Abstract
Background:
Sepsis triggers dysfunction of coagulation and fibrinolytic systems leading to disseminated intravascular coagulation (DIC) that contributes to organ failure and death. Fondaparinux (FPX) is a synthetic pentasaccharide that binds to antithrombin (AT) and selectively inhibits factor (F) Xa and other upstream coagulation proteases but not thrombin (T).
Objectives:
We used a baboon model of lethal E. coli sepsis to investigate the effects of FPX treatment on DIC, organ function and outcome.
Methods:
Two experimental groups were studied: (i) E. coli challenge (n=4) and (ii) E. coli plus FPX (n=4). Bacteremia was modeled by intravenous infusion of pathogen (1–2 × 1010 cfu/kg). FPX (0.08 mg/kg) was administered subcutaneously, 3 hours prior to and 8 hours after bacteria infusion.
Results:
Bacteremia rapidly increased plasma levels of inhibitory complexes of AT with coagulation proteases. Activation markers of both intrinsic (FXIa-AT), and extrinsic (FVIIa-AT) pathways were significantly reduced in FPX treated animals. FXa-AT and TAT complexes were maximal at 4–8 hours post-challenge and reduced >50% in FPX-treated animals. Fibrinogen consumption, fibrin generation and degradation, neutrophil and complement activation, and cytokine production were strongly induced by sepsis. All above parameters were significantly reduced, while platelet count was unchanged by the treatment. FPX infusion attenuated organ dysfunction, prolonged survival and saved 2 out of 4 challenged animals (log-rank Mantel-Cox test, P=0.0067).
Conclusion:
Our data indicate that FPX mediated inhibition of coagulation prevents sepsis coagulopathy, protects against excessive complement activation, inflammation, organ dysfunction, and provides survival benefit in E. coli sepsis.
Keywords: disseminated intravascular coagulation, E. coli, non-human primates, pentasaccharide, sepsis
INTRODUCTION
Sepsis is a multistage, multi-factorial disease and major cause of morbidity and mortality worldwide. Despite the advancement in antibiotic therapy and supportive care, mortality rates of 30–50% have been reported in intensive care units[1]. Bacterial sepsis is associated with robust activation of host inflammatory response and plasma protease cascades such as coagulation, fibrinolysis and complement. Pathogens or pathogen associated molecular patterns (PAMPs) like lipopolysaccharides (LPS) and peptidoglycans could initiate the activation of these enzymatic cascades. While local activation of coagulation is considered beneficial to limit the spread of the pathogens, systemic activation of procoagulant mechanisms coupled with simultaneous down-regulation of natural anticoagulants and impaired fibrinolysis can lead to microvascular thrombosis with consumption of multiple clotting factors, resulting in disseminated intravascular coagulation (DIC) that can sequentially promote ischemia, tissue damage, multiple organ failure and finally death[2]. Since DIC is ubiquitous, systemic anticoagulation was considered for many years as a possible sepsis therapy but was not universally accepted due to increased risk of bleeding and possible interference with bacteria clearance[3].
Sepsis decreases the main three anticoagulant mechanisms, tissue factor pathway inhibitor (TFPI), thrombomodulin (TM) -protein C (PC) and antithrombin (AT), and anticoagulant replenishment was tested in multiple preclinical and clinical trials. While treatments with TFPI[4], AT[5, 6] and activated PC (APC)[7] showed some promise in preclinical studies, all ultimately failed in clinical trials[8–10] due to bleeding, despite showing benefit in subsets of patients with high risk of death. Similarly, anticoagulation with unfractionated or low molecular weight (LMW) heparins that promote mainly AT-mediated thrombin inhibition did not provide survival benefit in septic patients[11]. Beside the bleeding risk associated with all anticoagulants, heparins also have other detrimental effects, including heparin-induced thrombocytopenia (HIT) and allergic reactions. Heparin administration in patients treated with AT[9] or TFPI[10] reduced the efficacy of each experimental drug, suggesting that inhibition of thrombin may not be the best strategy to treat sepsis because the thrombin-TM system is necessary for generation of APC, a major antithrombotic, anti-inflammatory and cytoprotective molecule[12]. Unfortunately, many decades and tens of failed trials later, there is still no FDA approved antithrombotic treatment for sepsis DIC.
Here, we hypothesized that inhibition of coagulation at factor (F) Xa level could prevent sepsis DIC and provide survival benefit in Gram negative sepsis. For this purpose we used our established model of E. coli bacteremia in baboons[13] to test the protective effects of the synthetic pentasaccharide fondaparinux (FPX), which binds to AT and enhances FXa inhibition by 300-fold[14] without inhibiting thrombin[15]. The main endpoints of the study were the effect of FPX treatment on disease biomarkers, clinical condition and survival benefit. Our study provides proof-of-principle that FXa inhibition by FPX can decrease DIC, excessive complement activation, inflammation and multiple organ dysfunction, and improve survival in animals challenged with lethal doses of E. coli.
METHODS
Baboon model of E. coli sepsis
The study protocol received prior approvals from the Institutional Animal Care and Use Committees of both the Oklahoma Medical Research Foundation and the University of Oklahoma Health Sciences Center and it was performed in compliance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals[16], and the NIH Office of Laboratory Animal Welfare. The animals had free access to water and food; fruits and dietary enrichments were given during the course of the study. Tuberculosis-free, healthy Papio anubis baboons, 3–4 years old, 6–10 kg body weight, with hemoglobin greater than 10 mg/dL and WBC counts less than 12,000 were included in the study. Two experimental groups were studied: (i) E. coli challenge (n=4) and (ii) E. coli + FPX (n=4). E. coli (1–2 × 1010 CFU/kg, LD100 dose, serotype B7–086a:K61; ATCC) was infused intravenously (IV) over a 2 hours period (T0 to T+2). FPX (Arixtra®, 0.08 mg/kg, GlaxoSmithKline) was administered subcutaneously (SC) 3 hours prior to E. coli challenge (T-3) followed by a second SC injection after 8 hours post the E. coli (T+8). The time after n hours of E. coli infusion was referred as T+n hours. Animals were sedated with ketamine, 14–20 mg/kg initial intramuscular injection (IM) followed by 2 mg/kg pentobarbital, administered IV periodically to maintain a light level of anesthesia during the whole procedure. Gentamicin was given twice: first as IV infusion (9 mg/kg, 1h) after completion of E. coli challenge (T+2), then 4.5 mg/kg IM at T+8. Mean systemic arterial pressure (MSAP), core body temperature, oxygen saturation, as well as the heart and respiration rates were monitored with a Cardell Max-12 HD Duo monitor (Abaxis Veterinary Diagnostics, Union City, CA). Blood samples and physiological parameters were collected while the animal was under anesthesia (from T-3 to T+8). The clinical condition of the animals was continuously monitored during the duration of the study (7 days). Animals were humanely euthanized with Euthasol (50 mg/kg, IV) when their condition deteriorated, and tissue samples were collected from select organs for histopathology analysis. Formalin-fixed, paraffin embedded sections were stained with hematoxylin-eosine or phosphotungstic acid-hematoxylin to reveal the general morphology or fibrin deposition in organs, respectively. Microscopy specimens were analysed by a veterinary pathologist who was blinded for the experimental condition[17]. Based on our extensive previous experience with this model, the animals that reached 7 days were considered permanent survivors[13, 18, 19].
Bacteria count in blood
Blood bacteremia expressed as Colony forming units (CFU) was determined as described [17].
Biochemical assays
Plasma lactate was measured using Lactate Scout (EKF Diagnostics, San Antonio, TX). Serum metabolites blood urea nitrogen (BUN), creatinine and alanine aminotransferase (ALT) were measured by using a comprehensive diagnostic profile rotor on a VetScan VS2 (Abaxis Veterinary Diagnostics, Union City, CA) chemistry analyzer[17].
ELISA
Plasma cytokines were measured using the MILLIPLEX MAP Nonhuman Primate Cytokine Magnetic Bead Panel (EMD Millipore, Burlington, MA) as per the manufacturer’s instructions. Plasma levels of inhibitory complexes of AT with activated coagulation factors: FXa-AT, FXIa-AT, thrombin-AT (TAT) and FVIIa-AT were measured using sandwich ELISAs as described[20]. Results are expressed as fold change over T0 values. Total AT antigen was measured by a sandwich ELISA, as described[20]. APC complexes with α1-antitrypsin were determined with a sandwich ELISA using affinity purified antibodies, sheep anti-human protein C (2 μg/mL; Affinity Biologicals, Ancaster, ON) for capture and biotin-conjugated goat anti-human α1-antitrypsin (1 μg/mL; Affinity Biologicals) for detection. For D-dimer, mouse monoclonal antibody clone DD1 (Novus Biologicals, Littleton, CO) was used for capture and affinity purified HRP conjugated polyclonal sheep anti-human fibrinogen (Affinity Biologicals) was used as detection antibody. For plasmin-antiplasmin (PAP) complexes, affinity purified goat anti-plasminogen (2 μg/mL, Affinity Biologicals) was used as capture antibody and HRP conjugated goat polyclonal anti-antiplasmin antibody was used for detection. Soluble TM, tissue-type plasminogen activator (t-PA) and plasminogen activator inhibitor 1 (PAI-1) were measured with DuoSet ELISA kits (R&D System, Minneapolis, MN). C3b and C5b-9 were measured as previously described[17]. The ELISA kits for High Mobility Group Box 1 (HMGB1) and citrullinated histone H3 were from Tecan US, Inc., Morrisville, NC and Cayman Chemicals, Ann Arbor, MI, respectively.
Coagulation tests
Activated partial thromboplastin time (APTT) and prothrombin time (PT) were measured as described[18]. Fibrin degradation products (FDP) were measured by latex agglutination assay[19]. Functional fibrinogen was determined using a clotting based assay[19].
Myeloperoxidase activity assay
Plasma level of myeloperoxidase (MPO) was determined using Fluoro MPO detection kit (Cell Technology, Fremont, CA) as per the manufacturer’s instructions[17].
Statistical analysis
Statistical analysis was performed using Prism (GraphPad Software 7.0b). Values are given as mean ± SEM. Confidence intervals and p values for significance were determined by fitting the data to linear mixed effects model, using the lme function implemented in the nlme R package. This function is an extended version of the regular linear model (including ANOVA and linear regression) that can accommodate complex repeat measure data collection design trough proper specification of the random effect structure[21, 22].
Temporal changes, measured over a longer period, of the vital signs (O2 saturation, temperature, respiration rate, heart rate and MSAP) were modeled in the framework of Generalized Additive Models (GAM), as implemented in the GAM function of the R package mgcv. The function relies on smoothing splines for fitting the temporal curves and is able to deal with random effects and capture complex data collection design. Comparison of survival data was done using log-rank Mantel–Cox test. Results were considered significant at P < 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001).
RESULTS
Effects of FPX on sepsis-induced coagulopathy/DIC
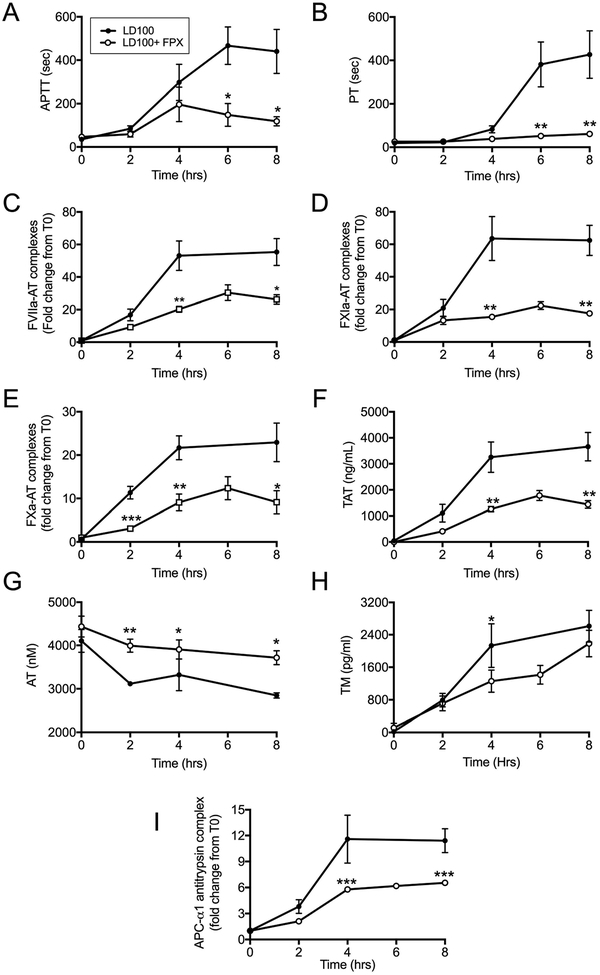
E. coli sepsis induced pronounced activation of coagulation as shown by prolongation of APTT and PT (Figure 1, A and B), and increased levels of FVIIa-AT, FXIa-AT, FXa-AT and TAT complexes (Figure 1, C–F). FPX treated animals showed shorter APTT and PT suggesting decreased consumption of clotting factors (Figure 1, A and B). The drug itself does not affect these assays directly[23]. Maximal formation of protease-AT complexes was observed 4–8 hours after E. coli challenge (Figure 1, C–F). FPX treatment reduced FVIIa-AT by ≈50% and FXIa-AT by ≈3 fold (Figure 1, C and D), which demonstrates a sustained inhibition of both coagulation initiation pathways. FPX significantly decreased FXa-AT and TAT (Figure 1, E and F) and prevented AT consumption (Figure 1 G), demonstrating inhibition of both the activation and the amplification phases of coagulation, without consumption of AT. FPX group also had lower amounts of soluble TM than the controls (Figure 1 H), thus indicating better endothelial protection. FPX treated animals displayed lower levels of complexes of APC with its heparin independent inhibitor alpha-1 antitrypsin[24] (Figure 1 I), which correlates well with the decreased thrombin generation measured as TAT (Figure 1 F).
Fig. 1.
Effect of FPX treatment on hemostatic biomarkers. Time course of: (A) APTT, (B) PT, (C) Factor VIIa-antithrombin complex (FVIIa-AT), (D) Factor XIa-antithrombin complex (FXIa-AT), (E) Factor Xa-antithrombin complex (FXa-AT), (F) Thrombin-antithrombin complex (TAT), (G) total antithrombin (AT), (H) thrombomodulin (TM), and (I) activated protein C (APC)-α1 antitrypsin complex, in septic baboons treated with or without FPX. Data are presented as mean ± SEM. For all graphs, asterisks indicate a statistically significant difference between LD100 and LD100+FPX, as calculated with the linear mixed effects model (*P < 0.05, **P <0.01, ***P < 0.001).
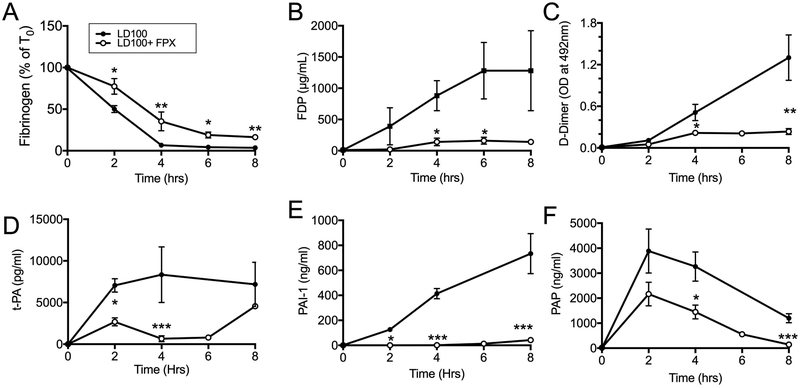
Although reduced, fibrinogen consumption still occurred in FPX treated animals (Figure 2 A), suggesting that part of the fibrinogen was consumed via thrombin-independent mechanisms. FPX treatment also reduced fibrin related biomarkers FDP and D-Dimer, which reflects decreased fibrin formation (Figure 2, B and C). FPX rebalanced the fibrinolytic mechanisms leading to plasmin generation by strongly reducing t-PA, PAI-1 and PAP in the plasma (Figure 2, D–F).
Fig. 2.
FPX treatment decreases fibrinogen consumption, fibrin formation and fibrin(ogen)olysis. (A) Functional fibrinogen, (B) fibrin(ogen) degradation products (FDP), (C) D-dimers, (D) tissue plasminogen activator (t-PA), (E) plasminogen activator inhibitor 1 (PAI-1), (F) plasmin-antiplasmin (PAP) complexes. Data are presented as mean ± SEM. For all graphs, asterisks indicate a statistically significant difference between LD100 and LD100+FPX as calculated with the linear mixed effects model (*P < 0.05, **P <0.01, ***P < 0.001).
Our data indicate that FPX treatment robustly decreased sepsis coagulopathy, without showing clinical signs of microvascular thrombosis, petechial hemorrhaging or gastrointestinal bleeding (not shown), despite displaying similar thrombocytopenia as LD100 controls (Supplemental Figure S1, D; see bellow).
Effects of FPX on hematologic parameters, complement activation and bacteria clearance
As illustrated in Supplemental Figure S1, during the first 8 hours post E. coli challenge hematocrit (A) and red blood cell (RBC, B) did not significantly change, white blood cell (WBC, C) sharply declined by 70–80% at T+2, and platelets (D) steady declined, with 60–70% decrease at T+8. Treatment with FPX did not change any of these parameters.
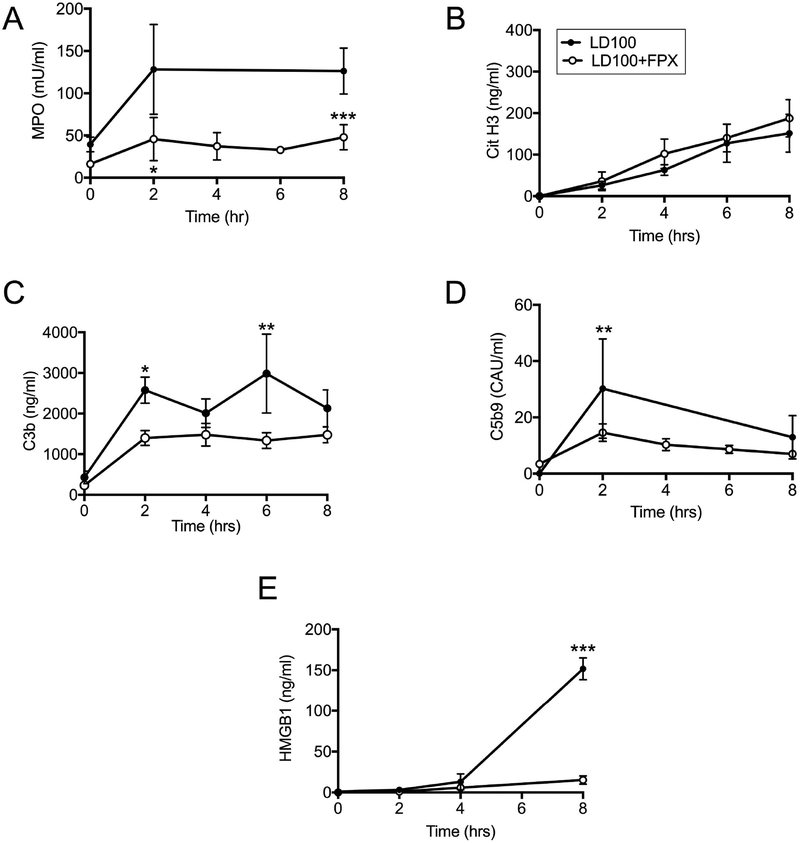
FPX treatment, however, decreased E. coli induced neutrophil degranulation, as revealed by plasma myeloperoxidase (MPO; Figure 3 A), but did not significantly change the release of neutrophil extracellular traps (NETs) quantified by circulating citrullinated histone H3 (Figure 3 B). This suggests selective effects of FPX treatment on neutrophils immune functions. FPX treated animals displayed lower complement activation as shown by plasma C3b (Figure 3 C) and C5b-9 (Figure 3 D) activation products. Similarly, the treatment decreased the plasma amounts of HMGB1 (Figure 3 E), a nuclear protein actively released from activated macrophages or passively from dying cells.
Fig. 3.
Effect of FPX on markers of leukocyte and complement activation. (A) Myeloperoxidase (MPO), (B) Citrullinated histone H3, (C) complement activation product C3b, (D) complement terminal complex C5b-9, and (E) high mobility group box 1 (HMGB1) in septic baboons without (LD100) or with FPX treatment (LD100+ FPX). Data are presented as mean ± SEM. For all graphs, asterisks indicate a statistically significant difference between LD100 and LD100+FPX, as calculated with the linear mixed effects model (*P < 0.05, **P <0.01, ***P < 0.001).
Importantly, the treatment did not impair bacterial clearance as shown by CFU counts in circulating blood (Supplemental Figure S2).
Effect of FPX on sepsis-induced cytokine production
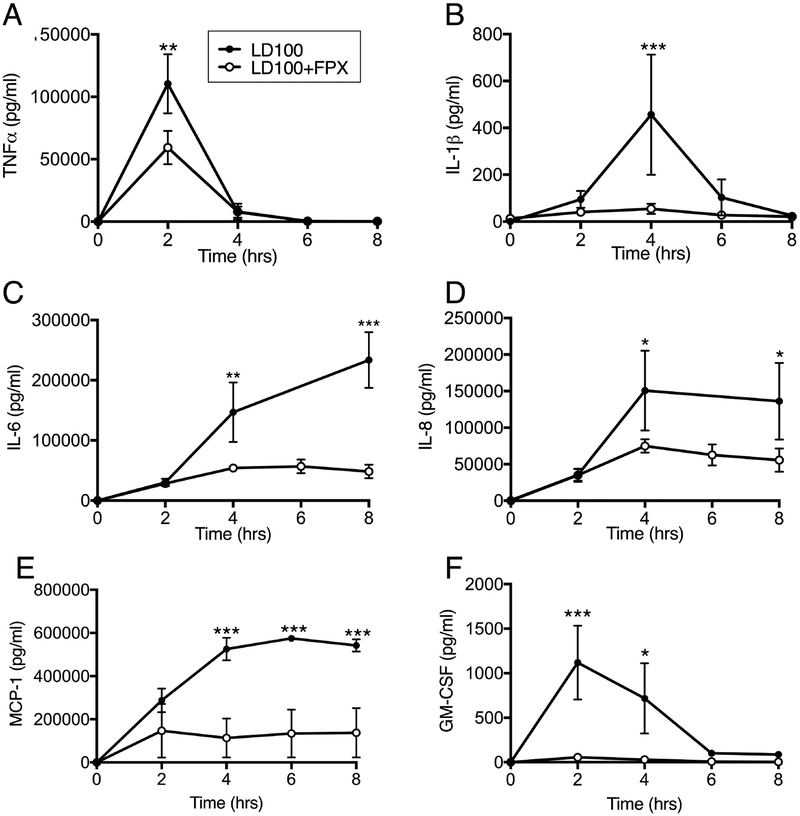
As shown in Figure 4, bacteremia challenge induced robust increase of plasma TNF, IL-1β, IL-6, IL-8, MCP-1 and GM-CSF. Maximal levels of TNF and GM-CSF occurred at T+2 and then returned to baseline (Figure 4, A and B). IL-1β peaked at T+4 and returned to baseline by T+8 (Figure 4 C). A sustained increase of IL-6, IL-8 and MCP1 was observed from T+2 and the levels were maximal at T+4 to T+8 (Figure 5, D–F). Treatment with FPX substantially reduced all of these cytokines.
Fig. 4.
Effect of FPX treatment on the production of pro-inflammatory cytokines. (A) TNF, (B) IL1b, (C) IL-6, (D) IL-8, (E) MCP1 and (F) GM-CSF in plasma of baboons treated or not with FPX. Data are presented as mean± SEM. For all graphs, asterisks indicate a statistically significant difference between LD100 and LD100+FPX, as calculated with the linear mixed effects model (*P < 0.05, **P <0.01, ***P < 0.001).
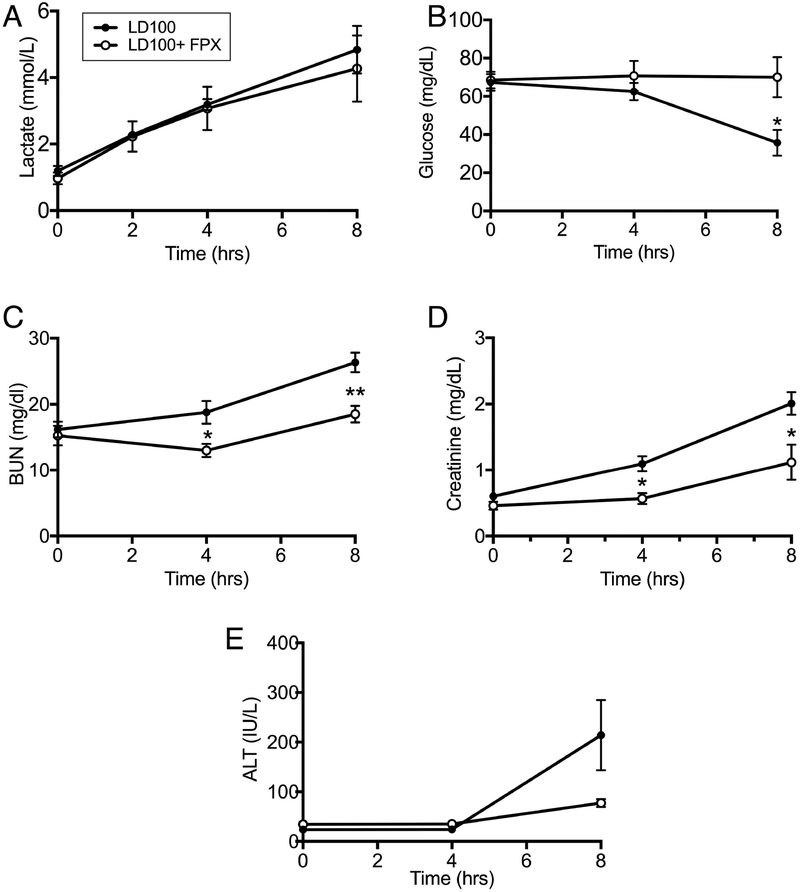
Fig. 5.
Effect of FPX on plasma markers of organ functions. (A) Lactate, (B) glucose, (C) blood urea nitrogen (BUN), (D) creatinine, and (E) alanine aminotransferase (ALT). Data are presented as mean ± SEM. For all graphs, “ns” indicates not statistically significant, and an asterisk and bracket indicate a statistically significant (*P < 0.05, **P <0.01, ***P < 0.001) difference between LD100 and LD100+FPX for each time point under the bracket, as calculated with the linear mixed effects model.
Effects of FPX on vital signs and organ function
Although blood pressure did not drop as low and showed an early recovery trend in FPX treated baboons, the time course of changes in MSAP and heart rate were not significantly different between groups (Supplemental Figure S3, A and B). Likewise, respiration rate was lower and oxygen saturation higher suggesting a slightly better respiratory function in the treated group (Supplemental Figure S3, C and D). Body core temperature was slightly lower in treated animals during the second half of the procedure (Supplemental Figure S3, E).
Sepsis induced cardiopulmonary impairment led to tissue hypoperfusion and organ dysfunction as shown by elevation of lactate (hypoxia marker; Figure 5 A), hypoglycemia (Figure 5 B), increased creatinine and BUN (renal function markers; Figure 5, C and D) and liver transaminase (ALT; Figure 5 E). FPX treatment prevented organ dysfunction.
Effect of FPX on sepsis-induced mortality
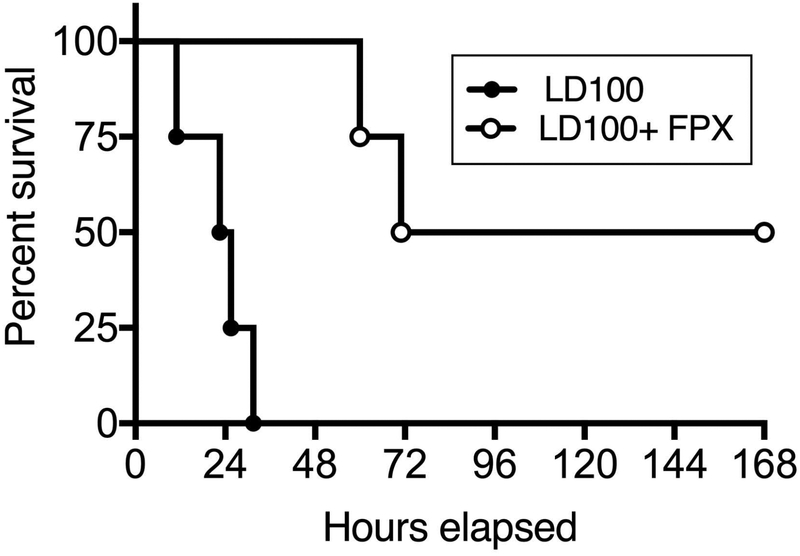
Intravenous infusion of live bacteria induced DIC and multiple organ dysfunction, especially cardiopulmonary failure that led to 100% lethality (LD100) within 24–36 hours of challenge. Histopathology analysis showed extensive microvascular thrombosis (DIC), congestion, capillary leakage and fibrin deposition in multiple organs, including the lung (Supplemental Figure S4). Treatment with FPX decreased fibrin deposition, prevented organ damage and provided survival benefit, with two out of four treated animals reaching the 7 days end-point, while all four animals in the control group died within 30 hours (Figure 6; Mantel–Cox test P = 0.0067). Two treated animals developed acute kidney failure, with creatinine >15 mg/dL and BUN >180 md/dL at the time of death, and were humanely euthanized at 60 and 72 hours post-challenge, respectively. The two survivors fully recovered by day 7. Based on our previous experience with this LD100 model, animals that survive 7 days are considered permanent survivors[13].
Fig. 6.
Effect of FPX on sepsis-induced mortality. Survival plot of animals challenged with LD100 vs. LD100 + FPX. Survival distribution of the two groups was determined using a log-rank Mantel-Cox test; the result is significant at P=0.0067.
DISCUSSION
Sepsis, a life-threatening organ dysfunction caused by deregulated host response to infection is a leading cause of morbidity and mortality worldwide[25]. DIC is a frequent complication and major contributor to organ dysfunction and mortality in sepsis[26]. Experimental and clinical studies aimed at the inhibition of thrombin by supplementation of AT[9], PC/APC[8] or anticoagulation with heparin[11] showed some benefit in patients with severe sepsis coagulopathy, but failed to improve survival in large randomized cohorts of septic shock patients. Currently, anticoagulation is not recommended due to increased risk of bleeding and possible impairment of pathogen clearance, which could outweigh the benefits[3]. Development of specific strategies to mitigate these risks could increase survival of septic shock patients and attenuate DIC-derived sequalae in survivors.
The aim of this study was to determine if inhibition of FXa using the heparin-derived pentasaccharide[15] fondaparinux (FPX) can protect against sepsis-induced DIC and inflammation, and provide survival benefit in our well-characterized baboon model of sepsis induced DIC and organ failure[13]. The LD100 challenge closely mimics in baboons the shock/DIC variant of severe sepsis[13]. While the model has limitations because its rapid progression limits the therapeutic window, it proved best suited to test anticoagulants[4, 6, 7] since it allows to determine the protective effects of a drug using minimal number of animals.
Our results demonstrate that FPX could attenuate sepsis-induced coagulopathy and inflammation, without impairing the clearance of live bacteria. Importantly, FPX provided 7-days survival benefit for two out of four treated animals.
Since commercial FPX (Arixtra) is formulated for subcutaneous injection, we administered the drug three hours before challenge, to achieve maximum bioavailability at the time of bacteremia peak. We administered 0.08 mg/kg FPX, twice at 11 hours interval; this dose is slightly higher than the thromboprofilaxis dose used in humans (0.05 mg/kg) but lower than the therapeutic dose used for venous thromboembolism post-surgery, which is the main indication of the drug.
FPX is a synthetic analog of the AT-binding heparin pentasaccharide sequence designed to potentiate AT-mediated inhibition of FXa, but not of thrombin[15]. In addition, FPX also accelerates AT inactivation of FVIIa and FIXa, and to lesser extent FXIa and FXIIa[27].
Targeting FXa therapeutically is attractive due to its position at crossroads between the intrinsic and extrinsic pathways of coagulation[28]. Moreover, FXa inactivation downregulates its signaling via protease activated receptor (PAR) 2, which plays a key role in fibro-proliferative responses[28] that contribute to the long-lasting fibrotic complications of sepsis. Therapeutic inhibition of thrombin has been tried and failed to show survival benefits in clinical trials[8, 9, 11] due to increased bleeding risk, which highlights the complex and critical effects of this molecule as both executioner of the coagulation cascade and activator of potent anticoagulant and antifibrinolytic proteases like APC and TAFI. Thrombin also has dual signaling effects: thrombin cleavage of PAR1 turns on pro-inflammatory and vascular disruptive effects; and, signaling via the APC-endothelial protein C receptor (EPCR)- PAR1 axis[29] or PAR1-PAR2 transactivation[30] mediate thrombin’s potent anti-inflammatory and vasoprotective effects. Therefore, we hypothesized that inhibition of the coagulation cascade at FXa level could be a better strategy to treat sepsis DIC.
Our data shows that FPX significantly reduces sepsis-induced DIC as indicated by shorter APTT and PT. Live E. coli is known to activate the intrinsic/contact phase via bacteria-derived long chain polyphosphates[31]. In parallel, bacterial wall LPS strongly induces expression of tissue factor (TF) by circulating monocytes and of pro-inflammatory cytokines TNF, IL1β and IL6, which further enhance TF-mediated coagulation and inflammation[32, 33]. FPX treatment suppressed both the intrinsic and extrinsic pathways of coagulation as shown by decreased FXIa-AT and FVIIa-AT inhibitory complexes. The dampening of the extrinsic pathway could occur through potentially lower TF expression due to decreased inflammatory cytokines or FPX-mediated inhibition of TF-FVIIa[34]. FPX prevented sepsis-induced drop in plasma AT by decreasing its consumption during complex formation with clotting factors or its degradation via neutrophil elastase[35], as suggested by less neutrophil activation marker. FPX also reduced fibrin deposition as reflected by the FDP, D-Dimer and PAP. Notably, the treatment strongly decreased sepsis induced production of PAI-1, a major plasma inhibitor of plasminogen activation, known to be induced by pro-inflammatory cytokines[26].
Notably, despite not preventing thrombocytopenia which is an independent risk factor for mortality in septic patients[36], FPX did not aggravate it and did not induce bleeding complications (e.g., skin or mucosal petechiae, bleeding at the site of catheter insertion, gastrointestinal bleeding) that are frequently seen in non treated animals. This suggests that either low levels of thrombin in FPX treated animals are sufficient to activate platelets, or other host defense events, such the complement-mediated opsonization[17, 18] could drive sepsis thrombocytopenia[37]. Differently from unfractionated or LMW heparins that promote platelet activation, aggregation and thrombocytopenia (HIT), FPX does not produce HIT[38], which could be an important advantage for its clinical use in sepsis patients.
Beside fulminant DIC, E. coli infusion leads to rapid and severe inflammation, robust complement activation and deadly organ failure within 24–36 hours post-challenge[13]. FPX treatment significantly suppressed the induction of pro-inflammatory cytokines and chemokines (TNF, IL1β, IL6, IL8, MCP1 and GM-CSF), and blunted the activation of complement with formation of C3b and C5b-9 terminal complex and diminished the release of pro-inflammatory C5a anaphylatoxin. These effects are consistent with reports showing that FPX and danaparoid (a FXa inhibitory LMWH) have anti-inflammatory and cytoprotective effects in models of ischemia reperfusion injury[39] or endotoxemia in rats[40–42]. Alternatively, FPX could diminish sepsis induced inflammation by inhibiting FXa mediated PAR2 signaling[43].
Whereas it has been postulated that fibrin clot can help controlling the invasion of the bacteria, FPX treatment did not impede the clearance of live E. coli from the blood, while efficiently reducing the coagulation biomarkers by 50–60%. Whether the lack of interference of FPX with pathogen clearance is valid for other bacteria species remains to be determined. In our model pathogen removal from blood is likely driven by neutrophil degranulation, netosis, complement activation and phagocytosis. While FPX reduced neutrophil degranulation and complement activation, it did not affect NETs release. It is possible that FPX achieves the low level of activation of innate immune mechanisms needed for pathogen clearance without the pathologic amplification of these pathways deleterious to the host. Alternatively, the FPX-mediated cytoprotection of vascular cells, indicated by reduced circulating HMGB1 levels, could prolong the half-life of professional phagocytes, such as monocyte/macrophages, which could contribute to pathogen clearance.
Therapies targeting FXa have been tried in the baboon model with mixed result[44, 45]. Our group showed that IV infusion of the active site blocked FXa (DEGR-FXa) attenuated DIC but did not reduce inflammation, organ injury and lethality in the same baboon model of E. coli sepsis, concluding that blocking fibrin generation is not sufficient to save the baboons[44]. Differently from DEGR-Xa, FPX blocks not only FXa but also upstream coagulation proteases FVIIa and FXIa involved in the initiation and amplification of the coagulation cascade, thus preventing sepsis induced overt DIC. In addition, FPX decreased inflammation and provided survival benefits, suggesting that besides anticoagulation, FPX has additional protective effects by reducing the production of pro-inflammatory cytokines and chemokines, and the activation of neutrophils and monocytes/macrophages. Our results are in line with the reported reduction by FPX of the coagulation, inflammation, and neutrophil accumulation in a rat model of kidney ischemia–reperfusion injury[46].
The current study is based on only four baboons per group, therefore is not robust enough to predict survival benefit in a clinical trial[47]. Since non-human primates are the closest species to humans, ethical considerations dictate us to use the smallest numbers possible to get valid scientific information, while taking every practical step to prevent or limit distress to the animal[48].
FPX has major advantages over heparins including longer half-life and bioavailability, subcutaneous administration, easier dosing and lack of HIT risk. As a drawback, FPX is not neutralized by protamine and there is no clinically approved antidote to reverse its anticoagulant effects yet, which could deter clinicians from prescribing it to sepsis patients; however, a promising antidote is currently under FDA review[49].
Despite limitations, our study provides proof-of-concept information that may be used in the design of future clinical trials, particularly on dosing and timing as well as the efficacy of the drug to prevent sepsis DIC.
To our knowledge, this is the first preclinical report of FPX use in a bacteremia sepsis model. Since FPX is FDA approved for prevention and therapy of thrombotic complications and it has well-characterized pharmacological properties, this drug is well positioned for further clinical testing for sepsis indication.
Supplementary Material
Essentials.
Activation of coagulation leading to microvascular thrombosis and consumptive coagulopathy is a major contributor to organ failure and death in sepsis patients.
We treated septic baboons with Fondaprinux, an anticoagulant that inhibits mainly Factor Xa.
Fondaparinux reduced sepsis coagulopathy, complement activation and inflammation, and improved survival outcome of septic baboons.
Fondaparinux can be useful for prevention and treatment of sepsis-induced disseminated intravascular coagulation.
ACKNOWLEDGMENTS
The authors thank Dr. Fletcher Taylor and Dr. Gary Kinasewitz for helpful discussions and Dr. Stanley Kosanke for help with pathology scoring.
This work was supported by grants from the National Institutes of Health, National Institute of General Medical Sciences (RC1GM091739 [FL and CTE], R01GM121601 [FL], R01GM122775 [FL], R01GM116184 [OJTM and FL], and P30GM114731 [FL]) and National Institute of Allergy and Infectious Diseases (U19AI062629 [FL]).
Footnotes
Presented in abstract form at the 59th Annual Meeting of the American Society of Hematology, December 9–12, 2017, Atlanta, GA.
The online version of the article contains data supplement.
CONFLICT OF INTEREST DISCLOSURE
The authors declare no competing financial interests.
REFERENCES
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. The New England journal of medicine. 2013; 369: 840–51. 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 2.Lupu F, Keshari RS, Lambris JD, Coggeshall KM. Crosstalk between the coagulation and complement systems in sepsis. Thrombosis research. 2014; 133 Suppl 1: S28–31. 10.1016/j.thromres.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Poll T, Opal SM. Should all septic patients be given systemic anticoagulation? No. Intensive Care Med. 2017; 43: 455–7. 10.1007/s00134-016-4607-x. [DOI] [PubMed] [Google Scholar]
- 4.Creasey AA, Chang AC, Feigen L, Wun TC, Taylor FB Jr., Hinshaw LB. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. The Journal of clinical investigation. 1993; 91: 2850–60. 10.1172/JCI116529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emerson TE Jr., Fournel MA, Redens TB, Taylor FB Jr. Efficacy of antithrombin III supplementation in animal models of fulminant Escherichia coli endotoxemia or bacteremia. Am J Med. 1989; 87: 27S–33S. [DOI] [PubMed] [Google Scholar]
- 6.Taylor FB Jr., Emerson TE Jr., Jordan R, Chang AK, Blick KE. Antithrombin-III prevents the lethal effects of Escherichia coli infusion in baboons. Circ Shock. 1988; 26: 227–35. [PubMed] [Google Scholar]
- 7.Taylor FB Jr., Chang A, Esmon CT, D’Angelo A, Vigano-D’Angelo S, Blick KE. Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboon. The Journal of clinical investigation. 1987; 79: 918–25. 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gardlund B, Marshall JC, Rhodes A, Artigas A, Payen D, Tenhunen J, Al-Khalidi HR, Thompson V, Janes J, Macias WL, Vangerow B, Williams MD, Group P-SS. Drotrecogin alfa (activated) in adults with septic shock. The New England journal of medicine. 2012; 366: 2055–64. 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 9.Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I, Chalupa P, Atherstone A, Penzes I, Kubler A, Knaub S, Keinecke HO, Heinrichs H, Schindel F, Juers M, Bone RC, Opal SM, KyberSept Trial Study G. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001; 286: 1869–78. [DOI] [PubMed] [Google Scholar]
- 10.Abraham E, Reinhart K, Opal S, Demeyer I, Doig C, Rodriguez AL, Beale R, Svoboda P, Laterre PF, Simon S, Light B, Spapen H, Stone J, Seibert A, Peckelsen C, De Deyne C, Postier R, Pettila V, Sprung CL, Artigas A, Percell SR, Shu V, Zwingelstein C, Tobias J, Poole L, Stolzenbach JC, Creasey AA, Group OTS. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003; 290: 238–47. 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- 11.Zarychanski R, Abou-Setta AM, Kanji S, Turgeon AF, Kumar A, Houston DS, Rimmer E, Houston BL, McIntyre L, Fox-Robichaud AE, Hebert P, Cook DJ, Fergusson DA, Canadian Critical Care Trials G. The efficacy and safety of heparin in patients with sepsis: a systematic review and metaanalysis. Crit Care Med. 2015; 43: 511–8. 10.1097/CCM.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 12.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007; 109: 3161–72. 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 13.Taylor FB Jr., Kinasewitz GT, Lupu F. Pathophysiology, staging and therapy of severe sepsis in baboon models. J Cell Mol Med. 2012; 16: 672–82. 10.1111/j.1582-4934.2011.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walenga JM, Jeske WP, Bara L, Samama MM, Fareed J. Biochemical and pharmacologic rationale for the development of a synthetic heparin pentasaccharide. Thrombosis research. 1997; 86: 1–36. [DOI] [PubMed] [Google Scholar]
- 15.Brandao GM, Junqueira DR, Rollo HA, Sobreira ML. Pentasaccharides for the treatment of deep vein thrombosis. Cochrane Database Syst Rev. 2017; 12: CD011782 10.1002/14651858.CD011782.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Council NR. Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press, 2011. [Google Scholar]
- 17.Keshari RS, Silasi R, Popescu NI, Patel MM, Chaaban H, Lupu C, Coggeshall KM, Mollnes TE, DeMarco SJ, Lupu F. Inhibition of complement C5 protects against organ failure and reduces mortality in a baboon model of Escherichia coli sepsis. Proc Natl Acad Sci U S A. 2017; 114: E6390–E9. 10.1073/pnas.1706818114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silasi-Mansat R, Zhu H, Popescu NI, Peer G, Sfyroera G, Magotti P, Ivanciu L, Lupu C, Mollnes TE, Taylor FB, Kinasewitz G, Lambris JD, Lupu F. Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of Escherichia coli sepsis. Blood. 2010; 116: 1002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor FB Jr., Stearns-Kurosawa DJ, Kurosawa S, Ferrell G, Chang AC, Laszik Z, Kosanke S, Peer G, Esmon CT. The endothelial cell protein C receptor aids in host defense against Escherichia coli sepsis. Blood. 2000; 95: 1680–6. [PubMed] [Google Scholar]
- 20.Silasi R, Keshari RS, Lupu C, Van Rensburg WJ, Chaaban H, Regmi G, Shamanaev A, Shatzel JJ, Puy C, Lorentz CU, Tucker EI, Gailani D, Gruber A, McCarty OJT, Lupu F. Inhibition of contact-mediated activation of factor XI protects baboons against S aureus-induced organ damage and death. Blood Adv. 2019; 3: 658–69. 10.1182/bloodadvances.2018029983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982; 38: 963–74. [PubMed] [Google Scholar]
- 22.Lindstrom ML, Bates DM. Nonlinear mixed effects models for repeated measures data. Biometrics. 1990; 46: 673–87. [PubMed] [Google Scholar]
- 23.Linkins LA, Julian JA, Rischke J, Hirsh J, Weitz JI. In vitro comparison of the effect of heparin, enoxaparin and fondaparinux on tests of coagulation. Thrombosis research. 2002; 107: 241–4. [DOI] [PubMed] [Google Scholar]
- 24.Heeb MJ, Griffin JH. Physiologic inhibition of human activated protein C by alpha 1-antitrypsin. J Biol Chem. 1988; 263: 11613–6. [PubMed] [Google Scholar]
- 25.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016; 315: 801–10. 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levi M, Poll T. Coagulation in patients with severe sepsis. Semin Thromb Hemost. 2015; 41: 9–15. 10.1055/s-0034-1398376. [DOI] [PubMed] [Google Scholar]
- 27.Olson ST, Swanson R, Raub-Segall E, Bedsted T, Sadri M, Petitou M, Herault JP, Herbert JM, Bjork I. Accelerating ability of synthetic oligosaccharides on antithrombin inhibition of proteinases of the clotting and fibrinolytic systems. Comparison with heparin and low-molecular-weight heparin. Thromb Haemost. 2004; 92: 929–39. 10.1160/TH04-06-0384. [DOI] [PubMed] [Google Scholar]
- 28.Borensztajn K, Spek CA. Blood coagulation factor Xa as an emerging drug target. Expert Opin Ther Targets. 2011; 15: 341–9. 10.1517/14728222.2011.553608. [DOI] [PubMed] [Google Scholar]
- 29.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002; 296: 1880–2. 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 30.Kaneider NC, Leger AJ, Agarwal A, Nguyen N, Perides G, Derian C, Covic L, Kuliopulos A. ‘Role reversal’ for the receptor PAR1 in sepsis-induced vascular damage. Nat Immunol. 2007; 8: 1303–12. 10.1038/ni1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zilberman-Rudenko J, Reitsma SE, Puy C, Rigg RA, Smith SA, Tucker EI, Silasi R, Merkulova A, McCrae KR, Maas C, Urbanus RT, Gailani D, Morrissey JH, Gruber A, Lupu F, Schmaier AH, McCarty OJT. Factor XII Activation Promotes Platelet Consumption in the Presence of Bacterial-Type Long-Chain Polyphosphate In Vitro and In Vivo. Arterioscler Thromb Vasc Biol. 2018; 38: 1748–60. 10.1161/ATVBAHA.118.311193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grover SP, Mackman N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler Thromb Vasc Biol. 2018; 38: 709–25. 10.1161/ATVBAHA.117.309846. [DOI] [PubMed] [Google Scholar]
- 33.Li A, Chang AC, Peer GT, Hinshaw LB, Taylor FB Jr. Comparison of the capacity of rhTNF-alpha and Escherichia coli to induce procoagulant activity by baboon mononuclear cells in vivo and in vitro. Shock. 1996; 5: 274–9. [DOI] [PubMed] [Google Scholar]
- 34.Lormeau JC, Herault JP, Herbert JM. Antithrombin-mediated inhibition of factor VIIa-tissue factor complex by the synthetic pentasaccharide representing the heparin binding site to antithrombin. Thromb Haemost. 1996; 76: 5–8. [PubMed] [Google Scholar]
- 35.Cohen JR, Sarfati I, Birnbaum E, Benacquista T, Wise L. The inactivation of antithrombin III by serum elastase in patients with surgical infections. Am Surg. 1990; 56: 665–7. [PubMed] [Google Scholar]
- 36.Venkata C, Kashyap R, Farmer JC, Afessa B. Thrombocytopenia in adult patients with sepsis: incidence, risk factors, and its association with clinical outcome. J Intensive Care. 2013; 1: 9 10.1186/2052-0492-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong CH, Jenne CN, Petri B, Chrobok NL, Kubes P. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat Immunol. 2013; 14: 785–92. 10.1038/ni.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savi P, Chong BH, Greinacher A, Gruel Y, Kelton JG, Warkentin TE, Eichler P, Meuleman D, Petitou M, Herault JP, Cariou R, Herbert JM. Effect of fondaparinux on platelet activation in the presence of heparin-dependent antibodies: a blinded comparative multicenter study with unfractionated heparin. Blood. 2005; 105: 139–44. 10.1182/blood-2004-05-2010. [DOI] [PubMed] [Google Scholar]
- 39.Guillou S, Tamareille S, Giraud S, Poitevin G, Prunier-Mirebeau D, Nguyen P, Prunier F, Macchi L. Fondaparinux upregulates thrombomodulin and the endothelial protein C receptor during early-stage reperfusion in a rat model of myocardial infarction. Thrombosis research. 2016; 141: 98–103. 10.1016/j.thromres.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Iba T, Okamoto K, Ohike T, Tajirika T, Aihara K, Watanabe S, Kayhanian H. Enoxaparin and fondaparinux attenuates endothelial damage in endotoxemic rats. J Trauma Acute Care Surg. 2012; 72: 177–82. 10.1097/TA.0b013e31821a83f0. [DOI] [PubMed] [Google Scholar]
- 41.Iba T, Miyasho T. Danaparoid sodium attenuates the increase in inflammatory cytokines and preserves organ function in endotoxemic rats. Crit Care. 2008; 12: R86 10.1186/cc6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagiwara S, Iwasaka H, Hidaka S, Hishiyama S, Noguchi T. Danaparoid sodium inhibits systemic inflammation and prevents endotoxin-induced acute lung injury in rats. Crit Care. 2008; 12: R43 10.1186/cc6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rallabhandi P, Nhu QM, Toshchakov VY, Piao W, Medvedev AE, Hollenberg MD, Fasano A, Vogel SN. Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: a novel paradigm for receptor cooperativity. J Biol Chem. 2008; 283: 24314–25. 10.1074/jbc.M804800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor FB Jr., Chang AC, Peer GT, Mather T, Blick K, Catlett R, Lockhart MS, Esmon CT. DEGR-factor Xa blocks disseminated intravascular coagulation initiated by Escherichia coli without preventing shock or organ damage. Blood. 1991; 78: 364–8. [PubMed] [Google Scholar]
- 45.Schochl H, van Griensven M, Heitmeier S, Laux V, Kipman U, Roodt J, Bahrami S, Redl H. Dual inhibition of thrombin and activated factor X attenuates disseminated intravascular coagulation and protects organ function in a baboon model of severe Gram-negative sepsis. Crit Care. 2017; 21: 51 10.1186/s13054-017-1636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frank RD, Schabbauer G, Holscher T, Sato Y, Tencati M, Pawlinski R, Mackman N. The synthetic pentasaccharide fondaparinux reduces coagulation, inflammation and neutrophil accumulation in kidney ischemia-reperfusion injury. J Thromb Haemost. 2005; 3: 531–40. 10.1111/j.1538-7836.2005.01188.x. [DOI] [PubMed] [Google Scholar]
- 47.Walsh M, Srinathan SK, McAuley DF, Mrkobrada M, Levine O, Ribic C, Molnar AO, Dattani ND, Burke A, Guyatt G, Thabane L, Walter SD, Pogue J, Devereaux PJ. The statistical significance of randomized controlled trial results is frequently fragile: a case for a Fragility Index. J Clin Epidemiol. 2014; 67: 622–8. 10.1016/j.jclinepi.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 48.Chen L, Welty-Wolf KE, Kraft BD. Nonhuman primate species as models of human bacterial sepsis. Lab Anim (NY). 2019; 48: 57–65. 10.1038/s41684-018-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siegal DM, Curnutte JT, Connolly SJ, Lu G, Conley PB, Wiens BL, Mathur VS, Castillo J, Bronson MD, Leeds JM, Mar FA, Gold A, Crowther MA. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. The New England journal of medicine. 2015; 373: 2413–24. 10.1056/NEJMoa1510991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.