Abstract
Purpose:
Some resources recommended Bouin solution for the fixation of testis biopsy specimens. We compared the histologic quality of rat testicular tissue using buffered formalin and Bouin solution as fixatives.
Methods:
We prospectively compared the histologic quality of rat testicular tissue fixed in Bouin solution versus formalin. Testicular tissue was harvested post-mortem from six rats. Each testis was removed and sectioned in half; one half was fixed in formalin and one half in Bouin solution. Testicular tissue histology (nuclear membrane detail, nuclear granularity, cytoplasmic granularity, cytoplasmic membrane detail, and basement membrane detail) was graded as high quality (2) or low quality (1). Sloughing of cells into the lumens of the seminiferous tubules was graded on a 0-3 scale (0=none, 1=mild, 2=moderate, 3=extensive).
Results:
All slides regardless of fixative were of appropriate quality for the histologic evaluation of spermatogenesis. The average sloughing score for formalin cases was 1.4 and for Bouin cases 1.6. Formalin fixed tissue was found to have high quality nuclear membrane detail (2), nuclear granularity (1.9), and basement membrane detail (2). Cytoplasmic granularity was of lesser but adequate quality (1.4). Cytoplasmic membrane detail was poor, (1). Tissue fixed with Bouin solution had high quality basement membrane detail (2) and adequate cytoplasmic granularity (1.5), nuclear membrane detail (1.3) and nuclear granularity (1.4). Cytoplasmic membrane detail was poor (1).
Conclusion:
Compared to Bouin solution, formalin fixation of rat testicular tissue produced adequate histology for the evaluation of spermatogenesis and may be superior to Bouin solution for certain cytologic features.
Keywords: Testis, biopsy, histology, pathology, fixation, quality assurance
Introduction
Fifteen percent of couples are affected by infertility, and approximately 50% of infertile couples have some male factor.1-3 Failure to conceive after 1 year of unprotected intercourse often leads both male and female partners to undergo fertility evaluations. The AUA Best Practice statement on male infertility summarizes the goal of the infertility evaluation
to recognize and treat reversible conditions, to categorize disorders potentially amenable to assisted reproductive techniques (ART), to identify syndromes and conditions which may be detrimental to the patient’s health, and to distinguish genetic abnormalities which can be transmitted to or affect the health of the offspring.4
The evaluation of an infertile man includes a comprehensive history and physical exam, two semen analyses, and assessment for any underlying endocrine dysfunction. In azoospermic men with normal testes, at least 1 palpable vas deferens, and normal follicle-stimulating hormone levels, a testicular biopsy is performed to differentiate between obstructive and nonobstructive azoospermia.2,5
Testicular biopsy interpretation is important to differentiate between obstruction and a disorder of spermatogenesis and in some instances, the findings are predictive of successful sperm retrieval.1,6,7 Due to concern that common fixatives, such as formalin, may distort the architecture of testicular tissues, Bouin solution has traditionally been the recommended fixative for testis biopsy specimens.1,2 Bouin solution is a preparation of 5% acetic acid, 9% formaldehyde, and 1.5% picric acid in aqueous solution. The picric acid in Bouin solution presents challenges for safe handling and disposal due to its mutagenic and explosive potential.8 Tissues fixed with Bouin solution require multiple rinses with alcohol to remove the picric acid to prevent compromised staining.8 The handling precautions and washes are very time-consuming for histology laboratories. Although buffered formalin is the most common fixative used in pathology, its use as a testicular fixative has been discouraged due to concern that it may cause shrinkage artifact and sloughing of luminal cells. This could potentially hamper assessment of the stages of sperm development.1,4 Formalin offers advantages over Bouin solution, however, in its ease of use, availability as a common fixative used in histology labs, and lack of explosive potential. For the past several years at our institution, formalin has been used as a fixative for testis biopsies due to regulations restricting the presence of Bouin solution in operating rooms. Herein, we compare the use of buffered formalin and Bouin solution for fixation of rat testes.
Materials and Methods
We obtained institutional review board approval for our study. A prospective evaluation was carried out to compare the histologic quality of rat testicular tissue fixed in Bouin solution versus formalin. Testicular tissue was harvested post-mortem from 6 rats. Tissue was collected within 15 minutes of death to prevent histologic changes secondary to ischemic injury. Each testis was removed and sectioned in half; one half was fixed in formalin and one half in Bouin solution, yielding a total of 12 specimens in each group. The rat testicular tissue was allowed to fix in each respective solution for 6 hours and then processed. The following morphologic features were evaluated and graded as high quality (2) or low quality (1) based on microscopic appearance: nuclear membrane detail, nuclear granularity, cytoplasmic granularity, cytoplasmic membrane detail, and basement membrane detail. In addition, sloughing of cells into the lumnes of the seminiferous tubules was graded on a 0 to 3 scale (0 = none, 1 = mild, 2 = moderate, 3 = extensive). The histologic evaluation was performed by 4 surgical pathologists, 2 with genitourinary expertise and 2 general surgical pathologists, all blinded to the fixative used per tissue specimen. The scores from all pathologists were averaged to help give an idea of cytologic quality while taking into account interobserver variability.
Results
There was no spatial heterogeneity identified in the histologic quality of the tissue samples. All slides regardless of fixative were of appropriate quality for the histologic evaluation of spermatogenesis (Table 1). Some degree of sloughing of cells into the lumens of the seminiferous tubules was present in all cases. However, no cases had extensive sloughing of cells and on average there was minimal to moderate sloughing. The average sloughing score for formalin cases was 1.4 and for Bouin cases 1.6.
Table 1.
Histologic findings in rat testicular tissue utilizing different fixatives.
| Average pathologic score |
||
|---|---|---|
| Formalin | Bouin solution | |
| Nuclear membrane detail | 2 | 1.3 |
| Nuclear granularity | 1.9 | 1.4 |
| Cytoplasmic granularity | 1.4 | 1.5 |
| Cytoplasmic membrane detail | 1 | 1 |
| Basement membrane detail | 2 | 2 |
| Sloughing of cells into lumens | 1.6 | 1.4 |
For nuclear membrane detail, nuclear granularity, cytoplasmic granularity, cytoplasmic detail, and basement membrane detail 2 = highest quality. For sloughing of cells 3 = most sloughing.
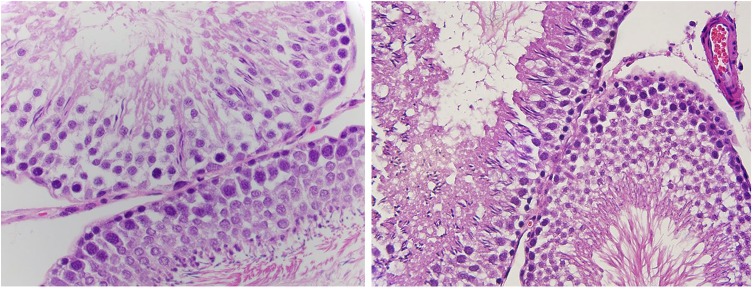
Formalin-fixed tissue was found to be of high quality with regard to nuclear membrane detail (average score = 2), nuclear granularity (average score = 1.9), and basement membrane detail (average score = 2) (Figure 1, 2). Indeed, in regard to these cytologic variables, the formalin-fixed tissue showed high-quality histology with a high consensus among all pathologists. In addition, cytoplasmic granularity was of lesser but adequate quality with an average score of 1.4. Cytoplasmic membrane detail was poor, with an average score of 1 and a complete agreement almost pathologists.
Figure 1 and 2.
High-power magnification of a hematoxylin and eosin stained slide from rat testicular tissue fixed in formalin showing high quality morphologic features, especially nuclear detail.
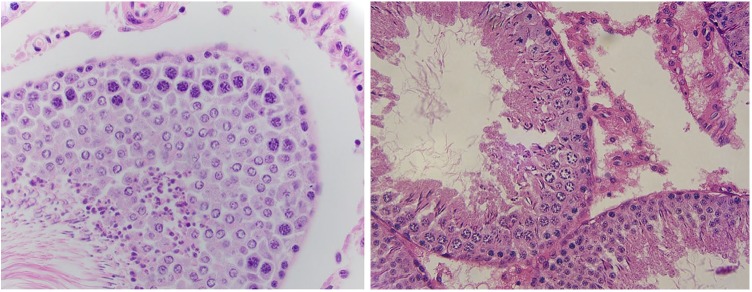
Tissue fixed with Bouin solution had high-quality basement membrane detail with an average score of 2 and complete agreement among pathologists. Bouin solution also produced adequate cytoplasmic granularity, with an average score of 1.5. However, nuclear membrane detail (1.3) and nuclear granularity (1.4) received lower scores on average than tissue fixed in formalin (Figure 3, 4). Interestingly, Bouin solution also produced poor cytoplasmic membrane detail, similar to what was seen in formalin-fixed tissue.
Figure 3 and 4.
High-power magnification of a hematoxylin and eosin stained slide from rat testicular tissue fixed in Bouin solution showing nuclear clearing and clumping of chromatin in cells.
Discussion
Testis biopsy plays a critical role in the evaluation of azoospermic men by differentiating between obstructive azoospermia and disorders of spermatogenesis. In addition, testicular biopsy findings can help guide subsequent interventions and predict future success of sperm retrieval.1,6,7,9 Pathologists assess for the presence of sertoli cells, germ cells, and leydig cells as well as appropriate maturation of germ cells and appropriate spermatogenesis for age. Presence of germ cell neoplasia in situ (GCNIS) must also be evaluated as the infertility population is at higher risk for testicular tumors and GCNIS is a precursor to most germ cell tumors.2
Histologic artifact, such as poor morphologic detail and sloughing of cells into the lumens of the seminiferous tubules, can make evaluation of testicular biopsy specimens challenging for pathologists. Bouin solution has historically been recommended as a fixative for testicular biopsy specimens. At our institution, due to safety concerns, Bouin solution is not allowed in operating rooms. This necessitated that we find an alternative fixative. Campbell-Walsh Urology recommends use of either Bouin, Zenker, or collidine-buffered glutaraldehyde solution for testis fixation and specifically states that formalin should not be used.2 Other studies from the urologic pathology literature recommend against the use of formalin as a fixative for testicular tissue due to concern that shrinkage artifact and sloughing of luminal cells will prevent accurate histologic interpretation.1,2,6 To our knowledge, however, there have been no studies that directly compare Bouin solution with formalin for testis biopsy fixation.
Formalin has advantages over Bouin solution in that it is widely available and easy to use. In the United States, formalin is used for the vast majority of histologic tissue processing. While both Formalin and Bouin solution have their own risks with regard to occupational exposure, Bouin solution carries additional risk due to the explosive nature to one of its components, picric acid. In addition, it is not clear whether Bouin solution is superior with regard to tissue fixation. Latendresse et al compared Bouin solution with a modified Davidson solution, a solution of formaldehyde, ethanol, glacial acetic acid, and water, for fixation of rat testis. They assessed for clarity of morphologic detail, shrinkage of seminiferous tubules, cytoplasmic graininess, nuclear chromatin aggregation, sharpness of acrosomal staining, and immunohistochemical staining and found that shrinkage of seminiferous tubules was more pronounced in testis fixed with Bouin solution.8 However, they did not compare Bouin solution with formalin directly.
In our study of rat testicular tissue, tissue histology by both fixation methods was of adequate quality for the evaluation of primary versus secondary causes of azoospermia. It is difficult to explain the observed variation in histologic quality among samples fixed using the same solution. All testes were harvested at roughly the same interval after death, thus controlling for ischemic time. However, some testes were larger specimens than others and the containers all had a standard volume of fixative, as is common with prefilled containers that one would utilize in the clinical setting. It is possible that specimen size could have impacted the quality of fixation. However, all specimens were adequately submerged in fixative for 6 hours prior to processing and final tissue histology can show variation even when using the same processing conditions.
Slides from specimens fixed in formalin were found to be of uniformly high quality with respect to nuclear membrane detail, nuclear granularity, and basement membrane detail. Bouin solution also produced uniform high quality for basement membrane detail and had slightly less sloughing of cells on average. However, slides from Bouin solution–fixed specimens demonstrated more variability, with lower quality histology for nuclear membrane detail, nuclear granularity, and cytoplasmic granularity.
The authors recognize that there will always be interobserver variability in the analysis of cytologic features. As such, we performed the histologic analysis by 4 surgical pathologists to help create an overall consensus for the results. Based on our findings with rat testis tissue, both formalin and Bouin solution are appropriate to use for fixation of testis biopsy specimens. Formalin’s ubiquitous use in pathology makes it an attractive alternative to Bouin solution and circumvents the handling precautions and lengthy process involved when working with Bouin solution. A prospective study comparing formalin and Bouin solution could further support the use of formalin for human testis tissue.
Conclusion
Compared with Bouin solution, formalin fixation of rat testicular tissue produced adequate histology for the evaluation of spermatogenesis and may be superior to Bouin solution for certain cytologic features, including nuclear membrane detail and nuclear granularity. We therefore conclude that formalin solution is an appropriate alternative to Bouin solution for fixation of testis biopsy specimens.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: James L Ellenburg  https://orcid.org/0000-0001-6174-1361
https://orcid.org/0000-0001-6174-1361
Giovanna Giannico  https://orcid.org/0000-0002-0326-3207
https://orcid.org/0000-0002-0326-3207
References
- 1. Cerilli LA, Kuang W, Rogers D. A practical approach to testicular biopsy interpretation for male infertility. Arch Pathol Lab Med. 2010;134:1197-1204. [DOI] [PubMed] [Google Scholar]
- 2. Wein AJ, Kavoussi LR, Campbell MF. Campbell-Walsh Urology. 11th ed. Philadelphia, PA: Elsevier Saunders; 2016. [Google Scholar]
- 3. Olesen IA, Andersson AM, Aksglaede L, et al. Clinical, genetic, biochemical, and testicular biopsy findings among 1,213 men evaluated for infertility. Fertil Steril. 2017;107:74-82.e7. [DOI] [PubMed] [Google Scholar]
- 4. Male Infertility Best Practice Policy Committee of the American Urological Association; Practice Committee of the American Society for Reproductive Medicine. Report on optimal evaluation of the infertile male. Fertil Steril. 2006;86:S202-S209. [DOI] [PubMed] [Google Scholar]
- 5. Gordetsky J, van Wijngaarden E, O’Brien J. Redefining abnormal follicle-stimulating hormone in the male infertility population. BJU Int. 2012;110: 568-572. [DOI] [PubMed] [Google Scholar]
- 6. Dohle GR, Elzanaty S, van Casteren NJ. Testicular biopsy: clinical practice and interpretation. Asian J Androl. 2012;14:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hess RA, Moore BJ. Histological methods for evaluation of the testes. In: Chapin RE, Heindel JJ, eds. Methods in Toxicology, Vol. 3: Part A. Male Reproductive Toxicology. San Diego, CA: Academic Press; 1993:86-94. [Google Scholar]
- 8. Latendresse JR, Warbrittion AR, Jonassen H, Creasy DM. Fixation of testes and eyes using a modified Davidson’s fluid: comparison with Bouin’s fluid and conventional Davidson’s fluid. Toxicol Pathol. 2002;30:524-533. [DOI] [PubMed] [Google Scholar]
- 9. Su LM, Palermo GD, Goldstein M, Veeck LL, Rosenwaks Z, Schlegel PN. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia: testicular histology can predict success of sperm retrieval. J Urol. 1999;161:112-116. [PubMed] [Google Scholar]