Abstract
Continuous positive airway pressure (CPAP) is highly effective in treating sleep-disordered breathing (SDB). However, unlike surgical interventions, this treatment modality relies heavily on patient acceptance and adherence. The current definition of adherence is largely arbitrary and is mainly used by third-party payers to determine CPAP reimbursement but CPAP adherence remains sub-optimal. Strategies to augment adherence, especially early in the course of a CPAP trial, are needed in the management of SDB. An understanding of the basis for observed differences in CPAP and oral appliance (OA) use is necessary in developing these strategies, but to date no single factor has been consistently identified. Consequently, a multidimensional approach using educational, behavioural, technological and potentially pharmacological strategies to target (i) disease characteristics, (ii) patient characteristics including psychosocial factors, (iii) treatment protocols and (iv) technological devices and side effects that may influence adherence, is likely required to augment the complex behaviour of CPAP and OA use. In the near future, we envision a personalized medicine approach to determine the risk of non-adherence and set individualized adherence goals aimed at treating specific symptoms (e.g. excessive day-time sleepiness) and reducing the risk of patient-specific SDB consequences (e.g. atherosclerosis). Resources for interventions to improve adherence such as educational programmes and telemedicine encounters could then be more efficiently allocated.
Keywords: compliance, continuous positive airway pressure adherence, lung, sleep apnoea, sleep-disordered breathing
INTRODUCTION
Sleep-disordered breathing (SDB) is highly prevalent and associated with considerable morbidity and mortality. Positive airway pressure (PAP) is currently the mainstay of treatment, but unlike surgical interventions, efficacy relies heavily on acceptance and adherence. The stakes are high; on an individual level, SDB results in a number of disease consequences including cardiovascular and metabolic disease. From a societal perspective, excessive daytime sleepiness and impaired functioning during wakefulness have profound socioeconomic implications.
DEFINING ADHERENCE TO THERAPY
While somewhat arbitrary, a threshold of less than 4 h of nightly PAP use on 70% of nights has been adopted to define non-adherence. This threshold has been debated since a dose-response relationship between PAP usage and clinical outcomes in obstructive sleep apnoea (OSA) has been demonstrated.1–4 That being said, specific adherence thresholds to treat OSA consequences are moving targets and vary depending on the outcomes of interest. In a cohort of 149 patients with severe OSA, Weaver et al. demonstrated that 4 h of nightly continuous PAP (CPAP) use was associated with improvement in the Epworth Sleepiness Scale but 7.5 h of nightly use was required for improvement in the Multiple Sleep Latency Test and Functional Outcomes associated with Sleepiness Questionnaire.1 For treatment of hypertension, CPAP usage >3.5 h was associated with greater reduction in 24-h diastolic blood pressure after 4 weeks of treatment.5 There is, however, general acknowledgement that more is better with respect to CPAP usage.
In the USA, this concept of an adherence threshold has not been helped by third-party payers who have developed strict criteria for PAP reimbursement mandating the use of PAP adherence tracking systems. The Centers for Medicare and Medicaid Services (CMS) requires that for continued PAP coverage beyond the first 3 months of therapy, the treating physician must conduct a face-to-face clinical re-evaluation no sooner than 31 days but no later than 91 days after initiating therapy. In addition, there must be documentation that the patient is benefiting from PAP therapy with objective evidence of PAP adherence based on download data showing ≥4 h of nightly use for ≥70% of nights monitored during a consecutive 30-day period. These criteria reinforce the erroneous notion of a therapeutic threshold and do not take into consideration the evidence suggesting that some PAP use is better than no use. In an outpatient cohort of 227 patients with concurrent chronic obstructive pulmonary disease and OSA or the overlap syndrome, any level of CPAP use was associated with some mortality benefit over no CPAP use.6
ADHERENCE ESTIMATES, SELFREPORTING AND OBJECTIVE USAGE MEASUREMENTS
Adherence, the main limitation to PAP therapy, is highly variable but remains sub-optimal in many patient populations. Using a criterion of 4 h or less of nightly use, CPAP non-adherence has been estimated between 29% and 83%.3,7,8 We have recently observed evidence of improving adherence over time, with one Big Data analysis showing up to 87% adherence using modern technology.9 The pattern of PAP usage is established early, typically during the first week of therapy, and has been shown to predict long-term use.3,4,10–12 Compared with PAP, oral appliance (OA) adherence is usually higher but is also highly variable with 1-year compliance estimates between 32% and 82%. Comparison of the two modalities is limited, however, as the majority of studies compare subjective reports of OA use with objective CPAP usage data.13–16
In the past, the accuracy of PAP adherence estimates has been limited by self-reporting, which was often unreliable. This situation has changed as device technology now allows for objective measurement of use. Microprocessors embedded within PAP units monitor cumulative time that the PAP device is turned on at the effective pressure. While this technology is not yet able to discriminate who is wearing the device when turned on, it does reliably track PAP use.17 This information can then be viewed using various transmission systems including smartcards or SD cards, memory sticks and via modem or wireless transmission. The information is not standardized between the different proprietary tracking systems but adherence data are available for all (Fig. 1). Of note, self-reported PAP use overestimates actual use (as determined by machine download) by approximately 1 h.3,18 A meta-analysis of 11 randomized controlled trials found that mean subjective CPAP use time was 0.70 (95% CI: 0.11–1.30) more hours per night than objective measures among treated OSA patients.14 Like CPAP, efficacy of mandibular advancement devices (MAD) is also reliant on adherence, and although there appears to be less reporting bias than with PAP, treatment tracking systems are being developed to limit discrepancies. An MAD with a built-in thermal sensor was studied in 80 patients with varying OSA severity.19 In this study, sensor-reported use was shown to be consistent with self-reported use. Meanwhile, Castillo et al. integrated a tooth microphone with an oral appliance device and monitored adherence through audio recording of night-time respiratory sounds.20 Whether such efforts to monitor use objectively alters adherence remains unclear.
Figure 1.
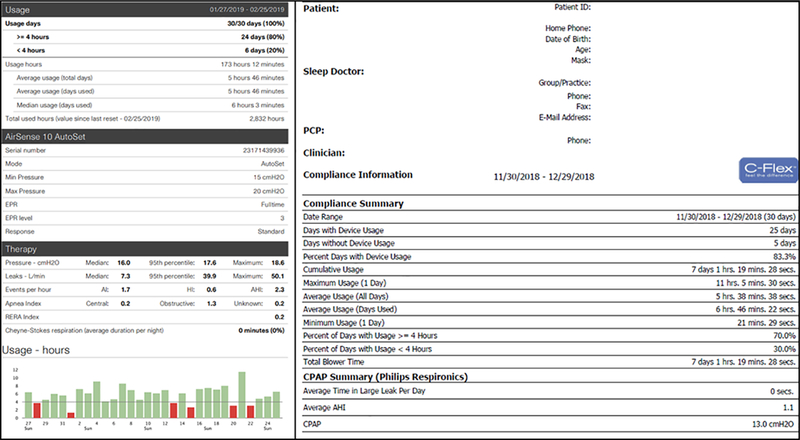
Examples of data downloaded from two different positive airway pressure (PAP) units.
AUGMENTING ADHERENCE
Given the challenges with adherence, strategies to augment adherence are needed in the management of SDB. Investigators have mainly focused on identifying and targeting factors that may predict or influence adherence. An understanding of the basis for observed differences in PAP and OA use is necessary in developing strategies to improve adherence. Yet, to date, no single factor has been consistently identified, and commonly explored anthropometric, symptomatic or polysomnographic severity was found to explain just 4–25% of variance in CPAP use.21 Consequently, a multidimensional approach using educational, behavioural, technological and potentially pharmacological strategies to target (i) disease characteristics, (ii) patient characteristics including psychosocial factors, (iii) treatment protocols and (iv) technological devices and side effects that may influence adherence, is likely required to augment the complex behaviour of PAP and OA use.7
Disease characteristics
SDB characteristics are not likely to be modifiable targets to improve adherence. OSA severity, typically measured using the apnoea-hypopnoea index, has not been consistently identified as a predictor of CPAP adherence.3,11,12,22,23 Similarly, studies looking at the association between REM-related OSA and CPAP adherence have been mixed.24,25 A more convincing association has been observed between initial severity of daytime sleepiness and PAP use, and an Epworth Sleepiness score >10 was shown to be an independent predictor of long-term CPAP use in 1211 consecutive OSA patients.3,12 Patients with the greatest level of CPAP adherence typically report the greatest improvement in OSA symptoms including daytime energy and greater satisfaction with CPAP use, but in a possible bidirectional relationship this improvement in OSA symptoms has also been shown to correlate positively with CPAP use.26
Patient characteristics
Anthropometric variables including age, sex and marital status have not been consistently associated with CPAP adherence nor are they readily modifiable strategic targets to improve adherence.8 Some (but not all) studies have suggested worse CPAP adherence in African-Americans,27–30 although reasons for this disparity are unclear, but may be a function primarily of socio-economic status. Very little data exist for other races and there is a need for future research on CPAP adherence among different demographics, including women, older patients and in different ethnic groups. In a sample of 126 New Zealand patients with OSA initiating CPAP therapy, 19.8% were Maori. This group had significantly lower CPAP usage than non-Maori but in a multiple regression model including ethnicity, socio-economic status, annual income, level of formal education and eligibility for government-subsidized health care, only non-completion of tertiary education and socio-economic deprivation remained as significant independent predictors of CPAP non-adherence.31 In a retrospective cohort of 260 veterans with newly diagnosed OSA, initial CPAP adherence was closely associated with higher neighbourhood socioeconomic factors.32 After adjustment for individual socio-demographic characteristics and medical comorbidity, the probability of daily CPAP use of 4 h ranged from 34.1% (95% CI: 26.4–42.7) for subjects from a low socio-economic neighbourhood to 62.3% (95% CI: 53.8–70.1) for subjects from a high socioeconomic neighbourhood. CPAP users have been shown to have more years of education and be more likely to work in professional occupations.3 To this extent, a social history may be helpful in developing a successful strategy to improve PAP and OA adherence.
Both PAP and OA adherence have been shown to be associated with psychological traits and disposition. The Type D (distressed) personality, defined as a combination of negative affectivity and social inhibition, was found in 30% of 247 OSA patients treated with CPAP for longer than 60 months. This personality type significantly increased the perceived frequency and severity of a range of side effects and had lower objective CPAP adherence compared to patients without Type D personality.33 Type D personality patients also reported a significantly higher discontinuation rate using MAD when compared to patients without Type D personality.34 The authors argued that identification of this personality could be used by healthcare personnel when evaluating patients awaiting treatment.
CPAP use has been associated with a patient’s perception of OSA symptoms and risks of SDB as well as perceived benefits to therapy. CPAP use may also relate to treatment outcome expectations, self-efficacy and coping mechanisms.23 These psychological factors may be more important than any other patient characteristic in determining patterns of CPAP and OA use. Patients who self-initiate their referral have been shown to have greater CPAP use.35 Very high treatment outcome expectations may be associated with worse adherence. Higher self-efficacy or confidence in the ability to use CPAP when faced with difficulties can improve adherence and positive effect.36 CPAP adherence has also been associated with engagement in active coping strategies with new and difficult situations.37
Despite the influence of these psychological factors, depression and anxiety do not seem to influence CPAP adherence significantly, and no association was demonstrated between the Hospital Anxiety and Depression scale and CPAP use.38 Claustrophobia, however, may be associated with poor adherence. A 15-item subscale measuring claustrophobic tendencies was measured pre-CPAP and after 3 months of CPAP in a secondary analysis of data from a prospective study of 153 OSA participants that completed 3 months of CPAP therapy.39 Poor CPAP adherence (<2 h per night) was more than two times higher in participants with a claustrophobia score of ≥25. Identification of patients with claustrophobic tendencies and targeted interventions designed to reduce the fear and intrusiveness of SDB therapies may be beneficial. Our clinical experience with modern masks including nasal pillows suggests that claustrophobia may be less of an issue in 2018 than in the past years.
Cognitive perception has been shown to influence PAP use, emphasizing the importance of education in patient formulation of accurate and realistic perceptions and expectations, both regarding SDB and treatments.7 Knowledge and support are likely of greater importance in those with less background education as discussed above. Patient education is recognized as a standard of care in the treatment of SDB.40 Despite this recognition, studies investigating the effects of educational strategies alone on PAP adherence have been mixed and generally shown minimal effect.41 The heterogeneity of the educational strategies used makes generalizations difficult and many do not measure the mediating variable of knowledge. Meurice et al. studied the effects of four educational strategies on CPAP compliance in 112 severe OSA patients in seven centres in the French ANTADIR homecare network.42 Patients received either a simple oral explanation or an oral and written explanation of CPAP use. In addition, they received, from homecare technicians, either a single home visit at CPAP onset, or repeated home visits at CPAP onset and at 1 week, 1 month and 3 months after. There was no significant difference in adherence between all four education groups. In contrast, Lai et al. randomized 100 OSA patients to a brief motivational enhancement education programme or usual care.43 The intervention group received usual care plus a brief motivational education programme directed at enhancing the subjects’ knowledge, motivation and self-efficacy to CPAP through the use of a 25-min video, a 20-min patient-centred interview and a 10-min telephone follow-up. The intervention group had better CPAP use (higher daily CPAP usage by 2 h per day (P < 0.001)) and a fourfold increase in the number of patients using CPAP for ≥70% of days with ≥4 h/day. These studies demonstrate the differences and frequent overlap in interventions. The equivalent amount of education and support employed as the control in one study may serve as the intervention in another, limiting interpretation of educational strategies on PAP adherence.8 More recently, with technological advances, there has been interest in alternative platforms for delivery of educational materials including video as discussed below. Guralnick et al. randomized 212 patients referred to a sleep laboratory that served a predominantly minority population for suspected OSA to view an educational video about OSA and CPAP therapy before the sleep study or to usual care.44 No difference in CPAP adherence was observed at 30 days.
Greater success has been described with cognitive behavioural or motivational strategies in improving adherence.35,45–51 These behavioural strategies again recognize the psychosocial influences on PAP adherence. In a meta-analysis assessing the effectiveness of educational, supportive or behavioural strategies in encouraging CPAP use, most studies incorporated elements of more than one intervention.48 Low-to moderate-quality evidence showed that all three types of interventions led to increased CPAP usage in CPAP-naive participants with moderate to severe OSA. Behavioural therapies led to improvement in average CPAP usage by 1.44h/night (95% CI: 0.43–2.45, n = 584, six studies: low-quality evidence) and increased the number of participants who used their machines for longer than 4 h per nights from 28 to 47 per 100 (OR: 2.23, 95% CI: 1.45–3.45, n = 358, 3 studies, low-quality evidence). These behavioural strategies have been shown to have greater effect size than support or educational interventions, but studies on behavioural strategies are again limited by heterogeneity of interventions with most interventions being a composite of various strategies.41 Similar to educational strategies, technologies are also being incorporated into behavioural strategies, discussed below. Cognitive behavioural therapies may increase CPAP use by improving self-efficacy and social support.51
Social support has been shown to have a positive influence on adherence in a few studies.8 Lewis et al. found CPAP use was higher in those living with someone as compared with those living alone.38 SDB treatments may raise concerns among patients regarding less intimacy with their bed partner and discussions regarding these concerns may influence adherence.30 In a study assessing the impact of OSA and CPAP treatment on patients’ partners, reported change in the partners’ sleep quality between pre-sleep study and CPAP treatment correlated positively with CPAP use (r = 0.5, P = 0.01).52 Baron et al. also found that perception of wives’ support for CPAP treatment predicted increased adherence, but only in patients with high disease severity.36 Spousal pressure to use CPAP was not found to be beneficial for adherence.
In completing the biopsychosocial model and returning to biological predictors of PAP, nasal patency is a potential patient target to improve PAP adherence. PAP devices rely on delivery of pressurized air via flexible tubing connecting to an external mask that interfaces with the patient. While various types of interfaces exist, nasal patency and resistance as measured using various techniques including active anterior rhino-manometry and acoustic rhinometry have been shown to have a significant effect on PAP adherence.53–56 CPAP use is lower in patients with smaller nasal passages, and nasal congestion has been associated with a decrease in mean daily CPAP use.53,54 Addressing nasal patency, often with the input of an otolaryngologist, is one strategy to improve PAP adherence. If symptoms persist despite medical therapies, surgery may be required.57,58 Surgical correction of severe nasal obstruction in 12 patients with severe OSA refractory to CPAP treatment resulted in a significant decrease in nasal resistance and rendered all patients tolerant to CPAP.58 In fact, some otolaryngologists have suggested that nasal septoplasty to facilitate PAP adherence is the most common surgery for OSA.
Treatment protocols
Early adoption of CPAP use has been associated with long-term adherence and consequently the initial period of CPAP prescription and initiation has been a target of interest.8,10,12 PAP can now be introduced in-laboratory or at home using auto-titrating PAP technologies. Several randomized trials have compared home testing with auto-titrating PAP to traditional CPAP prescription following in-laboratory diagnostic and titration polysomnography. In patients with a high pretest probability of moderate to severe OSA without major co-morbid medical conditions, this ambulatory model of care can provide excellent results, comparable to the traditional model.59–61 A meta-analysis and meta-regression of nine randomized trials studying a total of 282 patients found auto-titrating PAP was associated with a reduction in the mean applied pressure across the night by 2.2 cm H2O compared to CPAP and with similar adherence.62 Other investigators have suggested greater comfort and improved adherence with auto-titrating CPAP in patients requiring CPAP levels higher than 10 cm H2O and in patients reporting side effects on conventional CPAP.63,64 Reporting problems after the first night of CPAP has been shown to be an important predictor of ensuing CPAP use.38 In a population-based comprehensive CPAP programme utilizing daily telephone contact within the first week, ‘troubleshooting’ and regular feedback to both patients and physicians was shown to achieve CPAP compliance rates >85% over 6 months, again emphasizing the potential importance of early interventions in improving adherence.65
Others have assessed the use of a sedative-hypnotic early on during CPAP initiation in improving long-term PAP adherence with mixed results.66–70 Both the approach of using a single sedative dose, typically a Z-hypnotic, prior to split-night or titration polysomnography and regular use of a sedative for the first 14 days of CPAP therapy have been studied, but again no consistent improvement in adherence has been shown.
Following treatment initiation, the American Thoracic Society has recommended measuring outcomes 1 week, 4–6 weeks, 12 weeks, 6 months, 1 year after and then yearly monitoring thereafter.17 However, access to sleep providers remains limited and in reality follow-up monitoring is, at least in the USA, heavily influenced by third-party payers’ reimbursement criteria for PAP and OA devices. The arbitrary 31–90-day follow-up requirement imposed by CMS for PAP as discussed above may result in adverse consequences for some patients, adding inconvenience and expense for patients. Anecdotally, we feel that the fear of costs related to not meeting CMS reimbursement criteria has become a major determinant of PAP and OA acceptance and adherence, potentially improving adherence. Moreover, patients of disadvantaged socioeconomic status, unmarried and those with psychiatric disease may have difficulty meeting these reimbursement criteria, potentially resulting in unintended discrimination in provision of healthcare services.71
While follow-up is necessary in ensuring treatment adherence, the frequency, intensity and modality of follow-up to optimize adherence remain unknown. Adherence during the week prior to a clinic visit has been shown to be higher than the average adherence during the 2-month period prior to the clinic visit, suggesting importance of regular follow-up.53 Follow-up visits allow for identification and troubleshooting of treatment side effects. As discussed, studies on follow-up are now incorporating educational and behavioural interventions but again these interventions vary in specific constructs.
Novel technologies including smartphones are also increasingly being studied in treatment protocols, fostering active patient engagement (APE) and accountability, but whether these strategies result in improved adherence remains to be determined. PAP devices have the capability of displaying the previous night’s usage and providing direct feedback. Cloud-based platforms receive regular data updates from PAP machines and allow for real-time monitoring of adherence by providers. Malhotra et al. compared APE technology, a real-time internet-based patient engagement tool, to usual care monitoring in a retrospective analysis of two cloud-based databases (AirView and myAir).9 In 128 037 patients, APE was associated with more patients achieving adherence defined by US Medicare criteria compared to usual care with remote monitoring of PAP adherence (87.3% compared to 70.4%). Average therapy usage was 5.9 h in the APE group versus 4.9 h in the matched usual care group and patients ‘struggling’ with CPAP therapy adherence had a 17.6% absolute improvement in adherence using APE compared with usual care.
Technological advances and the availability of wireless capabilities and cloud-based databases in transferring data have also placed sleep medicine in a unique position for adopting telemedicine. Studies using telemedicine have shown mixed results on PAP adherence.72–77 In a four-arm randomized, factorial design clinical trial of 1455 patients referred for suspected OSA (Tele-OSA), two telemedicine interventions were implemented: (i) web-based OSA education (Tel-Ed) and (ii) CPAP telemonitoring with automated patient-messaging feedback (Tel-TM).73 Patients were randomized to (i) usual care, (ii) Tel-Ed added, (iii) Tel-TM added or (iv) Tel-Ed and Tel-TM (Tel-both). Average daily CPAP use at 90 days was 3.8 ± 2.5, 4.0 ± 2.4, 4.4 ± 2.2 and 4.8 ± 2.3 h in usual care, Tel-Ed, Tel-TM and Tel-both groups, respectively. Usage was significantly higher in the Tel-TM and Tel-both groups versus usual care but not for Tel-Ed (P = 0.10). Similar to prior studies on education, this study found that even with a telemedicine platform, education alone had no significant influence on PAP use and it again suggested that accountability may be more effective at inducing changes in adherence behaviour. Tele-medicine was less expensive than standard management, suggesting ongoing research on advanced technologies in SDB should be encouraged.
Technological factors
Side effects are not uncommon with PAP (and oral appliances) and technological advances are attempting to address these side effects. Few added technological features, however, have been shown to improve adherence, and in fact while it seems intuitive, side effects have not been shown to impact adherence significantly.7 Nonetheless, it is recommended that treatment side effects be identified and addressed, including both mask- and air pressure-related side effects.
Mask-related side effects include poor fit with leak, skin pressure and irritation, claustrophobia, dry mouth and nasal congestion. It does not appear that the PAP mask interface at treatment initiation significantly influences adherence but there are now a wide variety of interfaces with few studies directly comparing them.7,78 Heated humidification was developed to try to minimize dryness but the evidence to date does not consistently support improved adherence with the addition of this feature.79,80 Heated humidification may reduce symptoms of dry nose, mouth and throat, and should be individualized. Condensation in the tubing or rainout can be minimized using heated tubing or tube covers to reduce exposure of the tubing air to the cooler surrounding environment.
Pressure-related discomfort has led to the development of expiratory pressure relief technologies (e.g. C-flex in Respironics [Murrysville, Pennsylvania, USA], EPR in Resmed [San Diego, California, USA]), which reduces airway pressure during early expiration with a return to the prescribed pressure at the end of expiration to varying degrees. It too has not been shown to improve adherence reliably.81–84 The ramp feature reduces the initial PAP level, and then gradually increases the pressure over a set time period to the prescribed target, but no improvement in adherence has been shown with the addition of the ramp. Again, studies comparing auto-titrating units to traditional CPAP units have shown similar adherence. In 62 OSA patients randomized to CPAP or bilevel PAP, there was no significant difference between hourly use, the percentage of time that the device was running and the prescribed pressure that was being delivered at 1 year. Thus, bilevel PAP cannot be routinely recommended as a strategy to improvement adherence in OSA.85
FUTURE DIRECTIONS
Our vision for the future is that patients would undergo diagnostic testing using a wearable technology and/or blood biomarker, and these data would be used to assess disease severity and predict risk of complications. In some patients, administration of auto-titrating PAP with follow-up as needed may be sufficient. In such cases, remote monitoring via cloud may be sufficient if patients were empowered to call for help when needed. In other patients who may struggle with the interface or other issues, troubleshooting could be offered via telemedicine or face-to-face via a nurse practitioner or respiratory therapist. This approach would allow sleep specialists to focus on the most complex patients who are struggling with therapy or who have major co-morbidities that need to be addressed. We strongly believe that innovative solutions using technology to advantage will be required to deliver care to the estimated 1 billion OSA patients worldwide. More data are required to determine optimal management of OSA.
CONCLUSION
Strategies are necessary to improve adherence to non-surgical OSA treatment modalities, namely PAP. While the current definition of adherence is mandated by CMS to determine reimbursement, in the future patients may benefit from more individualized treatment targets with the goal of reducing risk of OSA consequences on a patient-to-patient basis. It seems clear that certain patient characteristics (e.g. psychological traits) and disease phenotypes (e.g. degree of EDS) impact PAP adherence and as such are important factors for the treating physician to note in assessing a patient’s risk of non-adherence. In initiating treatment, more support seems superior to less, and although studies are difficult to compare, OSA centres should prioritize prescribing a support regimen based on available data and available resources early in the course of a treatment trial. Development of new technologies, specifically those that give direct patient feedback, is ongoing and will hopefully improve adherence in motivated patients. Overall, given a multitude of available options with more in the pipeline, clinicians should be optimistic about achieving adherence to OSA therapies in their patients.
Abbreviations:
- APE
active patient engagement
- CMS
Centers Medicare and Medicaid Services
- CPAP
continuous PAP
- MAD
mandibular advancement device
- OR
odds ratio
- OSA
obstructive sleep apnoea
- PAP
positive airway pressure
- SDB
sleep-disordered breathing
REFERENCES
- 1.Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, Kader G, Mahowald M, Younger J, Pack AI. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007; 30: 711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antic NA, Catcheside P, Buchan C, Hensley M, Naughton MT, Rowland S, Williamson B, Windler S, McEvoy RD. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep 2011; 34: 111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, Redline S, Henry JN, Getsy JE, Dinges DF. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am. Rev. Respir. Dis. 1993; 147: 887–95. [DOI] [PubMed] [Google Scholar]
- 4.Reeves-Hoche MK, Meck R, Zwillich CW. Nasal CPAP: an objective evaluation of patient compliance. Am. J. Respir. Crit. Care Med. 1994; 149: 149–54. [DOI] [PubMed] [Google Scholar]
- 5.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am. J. Respir. Crit. Care Med. 2001; 163: 344–8. [DOI] [PubMed] [Google Scholar]
- 6.Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J. Clin. Sleep Med. 2013; 9: 767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med. Rev. 2011; 15: 343–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc. Am. Thorac. Soc. 2008; 5: 173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV. Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis. Chest 2018; 153: 843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Budhiraja R, Parthasarathy S, Drake CL, Roth T, Sharief I, Budhiraja P, Saunders V, Hudgel DW. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep 2007; 30: 320–4. [PubMed] [Google Scholar]
- 11.Krieger J Long-term compliance with nasal continuous positive airway pressure (CPAP) in obstructive sleep apnea patients and nonapneic snorers. Sleep 1992; 15: S42–6. [DOI] [PubMed] [Google Scholar]
- 12.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am. J. Respir. Crit. Care Med. 1999; 159: 1108–4. [DOI] [PubMed] [Google Scholar]
- 13.Bachour P, Bachour A, Kauppi P, Maasilta P, Makitie A, Palotie T. Oral appliance in sleep apnea treatment: respiratory and clinical effects and long-term adherence. Sleep Breath. 2016; 20: 805–12. [DOI] [PubMed] [Google Scholar]
- 14.Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, Chervin RD. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with Oral appliance therapy: an update for 2015. J. Clin. Sleep Med. 2015; 11: 773–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cistulli PA, Gotsopoulos H, Marklund M, Lowe AA. Treatment of snoring and obstructive sleep apnea with mandibular repositioning appliances. Sleep Med. Rev. 2004; 8: 443–57. [DOI] [PubMed] [Google Scholar]
- 16.Saglam-Aydinatay B, Taner T. Oral appliance therapy in obstructive sleep apnea: long-term adherence and patients experiences. Med. Oral Patol. Oral Cir. Bucal 2018; 23: e72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwab RJ, Badr SM, Epstein LJ, Gay PC, Gozal D, Kohler M, Levy P, Malhotra A, Phillips BA, Rosen IM et al. ; ATS Subcommittee on CPAP Adherence Tracking Systems. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am. J. Respir. Crit. Care Med. 2013; 188: 613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rauscher H, Formanek D, Popp W, Zwick H. Self-reported vs measured compliance with nasal CPAP for obstructive sleep apnea. Chest 1993; 103: 1675–80. [DOI] [PubMed] [Google Scholar]
- 19.Gjerde K, Lehmann S, Naterstad IF, Berge ME, Johansson A. Reliability of an adherence monitoring sensor embedded in an oral appliance used for treatment of obstructive sleep apnoea. J. Oral Rehabil. 2018; 45: 110–5. [DOI] [PubMed] [Google Scholar]
- 20.Castillo Y, Blanco-Almazan D, Whitney J, Mersky B, Jane R. Characterization of a tooth microphone coupled to an oral appliance device: a new system for monitoring OSA patients. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2017; 2017: 1543–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS). Sleep Med. Rev. 2003; 7: 81–99. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen AR, Eriksen F, Hansen RW, Erlandsen M, Thorup L, Damgard MB, Kirkegaard MG, Hansen KW. Determinants for adherence to continuous positive airway pressure therapy in obstructive sleep apnea. PLoS One 2017; 12: e0189614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawyer AM, Deatrick JA, Kuna ST, Weaver TE. Differences in perceptions of the diagnosis and treatment of obstructive sleep apnea and continuous positive airway pressure therapy among adherers and nonadherers. Qual. Health Res. 2010; 20: 873–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoshino T, Sasanabe R, Tanigawa T, Murotani K, Arimoto M, Ueda H, Shiomi T. Effect of rapid eye movement-related obstructive sleep apnea on adherence to continuous positive airway pressure. J. Int. Med. Res. 2018; 46: 2238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conwell W, Patel B, Doeing D, Pamidi S, Knutson KL, Ghods F, Mokhlesi B. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath. 2012; 16: 519–26. [DOI] [PubMed] [Google Scholar]
- 26.Wells RD, Freedland KE, Carney RM, Duntley SP, Stepanski EJ. Adherence, reports of benefits, and depression among patients treated with continuous positive airway pressure. Psychosom. Med. 2007; 69: 449–54. [DOI] [PubMed] [Google Scholar]
- 27.Billings ME, Auckley D, Benca R, Foldvary-Schaefer N, Iber C, Redline S, Rosen CL, Zee P, Kapur VK. Race and residential socioeconomics as predictors of CPAP adherence. Sleep 2011; 34: 1653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joo MJ, Herdegen JJ. Sleep apnea in an urban public hospital: assessment of severity and treatment adherence. J. Clin. Sleep Med. 2007; 3: 285–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Scharf SM, Seiden L, DeMore J, Carter-Pokras O. Racial differences in clinical presentation of patients with sleep-disordered breathing. Sleep Breath. 2004; 8: 173–83. [DOI] [PubMed] [Google Scholar]
- 30.Ye L, Pack AI, Maislin G, Dinges D, Hurley S, McCloskey S, Weaver TE. Predictors of continuous positive airway pressure use during the first week of treatment. J. Sleep Res. 2012; 21: 419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakker JP, O’Keeffe KM, Neill AM, Campbell AJ. Ethnic disparities in CPAP adherence in New Zealand: effects of socioeconomic status, health literacy and self-efficacy. Sleep 2011; 34: 1595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Platt AB, Field SH, Asch DA, Chen Z, Patel NP, Gupta R, Roche DF, Gurubhagavatula I, Christie JD, Kuna ST. Neighborhood of residence is associated with daily adherence to CPAP therapy. Sleep 2009; 32: 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brostrom A, Stromberg A, Martensson J, Ulander M, Harder L, Svanborg E. Association of Type D personality to perceived side effects and adherence in CPAP-treated patients with OSAS. J. Sleep Res. 2007; 16: 439–47. [DOI] [PubMed] [Google Scholar]
- 34.Dieltjens M, Vanderveken OM, Van den Bosch D, Wouters K, Denollet J, Verbraecken JA, Van de Heyning PH, Braem MJ. Impact of type D personality on adherence to oral appliance therapy for sleep-disordered breathing. Sleep Breath. 2013; 17: 985–91. [DOI] [PubMed] [Google Scholar]
- 35.Hoy CJVM, Kingshott R, Engleman HM, Douglas NJ. Can intensive support improve continuous positive airway pressure use in patients with the sleep apnea/hypopnea syndrome? Am. J. Respir. Crit. Care Med. 1999; 159: 1096–100. [DOI] [PubMed] [Google Scholar]
- 36.Baron KG, Smith TW, Berg CA, Czajkowski LA, Gunn H, Jones CR. Spousal involvement in CPAP adherence among patients with obstructive sleep apnea. Sleep Breath. 2011; 15: 525–34. [DOI] [PubMed] [Google Scholar]
- 37.Stepnowsky CJ Jr, Bardwell WA, Moore PJ, Ancoli-Israel S, Dimsdale JE. Psychologic correlates of compliance with continuous positive airway pressure. Sleep 2002; 25: 758–62. [DOI] [PubMed] [Google Scholar]
- 38.Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep 2004; 27: 134–8. [DOI] [PubMed] [Google Scholar]
- 39.Chasens ER, Pack AI, Maislin G, Dinges DF, Weaver TE. Claustrophobia and adherence to CPAP treatment. West. J. Nurs. Res. 2005; 27: 307–21. [DOI] [PubMed] [Google Scholar]
- 40.Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM et al. ; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009; 5: 263–76. [PMC free article] [PubMed] [Google Scholar]
- 41.Crawford MR, Espie CA, Bartlett DJ, Grunstein RR. Integrating psychology and medicine in CPAP adherence - new concepts? Sleep Med. Rev. 2014; 18: 123–39. [DOI] [PubMed] [Google Scholar]
- 42.Meurice JC, Ingrand P, Portier F, Arnulf I, Rakotonanahari D, Fournier E, Philip-Joet F, Veale D; ANTADIR Working Group “PPC”, CMTS ANTADIR. A multicentre trial of education strategies at CPAP induction in the treatment of severe sleep apnoea-hypopnoea syndrome. Sleep Med. 2007; 8: 37–42. [DOI] [PubMed] [Google Scholar]
- 43.Lai AYK, Fong DYT, Lam JCM, Weaver TE, Ip MSM. The efficacy of a brief motivational enhancement education program on CPAP adherence in OSA: a randomized controlled trial. Chest 2014; 146:600–10. [DOI] [PubMed] [Google Scholar]
- 44.Guralnick AS, Balachandran JS, Szutenbach S, Adley K, Emami L, Mohammadi M, Farnan JM, Arora VM, Mokhlesi B. Educational video to improve CPAP use in patients with obstructive sleep apnoea at risk for poor adherence: a randomised controlled trial. Thorax 2017; 72: 1132–9. [DOI] [PubMed] [Google Scholar]
- 45.Aloia MS, Di Dio L, Ilniczky N, Perlis ML, Greenblatt DW, Giles DE. Improving compliance with nasal CPAP and vigilance in older adults with OAHS. Sleep Breath. 2001; 5: 13–21. [DOI] [PubMed] [Google Scholar]
- 46.Aloia MS, Smith K, Arnedt JT, Millman RP, Stanchina M, Carlisle C, Hecht J, Borrelli B. Brief behavioral therapies reduce early positive airway pressure discontinuation rates in sleep apnea syndrome: preliminary findings. Behav. Sleep Med. 2007; 5: 89–104. [DOI] [PubMed] [Google Scholar]
- 47.Sawyer AM, King TS, Weaver TE, Sawyer DA, Varrasse M, Franks J, Watach A, Kolanowski AM, Richards KC. A tailored intervention for PAP adherence: the SCIP-PA trial. Behav. Sleep Med. 2019; 17: 49–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wozniak DR, Lasserson TJ, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst. Rev. 2014; (1): CD007736. [DOI] [PubMed] [Google Scholar]
- 49.Bartlett D, Wong K, Richards D, Moy E, Espie CA, Cistulli PA, Grunstein R. Increasing adherence to obstructive sleep apnea treatment with a group social cognitive therapy treatment intervention: a randomized trial. Sleep 2013; 36: 1647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pengo MF, Czaban M, Berry MP, Nirmalan P, Brown R, Birdseye A, Woroszyl A, Chapman J, Kent BD, Hart N et al. The effect of positive and negative message framing on short term continuous positive airway pressure compliance in patients with obstructive sleep apnea. J. Thorac. Dis. 2018; 10: S160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richards D, Bartlett DJ, Wong K, Malouff J, Grunstein RR. Increased adherence to CPAP with a group cognitive behavioral treatment intervention: a randomized trial. Sleep 2007; 30: 635–40. [DOI] [PubMed] [Google Scholar]
- 52.McArdle N, Kingshott R, Engleman HM, Mackay TW, Douglas NJ. Partners of patients with sleep apnoea/hypopnoea syndrome: effect of CPAP treatment on sleep quality and quality of life. Thorax 2001; 56: 513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Budhiraja R, Kushida CA, Nichols DA, Walsh JK, Simon RD, Gottlieb DJ, Quan SF. Impact of randomization, clinic visits, and medical and psychiatric cormorbidities on continuous positive airway pressure adherence in obstructive sleep apnea. J. Clin. Sleep Med. 2016; 12: 333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li HY, Engleman H, Hsu CY, Izci B, Vennelle M, Cross M, Douglas NJ. Acoustic reflection for nasal airway measurement in patients with obstructive sleep apnea-hypopnea syndrome. Sleep 2005; 28: 1554–9. [DOI] [PubMed] [Google Scholar]
- 55.Sugiura T, Noda A, Nakata S, Yasuda Y, Soga T, Miyata S, Nakai S, Koike Y. Influence of nasal resistance on initial acceptance of continuous positive airway pressure in treatment for obstructive sleep apnea syndrome. Respiration 2007; 74: 56–60. [DOI] [PubMed] [Google Scholar]
- 56.Morris LG, Setlur J, Burschtin OE, Steward DL, Jacobs JB, Lee KC. Acoustic rhinometry predicts tolerance of nasal continuous positive airway pressure: a pilot study. Am. J. Rhinol. 2006; 20: 133–7. [PubMed] [Google Scholar]
- 57.Fiorita A, Scarano E, Mastrapasqua R, Picciotti PM, Loperfido A, Rizzotto G, Paludetti G. Moderate OSAS and turbinate decongestion: surgical efficacy in improving the quality of life and compliance of CPAP using Epworth score and SNOT-20 score. Acta Otorhinolaryngol. Ital. 2018; 38: 214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakata S, Noda A, Yagi H, Yanagi E, Mimura T, Okada T, Misawa H, Nakashima T. Nasal resistance for determinant factor of nasal surgery in CPAP failure patients with obstructive sleep apnea syndrome. Rhinology 2005; 43: 296–9. [PubMed] [Google Scholar]
- 59.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, Hudgel D, Sateia M, Schwab R; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2007; 3: 737–47. [PMC free article] [PubMed] [Google Scholar]
- 60.Morgenthaler TI, Aurora RN, Brown T, Zak R, Alessi C, Boehlecke B, Chesson AL Jr, Friedman L, Kapur V, Maganti R et al. ; Standards of Practice Committee of the AASM; American Academy of Sleep Medicine. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep 2008; 31: 141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sunwoo B, Kuna ST. Ambulatory management of patients with sleep apnea: is there a place for portable monitor testing? Clin. Chest Med. 2010; 31: 299–308. [DOI] [PubMed] [Google Scholar]
- 62.Ayas NT, Patel SR, Malhotra A, Schulzer M, Malhotra M, Jung D, Fleetham J, White DP. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep 2004; 27: 249–53. [DOI] [PubMed] [Google Scholar]
- 63.Hukins C Comparative study of autotitrating and fixed-pressure CPAP in the home: a randomized, single-blind crossover trial. Sleep 2004; 27: 1512–7. [DOI] [PubMed] [Google Scholar]
- 64.Massie CA, Hart RW. Clinical outcomes related to interface type in patients with obstructive sleep apnea/hypopnea syndrome who are using continuous positive airway pressure. Chest 2003; 123: 1112–8. [DOI] [PubMed] [Google Scholar]
- 65.Sin DD, Mayers I, Man GC, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest 2002; 121: 430–5. [DOI] [PubMed] [Google Scholar]
- 66.Bradshaw DA, Ruff GA, Murphy DP. An oral hypnotic medication does not improve continuous positive airway pressure compliance in men with obstructive sleep apnea. Chest 2006; 130: 1369–76. [DOI] [PubMed] [Google Scholar]
- 67.Lettieri CJ, Collen JF, Eliasson AH, Quast TM. Sedative use during continuous positive airway pressure titration improves subsequent compliance: a randomized, double-blind, placebo-controlled trial. Chest 2009; 136: 1263–8. [DOI] [PubMed] [Google Scholar]
- 68.Lettieri CJ, Shah AA, Holley AB, Kelly WF, Chang AS, Roop SA; CPAP Promotion and Prognosis-The Army Sleep Apnea Program Trial. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann. Intern. Med. 2009; 151: 696–702. [DOI] [PubMed] [Google Scholar]
- 69.Park JG, Olson EJ, Morgenthaler TI. Impact of zaleplon on continuous positive airway pressure therapy compliance. J. Clin. Sleep Med. 2013; 9: 439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holley AB, Londeree WA, Sheikh KL, Andrada TF, Powell TA, Khramtsov A, Hostler JM. Zolpidem and eszopiclone premedication for PSG: effects on staging, titration, and adherence. Mil. Med. 2018; 183: e251–6. [DOI] [PubMed] [Google Scholar]
- 71.Brown LK. Adherence-based coverage of positive airway pressure treatment for sleep apnea: the ‘brave new world’ of cost-saving strategies. Curr. Opin. Pulm. Med. 2011; 17: 403–5. [DOI] [PubMed] [Google Scholar]
- 72.DeMolles DA, Sparrow D, Gottlieb DJ, Friedman R. A pilot trial of a telecommunications system in sleep apnea management. Med. Care 2004; 42: 764–9. [DOI] [PubMed] [Google Scholar]
- 73.Hwang D, Chang JW, Benjafield AV, Crocker ME, Kelly C, Becker KA, Kim JB, Woodrum RR, Liang J, Derose SF. Effect of telemedicine education and telemonitoring on continuous positive airway pressure adherence. The tele-OSA randomized trial. Am. J. Respir. Crit. Care Med. 2018; 197: 117–26. [DOI] [PubMed] [Google Scholar]
- 74.Sparrow D, Aloia M, Demolles DA, Gottlieb DJ. A telemedicine intervention to improve adherence to continuous positive airway pressure: a randomised controlled trial. Thorax 2010; 65: 1061–6. [DOI] [PubMed] [Google Scholar]
- 75.Stepnowsky CJ, Palau JJ, Marler MR, Gifford AL. Pilot randomized trial of the effect of wireless telemonitoring on compliance and treatment efficacy in obstructive sleep apnea. J. Med. Internet Res. 2007; 9: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turino C, de Batlle J, Woehrle H, Mayoral A, Castro-Grattoni AL, Gomez S, Dalmases M, Sanchez-de-la-Torre M, Barbe F. Management of continuous positive airway pressure treatment compliance using telemonitoring in obstructive sleep apnoea. Eur. Respir. J. 2017; 49: pii: 1601128. [DOI] [PubMed] [Google Scholar]
- 77.Mendelson M, Vivodtzev I, Tamisier R, Laplaud D, Dias-Domingos S, Baguet JP, Moreau L, Koltes C, Chavez L, De Lamberterie G et al. CPAP treatment supported by telemedicine does not improve blood pressure in high cardiovascular risk OSA patients: a randomized, controlled trial. Sleep 2014; 37: 1863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chai CL, Pathinathan A, Smith B. Continuous positive airway pressure delivery interfaces for obstructive sleep apnoea. Cochrane Database Syst. Rev. 2006; (4): CD005308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mador MJ, Krauza M, Pervez A, Pierce D, Braun M. Effect of heated humidification on compliance and quality of life in patients with sleep apnea using nasal continuous positive airway pressure. Chest 2005; 128: 2151–8. [DOI] [PubMed] [Google Scholar]
- 80.Neill AM, Wai HS, Bannan SP, Beasley CR, Weatherall M, Campbell AJ. Humidified nasal continuous positive airway pressure in obstructive sleep apnoea. Eur. Respir. J. 2003; 22: 258–62. [DOI] [PubMed] [Google Scholar]
- 81.Aloia MS, Stanchina M, Arnedt JT, Malhotra A, Millman RP. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest 2005; 127: 2085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dolan DC, Okonkwo R, Gfullner F, Hansbrough JR, Strobel RJ, Rosenthal L. Longitudinal comparison study of pressure relief (C-Flex) vs CPAP in OSA patients. Sleep Breath. 2009; 13: 73–7. [DOI] [PubMed] [Google Scholar]
- 83.Pepin JL, Muir JF, Gentina T, Dauvilliers Y, Tamisier R, Sapene M, Escourrou P, Fleury B, Philip-Joet F, Philip P et al. Pressure reduction during exhalation in sleep apnea patients treated by continuous positive airway pressure. Chest 2009; 136: 490–7. [DOI] [PubMed] [Google Scholar]
- 84.Bakker JP, Marshall NS. Flexible pressure delivery modification of continuous positive airway pressure for obstructive sleep apnea does not improve compliance with therapy: systematic review and meta-analysis. Chest 2011; 139: 1322–30. [DOI] [PubMed] [Google Scholar]
- 85.Reeves-Hoche MK, Hudgel DW, Meck R, Witteman R, Ross A, Zwillich CW. Continuous versus bilevel positive airway pressure for obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1995; 151: 443–9. [DOI] [PubMed] [Google Scholar]


