Abstract
Mycobacterium shigaense has recently been recognized as an emerging human pathogen, and is well known as a skin pathogen in immunocompromised individuals. In this report we describe the first case of chronic pulmonary infectious disease caused by M. shigaense in an immunocompetent individual.
Keywords: Chest computed tomography (CT) images, emerging infectious diseases, immunocompetent, Mycobacterium shigaense, Mycobacterium simiae complex, Pulmonary disease, skin disease
Mycobacterium shigaense is an emerging human pathogen. It is slow-growing and scotochromogenic and we first reported it as an opportunistic mycobacterium isolated from the skin of an individual with a severe cellular immunodeficiency disorder [1]. Recently, we completed genome sequencing of M. shigaense and proposed this pathogen to be a new mycobacterial genus [2,3]. Eight patients with M. shigaense infections have been reported, most of them had skin and/or disseminated diseases associated with cellular immunodeficiency [1,2,[4], [5], [6]]. In contrast, we encountered a patient with a chronic pulmonary infection that was similar to Mycobacterium avium complex. Hence, this is the first case of an M. shigaense pulmonary infection in an immunocompetent patient.
A 58-year-old man was admitted for evaluation of an abnormal chest radiograph obtained during an annual health check-up in July 2014.
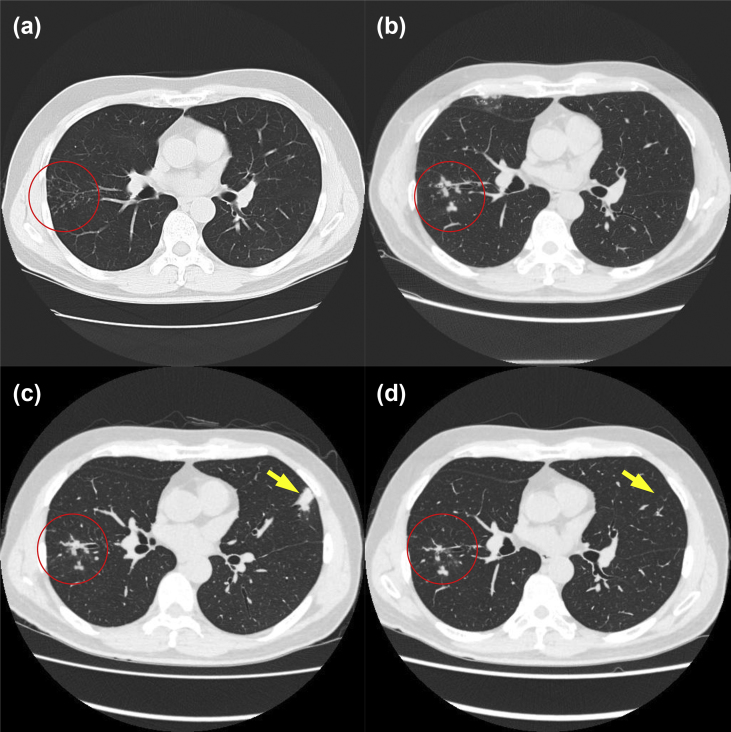
On physical examination, the body mass index was 24.1 kg/m2, the vital signs were normal, and there was no cervical or axillary lymphadenopathy. The man had no history of immunosuppressive therapy. The results of routine laboratory tests were unremarkable, including the erythrocyte sedimentation rate and C-reactive protein level. A chest computed tomography (CT) scan revealed multiple nodular intensities in the right S6 region and middle lobes (Fig. 1a). A QuantiFERON-TB Gold In-Tube test was negative.
Fig. 1.
Transition of chest computed tomography (CT) images. (a) Multiple small nodular shadows in the right lower lobe (circle; October 2014).(b) Worsening nodular shadows in the right middle lobe (circle; April 2017). (c) Chest CT showed further improvement of multiple nodular shadows in the right lower lobe (circle). New consolidation in the lingular lobe (arrow; October 2017). (d) Image 4 months after treatment. Shrinkage of nodular lesion in the right lower lobe (circle) and resolution of consolidation in the lingular lobe (arrow; February 2018).
At the time of the follow-up evaluation, there were increased nodular regions in the right upper and lower lobes (Fig. 1b). Bronchoalveolar lavage was performed for diagnosis. The samples were collected from bronchoalveolar lavage fluid/brushing and subjected to smear testing and culture. Ziehl–Neelsen staining of the smears for acid-fast bacilli was positive; the other microbial cultures were sterile. An isolate (strain OCH) streaked on an egg-slant at 37°C for 3 weeks formed smooth, yellow colonies, so a mycobacterial infection was suspected. PCRs for Mycobacterium tuberculosis and M. avium complex were negative and a commercially available identification test for 18 common species was also negative.
Partial DNA sequences of the 16S rRNA, hsp65 and rpoB genes were determined (the GenBank/EMBL/DDBJ accession numbers are LC508988, LC508986 and LC508985, respectively). The sequences of these three genes were identical to the type strain of M. shigaense (UN-152).
In vitro drug susceptibility was determined using a microdilution method according to established guidelines (CLSI M24A). The isolate was susceptible to clarithromycin, rifampicin, tedizolid and bedaquilin, and resistant to carbapenem (faropenem, imipenem and meropenem).
After the diagnosis, a follow-up chest CT scan revealed improvement in the right middle lobe and worsening in the left lingular lobe (Fig. 1c). The patient was treated with rifampin (450 mg once a day), ethambutol (750 mg once a day) and clarithromycin (600 mg once a day) orally for 4 months. The consolidations in the left lingular lobe and some of the nodules in the right lower lobe subsided (Fig. 1d). New nodules did not recur, and no notable side effects occurred.
We have provided further evidence that M. shigaense should be classified as a pathogenic respiratory microorganism in immunocompetent individuals. The lesions improved after triple therapy.
Conflicts of interest
There is no conflict of interest.
Acknowledgements
This work was in part supported by a grant from the Japan Agency for Medical Research and Development/Japan International Cooperation Agency (AMED) to Y. Hoshino (jp18fk0108043, jp18fk0108064, jp18fk0108075 and jp18jm0510004), by Grants-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (JSPS) to Y. Hoshino (jp18K08312), and by Grants-in-Aid for Early-Career Scientists to H. Fukano (jp18K15966). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nakanaga K., Hoshino Y., Wakabayashi M., Fujimoto N., Tortoli E., Makino M. Mycobacterium shigaense sp. nov., a novel slowly growing scotochromogenic mycobacterium that produced nodules in an erythroderma patient with severe cellular immunodeficiency and a history of Hodgkin’s disease. J Dermatol. 2012;39:389–396. doi: 10.1111/j.1346-8138.2011.01355.x. [DOI] [PubMed] [Google Scholar]
- 2.Fukano H., Yoshida M., Kazumi Y., Fujiwara N., Katayama K., Ogura Y. Mycobacterium shigaense sp. nov., a slow-growing, scotochromogenic species, is a member of the Mycobacterium simiae complex. Int J Syst Evol Microbiol. 2018;68:2437–2442. doi: 10.1099/ijsem.0.002845. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida M., Fukano H., Ogura Y., Kazumi Y., Mitarai S., Hayashi T. Complete genome sequence of Mycobacterium shigaense. Genome A. 2018;6 doi: 10.1128/genomeA.00552-18. e00552–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui P., Vissa V., Li W., Zhang X., Lin L., Wang H. Cutaneous Mycobacterium shigaense infection in immunocompetent woman, China. Emerg Infect Dis. 2013;19:819–820. doi: 10.3201/eid1905.121022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koizumi Y., Shimizu K., Shigeta M., Minamiguchi H., Hodohara K., Andoh A. Mycobacterium shigaense causes lymph node and cutaneous lesions as immune reconstitution syndrome in an AIDS patient: the third case report of a novel strain non-tuberculous Mycobacterium. Intern Med. 2016;55:3375–3381. doi: 10.2169/internalmedicine.55.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naito D., Mizumoto C., Takeoka T., Tsuji M., Tomo K., Kazumi Y. Case report; a case of disseminated Mycobacterium shigaense infection. Nihon Naika Gakkai Zasshi. 2016;105:717–722. doi: 10.2169/naika.105.717. [DOI] [PubMed] [Google Scholar]