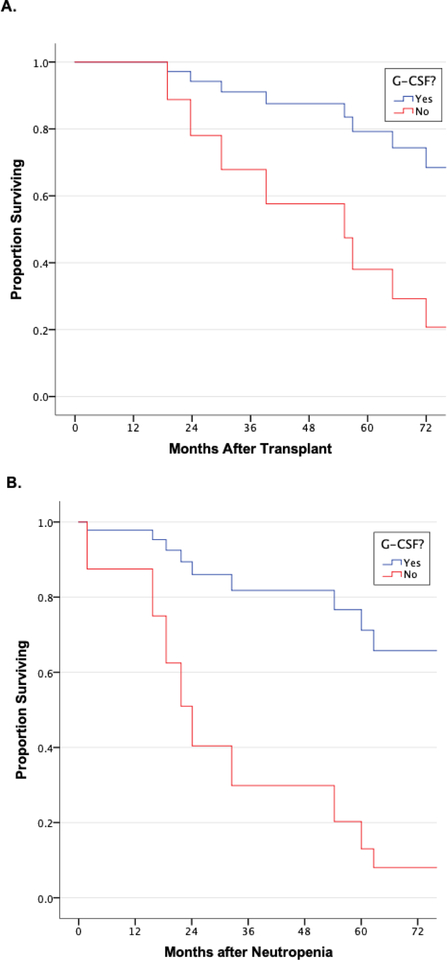
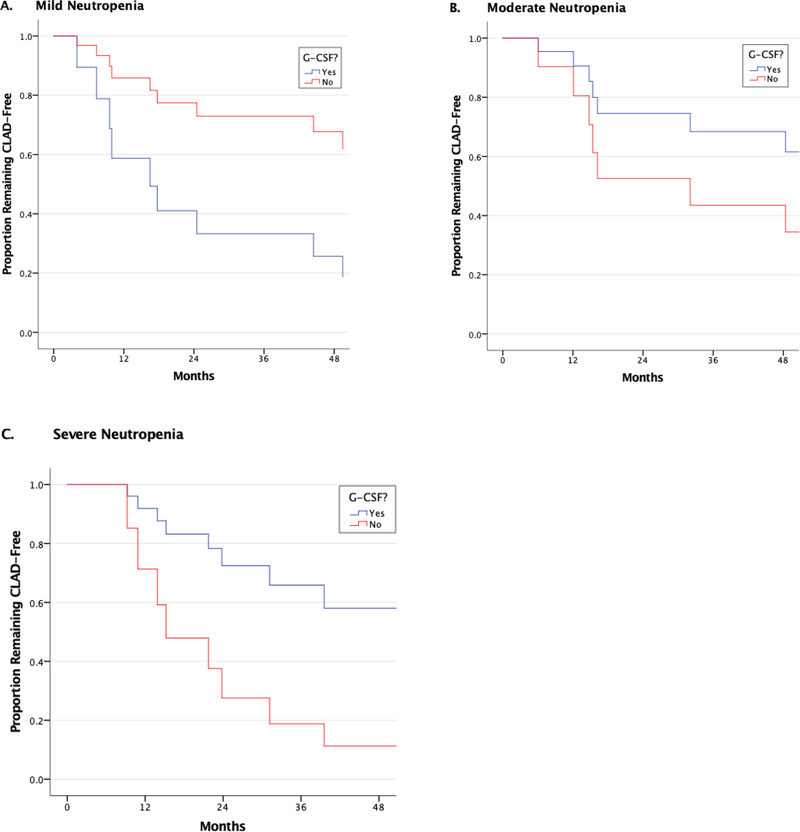
Propensity matching resulted in a matched cohort of 51 patients. Supplemental Tables 3 and 4 reveal pre- and post-matching demographics for the cohorts. For patients with mild and moderate neutropenia, GCSF administration had no meaningful association with survival (data not shown). However, for patients with severe neutropenia, GCSF administration was associated with significantly improved overall survival and improved survival after the first episode of neutropenia (overall aHR for death 0.24, 95% CI 0.07–0.88, p=0.031, Figure 2). GCSF had no significant association with the development of ACR in patients with any degree of neutropenia (data not shown). However, for CLAD, GCSF administration was associated with a strong trend towards increased CLAD for patients with mild neutropenia (aHR 3.49 (0.93–13.04), p=0.063) and decreased CLAD for patients with severe neutropenia (aHR 0.25 (0.06–1.02, p=0.053), although these did not reach statistical significance (Figure 3). For infection, stratification according to GCSF use revealed that patients with severe neutropenia had significantly higher rates of aspergillus infection when they did not receive GCSF (83.3% vs. 33.3%, p=0.046) (Supplemental Table 5). There were no other significant differences in infection outcomes among patients within each severity class of neutropenia based on whether or not they received GCSF.
Figure 2: Association Between GCSF use and Survival Among Propensity-Matched Cohort of Severely Neutropenic Patients.
A) Severe neutropenia patients who received GCSF had significantly improved overall survival than those who did not (aHR for death 0.24, 95% CI 0.07–0.88, p=0.031). B) Severe neutropenia patients who received GCSF had significantly improved post-neutropenia survival than severe neutropenia patients who did not (aHR for death 0.17, 95% CI 0.04–0.63, p=0.008).
Figure 3: Association Between GCSF use and CLAD Development Among Propensity-Matched Cohort of Neutropenic Patients.
Neutropenia groups represent severity of first episode of neutropenia. Time to CLAD is shown for patients with A) mild, b) moderate and c) severe neutropenia. For patients with mild neutropenia, GCSF administration was associated with a near significant increased risk for CLAD development (aHR 3.49, 95% CI 0.93–13.04, p=0.063). There were no significant differences in CLAD development for patients with moderate neutropenia, but patients with severe neutropenia who received GCSF had a near significant decreased risk for CLAD development according to whether or not they received GCSF (aHR 0.25, 95% CI 0.06–1.02, p=0.053).
Predictors of Neutropenia
In univariable analysis, high-risk CMV mismatch (seronegative recipient/seropositive donor) was strongly associated with development of post-transplant neutropenia and this relationship remained significant in multivariable analysis (aHR 2.464, 95% CI1.293–4.696, p=0.006) and male recipient gender was protective (HR 0.654, 95% CI 0.431–0.992, p=0.046, Table 5). No other demographic or clinical factors were found to significantly predict neutropenia.
Table 5:
Cox Regression Analysis for Predictors of Neutropenia
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value |
| Recipient Age at Transplant | 0.989 (0.975–1.003) | 0.120 | ||
| Recipient Male Gender | 0.700 (0.473–1.035) | 0.074 | 0.654 (0.431–0.992) | 0.046 |
| Recipient Race (Black vs. White) | 1.525 (0.668–3.485) | 0.316 | ||
| Induction Immunosuppression (ATGAM vs. Simulect) | 0.610 (0.370–1.004) | 0.052 | ||
| Recipient Diagnosis (Reference: COPD) |
0.383 | |||
| Interstitial Lung Disease | 1.066 (0.640–1.776) | 0.805 | ||
| Cystic Fibrosis | 1.696 (0.950–3.028) | 0.074 | ||
| Alpha-1 Antitrypsin | 0.878 (0.263–2.938) | 0.833 | ||
| Pulmonary Hypertension | 0.658 (0.089–4.880) | 0.682 | ||
| Other | 0.870 (0.353–2.146) | 0.762 | ||
| Donor Age | 1.001 (0.989–1.015) | 0.822 | ||
| Donor Male Gender | 0.786 (0.527–1.171) | 0.236 | ||
| Donor Race (Black v. White) | 1.464 (0.947–2.264) | 0.087 | ||
| CMV Mismatch Status (Reference R−/D−) |
0.005 | 0.007 | ||
| R+/D+ | 1.531 (0.819–2.864) | 0.182 | 1.319 (0.694–2.509) | 0.398 |
| R+/D− | 1.016 (0.463–2.227) | 0.969 | 1.016 0.463–2.228) | 0.969 |
| R−/D+ | 2.526 (1.337–4.771) | 0.004 | 2.464 (1.293–4.696) | 0.006 |
| Transplant Ischemic Time | 1.000 (0.998–1.003) | 0.732 | ||
| Transplant Type (Bilateral vs. Single) | 1.526 (0.376–6.191) | 0.554 | ||
| PGD Grade at 72 hours (Reference: 0) | 0.525 | |||
| Grade 1 | 1.355 (0.875–2.099) | 0.173 | ||
| Grade 2 | 1.423 (0.677–2.990) | 0.352 | ||
| Grade 3 | 1.534 (0.541–4.348) | 0.420 | ||
| Calcineurin Inhibitor (cyclosporine vs. tacrolimus) | 1.817 (0.575–5.746) | 0.309 | ||
DISCUSSION
We find here that patients who develop severe neutropenia have decreased survival after transplant when compared to patients with no, mild or moderate neutropenia. GCSF use was associated with improved outcomes in patients with severe neutropenia, specifically a significantly decreased risk of death. Conversely, in patients with mild neutropenia, GCSF was associated with higher rates of CLAD without any meaningful association with survival or infection outcomes.
This is, to our knowledge, the largest clinical study in lung transplant recipients to evaluate the association between neutropenia and survival and how administration of GCSF may attenuate that association. A similar study among pediatric renal transplant recipients found neutropenia to be similarly frequent (~64%); however, they did not evaluate the effect of neutropenia on survival or other clinical outcomes.19 Likewise, a large Medicare database study of adult renal transplant recipients found that neutropenia was associated with increased risk of allograft loss and death, and that treatment with GCSF appeared to attenuate that effect2, but did not evaluate the differential impact of varying severity of neutropenia.
Neutropenia has been most extensively studied among hematopoietic transplant recipients, where increasing severity and duration of neutropenia is associated with increased infectious outcomes and death.20–22 Neutrophils are particularly crucial effector cells in the body’s defense against bacterial pathogens, migrating along chemokine gradients to areas of infection and/or inflammation.23–25 In patients receiving MPA for immunosuppression following lung transplantation, the effects of severe neutropenia are likely exacerbated. This is because MPA, in addition to its primary target, also interferes with antigen presentation by dendritic cells, promotes monocyte apoptosis and disrupts leukocyte adhesion.26–29 As such, the significantly higher rates of bacterial infections among severely neutropenic patients are to be expected. Additionally, as there were no significant differences in ACR or CLAD for severely neutropenic patients, it is likely that the increase in infectious complications is primarily responsible for the decrement in survival seen in these patients. This supports ISHLT registry data demonstrating that one of the leading causes of death, particularly after the first year post-lung transplant, is infection.5
Conversely, patients with mild neutropenia had better survival than all other patients. However, contrary to what was seen in patients with severe neutropenia, GCSF administration was associated with worsened CLAD outcomes among patients with mild neutropenia. GCSF is a cytokine that drives both neutrophil generation from myeloid progenitors within the bone marrow and neutrophil mobilization into the blood.30 However, GCSF promotes immunomodulatory effects on adaptive immune responses that could promote allograft tolerance. There is evidence that GCSF promotes the reduction of alloreactive T lymphocytes and the expansion of regulatory populations of T lymphocytes and antigen presenting cells.31–33 Although this might appear to be a paradoxical finding, recent evidence from experimental models of lung transplantation support the notion that GCSF under certain circumstances can promote alloimmunity.34 For example, previous work has shown that GCSF administration to a mouse model of lung transplantation induced neutrophils to activate antigen presenting cells, which in turn, caused the expansion of alloreactive T cells and prevented immunosuppression-mediated tolerance.35 Similarly, GCSF-dependent neutrophilia has been shown to stimulate rejection through neutrophil-mediated T-cell activation during a pseudomonas infection.36 Finally, it has been demonstrated that blocking GCSF-mediated emergency granulopoiesis prevents acute inflammatory injury.37 Given that patients with mild neutropenia showed no increased risk of infection, and increased rates of CLAD following GCSF administration, it is possible that the emergency granulopoiesis stimulated by GCSF administration in these patients leads to harmful neutrophil infiltration and damage to the allograft. Additionally, this might also explain why patients with mild neutropenia appeared to do better than patients with no neutropenia. This area requires more investigation as prior reports of the impact of GCSF on clinical transplant outcomes have been conflicting, likely related to the heterogeneity both of the patient populations and of the severity of neutropenia.7,8,38
The only significant predictors of neutropenia after transplant in multivariable modelling was recipient female gender and high-risk CMV mismatch recipients, recipients who were seronegative and received lungs from a seropositive donor. The exact relationship between gender and neutropenia is unclear, however the increased risk associated with females might be related to the known increased risk of alloimmunity particularly in women who have had children. This is a phenomenon that requires more investigation. In our institution, the standard protocol for high risk CMV mismatch status is valganciclovir treatment for CMV prophylaxis for the first six months following transplant. Thus, it is interesting to note that pharmacokinetic interactions between valganciclovir and MPA39 have been related to increase rates of neutropenia.40,41 It is also possible that there are different etiologies of neutropenia depending on time after transplant. Neutropenia early after transplant, although more common, was shorter and was not associated significantly with overall survival. Given that combination of medications used early after transplant results in higher effective immunosuppression, the transitory nature of early neutropenia could be related to the immunosuppression treatment. In contrast, late neutropenia was significantly longer, was not associated with valganciclovir use, and was associated with significantly decreased survival. Given that prolonged exposure to MPA and other commonly used immunosuppression agents can be cytotoxic, late neutropenia might represent a different phenomenon related to changes in neutrophil biology, production and maturation.42
This study has several limitations. The retrospective design limited the data available to pre-existing patient records. Therefore, incomplete records, or records not in our system (for non-local transplant recipients) may have led to missed episodes of neutropenia or infection. Additionally, there is a selection bias for patients previously enrolled in our lung transplant database. Secondly, the relatively small sample size resulted in fewer numbers of patients available for subgroup analyses. It is possible that type II errors exist in some of the subgroup analyses. In particular, we were unable to assess how changes to clinical management in response to neutropenia altered the association with survival. Additionally, rejection episodes were recorded without controlling for interpatient variability in the number of bronchoscopies performed. Finally, again due to the retrospective nature of the study, it was not possible to control for all potential confounders.
Despite these limitations, we conclude that severe neutropenia is associated with decreased survival and increased rates of infection. Among severely neutropenic patients, GCSF administration may be helpful to attenuate these risks. However, administration to mildly neutropenic patients appears to be detrimental as it was associated with increased rates of CLAD without any meaningful improvement in allograft survival or reduction in infectious complications. Thus, further investigation into proper patient selection for GCSF therapy is warranted to better optimize neutropenia treatment protocols for lung transplant recipients.
Supplementary Material
ACKNOWLEDGMENTS
LK Tague is supported by the Washington University Division of Pulmonary and Critical Care Medicine grant T32HL007317–39 from the National Institutes of Health (NIH). AE Gelman is supported by grants from the Barnes Jewish Foundation and NIH Grants R01HL113436–01A1, 2RHL094601, R01HL121218–01 and P01AI116501–01. Dr. Gage is supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR002345. The content is solely the responsibility of the authors and does not necessarily represent the official view of Washington University, Barnes Jewish Foundation or the NIH.
ABBREVIATIONS
- ACR
Acute Cellular Rejection
- AHR
Adjusted Hazard Ratio
- ANC
Absolute Neutrophil Count
- ATG
Anti-thymocyte globulin
- CLAD
Chronic Lung Allograft Dysfunction
- CMV
Cytomegalovirus
- FEV1
Forced Expiratory Volume in One Second
- FVC
Forced Vital Capacity
- GCSF
Granulocyte Colony Stimulating Factor
- IQR
Interquartile Range
- ISHLT
International Society for Heart and Lung Transplantation
- MPA
Mycophenolic Acid
- PSM
Propensity Score Matching
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Zafrani L, Truffaut L, Kreis H, et al. Incidence, risk factors and clinical consequences of neutropenia following kidney transplantation: a retrospective study. Am J Transplant. 2009;9(8):1816–1825. [DOI] [PubMed] [Google Scholar]
- 2.Hurst FP, Belur P, Nee R, et al. Poor outcomes associated with neutropenia after kidney transplantation: analysis of United States Renal Data System. Transplantation. 2011;92(1):36–40. [DOI] [PubMed] [Google Scholar]
- 3.Alraddadi B, Nierenberg NE, Price LL, et al. Characteristics and outcomes of neutropenia after orthotopic liver transplantation. Liver Transpl. 2016;22(2):217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danziger-Isakov L, Mark Baillie G. Hematologic complications of anti-CMV therapy in solid organ transplant recipients. Clin Transplant. 2009;23(3):295–304. [DOI] [PubMed] [Google Scholar]
- 5.Yusen RD, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant. 2016;35(10):1170–1184. [DOI] [PubMed] [Google Scholar]
- 6.Vanhove T, Kuypers D, Claes KJ, et al. Reasons for dose reduction of mycophenolate mofetil during the first year after renal transplantation and its impact on graft outcome. Transpl Int. 2013;26(8):813–821. [DOI] [PubMed] [Google Scholar]
- 7.Casciello N, Hulbert A, Snyder L, Byrns J. Incidence of acute cellular rejection following granulocyte colony-stimulating factor administration in lung transplantation: A retrospective case-cohort analysis. Clin Transplant. 2017;31(5). [DOI] [PubMed] [Google Scholar]
- 8.Vrtovec B, Haddad F, Pham M, et al. Granulocyte colony-stimulating factor therapy is associated with a reduced incidence of acute rejection episodes or allograft vasculopathy in heart transplant recipients. Transplant Proc. 2013;45(6):2406–2409. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen AB, Lourenco L, Chung BB, et al. Increase in short-term risk of rejection in heart transplant patients receiving granulocyte colony-stimulating factor. J Heart Lung Transplant. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tett SE, Saint-Marcoux F, Staatz CE, et al. Mycophenolate, clinical pharmacokinetics, formulations, and methods for assessing drug exposure. Transplant Rev (Orlando). 2011;25(2):47–57. [DOI] [PubMed] [Google Scholar]
- 11.Berastegui C, Roman J, Monforte V, et al. Biomarkers of pulmonary rejection. Transplant Proc. 2013;45(9):3163–3169. [DOI] [PubMed] [Google Scholar]
- 12.Tague LK, Byers DE, Hachem R, et al. Impact of SLCO1B3 polymorphisms on clinical outcomes in lung allograft recipients receiving mycophenolic acid. Pharmacogenomics J. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):427–431. [DOI] [PubMed] [Google Scholar]
- 14.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26(12):1229–1242. [DOI] [PubMed] [Google Scholar]
- 15.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297–310. [DOI] [PubMed] [Google Scholar]
- 16.Verleden GM, Glanville AR, Lease ED, et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant. 2019;38(5):493–503. [DOI] [PubMed] [Google Scholar]
- 17.Meyer KC, Raghu G, Verleden GM, et al. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J. 2014;44(6):1479–1503. [DOI] [PubMed] [Google Scholar]
- 18.Lederer DJ, Bell SC, Branson RD, et al. Control of Confounding and Reporting of Results in Causal Inference Studies. Guidance for Authors from Editors of Respiratory, Sleep, and Critical Care Journals. Ann Am Thorac Soc. 2019;16(1):22–28. [DOI] [PubMed] [Google Scholar]
- 19.Becker-Cohen R, Ben-Shalom E, Rinat C, Feinstein S, Geylis M, Frishberg Y. Severe neutropenia in children after renal transplantation: incidence, course, and treatment with granulocyte colony-stimulating factor. Pediatr Nephrol. 2015;30(11):2029–2036. [DOI] [PubMed] [Google Scholar]
- 20.Kameda K, Kimura SI, Misaki Y, et al. Associations between febrile neutropenia-related parameters and the risk of acute GVHD or non-relapse mortality after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2018. [DOI] [PubMed] [Google Scholar]
- 21.Lohmann DJA, Asdahl PH, Abrahamsson J, et al. Associations between neutrophil recovery time, infections and relapse in pediatric acute myeloid leukemia. Pediatr Blood Cancer. 2018;65(9):e27231. [DOI] [PubMed] [Google Scholar]
- 22.Barnes G, Pathak A, Schwartzberg L. G-CSF utilization rate and prescribing patterns in United States: associations between physician and patient factors and GCSF use. Cancer Med. 2014;3(6):1477–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galdames JA, Zuniga-Traslavina C, Reyes AE, Feijoo CG. Gcsf-Chr19 promotes neutrophil migration to damaged tissue through blood vessels in zebrafish. J Immunol. 2014;193(1):372–378. [DOI] [PubMed] [Google Scholar]
- 24.Teng TS, Ji AL, Ji XY, Li YZ. Neutrophils and Immunity: From Bactericidal Action to Being Conquered. J Immunol Res. 2017;2017:9671604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warren E, Teskey G, Venketaraman V. Effector Mechanisms of Neutrophils within the Innate Immune System in Response to Mycobacterium tuberculosis Infection. J Clin Med. 2017;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cicinnati VR, Hou J, Lindemann M, et al. Mycophenolic acid impedes the antigen presenting and lymph node homing capacities of human blood myeloid dendritic cells. Transplantation. 2009;88(4):504–513. [DOI] [PubMed] [Google Scholar]
- 27.Ritter ML, Pirofski L. Mycophenolate mofetil: effects on cellular immune subsets, infectious complications, and antimicrobial activity. Transpl Infect Dis. 2009;11(4):290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehling A, Grabbe S, Voskort M, Schwarz T, Luger TA, Beissert S. Mycophenolate mofetil impairs the maturation and function of murine dendritic cells. J Immunol. 2000;165(5):2374–2381. [DOI] [PubMed] [Google Scholar]
- 29.Allison AC, Eugui EM. Mechanisms of action of mycophenolate mofetil in preventing acute and chronic allograft rejection. Transplantation. 2005;80(2 Suppl):S181–190. [DOI] [PubMed] [Google Scholar]
- 30.Bennett CL, Djulbegovic B, Norris LB, Armitage JO. Colony-stimulating factors for febrile neutropenia during cancer therapy. N Engl J Med. 2013;368(12):1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geng D, Joshi SK, Podolsky R, She JX. GCSF receptor regulates antigen uptake and expression of cytokines and costimulatory molecules in dendritic cells. Mol Immunol. 2007;44(4):521–529. [DOI] [PubMed] [Google Scholar]
- 32.Malashchenko VV, Meniailo ME, Shmarov VA, et al. Direct anti-inflammatory effects of granulocyte colony-stimulating factor (G-CSF) on activation and functional properties of human T cell subpopulations in vitro. Cell Immunol. 2018;325:23–32. [DOI] [PubMed] [Google Scholar]
- 33.Perobelli SM, Mercadante AC, Galvani RG, et al. G-CSF-Induced Suppressor IL-10+ Neutrophils Promote Regulatory T Cells That Inhibit Graft-Versus-Host Disease in a Long-Lasting and Specific Way. J Immunol. 2016;197(9):3725–3734. [DOI] [PubMed] [Google Scholar]
- 34.Scozzi D, Ibrahim M, Menna C, Krupnick AS, Kreisel D, Gelman AE. The Role of Neutrophils in Transplanted Organs. Am J Transplant. 2017;17(2):328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreisel D, Sugimoto S, Zhu J, et al. Emergency granulopoiesis promotes neutrophil-dendritic cell encounters that prevent mouse lung allograft acceptance. Blood. 2011;118(23):6172–6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto S, Nava RG, Zhu J, et al. Cutting edge: Pseudomonas aeruginosa abolishes established lung transplant tolerance by stimulating B7 expression on neutrophils. J Immunol. 2012;189(9):4221–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreisel D, Sugimoto S, Tietjens J, et al. Bcl3 prevents acute inflammatory lung injury in mice by restraining emergency granulopoiesis. J Clin Invest. 2011;121(1):265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winston DJ, Foster PF, Somberg KA, et al. Randomized, placebo-controlled, double-blind, multicenter trial of efficacy and safety of granulocyte colony-stimulating factor in liver transplant recipients. Transplantation. 1999;68(9):1298–1304. [DOI] [PubMed] [Google Scholar]
- 39.Gimenez F, Foeillet E, Bourdon O, et al. Evaluation of pharmacokinetic interactions after oral administration of mycophenolate mofetil and valaciclovir or aciclovir to healthy subjects. Clin Pharmacokinet. 2004;43(10):685–692. [DOI] [PubMed] [Google Scholar]
- 40.Rerolle JP, Szelag JC, Le Meur Y. Unexpected rate of severe leucopenia with the association of mycophenolate mofetil and valganciclovir in kidney transplant recipients. Nephrol Dial Transplant. 2007;22(2):671–672. [DOI] [PubMed] [Google Scholar]
- 41.Brum S, Nolasco F, Sousa J, et al. Leukopenia in kidney transplant patients with the association of valganciclovir and mycophenolate mofetil. Transplant Proc. 2008;40(3):752–754. [DOI] [PubMed] [Google Scholar]
- 42.Ferjani H, Draz H, Abid S, Achour A, Bacha H, Boussema-Ayed I. Combination of tacrolimus and mycophenolate mofetil induces oxidative stress and genotoxicity in spleen and bone marrow of Wistar rats. Mutat Res. 2016;810:48–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.