Abstract
Objective: There is an unmet need to improve immediate burn care, particularly when definitive treatment is delayed. Therefore, the purpose of this project was to formulate a hydrogel that contains very high concentrations of antibiotics and validate its use together with a platform wound device (PWD) for the immediate care of burns.
Approach: The hydrogel properties were optimized by using a rheometer, differential scanning calorimetry, and liquid chromatography–mass spectrometry and were tested in an infected porcine burn model. Immediately, after burn creation, the burns were infected with different bacteria. Subsequently, the burns infected with Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter baumannii were covered with the PWD and treated with a single dose of hydrogel containing 1000 × minimum inhibitory concentration of vancomycin, gentamicin, and minocycline, respectively. On day 7 or 45, the animals were euthanized, and the burns were harvested for histology and quantitative bacteriology.
Results: 0.625% was the best alginate concentration for the hydrogel in terms of viscosity, stability, and drug release. The porcine studies demonstrated that vancomycin-, gentamicin-, and minocycline-treated tissues contained significantly less bacteria and reduced depth of tissue necrosis in comparison to controls.
Innovation: The PWD represents a platform technology that begins at the point of the first treatment by protecting the wound and allowing administration of topical therapeutics. The device can be adapted to enclose any size burn over any contour of the body.
Conclusion: Antibiotics can be delivered safely in very high concentrations in a hydrogel using the PWD, and burn infections can be treated successfully with this method.
Keywords: alginate, burns, hydrogel, topical treatment, platform wound device, wound healing
Kristo Nuutila, MSc, PhD.

Introduction
Acute burns caused by explosions and fires are severe and often challenging injuries to treat. Burns are the fourth most common type of trauma worldwide, and every year in the United States only, over millions of people require medical attention due to a burn injury.1 Burns often show a prolonged, intense inflammatory reaction and are likely to become contaminated and infected. Consequently, burn patients are at risk of developing an invasive burn wound infection that can progress to sepsis.2 Fortunately, the survival rates for burn patients have improved considerably over the years because of developments in medical care in specialized burn centers.3,4 However, many burns occur in regions that are far away from a specialized burn unit.5,6 Therefore, there is an unmet critical need to improve immediate burn treatment, particularly when the definitive treatment is delayed.
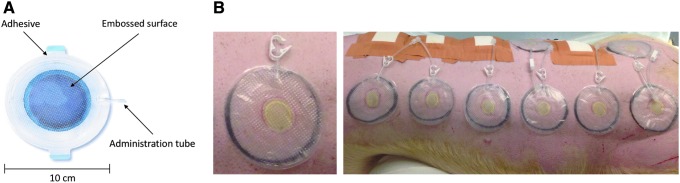
To enhance the initial care and treatment of burn injuries, we have developed a platform wound device (PWD) for immediate burn care. The PWD is a treatment platform that can be used to encapsulate a serious burn wound at the first point of care and protect it against contamination during transit to a medical facility. When placed on the injured area, the PWD creates an incubator-like environment and enables an immediate topical delivery of drugs such as antibiotics and analgesics to prevent and treat infection and reduce pain. It is made of polyurethane and can be designed to cover any kind of burn wound, even an entire limb (Fig. 1).
Figure 1.
(A) The PWD consists of a transparent polyurethane chamber with an adhesive flexible ring and access port to allow injection of active treatment in liquid or gel and withdrawal of wound fluid. The PWD is impermeable and embossed on the skin-facing side with a pattern of small pyramids that promote even distribution of liquid or hydrogel formulated medications. The PWDs used were 10 cm in diameter. (B) The PWD as applied on the pig. Up to 12 deep partial-thickness burn wounds were created on the dorsum of each pig using a custom burn device to create standardized partial-thickness burns (3 cm in diameter). The pictures depict the burn wound on day 0. PWD, platform wound device.
Topical delivery of drugs is an appealing treatment modality in burn and wound care because it increases the effective drug concentration at the target site compared with systemic administration. Thus, when delivered topically, very high concentrations of the drug can be achieved with relatively small volumes of liquid or gel.7,8 Topical delivery treats infection and reduces the risk of adverse effects such as nephropathy, neuropathy, and gastrointestinal disturbances that are all related to prolonged use of systemic antibiotics.9,10 Today, burns are treated locally with various antimicrobial dressings, creams, ointments, and hydrogels such as silver sulfadiazine, mafenide acetate, and honey-based products.11
Hydrogels are three-dimensionally cross-linked networks composed of hydrophilic polymers with high water content.12 They have many good properties as wound dressings and have been found practical in the treatment of all kinds of burns and wounds. Hydrogels provide a wound healing promoting moist environment, and concurrently, their tight mesh size protects the wound from microbes.13 Conveniently, hydrogels' porous structure also enables loading and releasing of bioactive molecules such as drugs to the wound.14 In addition, hydrogels can absorb and retain wound exudate that contains important molecules such as growth factors for wound healing.15
The purpose of this study was to validate the use of the PWD with topical antibiotics in a hydrogel for immediate treatment of burn wounds. For the present study, we formulated an antibiotic containing alginate hydrogel to be used in conjunction with the PWD. Alginate is a biomaterial that is used in many applications in biotechnology due to its advantageous properties, including biocompatibility and ease of gelation.16 For this study, alginate was chosen as the carrier for drug delivery because alginate hydrogels have been particularly attractive in wound healing and tissue engineering applications since they retain structural similarity to the extracellular matrices in tissues.13
High concentrations of gentamicin, minocycline, and vancomycin were formulated in the alginate hydrogel. These antibiotics were chosen because they are all common first- and second-generation antibiotics with a broad therapeutic spectrum. Also, importantly, they are all highly soluble in a hydrogel and have low toxicity to the injured skin.17–19 The hydrogel properties for burn treatment were optimized by using a rheometer, differential scanning calorimetry (DSC), and liquid chromatography–mass spectrometry (LCMS). Subsequently, the efficacy of the hydrogel treatment on injury progression and number of bacteria was studied in an infected deep partial-thickness porcine burn wound model. The burn wounds were infected with Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter baumannii and immediately treated with the selected concentrations of vancomycin, gentamicin, and minocycline, respectively. On day 7, the animals were euthanized, and the burns were harvested for histology and quantitative bacteriology. In addition, the outcomes of the PWD with the antibiotic alginate hydrogel treatment for the long-term quality of healing were evaluated by following the burn wounds for 45 days.
Clinical Problem Addressed
The majority of the burn injuries occur in low- to middle-income countries, in regions that are far away from a specialized burn unit. The objective of this project was to address the unmet need to improve immediate burn treatment, particularly when the definitive treatment is delayed.
Materials and Methods
Hydrogel formulation
FMC Pronova Ultrapure MVG alginate (Dupont, Wilmington, DE) was dissolved in Millipore-purified water (Millipore, Burlington, MA) at 1% mass overnight and sterile-filtered with a 0.22-μm filter. The resulting solution was frozen overnight in a −30°C freezer and lyophilized over 4 days at a vacuum pressure of 0.035 Torr. The 6.625 g of dry alginate was added to sterile glass vials along with TheraTears Solution (Akorn, Lake Forest, IL) to produce a 2.5% weight solution of alginate. These aliquots were vigorously vortex-mixed for 16 h at room temperature, and subsequently, sterile gentamicin, minocycline, and vancomycin solutions were added during the vortexing.
Mechanical properties of the hydrogel
Mechanical properties of the hydrogel were studied with a TA Instruments OSP-ARES-GT strain-controlled rheometer (TA Instruments, New Castle, DE). Hydrogel samples that were homogenized by vortexing were injected at room temperature onto a Peltier stage plate with a rough surface of 20 mm diameter, 0°59′42′′ cone geometry with a gap of 27 μm, at 25°C. Rheology measurements were run in triplicate.
Thermal stability
DSC measurements of the hydrogel samples from room temperature to −45°C at a rate of 0.1°C per minute were performed.
Drug release properties of the hydrogel
To study antibiotics (gentamicin, minocycline, and vancomycin) release characteristics over time from the 0.625% weight alginate hydrogels, LCMS (Agilent 1290/6140 with an Agilent 6140 Quadrupole MSD system; Agilent Technologies, Santa Clara, CA) was used. Homogenized hydrogel samples of 500 μL were placed in Trans-well membrane plates (0.4-μm membrane pore size), with 500 μL of phosphate-buffered saline (PBS) in the bottom well. At each time point (1, 2, 3, and 4 days), 100 μL of aliquots was taken from the bottom well, whereas 100 μL of fresh PBS was added simultaneously. Aliquots were run on LCMS to quantify the concentrations of the respective antibiotics. Mobile phase consisted of 20 mM heptafluorobutyric acid in 95% water and 5% acetonitrile flowing at 0.3 mL/min in a C18 column (Agilent Poroshell 120, 2.1 × 100 mm). Standard curves for the antibiotics were created to calculate the concentration of antibiotics present in the aliquot and therefore the amount of antibiotic released from the hydrogel. Replicates of three were conducted for each condition.
Platform wound device
The PWD (Applied Tissue Technologies LLC, Hingham, MA) consists of a transparent polyurethane chamber enclosing the injured area. It is sealed with an adhesive to the intact skin surrounding the wound. The polyurethane membrane of the device is flexible, impermeable, and embossed on the skin-facing side with a pattern of small pyramids that promote even distribution of liquid or hydrogel-formulated medications. In the present study, the PWDs that were 10 cm in diameter were used to cover the burn wound and subsequently deliver high concentrations of antibiotics formulated in a hydrogel (Fig. 1).
Animals, anesthesia, and analgesia
All the animal experiments were conducted under good laboratory practice at Toxikon Corporation (Bedford, MA) and were accepted by the Animal Care and Use Committee (IACUC, #2016-NR-38). In total, 12 female Yorkshire pigs, weighing at least 45 kg, were used in the study. Animals were acclimated for at least 5 days and assigned to the study. All animals were fasted overnight. Body weights and clinical observations were conducted before the surgical procedure on day 0. The pigs' back had hair removal performed by applying depilatory wax or agent such as NAIRTM (Church & Dwight, Ewing, NJ). If necessary, animals were also shaved using standard animal clippers. On the day of surgery, the animals were anesthetized using ketamine ∼33 mg/kg, acepromazine ∼1.1 mg/kg, xylazine ∼2.2 mg/kg, and atropine ∼0.02 mg/kg. Anesthesia was maintained with isoflurane 0–5% via face mask or endotracheal tube. A catheter was placed in the ear vein or other peripheral vein. The animals were then moved to the operating room. The level of anesthesia (respiratory rate, heart rate, peripheral capillary oxygen saturation [SpO2], muscular relaxation) was monitored. The skin was aseptically prepared for surgery. Buprenorphine was given pre-surgery at 0.03 mg/mL. Fentanyl patches (sufficient to deliver 2–3 mcg/kg/h) were placed for pain medication.
Infected deep partial-thickness burn model
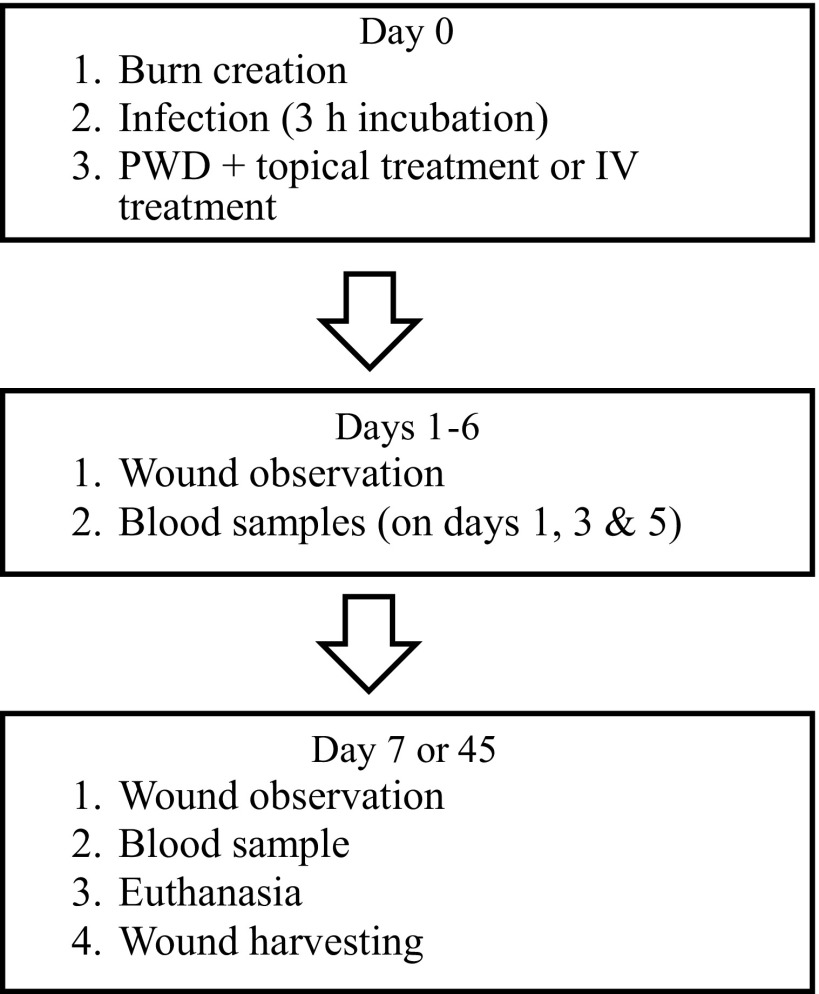
In total, 12 pigs were used in this study. On day 0, up to 12 deep partial-thickness burn wounds were created on the dorsum of each pig using a custom burn device to create standardized partial-thickness burns (3 cm in diameter). The custom burn device consists of an aluminum disk that is insulated with cork on all sides except the base, which comes in direct contact with the skin.20 The device was heated up to 100°C and applied to the skin for 20 s. This way, the depth of injury at 72 h post-burning will be ∼1.61 mm, which in Yorkshire pigs represents a deep partial-thickness burn. To ensure equal force of application among all burns during the burn creation, a custom plunger device calibrated to 10 Newton of force was used to apply the burn device to the skin, helping to eliminate operator variance. After the burn creation, the burns were enclosed with the PWDs and topically infected with 108 colony-forming unit (CFU) of S. aureus, P. aeruginosa, or A. baumannii (Fig. 1). After a 3-h incubation, 10 mL of an alginate hydrogel containing antibiotics was administered into the PWDs. The S. aureus-infected burns were treated with vancomycin, P. aeruginosa with gentamicin, and A. baumannii with minocycline. Silvadene cream, blank hydrogel, empty PWD, and intravenous (IV) antibiotic treatment were used as controls. On postoperative days 1, 3, 5, and 7, blood samples (1/pig/time point) were drawn to measure the antibiotic concentration in the blood. Minocycline concentration in the blood serum samples were detected using an Agar diffusion assay.21 Vancomycin and gentamicin concentrations were detected using cobas® 8000 modular analyzer series (Roche, Basel, Switzerland). Minocycline, vancomycin, and gentamicin samples were analyzed at the Clinical Microbiology Laboratory of Brigham and Women's Hospital. On day 7 or 45, after burn creation, the PWDs were removed, the burn wounds were photographed, the animals euthanized, and subsequently, the burn wounds were harvested for histology and quantitative bacteriology. The wounds were excised using a scalpel and fixed in formalin for histological analysis. The biopsies were embedded in paraffin, cut in sections, and stained with hematoxylin and eosin. For quantitative bacteriology, a 6-mm punch biopsy was obtained from each burn. Biopsies were placed in dry, sterile cryogenic vials and flash frozen for analysis. Figure 2 demonstrates the study design, and Table 1 lists the different treatment groups and n-numbers.
Figure 2.
Animal study design.
Table 1.
Treatment groups and numbers
| 7-Day Study: 8 Pigs (4 Topical, 4 IV) | ||
|---|---|---|
| Treatment | Pathogen | n |
| Gentamicin 1000 × MIC hydrogel [2 μg/mL] | Pseudomonas aeruginosa | 5 |
| Minocycline 1000 × MIC hydrogel [8 μg/mL] | Acinetobacter baumannii | 5 |
| Vancomycin 1000 × MIC hydrogel [1 μg/mL] | Staphylococcus aureus | 5 |
| Silvadene cream | P. aeruginosa/A. baumannii/S. aureus | 5 |
| Blank hydrogel | P. aeruginosa/A. baumannii/S. aureus | 4 |
| Empty PWD | P. aeruginosa/A. baumannii/S. aureus | 4 |
| IV gentamicin | P. aeruginosa | 6 |
| IV minocycline | A. baumannii | 6 |
| IV vancomycin | S. aureus | 6 |
| 45-Day Study: 4 Pigs (2 Topical, 2 IV) | ||
|---|---|---|
| Treatment | Pathogen | n |
| Minocycline 1000 × MIC hydrogel [8 μg/mL] | S. aureus | 6 |
| Vancomycin 1000 × MIC hydrogel [1 μg/mL] | S. aureus | 6 |
| Silvadene cream | S. aureus | 8 |
| Empty PWD | S. aureus | 8 |
| IV minocycline | S. aureus | 6 |
| IV vancomycin | S. aureus | 6 |
IV, intravenous; MIC, minimum inhibitory concentration; PWD, platform wound device.
Statistical analyses
Only outcomes within the same pathogen inoculation and antibiotic treatments were compared. All treatment groups were compared with controls. All statistical analyses were performed in GraphPad Prism (GraphPad, La Jolla, CA). Data are presented as mean ± standard error of mean. The Shapiro–Wilk normality test was used to test whether the data are normal. All the samples passed the normality test allowing us to state that no significant departure from normality was found. Comparison of groups was performed using t-test, and p-values <0.05 were considered statistically significant (*0.01 ≤ p < 0.05, **0.001 ≤ p < 0.01, ***0.0001 ≤ p < 0.001, p < 0.0001). The unpaired t method tests the null hypothesis that the population means related to two independent random samples from an approximately normal distribution are equal. The parametric statistical test is one that makes assumptions about the parameters of the population distribution(s) from which one's data are drawn.22,23 The n-number for each treatment and time point in the experiments was 4–8. All samples were evaluated by blinded experienced investigators.
Results
Rheological characterization of hydrogel mechanical properties
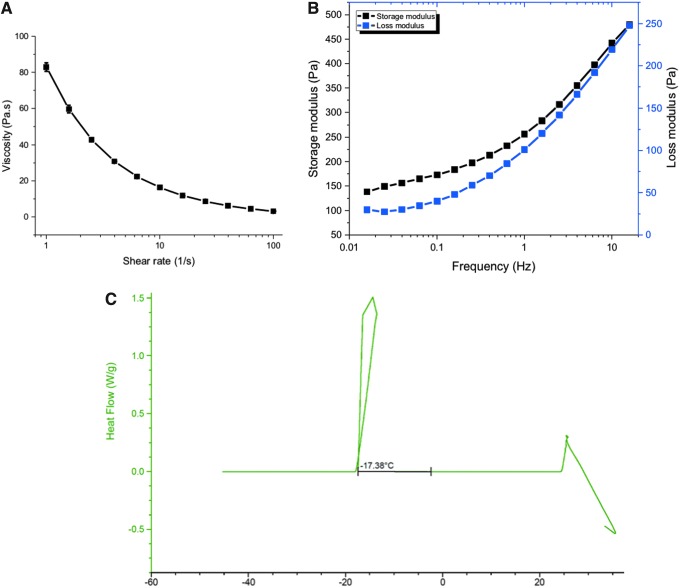
To characterize the injectability of the hydrogel formulations into the PWDs, shear rate sweeps to probe the viscosity were conducted from 1 to 100 s−1. The geometry used were rough-surfaced 1 degree 40 mm Cone-and-Plate at a gap of 400 μm. For 30-mL syringes, the shear rates can range from 5 to 100,000 s−1, depending on the presence and size of the injection needle. Field injectability is commonly thought of in terms of viscosities below 100 mPa*s, which these hydrogels fall well within, even at lower shear rates. Another design parameter for the formulation was for the hydrogels to be self-standing to prevent pooling of the material due to gravity, therefore having a higher storage modulus than loss modulus. Frequency sweeps of the storage and loss modulus satisfy these requirements and indicate the nonlinear viscoelasticity of the alginate hydrogel networks that are soft gels and not viscous liquids (Fig. 3A, B).
Figure 3.
(A) On a stress-controlled rheometer, homogenized hydrogels were analyzed via shear rate sweeps to calculate the viscosity, as an indication of injectability into the PWDs. The geometry used were rough-surfaced 1 degree 40 mm Cone-and-Plate at a gap of 400 μm. (B) The storage modulus is larger than the loss modulus; it is indicative that we have a gel and not a viscous liquid. Also, the moduli are quite low, demonstrating that we have a very soft hydrogel. A strain sweep is used to determine the linear viscoelastic area of the hydrogel, and a frequency sweep is used to determine the linear equilibrium modulus plateau of the hydrogel. (C) DSC measurements of the hydrogel sample from room temperature to −45°C at a rate of 0.1°C per minute. The freezing point is indicated by a positive heat flow into the hydrogel sample during the cooling cycle beginning at −17.38°C. DSC, differential scanning calorimetry.
Thermal stability in low temperature storage
Because these hydrogels are planned to be stored at low temperatures until point-of-care use, the low temperature stability of the hydrogels was measured by DSC. Freezing the hydrogels would dramatically change the polymeric architecture and thus the release kinetics by creating pores due to ice crystal formation. By slowly decreasing the temperature from 25°C to −40°C, we were able to determine the freezing point of the alginate hydrogels containing the antibiotics to be −17.4°C. The freezing point is indicated by a positive heat flow into the hydrogel sample during the cooling cycle (Fig. 3C).
Burst release of antibiotics from alginate hydrogels
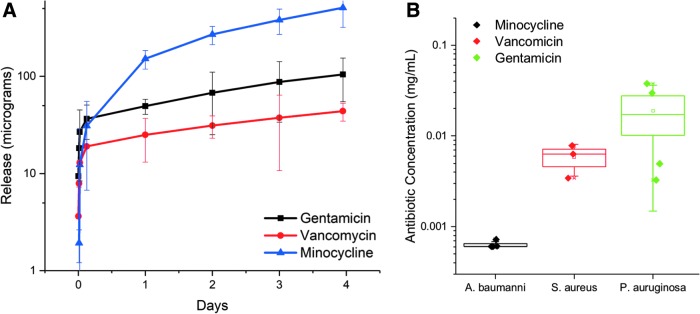
Using LCMS, we were able to quantify the amount of cumulative drug being released from the alginate hydrogel formulations over the course of 4 days. Overall, all hydrogels displayed burst release kinetics, with the majority of the drugs being released into the simulated wound supernatant within the first few hours of contact. This is desirable in this application as the imperative is to reach high levels of antibiotic concentrations to immediately suppress bacterial growth. These results also show that the minocycline-laden hydrogels exhibited the fastest release kinetics, although these were also the samples that had the highest molar concentration of antibiotics, as these were formulated to be 1000 × minimum inhibitory concentration (MIC). The release curves are consistent with Fickian diffusion-based release mechanisms (Fig. 4A).
Figure 4.
Hydrogel samples of 500 μL were placed in Trans-well membrane plates, with 500 μL of PBS in the bottom well. At each time point, 100 μL of aliquots was taken from the bottom well, whereas 100 μL of fresh PBS was added simultaneously. Aliquots were run on LCMS to quantify the concentrations of the respective antibiotics. (A) Release of minocycline, vancomycin, and gentamicin from the 0.625% alginate hydrogel over time. (B) The amount of remaining minocycline, vancomycin, gentamicin, and fluconazole in the alginate hydrogel after 7 days on deep-partial thickness porcine burn that had been inoculated with different microorganisms (Acinetobacter baumannii, Staphylococcus aureus, Pseudomonas aeruginosa). LCMS, liquid chromatography–mass spectrometry. PBS, phosphate-buffered saline.
Remaining antibiotics in the hydrogel post-therapy
To determine the extent to which the antibiotics have been adsorbed or used up at the wound site, the hydrogels were collected from the animals following a 7-day treatment. These were from induced burn wounds that were inoculated with three different bacteria: A. baumannii, S. aureus, and P. aeruginosa, corresponding to minocycline, vancomycin, and gentamicin, respectively. The hydrogels were digested using 34 U/mL of alginate lyase for 20 min at 37°C, and the resultant solution was analyzed for antibiotic concentration via LCMS. Despite having the highest initial antibiotic concentration, the minocycline hydrogels showed less than 1 μg/mL of the original 8,000 μg/mL available. This suggests that there is substantially more uptake in minocycline compared with vancomycin followed by gentamicin. Vancomycin, which had slower release kinetics from the hydrogel, had significantly more update than gentamicin (Fig. 4B).
Animal studies
Depth of tissue necrosis
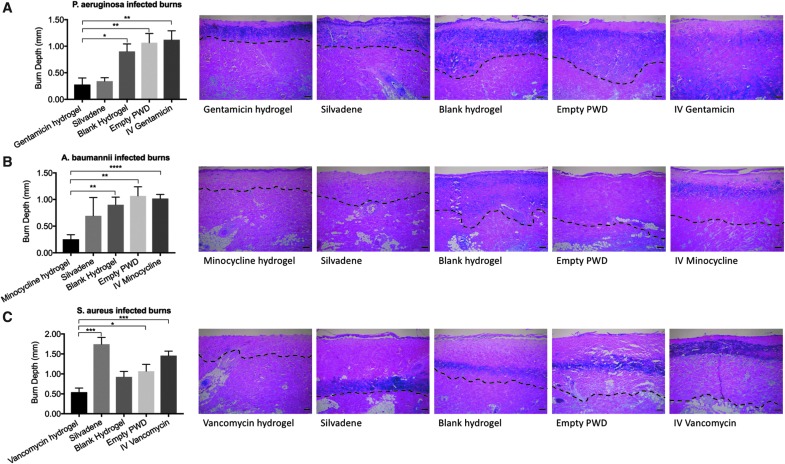
On day 7 post-burn, the results showed that topical gentamicin hydrogel (1000 × MIC) decreased the depth of tissue necrosis more efficiently in the P. aeruginosa-infected burns than blank hydrogel, empty PWD, and IV treatment with gentamicin (p < 0.05). The depth of tissue necrosis in the burn wounds treated with topical gentamicin hydrogel (1000 × MIC), Silvadene cream, blank hydrogel, empty PWD, and IV minocycline was 0.28 ± 0.12 mm, 0.34 ± 0.07 mm, 0.9 ± 0.14 mm, 1.1 ± 0.17 mm, and 1.21 ± 0.17 mm, respectively (Fig. 5A). Minocycline alginate hydrogel also reduced the depth of tissue necrosis statistically significantly in comparison to controls (p < 0.05), excluding Silvadene cream. The depth of tissue necrosis in the burn wounds treated with topical minocycline hydrogel (1000 × MIC), Silvadene cream, blank hydrogel, empty PWD, and IV minocycline was 0.25 ± 0.1 mm, 0.70 ± 0.34 mm, 0.9 ± 0.14 mm, 1.1 ± 0.17 mm, and 1.02 ± 0.1 mm, respectively (Fig. 5B). The depth of tissue necrosis in the burn wounds treated with topical vancomycin hydrogel (1000 × MIC), Silvadene cream, blank hydrogel, empty PWD, and IV vancomycin was 0.54 ± 0.1 mm, 1.74 ± 0.17 mm, 0.93 ± 0.14 mm, 1.1 ± 0.1 mm, and 1.5 ± 0.11 mm, respectively. The results showed that in the S. aureus-infected deep partial-thickness burns topical vancomycin hydrogel (1000 × MIC) reduced the depth of tissue necrosis the most efficiently. The difference between vancomycin hydrogel and Silvadene cream, empty PWD, and IV vancomycin was statistically significant (p < 0.05) (Fig. 5C).
Figure 5.
(A) Gentamicin: on day 7 post-burn, the results showed that topical gentamicin hydrogel (1000 × MIC) decreased the depth of tissue necrosis more efficiently in the P. aeruginosa-infected burns than blank hydrogel (p = 0.0135), empty PWD (p = 0.0065), and IV treatment (p = 0.0038) with gentamicin (p < 0.05). H&E-stained sections represent the burn wound after each treatment, and dashed lines are used to depict the depth of tissue necrosis. (B) Minocycline: minocycline alginate hydrogel also reduced the depth of tissue necrosis statistically significantly in comparison to controls (p < 0.05), excluding Silvadene cream (p-values: minocycline vs. blank hydrogel = 0.0049; minocycline vs. empty PWD = 0.0027; minocycline vs. IV minocycline = 0.0001). H&E-stained sections represent the burn wound after each treatment, and dashed lines are used to depict the depth of tissue necrosis. (C) Vancomycin: the results showed that in the S. aureus-infected deep partial-thickness burns topical vancomycin hydrogel (1000 × MIC) reduced the depth of tissue necrosis the most efficiently. The difference between vancomycin hydrogel and Silvadene cream (p = 0.0003), empty PWD (p = 0.0284), and IV vancomycin (p = 0.0002) was statistically significant. H&E-stained sections represent the burn wound after each treatment, and dashed lines are used to depict the depth of tissue necrosis. Comparison of groups was performed using t-test, and p-values <0.05 were considered statistically significant (*0.01 ≤ p < 0.05, **0.001 ≤ p < 0.01, ***0.0001 ≤ p < 0.001, p < 0.0001). H&E, hematoxylin and eosin; IV, intravenous; MIC, minimum inhibitory concentration.
Re-epithelialization
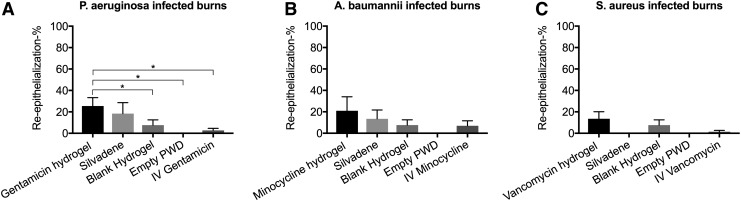
The burns treated with 1000 × MIC gentamicin hydrogel, Silvadene cream, blank hydrogel, empty PWD, and IV gentamicin were re-epithelialized 25.45% ± 7.83%, 18.35% ± 10.25%, 7.61% ± 4.95%, 0.00% ± 0.00%, and 2.73% ± 1.87%, respectively. The difference between topical gentamicin and blank hydrogel, empty PWD, and IV gentamicin was statistically significant (Fig. 6A). The burns treated with 1000 × MIC minocycline hydrogel, Silvadene cream, blank hydrogel, empty PWD, and IV minocycline were re-epithelialized 20.96% ± 13.1%, 13.45% ± 8.28%, 7.61% ± 4.95%, 0.00% ± 0.00%, and 6.95% ± 4.66%, respectively. No statistically significant differences were observed between the treatments (Fig. 6B). The burns treated with 1000 × MIC vancomycin hydrogel were re-epithelialized 13.55% ± 14.5%. The re-epithelialization percentage of the burns treated with Silvadene cream, blank hydrogel, empty PWD, and IV vancomycin was 0.00 ± 0.00, 7.61 ± 4.95, 0.00 ± 0.00, and 1.35 ± 1.35, respectively. No statistically significant differences were observed between the treatments (Fig. 6C).
Figure 6.
(A) Gentamicin: the burns treated with 1000 × MIC gentamicin hydrogel exhibited the highest re-epithelialization percentage on day 7 post-burn. The difference between topical gentamicin and empty PWD (p = 0.0241) as well as between IV gentamicin was statistically significant (p = 0.0130). (B) Minocycline: the burns treated with 1000 × MIC minocycline hydrogel had the highest re-epithelialization percentage in comparison to the other groups; however, no statistically significant differences were observed. (C) Vancomycin: the burns treated with 1000 × MIC vancomycin hydrogel were re-epithelialized more than the other groups, but no statistically significant differences were observed. Comparison of groups was performed using t-test, and p-values <0.05 were considered statistically significant (*0.01 p < 0.05, 0.001 p < 0.01, 0.0001 p < 0.001, p < 0.0001).
Quantitative bacteriology
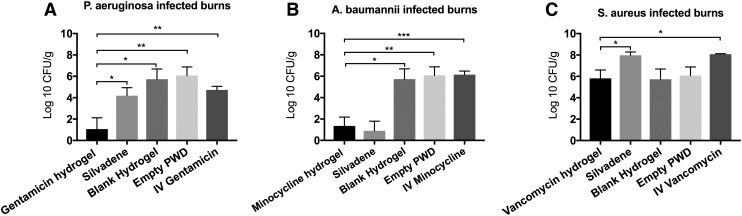
In the P. aeruginosa-infected burns, the 1000 × MIC gentamicin hydrogel reduced the number of bacteria in the injured tissue statistically significantly better than all the other groups (p < 0.05) (Fig. 7A). The bacterial counts in the burns treated with gentamicin hydrogel, Silvadene cream, blank hydrogel, empty PWD, and IV gentamicin were 1.06 ± 1.06 log 10 CFU/g, 4.18 ± 0.75 log 10 CFU/g, 5.73 ± 0.96 log 10 CFU/g, 6.08 ± 0.80 log 10 CFU/g, and 4.73 ± 0.33 log 10 CFU/g, respectively. The results also showed that both 1000 × MIC minocycline hydrogel and Silvadene cream reduced the number of A. baumannii in the injured tissue statistically significantly better than the IV minocycline (p < 0.05). Otherwise, no statistically significant differences were observed. The bacterial counts in the burns treated with minocycline hydrogel, Silvadene cream, blank hydrogel, empty PWD, and IV gentamicin were 1.35 ± 0.83 log 10 CFU/g, 0.90 ± 0.90 log 10 CFU/g, 5.73 ± 0.96 log 10 CFU/g, 6.08 ± 0.80 log 10 CFU/g, and 6.15 ± 0.33 log 10 CFU/g, respectively (Fig. 7B). In the S. aureus-infected burns, the 1000 × MIC vancomycin hydrogel reduced the number of bacteria in the injured tissue statistically significantly better than both Silvadene cream and IV vancomycin (p < 0.05) (Fig. 7C). The bacterial counts in the burns treated with vancomycin hydrogel, Silvadene cream, blank hydrogel, empty PWD, and IV gentamicin were 5.81 ± 0.79 log 10 CFU/g, 7.97 ± 0.31 log 10 CFU/g, 5.73 ± 0.96 log 10 CFU/g, 6.08 ± 0.80 log 10 CFU/g, and 8.07 ± 0.05 log 10 CFU/g, respectively. In addition to tissue samples, the number of bacteria in the serum was also studied on days 1, 3, 5, and 7. The analyses showed that no bacteria were detected in the serum of any of the pigs at any day.
Figure 7.
(A) Gentamicin: in the P. aeruginosa-infected burns, the 1000 × MIC gentamicin hydrogel reduced the number of bacteria in the injured tissue statistically significantly better than all the other groups (p-values: gentamicin vs. Silvadene = 0.0429; gentamicin vs. blank hydrogel = 0.0156; gentamicin vs. empty PWD = 0.0087; gentamicin vs. IV gentamicin = 0.0059). (B) Minocycline: in the A. baumannii-infected burns, the 1000 × MIC minocycline hydrogel reduced the number of bacteria in the injured tissue statistically significantly better than the blank hydrogel (p = 0.0106), empty PWD (p = 0.0051), and IV minocycline (p = 0.003). Otherwise, no statistically significant differences were observed. (C) Vancomycin: in the S. aureus-infected burns, the 1000 × MIC vancomycin hydrogel reduced the number of bacteria in the injured tissue statistically significantly better than both Silvadene (p = 0.0337) cream and IV vancomycin (p = 0.0113). Comparison of groups was performed using t-test, and p-values <0.05 were considered statistically significant (*0.01 p < 0.05, **0.001 p < 0.01, ***0.0001 p < 0.001, p < 0.0001).
Serum antibiotic levels
Serum samples were collected on days 1, 3, 5, and 7 to study the systemic antibiotic concentrations after topical treatment. The gentamicin concentrations on days 1, 3, 5, and 7 were 0.4, 0.00, 0.0, and 0.4 μg/mL, respectively. Concentrations <4.0 are not considered clinically relevant.24 For minocycline, the results demonstrated that no minocycline (0 μg/mL) was detected in any of the blood serum samples at any time point. In addition, the results showed that the vancomycin concentration in the blood serum samples on days 1, 3, 5, and 7 was 0.35, 0.00, 0.03, and 0.00 μg/mL, respectively. Concentrations <0.5 are not considered clinically relevant25 (Table 2).
Table 2.
Antibiotic concentration [μg/mL]
| Day | Gentamicin | Minocycline | Vancomycin |
|---|---|---|---|
| 1 | 0.35 | 0 | 0.4 |
| 3 | 0 | 0 | 0 |
| 5 | 0.03 | 0 | 0 |
| 7 | 0 | 0 | 0.4 |
Systemic antibiotic concentrations after topical treatment on days 1, 3, 5, and 7 after the topical treatment with high concentrations of antibiotics.
Depth, area, and volume of tissue necrosis on day 45 post-burn
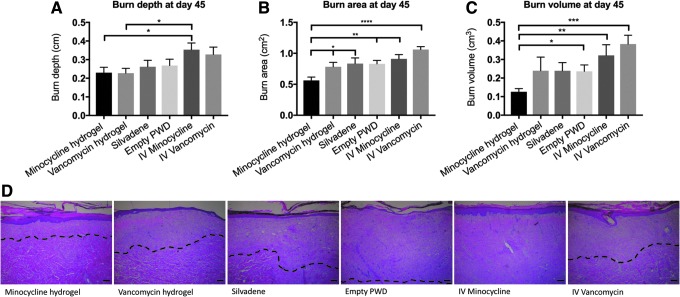
At day 45, the depth of tissue necrosis (in centimeters) in the burns treated with topical minocycline (1000 × MIC), topical vancomycin (1000 × MIC), Silvadene cream, empty PWD, IV minocycline, and IV vancomycin was 0.23 ± 0.07, 0.23 ± 0.06, 0.26 ± 0.09, 0.27 ± 0.09, 0.35 ± 0.08, and 0.33 ± 0.09, respectively. Both topical minocycline and vancomycin reduced the depth of tissue necrosis statistically significantly better than IV minocycline (p < 0.05). No other statistically significant differences were observed (Fig. 8A). At day 45, the area of tissue necrosis (in square centimeters) in the burns treated with topical minocycline (1000 × MIC), topical vancomycin (1000 × MIC), Silvadene cream, empty PWD, IV minocycline, and IV vancomycin was 0.56 ± 0.13, 0.78 ± 0.17, 0.83 ± 0.26, 0.83 ± 0.16, 0.91 ± 0.17, and 1.06 ± 0.11, respectively. The area of tissue necrosis in the burns that were treated with topical minocycline hydrogel was statistically significantly smaller than that in the other treatment groups (p < 0.05) (Fig. 8B). At day 45, the volume of tissue necrosis (in cubic centimeters) in the burns treated with topical minocycline (1000 × MIC), topical vancomycin (1000 × MIC), Silvadene cream, empty PWD, IV minocycline, and IV vancomycin was 0.13 ± 0.04, 0.24 ± 0.18, 0.24 ± 0.13, 0.24 ± 0.10, 0.32 ± 0.14, and 0.38 ± 0.12, respectively. The volume of tissue necrosis in the burns that were treated with topical minocycline hydrogel was statistically significantly smaller than that in burns that were treated with the empty PWD, IV minocycline, or IV vancomycin (p < 0.05) (Fig. 8C).
Figure 8.
(A) At day 45, both topical minocycline (p = 0.0284) and vancomycin (p = 0.0212) had reduced the depth of tissue necrosis statistically significantly better than IV minocycline. No other statistically significant differences were observed. (B) The area of tissue necrosis in the burns that were treated with topical minocycline hydrogel was statistically significantly smaller than that in the other treatment groups (p-values: minocycline vs. vancomycin = 0.0382; minocycline vs. Silvadene = 0.0382; minocycline vs. empty PWD = 0.055; minocycline vs. IV minocycline = 0.0024; minocycline vs. IV vancomycin = 0.0001). (C) The volume of tissue necrosis in the burns that were treated with topical minocycline hydrogel was statistically significantly smaller than that in the burns treated with the empty PWD (p = 0.024), IV minocycline (p = 0.0084), or IV vancomycin (p = 0.0005). (D) H&E-stained sections of the burn wounds on day 45 after each treatment. Dashed lines are used to depict the depth of tissue necrosis. Comparison of groups was performed using t-test, and p-values <0.05 were considered statistically significant (*0.01 p < 0.05, **0.001 p < 0.01, ***0.0001 p < 0.001, ****p < 0.0001).
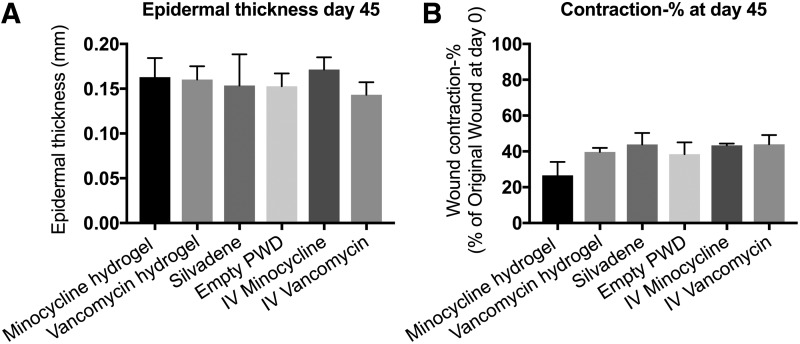
Re-epithelialization, epidermal thickness, and wound contraction on day 45 post-burn
All the burn wounds regardless of the treatment were 100% re-epithelialized by day 45 post-burn. The epidermal thickness in the burn wounds treated with topical minocycline (1000 × MIC), topical vancomycin (1000 × MIC), Silvadene cream, empty PWD, IV minocycline, and IV vancomycin was 0.16 ± 0.05, 0.16 ± 0.03, 0.15 ± 0.09, 0.15 ± 0.04, 0.17 ± 0.03, and 0.14 ± 0.03, respectively. No statistically significant differences were observed (Fig. 9A). Wound contraction was measured and expressed as a percentage of its original size on day 0. The results showed that the burns treated with minocycline hydrogel (26.64% ± 7.46%) had decreased contraction compared with the other treatments (vancomycin hydrogel: 39.72% ± 2.2%; Silvadene cream: 43.82% ± 6.4%; empty PWD: 38.5% ± 6.55%; IV minocycline: 43.34% ± 1.03%; vancomycin hydrogel: 43.96% ± 5.18%), but these differences were not statistically significant (Fig. 9B).
Figure 9.
(A) The epidermal thickness in the burn wounds treated with topical minocycline (1000 × MIC), topical vancomycin (1000 × MIC), Silvadene cream, empty PWD, IV minocycline, and IV vancomycin was measured on day 45 post-burn. No statistically significant differences were observed. (B) Wound contraction was measured and expressed as a percentage of its original size on day 0. No statistically significant differences were observed. Comparison of groups was performed using t-test, and p-values <0.05 were considered statistically significant (0.01 p < 0.05, 0.001 p < 0.01, 0.0001 p < 0.001, p < 0.0001).
Discussion
Over the years, burn care has advanced considerably due to improvements in both acute and long-term treatments. The areas that have improved the most include resuscitation, ventilation, nutrition, and sepsis control. As a result, the mortality has more than halved since the 1950s and the length of the hospital stay has also greatly reduced.4 In addition, many tissue engineering strategies have enhanced the long-term outcome of severely burnt patients.26 Despite notable advances in burn care, there is still a critical need to improve immediate burn treatment, particularly when the definitive treatment is delayed. This is important because majority of the burns occur in underdeveloped areas with a compromised access to a specialized burn care.5,27 It is important to start treatment of the burn immediately after the injury and continue until debridement and split-thickness skin grafting to stop injury progression and thus reduce the depth of tissue necrosis. Furthermore, efficient immediate burn care can promote healing and reduce scarring and other long-term consequences of severe burns.28
Alginate is a commonly used hydrophilic polymer in many biomedical applications, and it has many advantages over some other polymers. Alginate-based products have a very high water uptake, and they provide a good bacterial barrier. Alginate hydrogels also stay stable for a long time and have high mechanical stability, possessing good drug release characteristics. Furthermore, alginate products are nonadherent and easily removable from the wound surface by rinsing with saline. Therefore, alginate-based hydrogels have especially been recommended for the treatment of deep burns, surgical wounds, high exudate wounds, and chronic ulcers.16,29 Today, there are numerous commercially available alginate-based wound dressings, and besides hydrogels, they also exist in many other forms such as in films, foams, and sponges.30,31 Our results from the alginate hydrogel characterization experiments support the previous findings and demonstrated that the antibiotics released efficiently over time and that the gel stayed stable even in very low temperatures (Figs. 3 and 4).
In the animal studies, we evaluated the safety and efficacy of the PWD in a combination with an alginate hydrogel that contains high concentrations of antibiotics for immediate treatment of burn wounds. First, the alginate hydrogel was characterized and optimized for the application. Subsequently, infected burn wounds were treated and the effect on injury progression, bacterial counts, as well as on the quality of healing were studied over time. Our results from the porcine studies showed that enclosing the burn wounds with the PWD and treating them with high concentrations of antibiotics in the alginate hydrogel reduced the number of bacteria in the injured tissue and the burn depth in comparison to controls. This indicates that when applied topically on the burn, the antibiotics released efficiently from the alginate hydrogels and subsequently penetrate the burn eschar. Correspondingly, Yang et al. (2018) treated methicillin-resistant Staphylococcus aureus (MRSA)–infected full-thickness porcine burns with high concentrations of topical minocycline and likewise showed that minocycline infiltrated burn eschar and accelerated wound bed preparation. The study also indicated that high concentrations of topical minocycline was effective against MRSA infection.32 Importantly, our results also demonstrated that treating the burns topically with very high antibiotic concentrations was safe since no systemic toxicity or other adverse effects were observed in any of the animals during or after treatment. Previously, Junker et al. (2015), Daly et al. (2016), as well as Tsai et al. (2016) have similarly demonstrated in porcine models of full-thickness wounds and burns that ultrahigh concentrations of minocycline and gentamicin in liquid formulations could be safely and efficiently administered topically.10,33,34
The PWD is a treatment system for immediate burn care. It can be used to encapsulate serious burn wounds at the first point of care and protect them against contamination by delivering antibiotics formulated in a hydrogel during transit to a medical facility. In addition, we have shown that analgesics also can be delivered safely and efficiently either alone or together with antimicrobials using this treatment platform.33,35 Furthermore, the PWD can also be used to deliver negative pressure to the wound to accelerate healing. In our recently published study, the use of the PWD as a simplified negative pressure wound therapy device was introduced and validated.36
In conclusion, the purpose of this study was to produce a hydrogel that contains high concentrations of antibiotics and use the gel together with the PWD for immediate treatment of burn wounds. In the present study, we developed and characterized an alginate hydrogel and mixed various antibiotics into the formulation. The results from the deep partial-thickness porcine model demonstrated that by enclosing the burn wounds with the PWD, antibiotics can be delivered topically and safely in very high concentrations formulated in an alginate hydrogel. In addition, the study showed that the treatment reduced burn wound depth, and infection can be treated successfully with this method. Furthermore, we hypothesize that this treatment would also be efficient in the treatment of biofilm infection. However, further work is necessary to demonstrate this in practice.
Innovation
There is a need to improve immediate burn care, especially when the definitive treatment is delayed. We have developed the PWD and formulated an alginate hydrogel that contains very high concentrations of antibiotics for the immediate topical treatment of burns. The PWD is light weight and simple to use at the point of injury. It can be used to protect the burn, deliver antibiotics and other drugs in high concentrations that will stop the injury progression, and rapidly decontaminate the wound.
Key Findings
The PWD is a treatment system for immediate burn care that can be used to encapsulate serious burn wounds at the first point of care.
By enclosing the burn wounds with the PWD, antibiotics can be delivered topically and safely in very high concentrations formulated in an alginate hydrogel.
When applied on the burns, antibiotics released efficiently over time from the alginate hydrogel and subsequently penetrated the burn eschar.
Acknowledgments and Funding Sources
This material is based on work supported by the USAMRAA under Contract No. W81XWH-16-1-0784. The views, opinions, and/or findings contained in this report are those of the authors and should not be construed as an official Department of the Army position, policy, or decision unless so designated by other documentation.
Abbreviations and Acronyms
- CFU
colony-forming unit
- DSC
differential scanning calorimetry
- H&E
hematoxylin and eosin
- IV
intravenous
- LCMS
liquid chromatography–mass spectrometry
- MIC
minimum inhibitory concentration
- PBS
phosphate-buffered saline
- PWD
platform wound device
Author Disclosure and Ghostwriting
K.N., L.Y., M.B., and E.E. are employees of Applied Tissue Technologies that manufactures the PWDs. All the other authors declare no conflict of interest. No ghostwriters were used to write this article.
About the Authors
Kristo Nuutila, MSc, PhD, is a researcher and a biomedical engineer who currently works as the Director of Research at Applied Tissue Technologies. He is also a researcher at Brigham and Women's Hospital of Harvard Medical School and an Adjunct Professor at the University of Helsinki. Josh Grolman, PhD, is a postdoctoral researcher at Harvard University's Wyss Institute for Biologically Inspired Engineering and at John A. Paulson School of Engineering and Applied Sciences. Lu Yang, PhD, is a postdoctoral researcher at Applied Tissue Technologies. Michael Broomhead, MBA, is the CEO of Applied Tissue Technologies. Stuart Lipsitz, ScD, is the Director of Biostatistics at Brigham and Women's Center for Surgery and Public Health and a Professor at Harvard Medical School. Andrew Onderdonk, PhD, is a Professor of Pathology at Harvard Medical School and the Director of Clinical Microbiology at the Brigham and Women's Hospital. David Mooney, PhD, is the Robert P. Pinkas Family Professor of Bioengineering at Harvard University and the Core Faculty Member at Wyss Institute for Biologically Inspired Engineering. Elof Eriksson, MD, PhD, is a plastic surgeon and the Chief Medical Officer of Applied Tissue Technologies.
References
- 1. Stylianou N, Buchan I, Dunn KW. A review of the international Burn Injury Database (iBID) for England and Wales: descriptive analysis of burn injuries 2003–2011. BMJ Open 2015;5:e006184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev 2006;19:403–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tompkins RG. Survival from burns in the new millennium: 70 years' experience from a single institution. Ann Surg 2015;261:263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kearney L, Francis EC, Clover AJ. New technologies in global burn care—a review of recent advances. Int J Burns Trauma 2018;8:77–87 [PMC free article] [PubMed] [Google Scholar]
- 5. Peck MD. Epidemiology of burns throughout the world. Part I: distribution and risk factors. Burns 2011;37:1087–1100 [DOI] [PubMed] [Google Scholar]
- 6. Peck M, Pressman MA. The correlation between burn mortality rates from fire and flame and economic status of countries. Burns 2013;39:1054–1059 [DOI] [PubMed] [Google Scholar]
- 7. Williamson DA, Carter GP, Howden BP. Current and emerging topical antibacterials and antiseptics: agents, action, and resistance patterns. Clin Microbiol Rev 2017;30:827–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ng VW, Chan JM, Sardon H, et al. . Antimicrobial hydrogels: a new weapon in the arsenal against multidrug-resistant infections. Adv Drug Deliv Rev 2014;78:46–62 [DOI] [PubMed] [Google Scholar]
- 9. Brown TPLH, Cancio LC, McManus AT, Mason AD. Survival benefit conferred by topical antimicrobial preparations in burn patients: a historical perspective. J Trauma 2004;56:863–866 [DOI] [PubMed] [Google Scholar]
- 10. Tsai DM, Tracy LE, Lee CC, et al. . Full-thickness porcine burns infected with Staphylococcus aureus or Pseudomonas aeruginosa can be effectively treated with topical antibiotics. Wound Repair Regen 2016;24:356–365 [DOI] [PubMed] [Google Scholar]
- 11. Norman G, Christie J, Liu Z, et al. . Antiseptics for burns. Cochrane Database Syst Rev 2017;7:CD011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res 2015;6:105–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kamoun EA, Kenawy ES, Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res 2017;8:217–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater 2016;1:16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Madaghiele M, Demitri C, Sannino A, Ambrosio L. Polymeric hydrogels for burn wound care: advanced skin wound dressings and regenerative templates. Burns Trauma 2014;2:153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci 2012;37:106–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem 2014;6:25–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vranckx JJ, Slama J, Preuss S, et al. . Wet wound healing. Plast Reconstr Surg 2002;110:1680–1687 [DOI] [PubMed] [Google Scholar]
- 19. Eriksson E, Perez N, Slama J, et al. . Treatment of chronic, nonhealing abdominal wound in a liquid environment. Ann Plast Surg 1996;36:80–83 [DOI] [PubMed] [Google Scholar]
- 20. Singh M, Nuutila K, Minasian R, Kruse C, Eriksson E. Development of a precise experimental burn model. Burns 2016;42:1507–1512 [DOI] [PubMed] [Google Scholar]
- 21. Louie TJ, Tally FP, Bartlett JG, et al. . Rapid microbiological assay for chloramphenicol and tetracyclines. Antimicrob Agents Chemother 1976;9:874–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall, 1991 [Google Scholar]
- 23. Armitage P, Berry G, Matthews JNS. Statistical Methods in Medical Research (4th edition). Oxford: Blackwell Science, 2001 [Google Scholar]
- 24. Tan WH, Brown N, Kelsall AW, McClure RJ. Dose regimen for vancomycin not needing serum peak levels? Arch Dis Child Fetal Neonatal Ed 2002;87:F214–F216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hammett-Stabler CA, Johns T. Laboratory guidelines for monitoring of antimicrobial drugs. National Academy of Clinical Biochemistry. Clin Chem 1998;44:1129–1140 [PubMed] [Google Scholar]
- 26. Rowan MP, Cancio LC, Elster EA, et al. . Burn wound healing and treatment: review and advancements. Crit Care 2015;19:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keenan S, Riesberg JC. Prolonged field care: beyond the “Golden Hour.” Wilderness Environ Med 2017;28:S135–S139 [DOI] [PubMed] [Google Scholar]
- 28. Junker JP, Philip J, Kiwanuka E, Hackl F, Caterson EJ, Eriksson E. Assessing quality of healing in skin: review of available methods and devices. Wound Repair Regen 2014;22:2–10 [DOI] [PubMed] [Google Scholar]
- 29. Aderibigbe BA, Buyana B. Alginate in wound dressings. Pharmaceutics 2018;10:E42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Szekalska M, Puciłowska A, Szymańska E, Ciosek P, Winnicka K. Alginate: current use and future perspectives in pharmaceutical and biomedical applications. Int J Polym Sci 2016;8:1–17 [Google Scholar]
- 31. O'Meara S, Martyn-St James M, Adderley UJ. Alginate dressings for venous leg ulcers. Cochrane Database Syst Rev 2015;8:CD010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang L, Broomhead M, Nuutila K, et al. . Topically delivered minocycline penetrates a full-thickness burn eschar and reduces tissue bacterial counts. J Burn Care Res 2018;39:790–797 [DOI] [PubMed] [Google Scholar]
- 33. Junker JP, Lee CC, Samaan S, et al. . Topical delivery of ultrahigh concentrations of gentamicin is highly effective in reducing bacterial levels in infected porcine full-thickness wounds. Plast Reconstr Surg 2015;135:151–159 [DOI] [PubMed] [Google Scholar]
- 34. Daly LT, Tsai DM, Singh M, et al. . Topical minocycline effectively decontaminates and reduces inflammation in infected porcine wounds. Plast Reconstr Surg 2016;138:856e–868e [DOI] [PubMed] [Google Scholar]
- 35. Nuutila K, Yang L, Broomhead M, Proppe K, Eriksson E. PWD: treatment platform for both prolonged field care and definitive treatment of burn-injured warfighters. Mil Med 2019;184:e373–e380 [DOI] [PubMed] [Google Scholar]
- 36. Nuutila K, Yang L, Broomhead M, Proppe K, Eriksson E. Novel negative pressure wound therapy device without foam or gauze is effective at −50 mmHg. Wound Repair Regen 2019;27:162–169 [DOI] [PubMed] [Google Scholar]