Abstract
Introduction
Mild traumatic brain injury (TBI) is a global public health concern that affects millions of children annually. Mild TBI tends to result in subtle and diffuse alterations in brain tissue, which challenges accurate clinical detection and prognostication. Diffusion tensor imaging (DTI) holds promise as a diagnostic and prognostic tool, but little research has examined DTI in post-acute mild TBI. The current study compared post-acute white matter microstructure in children with mild TBI versus those with mild orthopedic injury (OI), and examined whether post-acute DTI metrics can predict post-acute and chronic post-concussive symptoms (PCS).
Materials and methods
Children aged 8–16.99 years with mild TBI (n = 132) or OI (n = 69) were recruited at emergency department visits to two children's hospitals, during which parents rated children's pre-injury symptoms retrospectively. Children completed a post-acute (<2 weeks post-injury) assessment, which included a 3T MRI, and 3- and 6-month post-injury assessments. Parents and children rated PCS at each assessment. Mean diffusivity (MD) and fractional anisotropy (FA) were derived from diffusion-weighted MRI using Automatic Fiber Quantification software. Multiple multivariable linear and negative binomial regression models were used to test study aims, with False Discovery Rate (FDR) correction for multiple comparisons.
Results
No significant group differences were found in any of the 20 white matter tracts after FDR correction. DTI metrics varied by age and sex, and site was a significant covariate. No interactions involving group, age, and sex were significant. DTI metrics in several tracts robustly predicted PCS ratings at 3- and 6-months post-injury, but only corpus callosum genu MD was significantly associated with post-acute PCS after FDR correction. Significant group by DTI metric interactions on chronic PCS ratings indicated that left cingulum hippocampus and thalamic radiation MD was positively associated with 3-month PCS in the OI group, but not in the mild TBI group.
Conclusions
Post-acute white matter microstructure did not differ for children with mild TBI versus OI after correcting for multiple comparisons, but was predictive of post-acute and chronic PCS in both injury groups. These findings support the potential prognostic utility of this advanced DTI technique.
Keywords: Pediatric traumatic brain injury, Concussion, Mild traumatic brain injury, Diffusion tensor imaging
1. Introduction
Pediatric traumatic brain injury (TBI) is a major global public health concern that affects millions of children annually (Ruff et al., 2009). Most TBI (i.e., ~ 85–90%) sustained by children are classified as mild in severity, including concussion (Gilchrist et al., 2011; Ruff et al., 2009). Outcomes from pediatric mild TBI can be highly heterogeneous and difficult to predict from one child to the next (Lumba-Brown et al., 2018; Taylor et al., 2010; Yeates et al., 2009). Although most children recover within several weeks or months following the injury, nearly one third have persistent complaints of post-concussive symptoms (PCS) after 1 month or longer (Barlow, 2016; Novak et al., 2016; Taylor et al., 2010; Yeates et al., 2009).
Neurobiological outcomes of pediatric mild TBI have proven equally complex. In contrast to more severe TBI, the subtle and diffuse effects of mild TBI are generally not visible on conventional clinical neuroimaging (Maugans et al., 2012; Wilde et al., 2008; Yallampalli et al., 2013). For that reason, the use of neuroimaging for accurate clinical diagnosis and prognostication of affected children is challenging (Lumba-Brown et al., 2018; Mayer et al., 2018; Papa et al., 2013; Yeates et al., 2017), especially within the context of the significant inter- and intra-individual variability that normally occurs with development across childhood (Vértes and Bullmore, 2015).
Advanced neuroimaging techniques such as diffusion tensor imaging (DTI) have provided promising insights into the neuropathology of mild TBI (Mayer et al., 2018). DTI techniques allow for non-invasive, in vivo investigation of brain microstructure and have demonstrated potentially high sensitivity to the subtle white matter alterations associated with pediatric mild TBI in both its early and late stages (Bigler, 2013; Bigler and Bazarian, 2010; Bigler and Maxwell, 2012; Dennis et al., 2017; Schmidt et al., 2018). Furthermore, DTI techniques have shown promise in predicting the outcomes of pediatric mild TBI (Bigler, 2015; Königs et al., 2017; Shenton et al., 2012). However, the few DTI studies of pediatric mild TBI to date have reported inconsistent findings, likely as a consequence of small sample sizes and highly variable methodologies. The post-acute effects of mild TBI on brain tissue remains particularly understudied (Chamard and Lichtenstein, 2018; Dennis et al., 2017; Schmidt et al., 2018).
1.1. Diffusion tensor imaging in pediatric mild TBI
DTI techniques are the most commonly utilized advanced MRI approach in studies of pediatric mild TBI (Schmidt et al., 2018). Most DTI studies have detected differences between youth with mild TBI and their respective comparison groups (Schmidt et al., 2018), even in the absence of visible trauma-related abnormalities (Chu et al., 2010; Mayer et al., 2012; Wilde et al., 2008; Yallampalli et al., 2013). However, findings have varied widely across studies. For example, both higher and lower fractional anisotropy (FA) have been reported in post-acute pediatric mild TBI (Babcock et al., 2015; Beek et al., 2015; Chu et al., 2010; Ewing-Cobbs et al., 2019; Mac Donald et al., 2019; Mayer et al., 2018; Murdaugh et al., 2018; Mustafi et al., 2018; Schmidt et al., 2018; Wilde et al., 2008), although a recent meta-analysis of mild TBI in children and adults suggested that FA is generally increased post-acutely but reduced in chronic injury phases (Eierud et al., 2014). Generally, mean diffusivity (MD) is reduced in children with mild TBI up to 6 months post-injury relative to controls (Babcock et al., 2015; Chu et al., 2010; Wilde et al., 2008), although differences have not been reported in all studies of chronic time periods (Bartnik-Olson et al., 2014; Königs et al., 2018). Alterations of white matter microstructure have been reported in many regions, including the corpus callosum, cortico-spinal tract, thalamic radiations, superior longitudinal fasciculus, and frontal white matter (Babcock et al., 2015; Beek et al., 2015; Chu et al., 2010; Ewing-Cobbs et al., 2019; Königs et al., 2018; Mac Donald et al., 2019; Mayer et al., 2018; Murdaugh et al., 2018; Mustafi et al., 2018; Schmidt et al., 2018; Wilde et al., 2008). These subcortical white matter tracts have shown the greatest strain and parenchymal deformation in association with mild TBI (Okamoto et al., 2019; Wu et al., 2004).
The prognostic utility of using early post-acute indices of white matter microstructure to predict early and chronic PCS severity in pediatric mild TBI is of both scientific and clinical interest. However, previous findings have been mixed. Post-acute microstructural characteristics have been related to post-acute PCS severity in children with mild TBI, with greater alterations associated with increased symptom severity in some (Chu et al., 2010; Mustafi et al., 2018; Wilde et al., 2008; Yallampalli et al., 2013; Yuan et al., 2015) but not all studies (Babcock et al., 2015; Mayer et al., 2012; Murdaugh et al., 2018). Notably, several of the papers demonstrating significant associations were based on the same sample (Chu et al., 2010; Wilde et al., 2008; Yallampalli et al., 2013). Further investigation into how early post-acute (i.e., within the first several weeks post-injury) white matter microstructure relates to early and later PCS is warranted to clarify these inconsistencies.
Although previous research has highlighted the potential sensitivity of DTI metrics to pediatric mild TBI and its associated outcomes, several limitations need to be addressed through further investigation. DTI studies of pediatric mild TBI have generally relied on small sample sizes that have been highly heterogeneous with regards to participant and injury characteristics (Dodd et al., 2014). Age ranges of participants have varied widely between studies (Babcock et al., 2015; Chu et al., 2010; Ewing-Cobbs et al., 2019; Mayer et al., 2010; Mustafi et al., 2018; Wilde et al., 2018b, 2008; Wozniak et al., 2007). Given that childhood is a period of dynamic maturation, further investigation of a broad age range in a single, larger sample is warranted to help determine whether mild TBI affects expected age-related trajectories of white matter development.
Comparison groups have also differed between studies. With few exceptions, most studies have compared children with mild TBI to typically developing children. Some studies have included non-concussed athletes or athletes who participate in noncontact sports (Mustafi et al., 2018; Spader et al., 2018). Less commonly, children with mild TBI have been compared to children with mild extracranial or orthopedic injuries (OI; Babcock et al., 2015; Ewing-Cobbs et al., 2019). The inclusion of typically developing children is problematic for several reasons (Mathias et al., 2013; Wilde et al., 2018a). First, it does not account for pre-injury characteristics that could predispose children to injury. It also does not control for post-injury factors such as psychological stress in response to trauma, pain, or effects related to medical treatment. A recent DTI study found that white matter microstructure characteristics of adolescents and young adults with mild TBI or OI were generally similar, but differed from a third group of healthy controls (Wilde et al., 2018b). Finally, practical reasons exist for including an extracranial or OI group; namely, they are more clinically relevant, given that health care providers must determine whether children with traumatic injuries sustained a mild TBI and are at risk for further complications. Thus, their clinical reference group is children with injuries, not healthy children.
DTI studies of pediatric mild TBI also have had varied widely in the timing of post-injury assessments (Dodd et al., 2014). Common terms used to describe post-injury timing, including acute, post-acute, semi-acute, and chronic, have been employed somewhat arbitrarily. For example, the terms “post-acute” and “semi-acute” have been used to characterize imaging obtained as early as 48 h and as late as 20 days post-injury (Chu et al., 2010; Mayer et al., 2012; Murdaugh et al., 2018; Mustafi et al., 2018; Wilde et al., 2008). Studies of chronic mild TBI have ranged from roughly 3 months to several years post-injury (e.g., Königs et al., 2018; Mayer et al., 2012). As previously mentioned, only a few studies have examined white matter microstructure in the post-acute period, with multiple reports from a single cohort (Chu et al., 2010; Wilde et al., 2008). Lastly, the majority of studies have used voxel-based analyses or included only specific ROIs. We recently showed that tractography-based approaches are superior to voxel-wise approaches that require co-registration to a template (Goodrich-Hunsaker et al., 2018).
1.2. Current study aims and hypotheses
The current study attempted to address the shortcomings of previous research by examining white matter microstructure in a larger, multisite cohort of children with mild TBI or OI. DTI was used to measure early post-acute (i.e., within 2-weeks post-injury) white matter microstructure. Few group differences (mild TBI vs. OI) in early post-acute DTI metrics (i.e., FA and MD) were expected given recent findings from a larger sample of adolescents and young adults (Wilde et al., 2018b). We also examined relations between post-acute DTI metrics and PCS ratings at approximately 2 weeks, 3 months, and 6 months post-injury. The severity of PCS was expected to be higher in children with mild TBI relative to those with OI at 2 weeks post-injury (Taylor et al., 2010), but not at the later timepoints, and to be predicted by DTI metrics in children with mild TBI, but not in children with OI.
2. Materials and methods
2.1. Study design and procedure
Data were drawn from a larger study of pediatric mild TBI that involved a prospective, concurrent cohort design with longitudinal follow-up. Children with mild TBI or OI between the ages of 8 and 16.99 years at the time of injury were recruited during emergency department (ED) visits within 24 h post-injury at Nationwide Children's Hospital (NCH) in Columbus, OH and Rainbow Babies and Children's Hospital (RBCH) in Cleveland, OH. Recruitment occurred over a period of 48 months to accrue the desired sample size. The study was conducted in compliance with internal review board standards for ethical clinical pediatric research at NCH and RBCH.
Information regarding the child's injury and acute clinical presentation was obtained at the ED visit, during which parents also provided retrospective ratings of their child's pre-injury symptoms. Children returned for three further assessments: post-acute (target within 2 weeks; range = 3–18 days, Mdn = 11.00, M = 10.31, SD = 2.81) and at 3- and 6-months post-injury. At the post-acute visit, children completed an MRI, as well as ratings of current PCS. Parents also rated the child's current PCS. Similar ratings were obtained at 3- and 6-months post-injury.
2.2. Participants and recruitment
Participants for the current study included children with mild TBI or mild OI who completed an MRI and other measures at the post-acute assessment. A comparison group of children with mild OI not involving the head was included to distinguish the effects of mild TBI from injury in general, to account for the stressful experience of suffering a traumatic injury, and to control for premorbid functioning and demographics (Loder et al., 1995; Mathias et al., 2013; Ozer et al., 2010; Uslu et al., 2007; Wilde et al., 2018b).
Children with mild TBI were included if they experienced a blunt head trauma that resulted in at least one of the following: (1) an observed loss of consciousness for less than 30 min, (2) Glasgow Coma Scale (GCS; Teasdale and Jennett, 1974) of 13 or greater (GCS was 13 or 14 in cases with no loss of consciousness or other signs or symptoms), or (3) at least two acute signs of symptoms of concussion as noted on standard case reports form (e.g., post-traumatic amnesia, focal neurological deficits, skull fracture, vomiting, headache, dizziness, or other mental status changes).
Children with OI were included if they experienced an upper or lower extremity fracture with an Abbreviated Injury Scale (AIS) score of 4 or less (Greenspan et al., 1985). Children with OI were excluded from the study if they showed any evidence of head trauma or any acute signs or symptoms of concussion. Children with mild TBI were eligible if they had a co-occurring OI.
Both groups were subject to the following exclusion criteria: (1) any other severe injury as defined by an AIS score greater than 4, (2) any associated injury likely to interfere with neuropsychological testing, (3) hypoxia, hypotension, or shock during or following the injury, (4) alcohol or drug ingestion involved with the injury, (5) history of previous TBI requiring hospitalization, (6) premorbid neurological disorder or intellectual disability, (7) injury resulting from abuse or assault, (8) history of severe psychiatric disorder requiring hospitalization, or (10) any contraindication to MRI. Children who were administered analgesic medication, including narcotics, were not excluded from either group. Additionally, children with a history of learning or attention problems were not excluded because they are at increased risk for sustaining traumatic injuries (Lee et al., 2008).
Overall, a total of 315 children (mild TBI = 195, OI = 120) were recruited for the study in the ED. Of those, a total of 217 children, 143 (73%) with mild TBI and 74 (62%) with OI, returned for the post-acute assessment. All but one of those who returned completed the post-acute MRI. Participants who returned for the post-acute assessment did not significantly differ from those who did not in acute symptoms as rated on the Standardized Assessment of Concussion (SAC; McCrea et al., 1998; F = 1.21, p = .273), sex (χ2 = 0.78, p = .799), premorbid cognitive (F = 0.02, p = .883) or somatic (F = 0.46, p = .498) symptoms ratings, or age at time of injury (F = 0.06, p = .807). However, socioeconomic status (SES), as estimated using the 2010 Census tract median family income, was significantly higher among children who returned for the post-acute assessment (M = $60,054.95, SD = $34,180.06) compared to those who did not return (M = $48,885.15, SD = $29,151.92), F = 7.88, p = .005, and significantly greater number of Caucasian and Asian children returned for the post-acute assessment than children with African-American, mixed, and another race (χ2 = 11.54, p < .001). Data from 15 participants who completed the post-acute MRI was excluded due to poor diffusion-weighted MRI data quality (i.e., severe motion or scanner-related artifact) or incomplete image acquisition (e.g., failure or refusal to complete the MRI).
Of the 201 (mild TBI = 132, OI = 69) participants with usable diffusion-weighted MRI data, 152 (mild TBI = 98, OI = 54) also completed the chronic assessment at 3 months post-injury and 134 (mild TBI = 92, OI = 42) completed the chronic assessment at 6 months post-injury. Participants who returned for the 3 month assessment did not significantly differ from those who did not in premorbid cognitive (F = 2.07, p = .151) or somatic (F = 2.36, p = .126) symptom ratings, post-acute cognitive (F < 1.00, p = .854) or somatic (F = 2.01, p = .157) PCS ratings, composite socioeconomic status (SES) index as described below (F < 1.00, p = .416), sex (χ2 < 1.00, p = .561), race (χ2 < 1.00, p = .965), or age at time of injury (F < 1.00, p = .359). The participants who returned at 3 months did have marginally significantly lower acute symptoms as rated on the SAC (M = 23.52, SD = 3.64) than those who did not return (M = 22.63, SD = 3.55), F = 3.88, p = .050. Participants who returned for the 6-month post-injury assessment did not significantly differ from those who did not in premorbid cognitive (F < 1.00, p = .984) or somatic (F = 1.27, p = .262) symptoms ratings, SES composite index (F < 1.00, p = .335), sex (χ2 = 3.24, p = .072), race (χ2 < 1.00, p = .990), age at time of injury (F < 1.00, p = .379), acute symptoms per the SAC (F < 1.00, p = .327), or post-acute somatic (F = 3.45, p = .065) PCS ratings. However, those who returned at 6 months did have significantly lower post-acute cognitive (M = 10.20, SD = 8.77) PCS ratings than those who did not return (M = 13.25, SD = 8.71), F = 5.43, p = .021.
2.3. Demographic information
Demographic information was collected during the post-acute assessment. A SES composite index was computed by averaging sample z-scores for years of maternal education, median family income for census tract, and the Duncan Socioeconomic Index (Stevens and Cho, 1985), a measure of occupational prestige. The two-subtest version of the Wechsler Abbreviated Scale of Intelligence - Second Edition (WASI-II) was used to estimate children's Full Scale IQ (Wechsler, 2011).
2.4. Post-concussive symptoms
Parent ratings of premorbid symptoms were collected during the ED visit, and post-injury PCS were rated by parents and children at each follow-up assessment, using the Health and Behavior Inventory (HBI; Ayr et al., 2009). The HBI has good internal consistency and inter-rater agreement, and has been adopted as a core measure in the Common Data Elements for Pediatric TBI (Adelson et al., 2012). It yields separate scores for cognitive and somatic symptoms.
2.5. Magnetic resonance imaging
Each participant completed a 3T MRI scan as part of the post-acute assessment. MRI scans were completed from 3–24 days post-injury (M = 10.82, Mdn = 11.00, SD = 3.21), with most scans (95%) completed between 3–15 days post-injury. Participants were not sedated during the MRI acquisition. Time between the injury and MRI scan did not differ by group, F(1, 196) = 1.94, p = .165, or site, F(1, 196) = 3.71, p = .056, and there was no group by site interaction, F(1, 196) < 1.00, p = .796. The MRI sequences were based on protocols recommended by the NINDS Common Data Elements group (Duhaime et al., 2012) and included: T1- and T2-weighted, axial T2-weighted fluid attenuated inversion recovery (FLAIR), axial susceptibility weighted (SWI), and axial diffusion-weighted sequences (Goodrich-Hunsaker et al., 2018). MRI at each site was accomplished in about 45–60 min. The same scanner was used for all studies at each site, to reduce within- and between-site variability. Periodic reliability checks were conducted by having randomly selected protocols from each site reviewed. Quality checks were also initially conducted through regular phantom scans.
MRI data were obtained at NCH using a Siemens Trio 3T scanner with a 32-channel head coil at the Research Institute at Nationwide Children's Hospital in Columbus, OH. High-resolution T1-weighted images were acquired using a 3D magnetization-prepared rapid gradient echo (MPRAGE) pulse sequence. The 3D MPRAGE T1-weighted sequence parameters were as follows: 192 contiguous sagittal slices with TR (repetition time) = 2200 ms; TE (echo time) = 4.37 ms; in-plane resolution = 0.90 mm2; slice thickness = 0.90 mm; flip angle = 78; field of view (FOV) = 230 mm2; and an acquisition matrix of 256 mm2. Diffusion-weighted images were acquired using a diffusion‐weighted spin echo‐planar imaging (EPI) pulse sequence with the following parameters: 64 interleaved axial slices with TR/TE = 6600/72.4 ms; in‐plane resolution = 1.8 mm2; slice thickness (with no gaps) = 1.8 mm; bandwidth = 1860 Hz/Px; FOV = 230 mm2; and with an acquisition matrix of 128 mm2. Diffusion gradients were applied in 30 directions with b = 700 s/mm2 with one b = 0 s/mm2.
MRI data were obtained at RBCH using a Philips 3.0T Achieva scanner with an 8-channel head coil at the University Hospitals Case Medical Center in Cleveland, OH. High-resolution T1-weighted images were acquired using a 3D magnetization-prepared rapid gradient echo (T1 3D TFE) pulse sequence. The 3D TFE T1-weighted sequence parameters were as follows: 170 contiguous sagittal slices with TR/TE = 8.9/4.1 ms; in-plane resolution = 0.90 mm2; slice thickness = 0.90 mm; flip angle = 88; FOV = 240 mm2; and acquisition matrix = 256 mm2. Diffusion-weighted images were acquired using a diffusion‐weighted spin‐echo EPI pulse sequence with the following parameters: 60 interleaved axial slices with TR/TE = 6634/76 ms; in‐plane resolution = 2 mm2; slice thickness (with no gaps) = 2 mm; bandwidth = 1786.9 Hz/Px in EPI freq direction; FOV = 224 mm2; and an acquisition matrix of 128 × 128 mm2. Diffusion gradients were applied in 32 directions with b = 800 s/mm2 with one b = 0 s/mm2.
2.5.1. Quality assurance and pre-processing
All images were inspected for visual artifact by an expert who was blind to group membership. Briefly, preprocessing and brain extraction procedures were completed on a remote Linux computing cluster. T1- and diffusion-weighted DICOM data were converted into NIfTI format using the dcm2niix tool in MRIcron (publicly available software; https://github.com/rordenlab/dcm2niix), and the bval and bvec files were automatically created from the raw diffusion-weighted DICOM headers. During conversion to NIfTI format, T1-weighted images were automatically reoriented to canonical space and auto-cropped. T1-weighted images were put into standard alignment using the Automatic Registration Toolbox acpcdetect tool (freely downloadable at https://www.nitrc.org/frs/?group_id=90), and were resampled with an isotropic voxel resolution of 1 mm using the Convert3D Medical Image Processing Tool (freely downloadable at http://www.itksnap.org/pmwiki/pmwiki.php?n=Downloads.C3D). Skull-stripped T1 images were acquired using the Advanced Normalization Tools version 2.1.0 volume-based cortical thickness estimation pipeline (antsCorticalThickness.sh; freely downloadable at https://github.com/stnava/ANTs/releases/tag/v2.1).
2.5.2. Automated fiber quantification
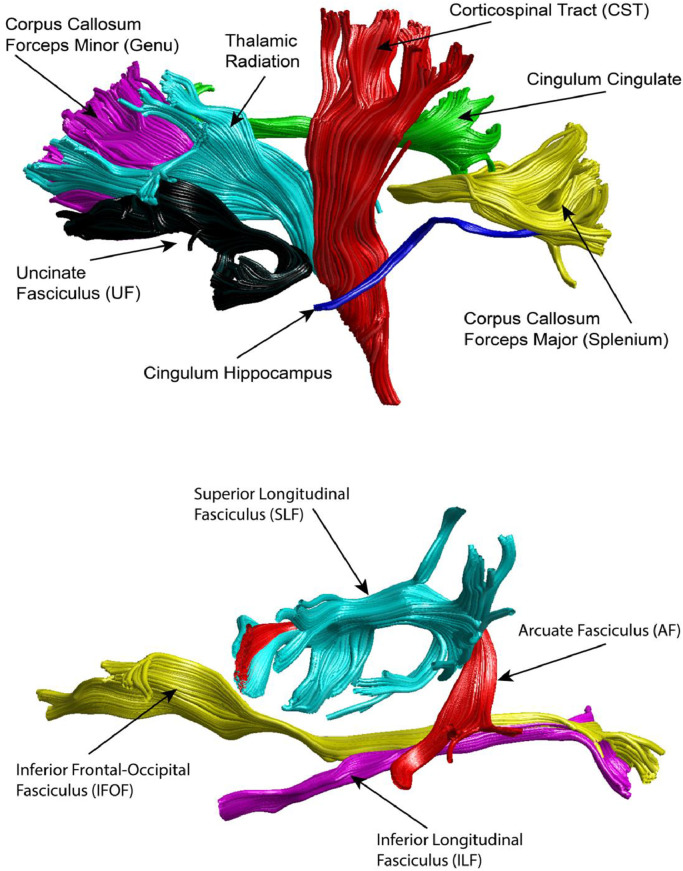
Automated global deterministic tractography was performed on diffusion-weighted images according to standard protocols. First, diffusion-weighted images were preprocessed using the dtiInit preprocessing pipeline wrapper from Stanford open-source VISTASOFT package version 1.0 (https://github.com/vistalab/vistasoft) running on MATLAB version 8.6.0 (R2015b; MathWorks Inc., Natick, MA). The diffusion-weighted images were corrected for eddy currents and head motion, skull-stripped, and tensor fitted. White matter pathways were automatically identified using an open-source, freely available software package, Automated Fiber Quantification (AFQ) version 1.2 (https://github.com/yeatmanlab/AFQ). The AFQ pipeline identifies 20 major fiber tracts, which are shown in Fig. 1 (Yeatman et al., 2012). FA and MD were extracted along 100 segments of each tract. Statistical analyses were performed on FA and MD averaged across the 100 segments of each tract.
Fig. 1.
The Automated Fiber Quantification (AFQ) pipeline was used to extract average fractional anisotropy (FA) and mean diffusivity (MD) from 20 identified white matter tracts, including the genu (forceps minor) and splenium (forceps major) of the corpus callosum and bilateral thalamic radiation, corticospinal tract, cingulum cingulate and hippocampal bundles, uncinate fasciculus, superior longitudinal fasciculus, inferior frontal occipital fasciculus, inferior longitudinal fasciculus, and arcuate fasciculus.
2.6. Statistical analyses
Demographic data were analyzed using analysis of variance (ANOVA) for continuous variables (e.g., age, SES) and chi-square techniques for categorical variables (e.g., sex).
For Aim 1, multiple multivariable regression analyses were used to investigate the relations of group, age, sex, and their interactions to FA and MD for each of the 20 white matter tracts, with site (i.e., NCH, RBCH) treated as a covariate. Non-significant interaction effects (adjusted for multiple comparisons) were trimmed from final models. For Aim 2, multiple multivariable negative binomial regression analyses were used to examine relations of DTI metrics for the average tract to post-acute and chronic (3- and 6-month post-injury) PCS ratings. Group, tract DTI metric (i.e., FA or MD), and their interaction were included as model predictors, with age, sex, and premorbid symptoms included as covariates, to test whether relations between post-acute white matter microstructure and residualized change in PCS severity differed in children with mild TBI versus OI.
The False Discovery Rate (FDR) was used to correct for multiple comparisons for all analyses (Benjamini and Hochberg, 1995). Continuous predictors in Aim 1 and 2 models were mean centered. A power analysis, conducted using G*Power v3.1 (Faul et al., 2009), indicated that the current sample size (N = 201) was sufficiently powered (1-β = 0.80) to detect a change in R2 of about 0.039 for a single tested predictor at α = 0.05, assuming 10 total predictors.
3. Results
3.1. Sample characteristics
Sample characteristics and demographic data are presented in Table 1. Across sites, the TBI and OI groups did not differ significantly in sex, age, or Full Scale IQ. However, SES was significantly higher in the group with mild TBI compared to the OI group and race significantly differed between the groups, with significantly greater number of white and Asian children in the mild TBI group compared to the OI group, across sites. No site differences were observed in sex, age, or Full Scale IQ, either across groups (sex, p = .241; age, p = .998; Full Scale IQ, p = .102) or within groups (TBI: sex, p = .297; age, p = .967; Full Scale IQ, p = .294; OI: sex, p = .850; age, p = .951; Full Scale IQ, p = .187).
Table 1.
Sample characteristics and demographic data.
| Variable | Group | |||||
|---|---|---|---|---|---|---|
| OI | TBI | |||||
| M/N | SD/% | M/N | SD/% | p | ||
| Age (M [SD] years) | 12.37 | 2.41 | 12.57 | 2.64 | .484 | |
| Full Scale IQ (M [SD]) | 97.81 | 14.62 | 98.51 | 14.32 | .745 | |
| Socioeconomic Status (M [SD] z-score) | −0.24 | 0.90 | 0.14 | 1.02 | .009 | |
| Race (N [%] White/Asian) | 27 | 39.13 | 72 | 54.54 | .038 | |
| Sex (N [%] male) | 47 | 68.12 | 87 | 65.91 | .753 | |
| Parent PCS Ratings | ||||||
| Premorbid Somatic Symptoms | 2.96 | 4.4 | 3.47 | 3.83 | .422 | |
| Premorbid Cognitive Symptoms | 9.99 | 7.82 | 10.03 | 7.58 | .917 | |
| Post-acute Somatic | 3.04 | 4.06 | 7.59 | 5.51 | <.001 | |
| Post-acute Cognitive | 9.07 | 8.37 | 12.34 | 8.91 | .018 | |
| 3-month Somatic | 2.98 | 4.12 | 3.44 | 3.98 | .299 | |
| 3-month Cognitive | 9.33 | 8.55 | 9.84 | 7.99 | .845 | |
| 6-month Somatic | 1.95 | 2.46 | 2.79 | 3.82 | .223 | |
| 6-month Cognitive | 8.93 | 8.73 | 9.07 | 8.35 | .906 | |
| Child PCS Ratings | ||||||
| Post-acute Somatic | 4.72 | 5.05 | 9.13 | 5.83 | <.001 | |
| Post-acute Cognitive | 9.78 | 8.13 | 13.74 | 8.23 | .016 | |
| 3-month Somatic | 4.30 | 4.22 | 4.89 | 4.90 | .656 | |
| 3-month Cognitive | 8.68 | 7.15 | 9.42 | 8.07 | .955 | |
| 6-month Somatic | 3.57 | 4.07 | 4.53 | 4.76 | .220 | |
| 6-month Cognitive | 8.40 | 7.48 | 8.10 | 7.60 | .945 | |
| Mechanism of Injury | .001 | |||||
| Fall | 29 | 42.02 | 43 | 32.58 | - | |
| Motor vehicle related | 2 | 2.89 | 6 | 4.55 | - | |
| Struck by an object | 16 | 23.19 | 35 | 26.52 | - | |
| Struck by a person | 7 | 10.14 | 34 | 25.76 | - | |
| Bicycle related | 5 | 7.25 | 12 | 9.09 | - | |
| Glasgow Coma Scale | – | – | 14.85 | 0.45 | – | |
3.2. White matter microstructure and time post injury
The number of days from injury to MRI scan was not associated with FA or MD in any of the tracts after FDR correction, across groups. However, average FA of the corpus callosum splenium (forceps major) was nominally, positively related to the number of days from injury to MRI in both groups, r = 0.17, p = .019.
3.3. Group differences in post-acute white matter microstructure
The results of multivariable regression analyses predicting average FA and MD of each tract segment are presented in Table 2. None of the 2- or 3-way interactions involving group, sex, and age were significant after FDR correction, so all interactions were trimmed from the final models. No group differences in average tract FA or MD were significant after FDR correction, suggesting no substantial difference between children with mild TBI versus OI. However, a nominally significant (i.e., unadjusted p < .05) group difference was found in average thalamic radiation MD bilaterally (TBI < OI).
Table 2.
Descriptive statistics and multivariable linear regression results examining group differences on white matter microstructure of examined tracts for Aim 1. Note. Italic = unadjusted p-value < .05; Bolded = FDR adjusted p-value < .05. AF = arcuate fasciculus; CST = Corticospinal Tract ; IFOF = inferior frontal-occipital fasciculus; SLF = superior longitudinal fasciculus; UF = uncinate fasciculus.
| Fractional anisotropy (FA) | Mean diffusivity (MD) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Descriptive statistics | Main effect | Descriptive statistics | Main effect | |||||||||||||||||||||
| Group | Age | Sex | Site | Group | Age | Sex | Site | |||||||||||||||||
| Mild TBI | OI | Parameter estimate | Mild TBI | OI | Parameter estimate | |||||||||||||||||||
| Tract | M | SD | M | SD | B | p | B | p | B | p | B | p | M | SD | M | SD | B | p | B | p | B | p | B | p |
| Left Hemisphere | ||||||||||||||||||||||||
| AF | 0.50 | 0.04 | 0.49 | 0.03 | <0.01 | .766 | <0.01 | .019 | <0.01 | .705 | −0.02 | <.001 | 0.75 | 0.03 | 0.75 | 0.03 | <0.01 | .601 | <0.01 | .002 | 0.01 | .086 | −0.03 | <.001 |
| Cingulum Cingulate | 0.49 | 0.06 | 0.47 | 0.06 | −0.01 | .098 | 0.01 | <.001 | 0.02 | .001 | −0.07 | <.001 | 0.78 | 0.03 | 0.79 | 0.04 | 0.01 | .058 | <0.01 | .006 | <0.01 | .706 | <0.01 | .570 |
| Cingulum Hippocampus | 0.36 | 0.04 | 0.37 | 0.04 | 0.01 | .447 | <0.01 | .065 | 0.01 | .403 | −0.05 | <.001 | 0.88 | 0.05 | 0.88 | 0.04 | 0.01 | .589 | <0.01 | .537 | <0.01 | .750 | 0.03 | .002 |
| CST | 0.63 | 0.03 | 0.63 | 0.03 | <0.01 | .822 | <0.01 | .003 | <0.01 | .769 | 0.01 | .040 | 0.77 | 0.04 | 0.77 | 0.03 | <0.01 | .403 | <0.01 | <.001 | <0.01 | .976 | −0.04 | <.001 |
| IFOF | 0.48 | 0.03 | 0.48 | 0.03 | <0.01 | .381 | <0.01 | .221 | −0.01 | .012 | −0.01 | .015 | 0.83 | 0.03 | 0.83 | 0.02 | <0.01 | .392 | <0.01 | .079 | 0.01 | .001 | <0.01 | .912 |
| ILF | 0.42 | 0.03 | 0.42 | 0.03 | <0.01 | .955 | <0.01 | .009 | <0.01 | .830 | −0.02 | <.001 | 0.87 | 0.04 | 0.88 | 0.03 | 0.01 | .135 | <0.01 | .117 | 0.01 | .133 | <0.01 | .829 |
| SLF | 0.43 | 0.05 | 0.44 | 0.05 | <0.01 | .533 | <0.01 | .019 | −0.01 | .296 | −0.06 | <.001 | 0.75 | 0.04 | 0.75 | 0.03 | <0.01 | .914 | <0.01 | .010 | 0.01 | .184 | −0.01 | .041 |
| Thalamic Radiation | 0.46 | 0.03 | 0.45 | 0.03 | <0.01 | .612 | <0.01 | .039 | 0.01 | .210 | −0.02 | <.001 | 0.76 | 0.03 | 0.77 | 0.02 | 0.01 | .033 | <0.01 | .030 | <0.01 | .647 | 0.01 | .025 |
| UF | 0.41 | 0.04 | 0.42 | 0.04 | <0.01 | .448 | <0.01 | .683 | −0.01 | .103 | −0.05 | <.001 | 0.86 | 0.03 | 0.86 | 0.03 | 0.01 | .183 | <0.01 | .232 | <0.01 | .856 | 0.03 | <.001 |
| Right Hemisphere | ||||||||||||||||||||||||
| AF | 0.47 | 0.04 | 0.47 | 0.04 | <0.01 | .940 | <0.01 | .122 | −0.01 | .223 | <0.01 | .531 | 0.77 | 0.05 | 0.76 | 0.05 | <0.01 | .783 | <0.01 | <.001 | 0.01 | .048 | −0.08 | <.001 |
| Cingulum Cingulate | 0.45 | 0.05 | 0.45 | 0.06 | <0.01 | .610 | 0.01 | <.001 | 0.01 | .311 | −0.06 | <.001 | 0.78 | 0.04 | 0.79 | 0.05 | <0.01 | .541 | <0.01 | .018 | 0.02 | .010 | −0.04 | <.001 |
| Cingulum Hippocampus | 0.38 | 0.04 | 0.37 | 0.04 | −0.01 | .386 | <0.01 | .269 | 0.01 | .268 | −0.04 | <.001 | 0.86 | 0.05 | 0.87 | 0.05 | 0.01 | .553 | <0.01 | .162 | <0.01 | .636 | 0.01 | .132 |
| CST | 0.63 | 0.03 | 0.63 | 0.03 | <0.01 | .688 | <0.01 | <.001 | <0.01 | .969 | 0.02 | <.001 | 0.76 | 0.04 | 0.76 | 0.03 | 0.01 | .250 | <0.01 | .022 | <0.01 | .820 | −0.04 | <.001 |
| IFOF | 0.49 | 0.03 | 0.50 | 0.03 | 0.01 | .084 | <0.01 | .028 | −0.01 | .031 | −0.01 | .001 | 0.83 | 0.04 | 0.84 | 0.04 | 0.01 | .117 | <0.01 | .104 | 0.01 | .019 | −0.04 | <.001 |
| ILF | 0.43 | 0.04 | 0.43 | 0.04 | <0.01 | .638 | <0.01 | .845 | <0.01 | .716 | −0.02 | .004 | 0.86 | 0.04 | 0.86 | 0.04 | <0.01 | .502 | <0.01 | .006 | 0.01 | .076 | −0.02 | .001 |
| SLF | 0.48 | 0.05 | 0.48 | 0.05 | <0.01 | .963 | <0.01 | <.001 | 0.01 | .188 | −0.04 | <.001 | 0.76 | 0.06 | 0.76 | 0.05 | <0.01 | .956 | <0.01 | .005 | 0.01 | .325 | −0.09 | <.001 |
| Thalamic Radiation | 0.47 | 0.03 | 0.47 | 0.03 | <0.01 | .861 | <0.01 | .001 | 0.01 | .110 | 0.01 | .005 | 0.76 | 0.03 | 0.77 | 0.03 | 0.01 | .020 | <0.01 | .444 | <0.01 | .409 | −0.03 | <.001 |
| UF | 0.43 | 0.03 | 0.43 | 0.03 | <0.01 | .683 | <0.01 | .090 | <0.01 | .384 | −0.02 | <.001 | 0.85 | 0.04 | 0.85 | 0.04 | 0.01 | .195 | <0.01 | .024 | <0.01 | .684 | −0.03 | <.001 |
| Corpus Callosum | ||||||||||||||||||||||||
| Splenium | 0.61 | 0.05 | 0.62 | 0.05 | 0.01 | .252 | <0.01 | .039 | <0.01 | .607 | −0.02 | .019 | 0.92 | 0.09 | 0.91 | 0.07 | −0.02 | .209 | −0.01 | .034 | 0.02 | .207 | 0.01 | .301 |
| Genu | 0.58 | 0.03 | 0.58 | 0.02 | <0.01 | .936 | <0.01 | .595 | <0.01 | .417 | −0.01 | .012 | 0.81 | 0.03 | 0.82 | 0.03 | <0.01 | .598 | <0.01 | .027 | <0.01 | .295 | −0.02 | <.001 |
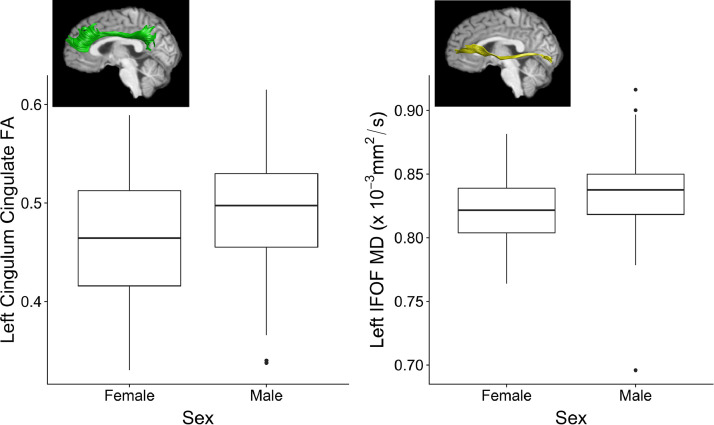
Age at time of injury was significantly positively associated with FA and negatively associated with MD in many of the tracts after FDR correction (see Table 2). As shown in Fig. 2, significant sex differences were found in the left cingulum cingulate FA (girls: M = 0.46, SD = 0.07, boys: M = 0.49, SD = 0.06) and left inferior frontal-occipital fasciculus MD (girls: M = 0.82, SD = 0.03, boys: M = 0.84, SD = 0.03) after FDR correction. Nominally significant sex differences also were found in bilateral inferior frontal-occipital fasciculus FA (left: girls, M = 0.49, SD = 0.03, boys, M = 0.47, SD = 0.04; right: girls, M = 0.50, SD = 0.03, boys, M = 0.49, SD = 0.03) and right arcuate fasciculus (girls: M = 0.75, SD = 0.04, boys: M = 0.77, SD = 0.05) and inferior frontal-occipital fasciculus MD (girls: M = 0.83, SD = 0.03, boys: M = 0.84, SD = 0.04), but did not survive FDR correction. Site was a significant covariate after FDR correction, although the effect of site varied by brain region (see Table 2).
Fig. 2.
Sex differences on white matter microstructure of examined tracts after FDR correction for Aim 1. Note. IFOF = inferior frontal-occipital fasciculus; FA = fractional anisotropy; MD = mean diffusivity.
3.4. Post-acute white matter microstructure and PCS
The negative binomial multivariable regression analyses predicting post-injury PCS ratings are summarized in Supplemental Tables 1–8.
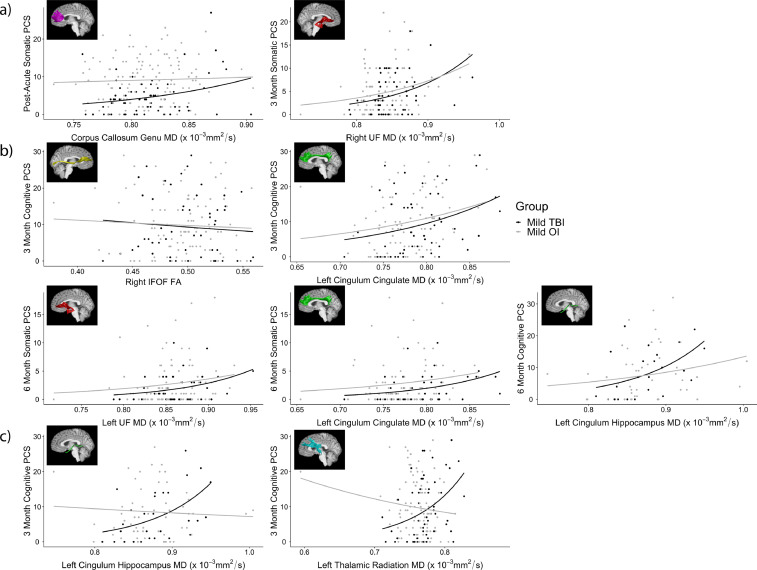
The groups showed significant differences on both parent and child ratings of post-acute PCS after FDR correction (mild TBI > OI), but not on 3- or 6-month post-injury PCS ratings (see Table 1). Nominally significant associations were found between FA (negative) and MD (positive) values in some tracts and parent's ratings of chronic somatic and cognitive PCS, and similar associations were found between DTI metrics and children's post-acute and chronic somatic PCS and post-acute cognitive PCS ratings, across groups. However, only a few of these associations survived FDR correction. As shown in Fig. 3a, average MD of the corpus callosum genu (forceps minor) was significantly positively associated with children's post-acute somatic PCS ratings and MD in the right uncinate fasciculus was significantly positively associated with children's 3-month somatic PCS ratings in both groups. In addition, average FA of the right inferior frontal-occipital fasciculus and average MD of the left cingulum cingulate, uncinate fasciculus, and cingulum hippocampus were significantly positively associated with parent's chronic somatic and cognitive PCS ratings (Fig. 3b). Two interactions of group by DTI metrics also were significant after FDR correction. As shown in Fig. 3c, average MD of the left cingulum hippocampus and thalamic radiation were significantly positively associated with parent's 3-month cognitive PCS ratings in the OI group (cingulum hippocampus: B = 11.96, p < .001; thalamic radiation: B = 10.18, p = .046), but not in the mild TBI group (cingulum hippocampus: B = −1.65, p = .563; thalamic radiation: B = −4.71, p = .164).
Fig. 3.
Relations among post-acute white matter microstructure and (a) children's and (b) parent's ratings of PCS severity, and (c) the group by DTI metric interaction effects that survived FDR correction for Aim 2. Note. MD = mean diffusivity, FA = fractional anisotropy, PCS = post-concussive symptoms, IFOF = inferior frontal-occipital fasciculus, UF = uncinate fasciculus.
Significant covariate effects of age did not survive FDR correction, but premorbid (i.e., pre-injury) PCS was consistently a significant covariate after FDR correction.
4. Discussion
The current study used data from a multisite study of children with mild TBI or mild OI to investigate post-acute white matter microstructure, as indexed using DTI, and its relationship to PCS. As expected, no statistically robust (i.e., significant after correcting for multiple comparisons using FDR) difference in post-acute white matter microstructure was observed between children with mild TBI and those with OI. The limited finding of reduced microstructure of bilateral thalamic radiation white matter in mild TBI should be interpreted with caution given that it did not survive correction for multiple comparison, although similar reductions have previously been reported in post-acute pediatric mild TBI (Mayer et al., 2012; Myer et al., 2016; Spader et al., 2018). Post-acute diffusion metrics also did not differentially predict PCS in the two injury groups, with the exception of left cingulum hippocampus and thalamic radiation MD values, which only predicted cognitive PCS at 3 months post-injury in children with OI. In both groups, lower post-acute FA and higher post-acute MD in the right inferior frontal-occipital fasciculus, left cingulum tracts, and bilateral uncinate fasciculus robustly predicted greater PCS severity at 3 and 6 months post-injury, respectively; and higher MD in the genu of the corpus callosum was related to greater post-acute somatic PCS.
The lack of robust differences between the mild TBI and OI groups in post-acute white matter microstructure was expected. We previously reported similar findings based on a subset of the current sample (Goodrich-Hunsaker et al., 2018). Overall diffusion results are consistent with other recent DTI studies that also showed no differences between mild TBI and OI groups (Wilde et al., 2018b) or healthy controls (Mac Donald et al., 2019; Maugans et al., 2012). However, some previous studies that compared children with mild TBI to children with OI did find differences in a number of regions, including frontal, temporal, and parietal white matter (Babcock et al., 2015; Ewing-Cobbs et al., 2019; Yuan et al., 2015). The inconsistencies between the current and prior results could reflect differences in injury characteristics, age range of participants, and data processing approach. For instance, post-acute assessment times have ranged from within 2 days to 7 weeks post-injury across studies (Babcock et al., 2015; Ewing-Cobbs et al., 2019; Wilde et al., 2018b), and children with mild TBI have not always been compared to children with OI separately from children with more severe injuries (i.e., moderate/severe TBI; Ewing-Cobbs et al., 2019). The age of participants included across studies of pediatric mild TBI and OI have ranged from childhood to young adulthood (e.g., 8–30 years), and most studies included slightly older cohorts (i.e., 11–16 years) than ours (i.e., 8–16 years; Babcock et al., 2015; Ewing-Cobbs et al., 2019; Wilde et al., 2018b; Yuan et al., 2015). Finally, diffusion-weighted MRI data processing approaches have varied widely between studies (Babcock et al., 2015; Ewing-Cobbs et al., 2019; Goodrich-Hunsaker et al., 2018; Wilde et al., 2018b; Yuan et al., 2015). This is the first sample to which automated tractography approaches have been applied to study differences in white matter microstructure following pediatric mild TBI (Dennis et al., 2017; Goodrich-Hunsaker et al., 2018).
Another potential explanation for the lack of group differences in white matter microstructure includes the use of children with OI as a comparison group. Recent evidence indicates that youth and young adults with mild TBI and OI are more similar on white matter microstructure than either group is to healthy controls (Wilde et al., 2018b). Their similarity may reflect several factors, including the effects of pain and the potential for immune changes, blood-brain barrier disruption, and neuroinflammation following systemic injury (Ghodadra et al., 2016; Lord et al., 2014; Morioka et al., 2019; Reikerås, 2010). Another possibility is that children with OI may have also experienced subconcussive impacts to the head at the time of injury (Hirad et al., 2019). Despite this, an OI group is more clinically relevant than a typically developing group, which does not serve the practical need for distinguishing between patients who sustained a TBI versus other injuries.
In contrast to the lack of group differences, we did find the expected variations in white matter diffusion that occur with maturation. Increases in FA and decreases in MD in most white matter regions during childhood and adolescence have been reported in studies of typical development (Lebel et al., 2019). While not always linear, changes can vary regionally and temporally (Lebel et al., 2019; Tamnes et al., 2018), consistent with the current findings. The sex differences we found (see Fig. 2), including reduced MD in the left cingulum cingulate and inferior frontal-occipital fasciculus in girls compared to boys, also have been demonstrated in typical development (Lebel et al., 2019; Tamnes et al., 2018). However, our analyses indicated that neither age nor sex moderated post-acute outcomes in white matter microstructure following mild TBI compared to OI. Further assessment of white matter microstructure at a later time post-injury, especially using a longitudinal approach, would more clearly establish whether mild TBI disrupts typical white matter development.
Children with mild TBI had more severe PCS than children with OI within 2 weeks post-injury, but not at 3- or 6-months post-injury. Post-acute white matter microstructure in a number of different tracts robustly and positively predicted chronic (i.e., 3 and 6 month post-injury) PCS (see Fig. 3). However, post-acute diffusion metrics were less robustly associated with post-acute PCS, with only one association remaining significant after correction for multiple comparisons. Post-acute diffusion metrics have not consistently been correlated with early post-injury symptoms (Babcock et al., 2015; Chu et al., 2010; Mac Donald et al., 2019; Murdaugh et al., 2018; Mustafi et al., 2018; Wilde et al., 2008). In contrast, DTI metrics have proven to be more predictive of long-term outcomes in pediatric TBI than conventional neuroimaging (Galloway et al., 2008; Königs et al., 2018). This is the first published study to examine the relations between early post-acute DTI metrics and PCS severity after mild TBI at 3 and 6 months post-injury. Further investigation of longitudinal changes in DTI metrics would be beneficial in elucidating whether children with mild TBI who show more severe PCS at 6 months post-injury also demonstrated more persistent alterations in diffusion metrics across recovery.
While the current study provided a much-needed examination of post-acute white matter microstructure in children with mild TBI versus OI, several study limitations must be acknowledged. The variance in diffusion metrics across sites was unsurprising. Different collection platforms can introduce systematic differences that distort diffusion MRI and can confound results from subsequent, automated processing pipelines. Variability in diffusion data can be introduced in multi-site studies through the use of scanners and scanning platforms from different vendors (Lebel et al., 2019). Even data from single-site studies of a single MRI scanner can be influenced by changes in hardware or software upgrades that can occur over the course of data collection.
Although image quality was controlled in the current study, motion artifact is of considerable concern in MRI studies (Reuter et al., 2015), particularly those involving children with neurodevelopmental problems (Afacan et al., 2016; Brown et al., 2010; Rauch, 2005). Impulsivity and hyperactivity, both of which are associated with increased risk for TBI in children (Gerring et al., 1998; Lee et al., 2008), have been linked with more severe motion artifacts in youth with attention-deficit/hyperactivity disorder (ADHD; Rauch, 2005). Age is also related to extent of motion artifact in children, with younger children generally showing greater motion artifact than older children (Blumenthal et al., 2002; Goodrich-Hunsaker et al., 2018; Roalf et al., 2016). However, children with mild TBI did not demonstrate more motion from those with OI in a subset of the current sample (Goodrich-Hunsaker et al., 2018).
We attempted to account for multiple comparisons using FDR, but this reduced our power to detect differences. Future research with a larger sample size would be beneficial. Finally, the current study only examined white matter diffusion at a single time. Injury-related changes might be more readily detected at more remote times post-injury. Further research that included longitudinal neuroimaging would be beneficial.
In summary, the current results suggest that, at a group level, white matter microstructure is not disrupted by uncomplicated mild TBI in the first couple weeks following injury. However, early diffusion characteristics were predictive of post-injury PCS severity, suggesting the potential prognostic utility of this advanced MRI technique. The results highlight the need for longitudinal neuroimaging studies of pediatric mild TBI, to determine whether changes in white matter microstructure can be detected over time, especially among children who are slow to recover after mild TBI.
Declaration of Competing Interest
None.
Acknowledgements
Funding Information: National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Predicting Outcomes in Children with Mild Traumatic Brain Injury, 1R01HD076885.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102106.
Appendix. Supplementary materials
References
- Adelson P.D., Pineda J., Bell M.J., Abend N.S., Berger R.P., Giza C.C., Hotz G., Wainwright M.S. Common data elements for pediatric traumatic brain injury: recommendations from the working group on demographics and clinical assessment. J. Neurotrauma. 2012;29:639–653. doi: 10.1089/neu.2011.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afacan O., Erem B., Roby D.P., Roth N., Roth A., Prabhu S.P., Warfield S.K. Evaluation of motion and its effect on brain magnetic resonance image quality in children. Pediatr. Radiol. 2016;46:1728–1735. doi: 10.1007/s00247-016-3677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayr L.K., Yeates K.O., Taylor H.G., Browne M. Dimensions of postconcussive symptoms in children with mild traumatic brain injuries. J. Int. Neuropsychol. Soc. 2009;15:19–30. doi: 10.1017/S1355617708090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock L., Yuan W., Leach J., Nash T., Wade S. White matter alterations in youth with acute mild traumatic brain injury. J. Pediatr. Rehabil. Med. 2015;8:285–296. doi: 10.3233/PRM-150347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow K.M. Postconcussion syndrome: a review. J. Child Neurol. 2016;31:57–67. doi: 10.1177/0883073814543305. [DOI] [PubMed] [Google Scholar]
- Bartnik-Olson B.L., Holshouser B., Wang H., Grube M., Tong K., Wong V., Ashwal S. Impaired neurovascular unit function contributes to persistent symptoms after concussion: a pilot study. J. Neurotrauma. 2014;31:1497–1506. doi: 10.1089/neu.2013.3213. [DOI] [PubMed] [Google Scholar]
- Beek L.V., Vanderauwera J., Ghesquière P., Lagae L., Smedt B.D. Longitudinal changes in mathematical abilities and white matter following paediatric mild traumatic brain injury. Brain Inj. 2015;29:1701–1710. doi: 10.3109/02699052.2015.1075172. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Methodol. 1995;57:289–300. [Google Scholar]
- Bigler E.D. Neuropathology of mild traumatic brain injury: correlation to neurocognitive and neurobehavioral findings. In: Kobeissy F.H., editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects, Frontiers in Neuroengineering. CRC Press/Taylor & Francis; Boca RatonFL: 2015. [Google Scholar]
- Bigler E.D. Neuroimaging biomarkers in mild traumatic brain injury (mTBI) Neuropsychol. Rev. 2013;23:169–209. doi: 10.1007/s11065-013-9237-2. [DOI] [PubMed] [Google Scholar]
- Bigler E.D., Bazarian J.J. Diffusion tensor imaging: a biomarker for mild traumatic brain injury? Neurology. 2010;74:626–627. doi: 10.1212/WNL.0b013e3181d3e43a. [DOI] [PubMed] [Google Scholar]
- Bigler E.D., Maxwell W.L. Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging Behav. 2012;6:108–136. doi: 10.1007/s11682-011-9145-0. [DOI] [PubMed] [Google Scholar]
- Blumenthal J.D., Zijdenbos A., Molloy E., Giedd J.N. Motion artifact in magnetic resonance imaging: implications for automated analysis. Neuroimage. 2002;16:89–92. doi: 10.1006/nimg.2002.1076. [DOI] [PubMed] [Google Scholar]
- Brown T.T., Kuperman J.M., Erhart M., White N.S., Roddey J.C., Shankaranarayanan A., Han E.T., Rettmann D., Dale A.M. Prospective motion correction of high-resolution magnetic resonance imaging data in children. Neuroimage. 2010;53:139–145. doi: 10.1016/j.neuroimage.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamard E., Lichtenstein J.D. A systematic review of neuroimaging findings in children and adolescents with sports-related concussion. Brain Inj. 2018;32:816–831. doi: 10.1080/02699052.2018.1463106. [DOI] [PubMed] [Google Scholar]
- Chu Z., Wilde E.A., Hunter J.V., McCauley S.R., Bigler E.D., Troyanskaya M., Yallampalli R., Chia J.M., Levin H.S. Voxel-Based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. Am. J. Neuroradiol. 2010;31:340–346. doi: 10.3174/ajnr.A1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E.L., Babikian T., Giza C.C., Thompson P.M., Asarnow R.F. Diffusion MRI in pediatric brain injury. Child’s Nervous Syst. 2017;33:1683–1692. doi: 10.1007/s00381-017-3522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd A.B., Epstein K., Ling J.M., Mayer A.R. Diffusion tensor imaging findings in semi-acute mild traumatic brain injury. J. Neurotrauma. 2014;31:1235–1248. doi: 10.1089/neu.2014.3337. [DOI] [PubMed] [Google Scholar]
- Duhaime A.-C., Holshouser B., Hunter J.V., Tong K. Common data elements for neuroimaging of traumatic brain injury: pediatric considerations. J. Neurotrauma. 2012;29:629–633. doi: 10.1089/neu.2011.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eierud C., Craddock R.C., Fletcher S., Aulakh M., King-Casas B., Kuehl D., LaConte S.M. Neuroimaging after mild traumatic brain injury: review and meta-analysis. Neuroimage Clin. 2014;4:283–294. doi: 10.1016/j.nicl.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L., DeMaster D., Watson C.G., Prasad M.R., Cox C.S., Kramer L.A., Fischer J.T., Duque G., Swank P.R. Post-Traumatic stress symptoms after pediatric injury: relation to pre-frontal limbic circuitry. J. Neurotrauma. 2019 doi: 10.1089/neu.2018.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Buchner A., Lang A.-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Galloway N.R., Tong K.A., Ashwal S., Oyoyo U., Obenaus A. Diffusion-weighted imaging improves outcome prediction in pediatric traumatic brain injury. J. Neurotrauma. 2008;25:1153–1162. doi: 10.1089/neu.2007.0494. [DOI] [PubMed] [Google Scholar]
- Gerring J.P., Brady K.D., Chen A., Vasa R., Grados M., Bandeen-Roche K.J., Bryan R.N., Denckla M.B. Premorbid prevalence of ADHD and development of secondary ADHD after closed head injury. J. Am. Acad. Child Adolesc. Psychiatry. 1998;37:647–654. doi: 10.1097/00004583-199806000-00015. [DOI] [PubMed] [Google Scholar]
- Ghodadra A., Alhilali L., Fakhran S. Principal component analysis of diffusion tensor images to determine white matter injury patterns underlying postconcussive headache. Am. J. Neuroradiol. 2016;37:274–278. doi: 10.3174/ajnr.A4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist J., Thomas K., Xu L., McGuire L.C., Coronado V.G. Nonfatal traumatic brain injuries related to sports and recreation activities among persons aged ≤19 years — United States, 2001–2009. MMWR. 2011;60:1337–1342. [PubMed] [Google Scholar]
- Goodrich-Hunsaker N.J., Abildskov T.J., Black G., Bigler E.D., Cohen D.M., Mihalov L.K., Bangert B.A., Taylor H.G., Yeates K.O. Age- and sex-related effects in children with mild traumatic brain injury on diffusion magnetic resonance imaging properties: a comparison of voxelwise and tractography methods. J. Neurosci. Res. 2018;96:626–641. doi: 10.1002/jnr.24142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan L., McLellan B.A., Greig H. Abbreviated injury scale and injury severity score: a scoring chart. J. Trauma. 1985;25:60–64. doi: 10.1097/00005373-198501000-00010. [DOI] [PubMed] [Google Scholar]
- Hirad A.A., Bazarian J.J., Merchant-Borna K., Garcea F.E., Heilbronner S., Paul D., Hintz E.B., van Wijngaarden E., Schifitto G., Wright D.W., Espinoza T.R., Mahon B.Z. A common neural signature of brain injury in concussion and subconcussion. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aau3460. eaau3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Königs M., Pouwels P.J., Ernest van Heurn L., Bakx R., Jeroen Vermeulen R., Carel Goslings J., Poll-The B.T., van der Wees M., Catsman-Berrevoets C.E., Oosterlaan J. Relevance of neuroimaging for neurocognitive and behavioral outcome after pediatric traumatic brain injury. Brain Imaging Behav. 2018;12:29–43. doi: 10.1007/s11682-017-9673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Königs M., Pouwels P.J., Ernest van Heurn L., Bakx R., Jeroen Vermeulen R., Carel Goslings J., Poll-The B.T., van der Wees M., Catsman-Berrevoets C.E., Oosterlaan J. Relevance of neuroimaging for neurocognitive and behavioral outcome after pediatric traumatic brain injury. Brain Imaging Behav. 2017 doi: 10.1007/s11682-017-9673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Treit S., Beaulieu C. A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR Biomed. 2019;32:e3778. doi: 10.1002/nbm.3778. [DOI] [PubMed] [Google Scholar]
- Lee L.-C., Harrington R.A., Chang J.J., Connors S.L. Increased risk of injury in children with developmental disabilities. Res. Dev. Disabil. 2008;29:247–255. doi: 10.1016/j.ridd.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Loder R.T., Warschausky S., Schwartz E.M., Hensinger R.N., Greenfield M.L. The psychosocial characteristics of children with fractures. J. Pediatr. Orthop. 1995;15:41–46. doi: 10.1097/01241398-199501000-00010. [DOI] [PubMed] [Google Scholar]
- Lord J.M., Midwinter M.J., Chen Y.-F., Belli A., Brohi K., Kovacs E.J., Koenderman L., Kubes P., Lilford R.J. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. 2014;384:1455–1465. doi: 10.1016/S0140-6736(14)60687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumba-Brown A., Yeates K.O., Sarmiento K., Breiding M.J., Haegerich T.M., Gioia G.A., Turner M., Benzel E.C., Suskauer S.J., Giza C.C., Joseph M., Broomand C., Weissman B., Gordon W., Wright D.W., Moser R.S., McAvoy K., Ewing-Cobbs L., Duhaime A.-C., Putukian M., Holshouser B., Paulk D., Wade S.L., Herring S.A., Halstead M., Keenan H.T., Choe M., Christian C.W., Guskiewicz K., Raksin P.B., Gregory A., Mucha A., Taylor H.G., Callahan J.M., DeWitt J., Collins M.W., Kirkwood M.W., Ragheb J., Ellenbogen R.G., Spinks T.J., Ganiats T.G., Sabelhaus L.J., Altenhofen K., Hoffman R., Getchius T., Gronseth G., Donnell Z., O’Connor R.E., Timmons S.D. Diagnosis and management of mild traumatic brain injury in children: a systematic review. JAMA Pediatr. 2018;172 doi: 10.1001/jamapediatrics.2018.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald C.L., Barber J., Wright J., Coppel D., De Lacy N., Ottinger S., Peck S., Panks C., Sun S., Zalewski K., Temkin N. Longitudinal clinical and neuroimaging evaluation of symptomatic concussion in 10- to 14-Year-Old youth athletes. J. Neurotrauma. 2019;36:264–274. doi: 10.1089/neu.2018.5629. [DOI] [PubMed] [Google Scholar]
- Mathias J.L., Dennington V., Bowden S.C., Bigler E.D. Community versus orthopaedic controls in traumatic brain injury research: how comparable are they? Brain Inj. 2013;27:887–895. doi: 10.3109/02699052.2013.793398. [DOI] [PubMed] [Google Scholar]
- Maugans T.A., Farley C., Altaye M., Leach J., Cecil K.M. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics. 2012;129:28–37. doi: 10.1542/peds.2011-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.R., Kaushal M., Dodd A.B., Hanlon F.M., Shaff N.A., Mannix R., Master C.L., Leddy J.J., Stephenson D., Wertz C.J., Suelzer E.M., Arbogast K.B., Meier T.B. Advanced biomarkers of pediatric mild traumatic brain injury: progress and perils. Neurosci. Biobehav. Rev. 2018;94:149–165. doi: 10.1016/j.neubiorev.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.R., Ling J., Mannell M.V., Gasparovic C., Phillips J.P., Doezema D., Reichard R., Yeo R.A. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010;74:643–650. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.R., Ling J.M., Yang Z., Pena A., Yeo R.A., Klimaj S. Diffusion abnormalities in pediatric mild traumatic brain injury. J. Neurosci. 2012;32:17961–17969. doi: 10.1523/JNEUROSCI.3379-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea M., Kelly J.P., Randolph C., Kluge J., Bartolic E., Finn G., Baxter B. Standardized assessment of concussion (SAC): on-site mental status evaluation of the athlete. J. Head Trauma Rehabil. 1998;13:27–35. doi: 10.1097/00001199-199804000-00005. [DOI] [PubMed] [Google Scholar]
- Morioka K., Marmor Y., Sacramento J.A., Lin A., Shao T., Miclau K.R., Clark D.R., Beattie M.S., Marcucio R.S., Miclau T., Ferguson A.R., Bresnahan J.C., Bahney C.S. Differential fracture response to traumatic brain injury suggests dominance of neuroinflammatory response in polytrauma. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-48126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh D.L., King T.Z., Sun B., Jones R.A., Ono K.E., Reisner A., Burns T.G. Longitudinal changes in resting state connectivity and white matter integrity in adolescents with sports-related concussion. J. Int. Neuropsychol. Soc. 2018;24:781–792. doi: 10.1017/S1355617718000413. [DOI] [PubMed] [Google Scholar]
- Mustafi S.M., Harezlak J., Koch K.M., Nencka A.S., Meier T.B., West J.D., Giza C.C., DiFiori J.P., Guskiewicz K.M., Mihalik J.P., LaConte S.M., Duma S.M., Broglio S.P., Saykin A.J., McCrea M., McAllister T.W., Wu Y.-C. Acute white-matter abnormalities in sports-related concussion: a diffusion tensor imaging study from the ncaa-dod care consortium. J. Neurotrauma. 2018 doi: 10.1089/neu.2017.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer G.D., Yuan W., Barber Foss K.D., Thomas S., Smith D., Leach J., Kiefer A.W., Dicesare C., Adams J., Gubanich P.J., Kitchen K., Schneider D.K., Braswell D., Krueger D., Altaye M. Analysis of head impact exposure and brain microstructure response in a season-long application of a jugular vein compression collar: a prospective, neuroimaging investigation in American football. Br. J. Sports Med. 2016;50:1276–1285. doi: 10.1136/bjsports-2016-096134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak Z., Aglipay M., Barrowman N., Yeates K.O., Beauchamp M.H., Gravel J., Freedman S.B., Gagnon I., Gioia G., Boutis K., Burns E., Ledoux A.-A., Osmond M.H., Zemek R.L., Pediatric Emergency Research Canada Predicting Persistent Postconcussive Problems in Pediatrics (PERC 5P) Concussion Team Association of persistent postconcussion symptoms with pediatric quality of life. JAMA Pediatr. 2016;170 doi: 10.1001/jamapediatrics.2016.2900. [DOI] [PubMed] [Google Scholar]
- Okamoto R.J., Romano A.J., Johnson C.L., Bayly P.V. Insights into traumatic brain injury from MRI of harmonic brain motion. J. Exp. Neurosci. 2019;13 doi: 10.1177/1179069519840444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer K., Gillani S., Williams A., Hak D.J. Psychiatric risk factors in pediatric hand fractures. J. Pediatr. Orthop. 2010;30:324–327. doi: 10.1097/BPO.0b013e3181d8fa8c. [DOI] [PubMed] [Google Scholar]
- Papa L., Ramia M.M., Kelly J.M., Burks S.S., Pawlowicz A., Berger R.P. Systematic review of clinical research on biomarkers for pediatric traumatic brain injury. J. Neurotrauma. 2013;30:324–338. doi: 10.1089/neu.2012.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch S.L. Neuroimaging and attention-deficit/hyperactivity disorder in the 21st century: what to consider and how to proceed. Biol. Psychiatry. 2005;57:1261–1262. doi: 10.1016/j.biopsych.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Reikerås O. Immune depression in musculoskeletal trauma. Inflamm. Res. 2010;59:409–414. doi: 10.1007/s00011-010-0167-7. [DOI] [PubMed] [Google Scholar]
- Reuter M., Tisdall M.D., Qureshi A., Buckner R.L., van der Kouwe A.J.W., Fischl B. Head motion during MRI acquisition reduces gray matter volume and thickness estimates. Neuroimage. 2015;107:107–115. doi: 10.1016/j.neuroimage.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf D.R., Quarmley M., Elliott M.A., Satterthwaite T.D., Vandekar S.N., Ruparel K., Gennatas E.D., Calkins M.E., Moore T.M., Hopson R., Prabhakaran K., Jackson C.T., Verma R., Hakonarson H., Gur R.C., Gur R.E. The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage. 2016;125:903–919. doi: 10.1016/j.neuroimage.2015.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R.M., Iverson G.L., Barth J.T., Bush S.S., Broshek D.K., the NAN Policy and Planning Committee Recommendations for diagnosing a mild traumatic brain injury: a national academy of neuropsychology education paper. Arch. Clin. Neuropsychol. 2009;24:3–10. doi: 10.1093/arclin/acp006. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Hayward K.S., Brown K.E., Zwicker J.G., Ponsford J., van Donkelaar P., Babul S., Boyd L.A. Imaging in pediatric concussion: a systematic review. Pediatrics. 2018;141 doi: 10.1542/peds.2017-3406. [DOI] [PubMed] [Google Scholar]
- Shenton M.E., Hamoda H.M., Schneiderman J.S., Bouix S., Pasternak O., Rathi Y., Vu M.-A., Purohit M.P., Helmer K., Koerte I., Lin A.P., Westin C.-F., Kikinis R., Kubicki M., Stern R.A., Zafonte R. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6:137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spader H.S., Dean D.C., LaFrance W.C., Raukar N.P., Cosgrove G.R., Eyerly-Webb S.A., Ellermeier A., Correia S., Deoni S.C.L., Rogg J. Prospective study of myelin water fraction changes after mild traumatic brain injury in collegiate contact sports. J. Neurosurg. 2018;1:1–9. doi: 10.3171/2017.12.JNS171597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens G., Cho J.H. Socioeconomic indexes and the new 1980 census occupational classification scheme. Soc. Sci. Res. 1985;14:142–168. [Google Scholar]
- Tamnes C.K., Roalf D.R., Goddings A.-L., Lebel C. Diffusion mri of white matter microstructure development in childhood and adolescence: methods, challenges and progress. Dev. Cogn. Neurosci. 2018;33:161–175. doi: 10.1016/j.dcn.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H.G., Dietrich A., Nuss K., Wright M., Rusin J., Bangert B., Minich N., Yeates K.O. Post-concussive symptoms in children with mild traumatic brain injury. Neuropsychology. 2010;24:148–159. doi: 10.1037/a0018112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Uslu, M., Uslu, R., Eksioglu, F., Ozen, N.E., 2007. Children with fractures show higher levels of impulsive-hyperactive behavior: Clin. Orthop. Relat. Res. 10.1097/BLO.0b013e31805002da. [DOI] [PubMed]
- Vértes P.E., Bullmore E.T. Annual research review: growth connectomics - the organization and reorganization of brain networks during normal and abnormal development. J. Child. Psychol. Psychiatry. 2015;56:299–320. doi: 10.1111/jcpp.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Pearson; San Antonio, TX: 2011. Wechsler Abbreviated Scale of Intelligence–2nd Edition(WASI-II), Manual. [Google Scholar]
- Wilde E.A., McCauley S.R., Hunter J.V., Bigler E.D., Chu Z., Wang Z.J., Hanten G.R., Troyanskaya M., Yallampalli R., Li X., Chia J., Levin H.S. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- Wilde E.A., Provenzale J.M., Taylor B.A., Boss M., Zuccolotto A., Hachey R., Pathak S., Tate D.F., Abildskov T.J., Schneider W. Assessment of quantitative magnetic resonance imaging metrics in the brain through the use of a novel phantom. Brain Inj. 2018;32:1266–1276. doi: 10.1080/02699052.2018.1494855. [DOI] [PubMed] [Google Scholar]
- Wilde E.A., Ware A.L., Li X., Wu T.C., McCauley S.R., Barnes A., Newsome M., Biekman B., Hunter J.V., Chu Z., Levin H. Orthopedic injured versus uninjured comparison groups for neuroimaging research in mild traumatic brain injury. J. Neurotrauma. 2018 doi: 10.1089/neu.2017.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J., Krach L., Ward E., Mueller B., Muetzel R., Schnoebelen S., Kiragu A., Lim K. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch. Clin. Neuropsychol. 2007;22:555–568. doi: 10.1016/j.acn.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.Z., Dong R.G., Schopper A.W. Analysis of effects of friction on the deformation behavior of soft tissues in unconfined compression tests. J. Biomech. 2004;37:147–155. doi: 10.1016/s0021-9290(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Yallampalli R., Wilde E.A., Bigler E.D., McCauley S.R., Hanten G., Troyanskaya M., Hunter J.V., Chu Z., Li X., Levin H.S. Acute white matter differences in the fornix following mild traumatic brain injury using diffusion tensor imaging. J. Neuroimaging. 2013;23:224–227. doi: 10.1111/j.1552-6569.2010.00537.x. [DOI] [PubMed] [Google Scholar]
- Yeates K.O., Beauchamp M., Craig W., Doan Q., Zemek R., Bjornson B.H., Gravel J., Mikrogianakis A., Goodyear B., Abdeen N., Beaulieu C., Dehaes M., Deschenes S., Harris A., Lebel C., Lamont R., Williamson T., Barlow K.M., Bernier F., Brooks B.L., Emery C., Freedman S.B., Kowalski K., Mrklas K., Tomfohr-Madsen L., Schneider K.J. Advancing concussion assessment in pediatrics (A-CAP): a prospective, concurrent cohort, longitudinal study of mild traumatic brain injury in children: study protocol. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates K.O., Taylor H.G., Rusin J., Bangert B., Dietrich A., Nuss K., Wright M., Nagin D.S., Jones B.L. Longitudinal trajectories of postconcussive symptoms in children with mild traumatic brain injuries and their relationship to acute clinical status. Pediatrics. 2009;123:735–743. doi: 10.1542/peds.2008-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Myall N.J., Wandell B.A., Feldman H.M. Tract Profiles of White Matter Properties: Automating Fiber-Tract Quantification. PLoS ONE 7. 2012:e49790. doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W., Wade S.L., Babcock L. Structural connectivity abnormality in children with acute mild traumatic brain injury using graph theoretical analysis. Hum. Brain Mapp. 2015;36:779–792. doi: 10.1002/hbm.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.