Abstract
The Dengue, Chikungunya and Zika viruses are arboviruses predominantly transmitted to humans through the bite of the female mosquito Aedes aegypti. Currently, the vector represents a potential epidemiological risk in several Latin American and Pacific countries. However, little is known about the geographical distribution and bioclimatic suitability of this mosquito in the projected climate change scenarios in Colombia. Using a species distribution model of maximum entropy (MaxEnt) based on presence-only records obtained from Global Biodiversity Information Facility (GBIF), land elevation obtained from Shuttle Radar Topography Mission (SRTM) and bioclimatic variables (WorldClim), we produced environmental suitability maps of this mosquito vector for present and future geographic distribution. The future distribution were constructed based on the Community Climate System Model (CCSM4) for the years 2050 and 2070, projected according to the Representative Concentration Pathways (RCP) 2.6, 4.5 and 8.5 described by the Intergovernmental Panel on Climate Change (IPCC). For the current conditions, Colombia has ~140,612.8 square km of areas with the possible presence of the vector; however, for the future, this will be reduced by more than 30%. For the future conditions, the suitable areas for A. aegypti decreased compared to the present, mainly for the year 2070 under RCP scenarios 4.5 and 8.5, however, the probability of mosquito occurrence increases in some departments of Colombia. Areas susceptible to the presence of A. aegypti are affected by climate change. The Caribbean and Andean regions have a high probability of mosquito distribution; therefore, control and epidemiological surveillance are required in these areas. The results can serve as an input to define preventive and control measures, especially in areas with a higher risk of contracting the virus.
Keywords: Mathematical modeling, Climate change, Viral vector, Public health, Environmental change, Aedes aegypti, Environmental suitability, Geographical distribution, MaxEnt, Colombia
Mathematical modeling; Climate change; Viral vector; Public health; Environmental change; Aedes aegypti; Environmental suitability; Geographical distribution; MaxEnt; Colombia
1. Introduction
The climate change (CC) can affect the areas of geographic distribution, seasonal activities, migratory patterns, abundances and interactions of species due to sudden changes in precipitation and temperature (Haines et al., 2006; IPCC, 2014). Recently, changes related to extreme climatic and meteorological phenomena have been evidenced, e.g., extremely cold temperatures, increase in extremely warm temperatures, the rise in maximum sea levels and a significantly increased cases of intense rainfall in various regions (IPCC, 2014), all of which are determinants of the geographical distribution of vectors like A. aegypti.
The current distribution of A. aegypti is broadly defined by regional temperature and precipitation trends, but the global CC has the potential to significantly alter the future geographic range of mosquito vectors (Danis-Lozano et al., 2015; Andriamifidy et al., 2019). The A. aegypti mosquito is highly dependent on climatic conditions; for example, if the temperature of the water rises, the larvae take a much shorter time to mature and consequently, there is a greater capacity to produce more offspring during the transmission period (Rueda et al., 1990; Andriamifidy et al., 2019). Besides, in warmer climates, the adult female mosquito digests blood faster and feeds more frequently, therefore, there is an increased transmission of diseases (Gillies, 1953; Andriamifidy et al., 2019). On the other hand, changes in humidity and precipitation can affect the behavio of adult mosquitoes and the availability of aquatic habitats necessary for larval development (Kraemer et al., 2015). Also, if precipitation increases, the number and quality of sites for mosquito breeding also increase (Githeko et al., 2000; Andriamifidy et al., 2019).
The A. aegypti mosquito is a species distributed most predominantly in the tropical and subtropical areas of the Americas, between lattudes 35° north and 35° south (Nelson, 1986), although it has been reported at latitude 45° too, possibly due to invasions during the warm season (Nelson, 1986). In this sense, A. aegypti represents a potential epidemiological risk in countries of Latin America and the Pacific, since it is one of the most important vectors in the transmission of established and emerging viral diseases such as Dengue virus (DENV, genus Flavivirus), Chikungunya (CHIKV, genus Alphavirus), Zika (ZIKV, genus Flavivirus) and yellow fever virus (genus Flavivirus) (Kantor, 2016; Rückert et al., 2017). For example, in Colombia, from 2007 to the epidemiological week 20 of 2018, there were 745,257 cases of Dengue and severe Dengue, 69,572 of Chikungunya and 13,590 of Zika (Instituto Nacional de la salud, 2018). According to medical studies, A. aegypti can transmit a combination of the viruses simultaneously (Rückert et al., 2017). There are no antiviral agents to treat the infection by the virus, and there are no authorized vaccines, despite the existence of some promising possibilities for eradication of the diseases in their entirety (Githeko et al., 2000; Weaver et al., 2012; Messina et al., 2015).
The transmission of diseases through vectors such as A. aegypti is a permanent challenge to public health. Besides, the situation is more difficult in countries where mosquito proliferation is abundant, and resources for medical care and comprehensive vector control services are limited (Kouri et al., 2007), as in the case of Colombia. It is therefore vitally important to advance in understanding the geographical distribution of the species at the national level since public health resources can be better allocated in anticipation of the emergence of diseases in the most vulnerable and vector-suitable communities.
Bearing in mind that the presence of A. aegypti is closely related to environmental conditions (Grillet et al., 2010; Rogers et al., 2014), including climate, it is feasible to predict the geographical distribution of A. aegypti under climate change scenarios. Some models or tools allow estimating the possible distribution of species according to environmental requirements (Elith and Leathwick, 2009). Species Distribution Models (SDMs) are tools that correlate records of known occurrences and environmental conditions in the localities of occurrence to obtain ecological and evolutionary knowledge and to predict the distribution of species in the landscape, which sometimes require extrapolation in space and time (Elith and Leathwick, 2009).
Several modeling techniques are developed to predict the influence of climate change on the distribution of species. Most of the models correlate the current occurrence of the species with the climatic variables or through an understanding of the physiological responses of the species to climate change (Pearson and Dawson, 2003). Once the climatic variables that influence the distribution of a species are determined, it is possible to estimate the potential redistribution of the species for future climatic scenarios. The SDMs have been used to predict the current potential geographic distribution in insect populations, including mosquitoes, as well as changes in their distribution influenced by environmental and climatic changes (Peterson et al., 2005; Escobar et al., 2016). Some studies worldwide have used niche modeling techniques to determine the geographic distribution of A. aegypti in the face of climate change. Khan et al. (2016) could corroborate that niche modeling techniques are adequate to predict the distribution of risk by the Zika virus; this was supported by Kraemer et al. (2015), who found that the tropical areas of the world are suitable for the global distribution of vectors A. aegypti and A. albopictus. Recently, Alaniz et al. (2017) modeled the distribution of A. aegypti worldwide and found that the risk of contracting Zika for the Americas is concentrated in the Atlantic coast of South America and on the coastal zones of the Caribbean Sea in Central America and North America.
Despite the existence of studies on the distribution of A. aegypti at a global level, it is necessary to develop research at a national level, regional level, which will allow the identification and prioritization of the administrative divisions of Colombia (departments) and/or areas with the highest probability of the presence of the vector. Besides, in most studies the spatial resolution is low (for example, 5 or 10 km), which makes it impossible to identify with reasonable precision the local distribution of the vector and, therefore, the risk of contracting viruses and the application of mitigation strategies to them. In this sense, the main objective of our research is to predict the potential geographic distribution of the species A. aegypti under climate change scenarios in Colombia, taking into account a relatively precise spatial resolution (1 km2) with respect to other studies. The present modeling and future geographic distribution changes of A. aegypti will provide a useful tool for disease management and health service delivery planning in the country.
2. Methods
2.1. Study area
The study area comprised the departments of Colombia except the San Andrés and Providencia.
We excluded The San Andrés and Providencia islands because of their size and the spatial resolution (1km) of the environmental variables used for the present and future scenarios. The exclusion of these islands facilitated the analysis and design of the geographical distribution maps of the mosquito and promoted not to use different spatial scales to visualize the results.
Colombia is located in the northwestern tip of South America, with the Antilles sea in the north, the Pacific Ocean in the west, by Venezuela and Brazil in the east, by Panama in the northwest and by Peru and Ecuador in the south (Ministerio de Asuntos Exteriores y de Cooperación, 2017).
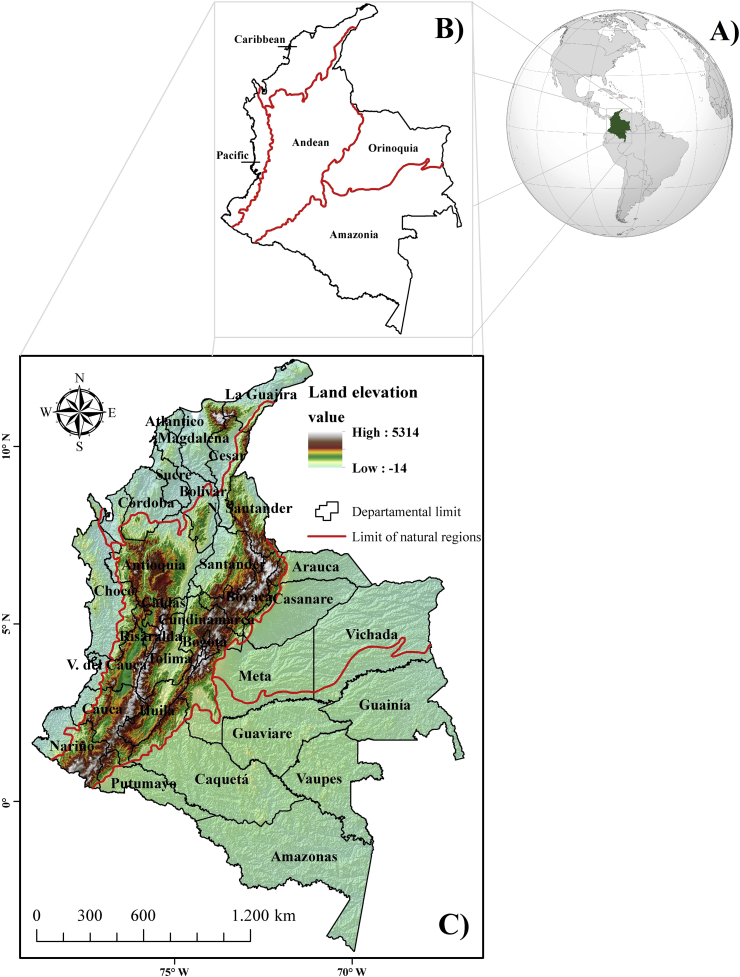
Colombia is divided into five geographical regions with different climatic and terrain conditions (Figure 1B): 1. The Caribbean region located in the north of the country receives an average annual rainfall between 500 and 2000 mm, with the maximum annual average temperature ranging between 28 and 32 °C (high and medium Guajira) and between 24 and 28 °C for the Sierra Nevada de Santa Martha and its surroundings (IDEAM, 2005). 2. The Andean Region located in the center of the country (northern ranges of the Andean mountain) records an average annual rainfall up to 2000 mm. Along the Eastern range and in the valleys of Alto Magdalena and Alto Cauca, the annual temperature averages between 24 and 28 °C (valleys of the main rivers such as Magdalena, Cauca, Patía and Sogamoso). The highlands of Cundinamarca, Boyacá and Nariño, the mountainous area of the center of Antioquia, Cauca and record between 12 and 16 °C, 8 °C in the high mountains (IDEAM, 2005). 3. The Orinoquía Region located to the east of the country receives on average an annual rainfall between 2000 to 3000 mm (central and eastern zone) and the average annual temperature ranges between 24 and 28 °C for the flat area and between 8 and 20 °C for the Piedmont area (IDEAM, 2005). 4. The region of the Amazon located in the south of the country receives on average an annual rainfall between 3000 and 4500 mm, with the average annual temperature ranging between 24 and 28 °C and in Piedmont between 12 and 20 °C (IDEAM, 2005). 5. The Pacific Region located in the western part of the country receives an average annual rainfall between 3000 and 12,000 mm, and the average annual temperature ranges between 24 and 28 °C (IDEAM, 2005).
Figure 1.
A) Worldwide location (wikipedia.org), B) Natural regions and C) land elevation of Colombia (Adapted from Digital Terrain Model (DTM) of the Radar Shuttle Topographic Mission (SRTM).
2.2. Species occurrences and environmental data
We downloaded 128 occurrence data (with replicas) for A. aegypti at country level from the Global Compendium of A. aegypti occurrence available at the Global Biodiversity Information Facility (GBIF, www.gbif.org, consulted on November 2017). The localities that are not spatially independent would bias the modeling with MaxEnt (Veloz, 2009; Anderson and Gonzalez, 2011; Boria et al., 2014; Phillips et al., 2016), Therefore, we filtered the localities and thus improved the performance of the ecological niche models (Boria et al., 2014). To reduce the spatial bias of the raw localities, the Spatially Rarefied Occurrence tool of the SDMtoolbox 2.0 (Brown et al., 2017), package was used, reducing occurrence locations to a single point within 10 km as the Euclidean distance (Pearson et al., 2007; Anderson and Raza, 2010), which resulted from the analysis of heterogeneity among the environmental variables filtered in this study (Figure 1 Supplementary material (SM)). To calculate climate heterogeneity the SDMtoolbox package was used following the steps below: 1) main analysis of components of bioclimatic variables, 2) calculation of heterogeneity and 3) selection of heterogeneity class and Euclidean distance (for example, if heterogeneity is classified as high, medium or low the localities of occurrence could be spatially filtered at 5 km2, 10 km 2 and 30 km2 respectively) (Brown et al., 2017).
We used bioclimatic variables of Worldclim for present and future with a spatial resolution of approximately (~) 30 s of arc (~0.86 km2) (Table 1). The variables for the present corresponding to the average of the years 1950–2000 (Hijmans et al., 2005), (http://worldclim.org/version2, consulted on 2017), for the future medium-term 2050 (average years 2041–2060) and long term 2070 (average years 2061–2080) (Hijmans et al., 2005), (http://www.worldclim.org/cmip5_30s, consulted on 2017). These variables were derived from monthly temperature and precipitation values to generate biologically significant variables (Fick and Hijmans, 2017). For future bioclimatic variables, we used the model of the Community Climate System (CCSM4) for the years 2050 and 2070 projected according to the RCP Representative Concentration Pathways 2.6, 4.5 and 8.5, which represent a radiative forcing of ~3 W/m2, 4.5 W/m2 and 8.5 W/m2 respectively. The Intergovernmental Panel on Climate Change (IPCC) described these scenarios, which is part of the simulations of the fifth phase of the Coupled Model Intercomparison Project Phase 5 (CMIP5). Global Circulation Model CCSM4 was selected because it has been used in several studies to predict the climate in Colombia, yielding excellent results (Rodríguez, 2012; Palomino et al., 2015).
Table 1.
Bioclimatic variables. Abbreviation and units of the environmental variables (Degrees Celcius (°C), millimeters (mm) and coefficient of variation (CV)).
| Code | Environmental variables | Unit |
|---|---|---|
| Bio1 | Annual mean temperature | °C |
| Bio2 | Mean diurnal range (mean of monthly max. and min. temp.) | °C |
| Bio3 | Isothermality ((Bio2/Bio7) × 100) | % |
| Bio4 | Temperature seasonality (standard deviation × 100) | CV |
| Bio5 | Maximum temperature of warmest month | °C |
| Bio6 | Minimum temperature of coldest month | °C |
| Bio7 | Temperature annual range (Bio5–Bio6) | °C |
| Bio8 | Mean temperature of wettest quarter | °C |
| Bio9 | Mean temperature of driest quarter | °C |
| Bio10 | Mean temperature of warmest quarter | °C |
| Bio11 | Mean temperature of coldest quarter | °C |
| Bio12 | Annual precipitation | mm |
| Bio13 | Precipitation of wettest period | mm |
| Bio14 | Precipitation of driest period | mm |
| Bio15 | Precipitation seasonality (CV) | CV |
| Bio16 | Precipitation of wettest quarter | mm |
| Bio17 | Precipitation of driest quarter | mm |
| Bio18 | Precipitation of warmest quarter | mm |
| Bio19 | Precipitation of coldest quarter | mm |
Generally, bioclimatic variables are highly correlated (Feilhauer et al., 2012). As a consequence, it can be a problem for parameter estimation, because it inflates the variance of the regression parameters, leading to the incorrect identification of relevant predictor variables in a statistical model (Dormann et al., 2013). To reduce the highly correlated bioclimatic variables, we used the tool Remove Highly Correlated Variables from the SDMtoolbox 2.0 package (Brown, 2014), with which we performed the multicollinearity test by using the Pearson correlation coefficient (r) to determine a set of independent variables (Sillero, 2011). Variables with a cross-correlation coefficient value equal to or greater than 0.8 were excluded (Yang et al., 2013; Beckham and Atkinson, 2017). It should be noted that to retain variables with a correlation less than or equal to 80%, the tool "Remove highly correlated variables" from the SDMtoolbox package was used (Brown et al., 2017). This tool evaluates correlations between all data in the input environment and then removes the layers that are correlated at the level specified by the user (Brown et al., 2017). This took into account layers that are frequently used in ecology and evolution studies and variables that best represent the original input climate data (as they directly reflect actual measurements) and are not derived from several layers or a subset of the data (Brown et al., 2017).
To explain the altitudinal variation of the A. aegypti distribution, we used altimetry data as another physical variable (~0.86 km2 resolution) extracted from the Shuttle Radar Topography Mission (SRTM) available at www.srtm.usgs.gov, consulted on 2017). It should be noted that the same altimetry of the land was used for both present and future modeling. Besides, elevation does not directly affect the distribution of the species but instead changes in temperature and air pressure that vary with altitude (Pearson, 2010). The environmental layers were processed in the ArcGIS10.6 software to obtain the information of the area of interest (Colombia).
2.3. Model selection, configurations and evaluation parameters
For A. aegypti distribution, we used the Maximum Entropy species distribution model (MaxEnt, version 3.4.1, available at http://biodiversityinformatics.amnh.org/open_source/maxent/, consulted on 2018). MaxEnt estimates a probability distribution of species of interest by finding the probability distribution of maximum entropy (Phillips et al., 2006). The model takes into account georeferenced presence data of a species at a geographical level and the different characteristics (environmental variables) that influence its distribution (Phillips et al., 2006).
MaxEnt was chosen because it is one of the most frequently and accurate methods of predicting the distribution of species (Elith et al., 2006; Phillips et al., 2006; Wisz et al., 2008; Ortega-Huerta and Peterson, 2008; Tognelli et al., 2009; Gomes et al., 2018) and is one of the most-used to predict the geographic distribution of insects, such as A. aegypti (Cardoso-Leite et al., 2014; Kraemer et al., 2015; Fatima et al., 2016; Khan et al., 2016; Alaniz et al., 2017). Besides, it is one of the models that presents the best prediction capabilities when different sample sizes are used, and even with sizes less than 25 occurrence data (Pearson et al., 2007; Phillips and Dudík, 2008; Wisz et al., 2008).
MaxEnt requires the use of background data or pseudo-presence data, that is, regions where the species does not have points of presence (Barbet-Massin et al., 2012). We used background data for MaxEnt calibration. It is essential that the background data used for training MaxEnt model does not relate to areas where the species is absent due to dispersion limitations or biotic interactions (Anderson and Raza, 2010; Barve et al., 2011). To avoid bias by the randomly constructed background (Phillips and Dudík, 2008), a sample of background points can be selected within a maximum radial distance of known occurrences (Thuiller et al., 2009). A maximum radial search distance of 200 km was used which covered the area of a Convex Minimum Polygon (MCP) of ~800,000 km2 which was constructed taking into account the occurrence data of the species and similar procedures proposed by other authors (Figure 3 SM) (VanDerWal et al., 2009; Brown and Yoder, 2015). MCP construction and background data used SDMtoolbox package 2.0 (Brown et al., 2017). The implementation of this method reduces the distribution of the species to the appropriate habitat within an area of known occurrence (Brown et al., 2017). The modalities for present and future climates are adjusted to the predetermined settings in MaxEnt, with the exception of the following parameters: random test percentage and training equal to 30% and 70% respectively; the output of the models was logistic, because it improves the calibration of the model and can be interpreted as a response to the probability of finding suitable areas for the species, based on environmental variables; the entity classes were: hinge, linear and quadratic, taking into account the number of filtered occurrences (Phillips and Dudík, 2008); the maximum number of background points equal 10 000 (Kramer-Schadt et al., 2013); the maximum number of interactions equal 5000 (Beckham and Atkinson, 2017); Regularization multiplier of 2, because it reduces the level of low levels (Radosavljevic and Anderson, 2014).
Since Colombia presents significant spatial variations (for example, elevation of terrain), it is probable that spatial groups of localities are too adjusted to environmental biases. Therefore, the performance values of the model are inflated (Veloz, 2009; Hijmans and Hall, 2012; Boria et al., 2014). This usually the case when using randomly partitioned occurrence data sets (Radosavljevic and Anderson, 2014) and a geographic division is indispensable (Osborne and Suarez-Seoane, 2002). Besides, such evaluations become more critical when modeling the distribution of species through space or time under CC (Anderson, 2013). To reduce the environmental biases related to spatial groups of localities, 100 replicas (Beckham and Atkinson, 2017) of Bootstrap type (replicated sets of samples chosen by sampling with replacement) taking into account the percentage of random and training tests of 30% and 70% respectively. This configuration causes the models to be calibrated and evaluated with different permutations of the points of occurrence and background data in different regions of the study area.
Jackknife test was performed to identify the relative importance of the environmental variables. During the Jackknife test, each variable was excluded in turn, and a model was created with the remaining variables. Then a model was created using each variable in isolation. Also, a model was created using all variables as before (Phillips, 2010). The environmental variable with the highest gain, when used in isolation, made the most significant influence in the mosquito modeled (Phillips, 2010). The environmental variable that decreases the gain the most when it is omitted appears to have the most information that is not present in other variables, therefore, it is likely to be highly influential (Phillips, 2010). In addition, the model showed response curves that relate the probability of finding suitable areas for mosquito distribution, taking into account the environmental variables used in the modeling.
The precision of the species distribution model was evaluated using the Area under Receiver Operating Characteristics (ROC) curve, better known as AUC (Fielding and Bell, 1997). This technique is one of the most used to evaluate the prediction of species distribution models (Elith et al., 2006). It is interpreted as the probability that a randomly chosen presence location has a higher classification than a randomly chosen background point, i.e. the AUC is used to determine how the model distinguishes between presences and absences (Merow et al., 2013). The ROC curve shows the false positive rate (the model predicts that a species will be present where it has not been observed) on the "X" axis and the true positive rate (the predictions may coincide with the observed presences of the species) on the "Y" axis represented in the range of probability threshold values (Phillips, 2010). The closer the ROC curve is to the axis, the higher the area below the curve (AUC) and therefore, the more accurate the model (Phillips, 2010). The AUC was evaluated from 0.0 to 1, and the precision of the model is better when approaching 1, that is, there is more significant discrimination of suitable areas than those not suitable for the species (Phillips et al., 2006). Models with an AUC value greater than 0.75 are classified as potentially useful for predicting the distribution of a species (Elith et al., 2006). Another way to validate the results was to contrast the maps of the potential distribution of the species with the real cases of Dengue, Chikungunya, and Zika historically reported in the country.
To determine the effect of climate change on the geographic distribution of A. aegypti, changes in distribution between current and future models were quantified. For that, the Distribution Changes Between Binary SDMs tool of the SDMtoolbox 2.0 package was used (Brown, 2014), which required binary maps (result of the 100 replications) of potential distribution with a logistic presence training threshold of 10 percentiles (Cardoso-Leite et al., 2014; Radosavljevic and Anderson, 2014). The distribution changes between the binary distribution models were determined to find the areas of potential distribution, range reduction, range expansion and zones without changes (Brown et al., 2017). We determined the distribution shift by estimating centroid changes in the distribution using the Centroid Changes tool of the SDMtoolbox 2.0 package (Brown et al., 2017). We determined the centroid changes to summarize the distribution changes of the nucleus in the ranges of A. aegypti, that is, by reducing the distribution of the mosquito to a single central point by creating a vector line that represents the magnitude and direction of the change in time (Brown et al., 2017).
3. Results and discussion
The bioclimatic variables used in the models are the following: average annual temperature (Bio1), Mean diurnal range (Mean of monthly (max temp - min temp)) (Bio2), isothermality ((Bio2/Bio7) × 100) (Bio3), Annual precipitation (Bio12) and precipitation of driest month (Bio14). These variables were selected because they had a correlation less than or equal to 0.8 (Table 1 SM) (Yang et al., 2013; Beckham and Atkinson, 2017). The variable temperature annual range (Bio 7) showed a correlation less than 80%; however, it was not included in the final modeling because it did not contribute significantly in previous modeling tests, this being a filter used in other studies (Zeng et al., 2016).
54 spatially independent occurrence data were used to power the MaxEnt model (Figure 2: SM). Out of 128 occurrence data downloaded from GBIF were reduced to 115, due to replicas (12) and points outside the study area (1). After applying Spatially Rarefy Occurrence to the points of presence (115), we obtained 54 spatially independent data separated with a minimum radial distance of 10 km2 (Brown et al., 2017).
The results revealed a good performance by MaxEnt in the model. The model had an excellent relationship with a random model (Figure 4A, SM) obtaining an AUC (average of 100 replications) of training ~0.812 (Figure 4B, SM) (Hosmer and Lemeshow, 2000). The AUC obtained allows classifying the model as potentially useful to predict the distribution of the species, because it exceeded a value of 0.75 (Elith et al., 2006).
The variables that presented the most significant contribution to the model were land elevation (~34.6%), isothermality ((Bio2/Bio7) × 100) (~30.5%), precipitation of the coldest quarter (~12.6%) and precipitation of the driest quarter (10.9%) (Table 2). The variables elevations, Bio3, and Bio12 attributed importance of permutation with 43.1, 25.8 and 14.2% respectively (Table 2).
Table 2.
Relative contributions of environmental variables to the MaxEnt model. The values shown are averages of 100 replicas.
| Variable | Percentage contribution | Importance of Permutation |
|---|---|---|
| Land Elevation | 34.6 | 43.1 |
| Bio3 | 30.5 | 25.8 |
| Bio12 | 12.6 | 14.2 |
| Bio14 | 10.9 | 8.9 |
| Bio2 | 5.7 | 2.7 |
| Bio1 | 5.6 | 5.3 |
Among the determining variables in the distribution of A. aegypti, elevation stands out, because it presented greater gain when used in isolation and, therefore, seems to have the most useful information in itself. The bioclimatic variable that reduces the gain when it is omitted is Bio3; therefore, it seems to have the most significant amount of information that is not present in the other variables, as evidenced in a distribution study of mosquito-carried out in Brazil (Cardoso-Leite et al., 2014).
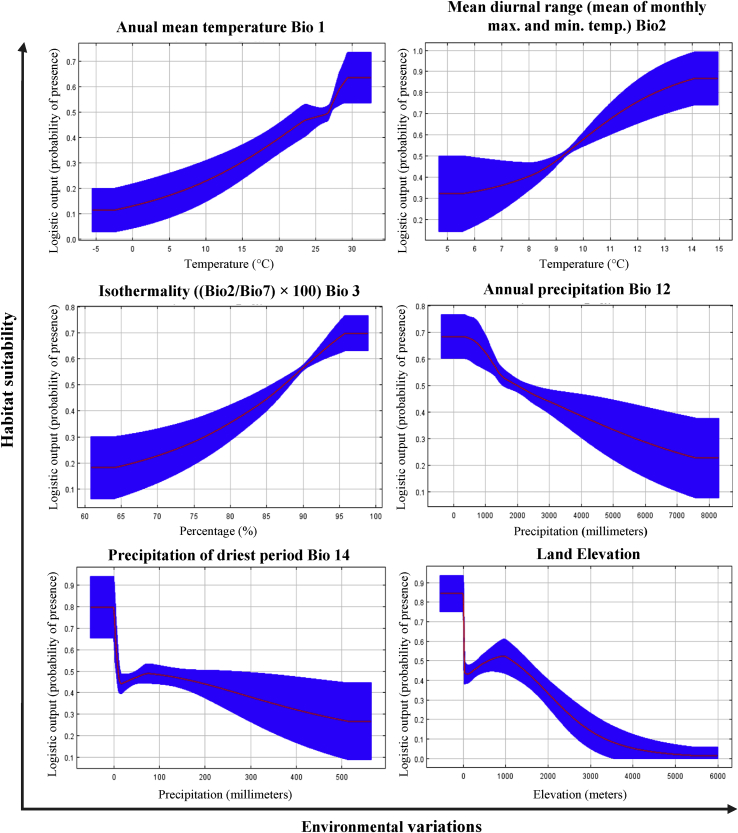
The response curves (Figure 2) reflect the environmental suitability of A. aegypti, considering a MaxEnt model created using only the corresponding variable. The ranges of environmental suitability with respect to the bioclimatic variables and the corresponding probability percentages are shown below: (Bio1) Annual Mean Temperature of ~28–32.5 °C with ~64%, the result is comparable with the optimum range for the species ranging from ~25 to 32 °C (Khormi and Kumar, 2014). It should be noted that if the temperature exceeds 30 °C, the adult reproductive capacity is affected (Carrington et al., 2013) and the extrinsic incubation period of the viruses decreases (Watts et al., 1987); however, (Attaway et al., 2016), pointed out that in laboratory tests, the mosquito has shown competition between vectors at 35 °C (Lozano-Fuentes et al., 2012). (Bio2) Mean diurnal range (mean of monthly (max temp - min temp) of ~14–15 °C with ~85%, this being consistent with other studies that suggest that the lower temperature range optimal for A. aegypti is ~14–18 °C) (Khormi and Kumar, 2014). (Bio3) Isothermality ((Bio2/Bio7) × 100) of ~96–97.5% with ~70%; (Bio12) Annual precipitation of ~0–500mm with 68%, highlighting the propensity of the mosquito to develop with a precipitation greater than or equal to 55 mm. Our mosquito develops well (Wiwanitkit, 2006) (Bio14) with Precipitation of the driest Month ~ smaller than 10mm with ~45% and ~80% when approaching 0mm, this is consistent with what (Wiwanitkit, 2006) stated, which highlighted that the ideal precipitation values are greater than 2mm.
Figure 2.
Response curves of the logistic prediction of the distribution of A. aegypti under environmental variables (bioclimatic variable). The Y axis shows the probability of presence expressed in logistic values (0–1). Each curve shows a unique model created using only the corresponding variable and represents the mean response of 100 repetitions in MaxEnt (blue) and the mean ±1 standard deviation (SD) (red).
On the other hand, the Figure 2 response curve related to the elevation of terrain shows that A. aegypti can distribute more predominantly at elevations lower than 1000 meters above sea level (~50% probability) and is more suitable close to 0 MASL (~85% probability), this being similar to what other studies showed (Lozano-Fuentes et al., 2012; Santos and Meneses, 2017). Besides, as the elevation increases, the probability of finding suitable sites decreases (Fatima et al., 2016), highlighting that A. aegypti mostly limits itself to 1500 meters above sea level (Suárez and Nelson, 1981); however, in Colombia, it has been reported at elevations close to 2200 meters above sea level (Suárez and Nelson, 1981); according to our model, because at that elevation, there is ~30% probability of finding suitable areas for the mosquito. This can be attributed to climate change, specifically to rising temperatures in high altitude areas with current temperature conditions below the innate mosquito thresholds (Lozano-Fuentes et al., 2012).
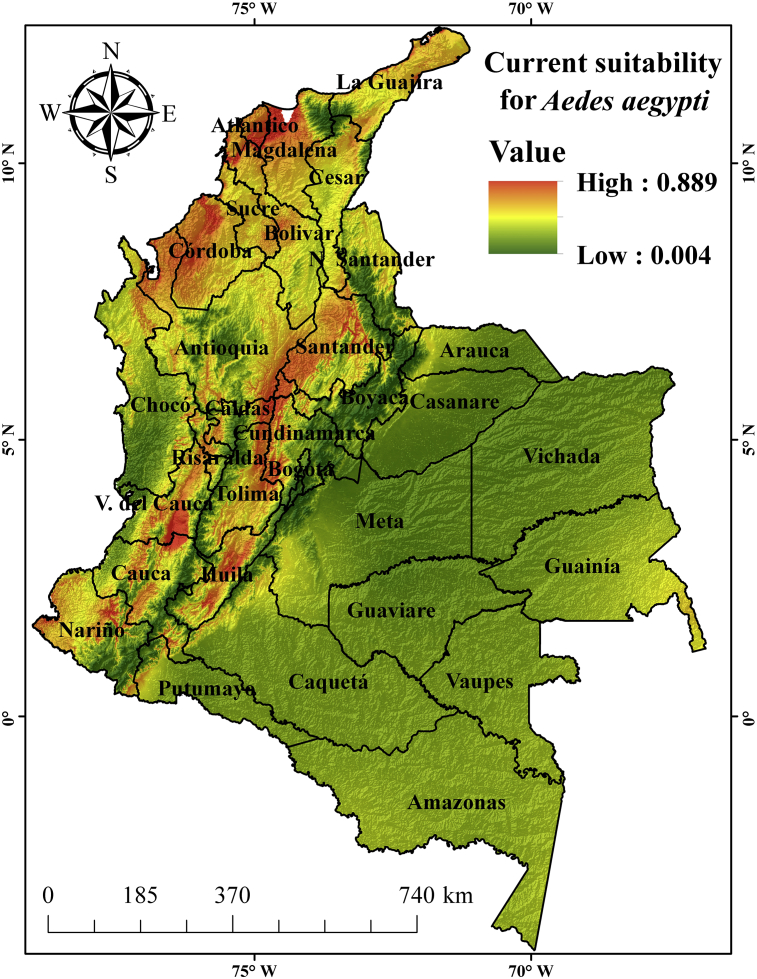
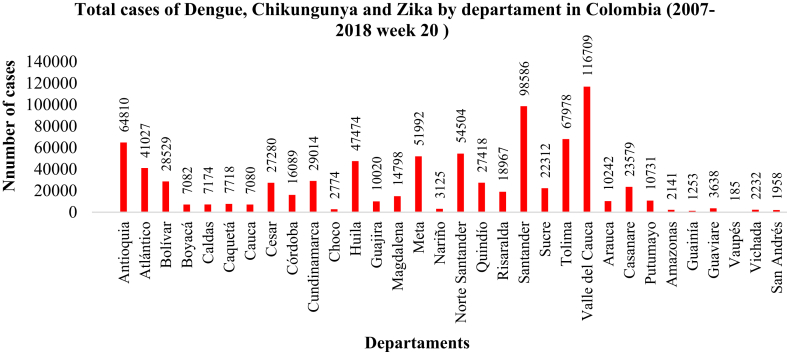
The geographical distribution of the A. egypti species varied spatially and temporally in the country. In the present conditions (Figure 3), the environmental suitability of the mosquito distribution is more likely in the Caribbean and Andean regions, especially in level areas, as shown by other studies (Alaniz et al., 2017; Tjaden et al., 2017). The distribution probability decreases in the Amazon region, due to the decrease in population density (Alaniz et al., 2017). In the Caribbean Region, the departments of Atlántico, Magdalena, Córdoba, Bolívar, Sucre and Cesar stand out in the distribution probability of the mosquito; as well as me plains of Valle del Cauca, Nariño, Cauca, Santander, Norte de Santander, Tolima, Antioquia, Huila, Cundinamarca, Caldas, and Risaralda in the Andean Region. The departments with a high probability of having suitable zones for the mosquito in the present, are largely consistent with the number of cases of Dengue, Chicungunya and Zika reported by the National Health Institute in Colombia (Figure 4). For example, from 2007 up to the epidemiological week 20 of 2018 in Colombia, 828,419 cases were presented due to the viruses, of which those in the Valle del Cauca, Santander, Tolima, Antioquia, and Norte de Santander accounted for 116,709, 98,586, 67,978, 64,810 and 54,504 cases respectively (Instituto Nacional de la salud, 2018). Also, a study that searched for dengue cases reported in Colombia between 2004 and 2013 showed that dengue outbreaks are reported mainly in departments of Norte de Santander, Santander, Huila, Tolima, Valle del Cauca and Antioquia (Castrillón et al., 2015). The probabilistic map of potential geographic distribution present for A. aegypti in Colombia is shown below.
Figure 3.
Probabilistic map of environmental suitability present for A. aegypti in Colombia. The red, yellow and green zones indicate high medium and low probability of mosquito distribution, respectively.
Figure 4.
Cumulative cases of Dengue, Chikungunya and Zika by department in Colombia, since 2007.
Below is the total number of cases of Dengue, Chikungunya and Zika reported in Colombia, sorted by department.
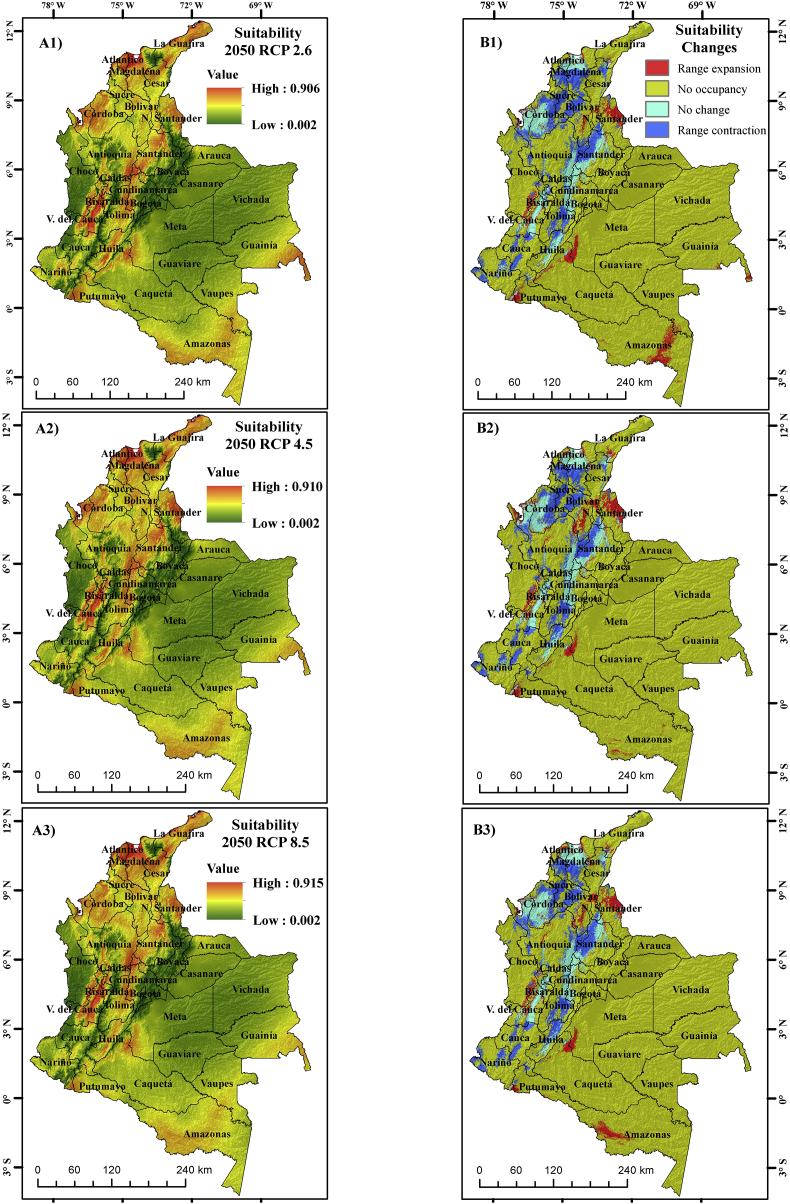
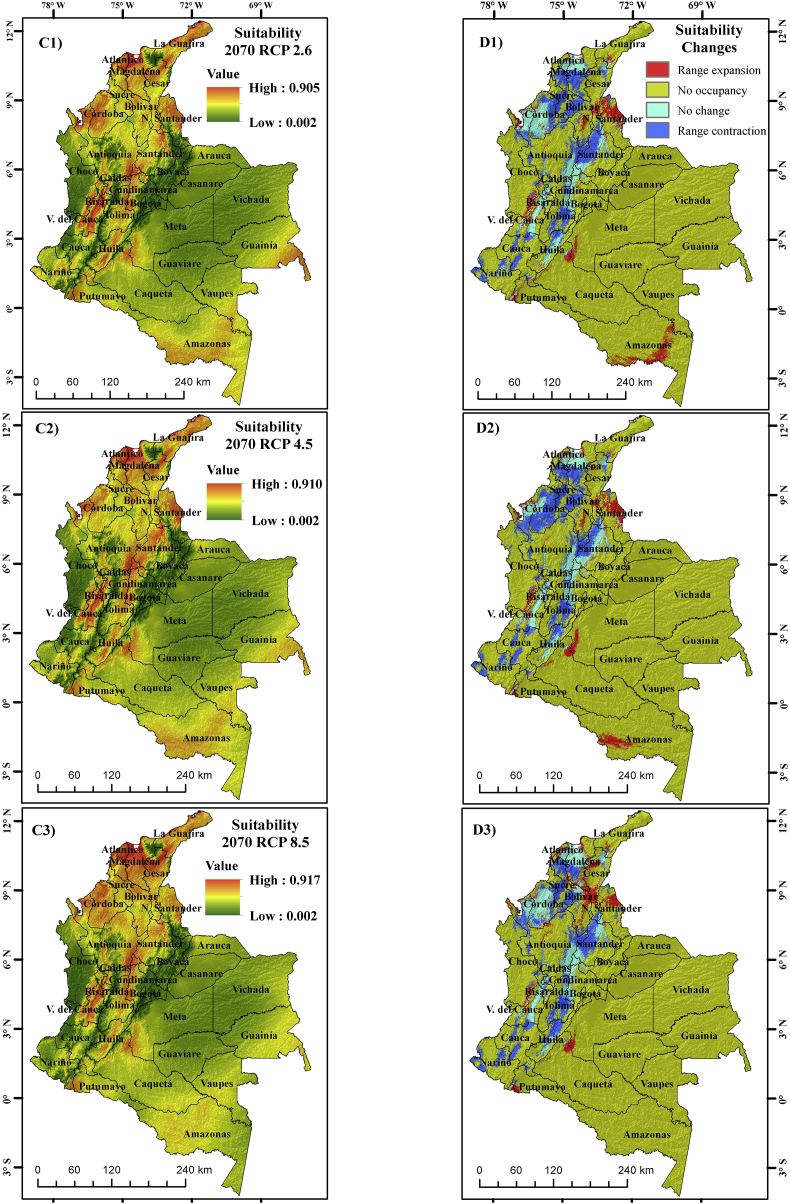
For the years 2050 and 2070, the geographical distribution of A. aegypti varied, compared to the present one. When quantifying the distribution areas under the climatic scenarios (Table 3), significant decreases in the ideal areas for the future were noticed. For the present climatic conditions, Colombia has ~140,612.8 km2 areas where the mosquito is possibly present; however, by 2050, they are reduced to ~92,233, ~94,231.9 and ~94,670.7 km2 under climatic scenarios RCP 2.6, 4.5 and 8.5 respectively, while for 2070 under the same scenarios, the areas of possible presence are ~93,809.2, ~84,116.2 and ~87,985.8 km2. It should be inferred that the situation is not straightforward but rather complicated as some areas will see an upsurge in mosquito distribution, while others are likely to see a decline in the future compared to the present. Also, the changes in species distribution area are not always linearly related to the intensity of emission scenarios (RCP) (Wilby and Dessai, 2010; Goberville et al., 2015). Thus, when modeling future distribution in our case, the same was observed for the 2070s RCP 4.5, as identified in other studies (Samy et al., 2016). This may be due to uncertainties arising from GCMs and emission scenarios.
Table 3.
Changes in future geographic distribution (2050 and 2070) (Km2) for A. aegypti due to climate scenario RCP 2.6, 4.5 and 8.5.
| Legend maps of changes | Changes in geographical distribution by year and climate scenario (km2) |
|||||
|---|---|---|---|---|---|---|
| 2050 |
2070 |
|||||
| RCP 2.6 | RCP 4.5 | RCP 8.5 | RCP 2.6 | RCP 4.5 | RCP 8.5 | |
| Range expansion | 32,344.8 | 31,519.8 | 31,738.8 | 34,729.9 | 30,822.1 | 28,755.4 |
| No occupancy | 963,801.3 | 964,626.3 | 964,407.3 | 961,416.3 | 965,324.1 | 967,390.8 |
| No change | 59,888.2 | 62,712.1 | 62,931.9 | 59,079.3 | 53,294.1 | 59,230.4 |
| Range contraction | 80,724.6 | 77,900.7 | 77,680.9 | 81,533.5 | 87,318.7 | 81,382.4 |
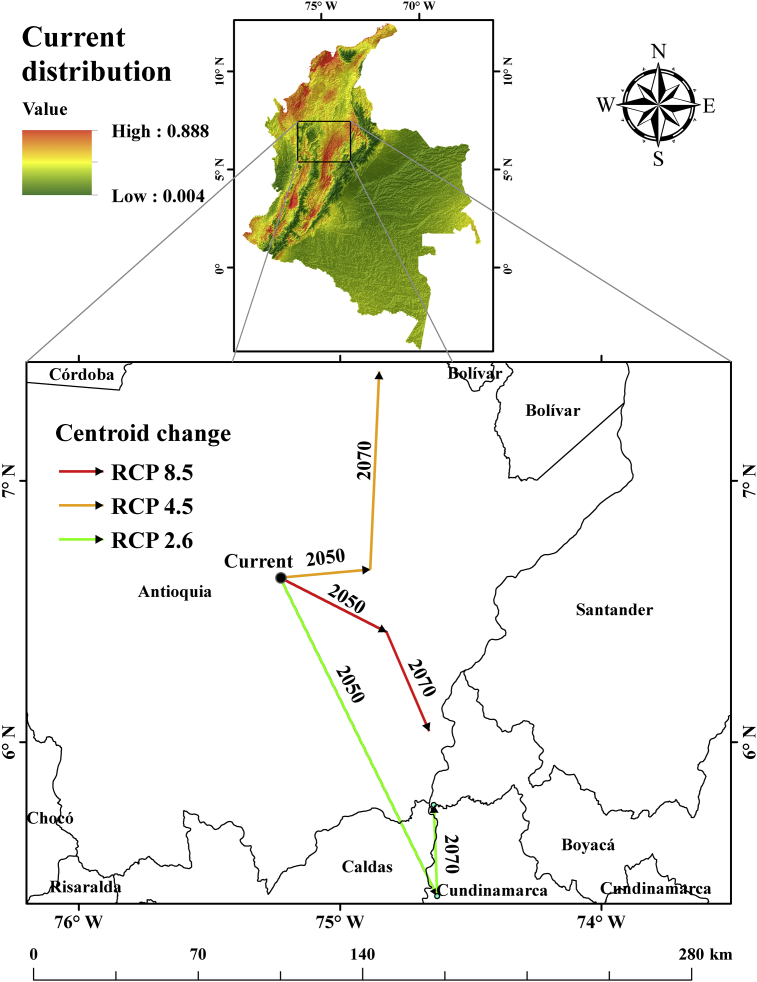
The results indicate that suitable distribution areas for mosquito would be reduced by more than 30% in the future (2050 and 2070). When comparing future scenarios, it is evident that by 2050, the suitable areas for mosquitos increase with warming (RCP 8.5), while by 2070, there were fluctuations. However, there were significant decreases mainly in RCP 4.5 and RCP 8.5 with respect to RCP 2.6. This change is possibly associated with extreme temperature increases in 2070 over 2050. When the warming becomes high, the areas where the mosquitos were present are no longer suitable in the future. Temperature over the optimum levels affects the life cycle of mosquitos and its reproductive capacity (Rueda et al., 1990; Khormi and Kumar, 2014; Andriamifidy et al., 2019). Although, by 2070, the ideal distribution area for the mosquito decreases, the distribution centroid shift tends to move towards the north of the country as warming increased (RCP 8.5) and towards the south when the warming is low (RCP 2.6) (Figures 5 and 6).
Figure 5.
Environmental suitability maps for A. aegypti by 2050 A1) RCP 2.6, A2) RCP 4.5 and A3) RCP 8.5 and changes in potential geographical distribution of A. aegypti by 2050 B1) RCP 2.6, B2) RCP 4.5 and B3) RCP 8.5.
Figure 6.
Environmental suitability maps for A. aegypti for 2070 C1) RCP 2.6, C2) RCP 4.5 and C3) RCP 8.5 and changes in potential geographical distribution of A. aegypti for 2070 D1) RCP 2.6, D2) RCP 4.5 and D3) RCP 8.5.
Despite the fact that for the future, the ideal areas for the mosquito presented a decreasing tendency with respect to the present, in departments such as Nariño and Cauca, in some areas of the departments such as Norte de Santander, Chocó, Meta, Caquetá, and Amazonas, there was an expansion of areas suitable for the mosquito. In addition, the probability of finding the species increased from ~89% in the present distribution to ~92% in some areas of the departments that present climatic conditions suitable for A. aegypti. The results are comparable with those of previous studies, where it was concluded that the optimum areas for the development of A. aegypti will decrease by the year 2070, mainly in some countries of South America, and among these, Colombia (Khormi and Kumar, 2014). On the other hand, to analyze the results presented in this study, it is necessary to take into account that when rainfall is high, possibly in RCP 2.6, the number and quantity of sites for mosquito breeding also increases (Githeko et al., 2000), but when the rainfall increases, the temperature (RCP 8.5) decreases. Further, when the temperature increases, there is a higher capacity to produce more offspring, because the larvae take less time to mature and besides, mosquitoes increase during the transmission period (Rueda et al., 1990) and the female A. aegypti feeds more frequently (Gillies, 1953).
Environmental suitability maps and potential distribution changes for A. aegypti for the years 2050 and 2070 are shown in Figure 5, Figure 6.
The distribution centroid changes of the environmental suitability of the mosquito for the future are shown in Figure 7. The results indicate that the direction and magnitude of the changes are different in the climatic scenarios studied. The distribution centroid of the current habitat is located at 75.230° W longitude and 6.630°N latitude in the department of Antioquia, Colombia. The distribution centroid of the suitable future area shifted towards 74.629 °W, 5.411 °N towards the southeastern location under RCP 2.6–2050, and 74.642 °W, 5.760 °N under RCP 2.6–2070. While under RCP4.5–2050, the distribution centroid of the future suitable area was located at 74.885 °W, 6.661°N, and southeastern position (74.659 °W, 6.042 °N) under RCP 4.5–2070. In the scenario RCP 8.5–2050, the centroid of the suitable future area moved towards southeastern at 74.823° W, 6.422° N, however, under RCP 8.5–2070 was located north of Colombia at 74.851 °W, 7.419 °N.
Figure 7.
Changes of environmental suitability centroid for A. aegypti for the years 2050 and 2070 in Colombia. The green, orange and red vectors indicate the direction and magnitude of distribution changes under RCP scenarios 2.6, 4.5 and 8.5 respectively.
Overall, the results indicated the core distributional shift expressed a relatively high magnitude (~150 km) towards the south when temperatures are low (RCP 2.6–2050), and towards north when temperatures are high (RCP 8.5–2070) distribute around 100 km respect to the current distribution centroid location 74.823 °W, 6.422 °N) (Figure 7).
Literature review shows that this is one of the first studies that predict the distribution and potential changes in the geographic range of A. aegypti in Colombia; taking into account current climatic conditions and future climate change scenarios (RCP 2.6, RCP 4.5 and RCP 8.5) for the years 2050 and 2070. Results provide information for public health for several reasons: 1) it predicts the probabilities of the presence of one of the most important primary arbovirus vectors worldwide, 2) it assesses the influence of climate change on the potential geographical distribution of A. Aegypti and 3) it provides a primary source of maps showing area changes and the shift in the mosquito distribution under climate change scenario.
This research has broad implications in the area of health, especially in the prediction of infectious diseases. For example, for the National Institute of Health, in charge of epidemiological surveillance and control in Colombia, these results are of vital importance for the taking of preventive and control measures, especially in the areas with a higher risk of the presence of vectors.
On the other hand, this study is a valuable input for the construction of predictive models with MaxEnt, since it describes in a general way the procedures to improve the performance of the model and to avoid some biases when configuring the parameters of the software, taking into account the environmental predictors and the presence data of the species.
4. Conclusions
It can be concluded that the modeling with MaxEnt allowed identifying the potential zones of Colombia where A. aegypti can be distributed. The areas susceptible to the presence of A. aegypti are affected by climate change. The Caribbean and Andean regions presented suitable areas with high probability of mosquito distribution in the present and future climatic conditions. In respect of the Caribbean Region, this included some areas of the departments of Atlántico, Magdalena, Córdoba, Bolívar, Sucre and Cesar stand out in the distribution probability of the mosquito; as well as some plains of Valle del Cauca, Nariño, Cauca, Santander, Norte de Santander, Tolima, Antioquia, Huila, Cundinamarca, Caldas, and Risaralda in the Andean Region.
In respect of the future (2050 and 2070), the geographical distribution of the mosquito varied from the present, ruling out significant decreases in the areas ideal for the insect. In the present conditions, Colombia has ~140,612.8 square km of areas with possible presence of the vector; however, for the future, this was reduced by more than 30%. On the other hand, when comparing the geographical distribution of the mosquito between 2050 and 2070, it was found that in 2050, there is a tendency for the ideal areas to increase, as the temperature increases (RCP8.5), while for 2070 under the same scenario, the areas suitable for the mosquito decreased.
The results found can serve as an input to take preventive and control measures, especially in areas with a higher risk of contracting the virus. Besides, some departments without evidence of being affected by A. aegypti in the present conditions could become areas of vulnerability in the future, with an active transmission. For this reason, it is necessary to implement or reinforce prevention measures for these areas.
We suggest modeling the distribution of A. aegypti taking into account other environmental variables, for example, population density, economic activities, water network, soil cover and among other variables which are related to the distribution and biology of the mosquito. Also, it is required to incorporate new occurrence data sets, for example, in the Orinoco region of Colombia.
Also, it is necessary to know the dynamics of the viruses transmitted by the vector to widely understand the distribution patterns of the same in Colombia and thus, take the relevant control measures to reduce the risk of transmission.
Finally, based on these models, we propose to create a GIS virtual platform for early warning system against communicable infectious diseases like Dengue, Zika, and Chikungunya in Colombia. Also, generate new predictions considering a more detailed spatial resolution to study the phenomena in the prioritized areas of the country.
Declarations
Author contribution statement
Cristiam Victoriano Portilla Cabrera: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
John Josephraj Selvaraj: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to thank Universidad Nacional de Colombia, Faculty of Engineering and Administration, Palmira campus for providing the lab facilities and software (ArcGIS) license.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Alaniz A.J., Bacigalupo A., Cattan P.E. Spatial quantification of the world population potentially exposed to Zika virus. Int. J. Epidemiol. 2017;46:966–975. doi: 10.1093/ije/dyw366. [DOI] [PubMed] [Google Scholar]
- Anderson R.P. A framework for using niche models to estimate impacts of climate change on species distributions. Ann. N. Y. Acad. Sci. 2013;1297:8–28. doi: 10.1111/nyas.12264. [DOI] [PubMed] [Google Scholar]
- Anderson R.P., Gonzalez I. Species-specific tuning increases robustness to sampling bias in models of species distributions: an implementation with Maxent. Ecol. Model. 2011;222:2796–2811. [Google Scholar]
- Anderson R.P., Raza A. The effect of the extent of the study region on GIS models of species geographic distributions and estimates of niche evolution: preliminary tests with montane rodents (genus Nephelomys) in Venezuela. J. Biogeogr. 2010;37:1378–1393. [Google Scholar]
- Andriamifidy R.F., Tjaden N.B., Beierkuhnlein C., Thomas S.M. Do we know how mosquito disease vectors will respond to climate change? Emerg. Top. Life Sci. 2019;3 doi: 10.1042/ETLS20180125. 115 LP-132. [DOI] [PubMed] [Google Scholar]
- Attaway D.F., Jacobsen K.H., Falconer A., Manca G., Waters N.M. Risk analysis for dengue suitability in Africa using the ArcGIS predictive analysis tools (PA tools) Acta Trop. 2016;158:248–257. doi: 10.1016/j.actatropica.2016.02.018. [DOI] [PubMed] [Google Scholar]
- Barbet-Massin M., Jiguet F., Albert C.H., Thuiller W. Selecting pseudo-absences for species distribution models: how, where and how many? Meth. Ecol. Evol. 2012;3:327–338. [Google Scholar]
- Barve N., Barve V., Jiménez-Valverde A., Lira-Noriega A., Maher S.P., Peterson A.T., Soberón J., Villalobos F. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol. Model. 2011;222:1810–1819. [Google Scholar]
- Beckham J.L., Atkinson S. An updated understanding of Texas bumble bee (Hymenoptera: Apidae) species presence and potential distributions in Texas, USA. PeerJ. 2017;5 doi: 10.7717/peerj.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boria R.A., Olson L.E., Goodman S.M., Anderson R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014;275:73–77. [Google Scholar]
- Brown J.L. SDMtoolbox: a python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Meth. Ecol. Evol. 2014;5:694–700. doi: 10.7717/peerj.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.L., Bennett J.R., French C.M. SDMtoolbox 2.0: the next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ. 2017;5 doi: 10.7717/peerj.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.L., Yoder A.D. Shifting ranges and conservation challenges for lemurs in the face of climate change. Ecol. Evol. 2015;5:1131–1142. doi: 10.1002/ece3.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso-Leite R., Vilarinho A.C., Novaes M.C., Tonetto A.F., Vilardi G.C., Guillermo-Ferreira R. Recent and future environmental suitability to dengue fever in Brazil using species distribution model. Trans. R. Soc. Trop. Med. Hyg. 2014;108:99–104. doi: 10.1093/trstmh/trt115. [DOI] [PubMed] [Google Scholar]
- Carrington L.B., Armijos M.V., Lambrechts L., Barker C.M., Scott T.W. Effects of fluctuating daily temperatures at critical thermal extremes on Aedes aegypti life-history traits. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillón J., Castaño J, Urcuqui S. Dengue en Colombia: diez años de evolución. Rev. Chil. Infectología. 2015;32:142–149. doi: 10.4067/S0716-10182015000300002. [DOI] [PubMed] [Google Scholar]
- Danis-Lozano R., Ramsey J.M., Luther C., Campbell L.P., Peterson A.T., Moo-Llanes D. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos. Trans. R. Soc. Biol. Sci. 2015;370:20140135. doi: 10.1098/rstb.2014.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann C.F., Elith J., Bacher S., Buchmann C., Carl G., Carré G., Marquéz J.R.G., Gruber B., Lafourcade B., Leitão P.J., Münkemüller T., Mcclean C., Osborne P.E., Reineking B., Schröder B., Skidmore A.K., Zurell D., Lautenbach S. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography (Cop.). 2013;36:27–46. [Google Scholar]
- Elith J., Graham C.H., Anderson R.P., Dudik M., Ferrier S., Guisan A., Hijmans R.J., Huettmann F., Leathwick J.R., Lehmann A., Li J., Lohmann L.G., Loiselle B.A., Manion G., Moritz C., Nakamura M., Nakazawa Y., Overton J.M., Peterson A.T., Phillips S.J., Richardson K., Scachetti-Pereira R., Schapire R.E., Soberon J., Williams S., Wisz M.S., Zimmermann N.E. Novel methods improve prediction of species’ distributions from occurrence data. Ecography (Cop.). 2006;29:129–151. [Google Scholar]
- Elith J., Leathwick J.R. Species distribution models: ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009;40:677–697. [Google Scholar]
- Escobar L.E., Romero-Alvarez D., Leon R., Lepe-Lopez M.A., Craft M.E., Borbor-Cordova M.J., Svenning J.C. Declining prevalence of disease vectors under climate change. Sci. Rep. 2016;6:39150. doi: 10.1038/srep39150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima S.H., Atif S., Rasheed S.B., Zaidi F., Hussain E. Species Distribution Modelling of Aedes aegypti in two dengue-endemic regions of Pakistan. Trop. Med. Int. Health. 2016;21:427–436. doi: 10.1111/tmi.12664. [DOI] [PubMed] [Google Scholar]
- Feilhauer H., He K.S., Rocchini D. Modeling species distribution using niche-based proxies derived from composite bioclimatic variables and MODIS NDVI. Remote Sens. 2012;4:2057–2075. [Google Scholar]
- Fick S.E., Hijmans R. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017;37:4302–4315. [Google Scholar]
- Fielding A.H., Bell J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997;24:38–49. [Google Scholar]
- Gillies M.T. The duration of the gonotrophic cycle in Anopheles gambiae and Anopheles funestus, with a note on the efficiency of hand catching. East Afr. Med. J. 1953;30:129–135. [PubMed] [Google Scholar]
- Githeko A.K., Lindsay S.W., Confalonieri U.E., Patz J.A. Climate change and vector-borne diseases: a regional analysis. Bull. World Health Organ. 2000;78:1136–1147. [PMC free article] [PubMed] [Google Scholar]
- Goberville E., Beaugrand G., Hautekèete N.C., Piquot Y., Luczak C. Uncertainties in the projection of species distributions related to general circulation models. Ecol. Evol. 2015;5:1100–1116. doi: 10.1002/ece3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes V.H.F., Ijff S.D., Raes N., Amaral I.L., Salomão R.P., Coelho L.D.S., Matos F.D.D.A., Castilho C.V., Filho D.D.A.L., López D.C., Guevara J.E., Magnusson W.E., Phillips O.L., Wittmann F., Carim M.D.J.V., Martins M.P., Irume M.V., Sabatier D., Molino J.F., Bánki O.S., Guimarães J.R.D.S., Pitman N.C.A., Piedade M.T.F., Mendoza A.M., Luize B.G., Venticinque E.M., Novo E.M.M.D.L., Vargas P.N., Silva T.S.F., Manzatto A.G., Terborgh J., Reis N.F.C., Montero J.C., Casula K.R., Marimon B.S., Marimon B.H., Coronado E.N.H., Feldpausch T.R., Duque A., Zartman C.E., Arboleda N.C., Killeen T.J., Mostacedo B., Vasquez R., Schöngart J., Assis R.L., Medeiros M.B., Simon M.F., Andrade A., Laurance W.F., Camargo J.L., Demarchi L.O., Laurance S.G.W., Farias E.D.S., Nascimento H.E.M., Revilla J.D.C., Quaresma A., Costa F.R.C., Vieira I.C.G., Cintra B.B.L., Castellanos H., Brienen R., Stevenson P.R., Feitosa Y., Duivenvoorden J.F., Aymard G.A.C., Mogollón H.F., Targhetta N., Comiskey J.A., Vicentini A., Lopes A., Damasco G., Dávila N., García-Villacorta R., Levis C., Schietti J., Souza P., Emilio T., Alonso A., Neill D., Dallmeier F., Ferreira L.V., Araujo-Murakami A., Praia D., Do Amaral D.D., Carvalho F.A., De Souza F.C., Feeley K., Arroyo L., Pansonato M.P., Gribel R., Villa B., Licona J.C., Fine P.V.A., Cerón C., Baraloto C., Jimenez E.M., Stropp J., Engel J., Silveira M., Mora M.C.P., Petronelli P., Maas P., Thomas-Caesar R., Henkel T.W., Daly D., Paredes M.R., Baker T.R., Fuentes A., Peres C.A., Chave J., Pena J.L.M., Dexter K.G., Silman M.R., Jørgensen P.M., Pennington T., Di Fiore A., Valverde F.C., Phillips J.F., Rivas-Torres G., Von Hildebrand P., Van Andel T.R., Ruschel A.R., Prieto A., Rudas A., Hoffman B., Vela C.I.A., Barbosa E.M., Zent E.L., Gonzales G.P.G., Doza H.P.D., Miranda I.P.D.A., Guillaumet J.L., Pinto L.F.M., Bonates L.C.D.M., Silva N., Gómez R.Z., Zent S., Gonzales T., Vos V.A., Malhi Y., Oliveira A.A., Cano A., Albuquerque B.W., Vriesendorp C., Correa D.F., Torre E.V., Van Der Heijden G., Ramirez-Angulo H., Ramos J.F., Young K.R., Rocha M., Nascimento M.T., Medina M.N.U., Tirado M., Wang O., Sierra R., Torres-Lezama A., Mendoza C., Ferreira C., Baider C., Villarroel D., Balslev H., Mesones I., Giraldo L.E.U., Casas L.F., Reategui M.A.A., Linares-Palomino R., Zagt R., Cárdenas S., Farfan-Rios W., Sampaio A.F., Pauletto D., Sandoval E.H.V., Arevalo F.R., Huamantupa-Chuquimaco I., Garcia-Cabrera K., Hernandez L., Gamarra L.V., Alexiades M.N., Pansini S., Cuenca W.P., Milliken W., Ricardo J., Lopez-Gonzalez G., Pos E., Ter Steege H. Species Distribution Modelling: contrasting presence-only models with plot abundance data. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-017-18927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillet M.E., Barrera R., Martínez J.E., Berti J., Fortin M.J. Disentangling the effect of local and global spatial variation on a mosquito-borne infection in a neotropical heterogeneous environment. Am. J. Trop. Med. Hyg. 2010;82:194–201. doi: 10.4269/ajtmh.2010.09-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines A., Kovats R.S., Campbell-Lendrum D., Corvalan C. Climate change and human health: impacts, vulnerability and public health. Public Health. 2006;120:585–596. doi: 10.1016/j.puhe.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Hijmans R.J., Cameron S.E., Parra J.L., Jones P.G., Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. [Google Scholar]
- Hijmans R.J., Hall W. Cross-validation of species distribution models : removing spatial sorting bias and calibration with a null model. Ecology. 2012;93:679–688. doi: 10.1890/11-0826.1. [DOI] [PubMed] [Google Scholar]
- Hosmer D.W., Lemeshow S. Wiley Ser. Probab. Sattistics; 2000. Applied Logistic Regression. [Google Scholar]
- IDEAM . Atlas Clim; Nac: 2005. Parte II Distribución Espacio-temporal de las variables del clima. [Google Scholar]
- Instituto Nacional de la salud Vigilancia Rutinaria [WWW Document] 2018. http://portalsivigila.ins.gov.co/sivigila/documentos/Docs_1.php
- IPCC . Informe de síntesis, Cambio climático 2014: Informe de síntesis. In: Pachauri R.K., Meyer L.A., editors. Contribución de los Grupos de trabajo I, II y III al Quinto Informe de Evaluación del Grupo Intergubernamental de Expertos sobre el Cambio Climático [Equipo principal de redacción. 2014. [Google Scholar]
- Kantor I.N. Dengue, Zika and chikungunya. Medicina (B. Aires) 2016:1–5. [PubMed] [Google Scholar]
- Khan K., Brady O.J., Marinho F., Cohn E., Hay S.I., Scott T.W., Pigott D.M., Shearer F.M., Gething P.W., Murray C.J., Brownstein J.S., Golding N., Jaenisch T., Kraemer M.U., Weiss D.J., Ruktanonchai C.W., Tatem A.J. Mapping global environmental suitability for Zika virus. Elife. 2016;5:1–19. doi: 10.7554/eLife.15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khormi H.M., Kumar L. Climate change and the potential global distribution of Aedes aegypti: spatial modelling using geographical information system and CLIMEX. Geospat. Health. 2014;8:405–415. doi: 10.4081/gh.2014.29. [DOI] [PubMed] [Google Scholar]
- Kouri G., Pelegrino J., Munster B.M., Guzmán M. Sociedad, economía, inequidades y dengue. Rev Cuba. Med. Trop. 2007;59:177–185. [PubMed] [Google Scholar]
- Kraemer M.U.G., Sinka M.E., Duda K.A., Mylne A.Q.N., Shearer F.M., Barker C.M., Moore C.G., Carvalho R.G., Coelho G.E., Van Bortel W., Hendrickx G., Schaffner F., Elyazar I.R., Teng H.J., Brady O.J., Messina J.P., Pigott D.M., Scott T.W., Smith D.L., William Wint G.R., Golding N., Hay S.I. The global distribution of the arbovirus vectors Aedes aegypti and Ae. Albopictus. Elife. 2015;4:1–18. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Schadt S., Niedballa J., Pilgrim J.D., Schröder B., Lindenborn J., Reinfelder V., Stillfried M., Heckmann I., Scharf A.K., Augeri D.M., Cheyne S.M., Hearn A.J., Ross J., Macdonald D.W., Mathai J., Eaton J., Marshall A.J., Semiadi G., Rustam R., Bernard H., Alfred R., Samejima H., Duckworth J.W., Breitenmoser-Wuersten C., Belant J.L., Hofer H., Wilting A. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013;19:1366–1379. [Google Scholar]
- Lozano-Fuentes S., Hayden M.H., Welsh-Rodriguez C., Ochoa-Martinez C., Tapia-Santos B., Kobylinski K.C., Uejio C.K., Zielinski-Gutierrez E., Delle Monache L., Monaghan A.J., Steinhoff D.F., Eisen L. The dengue virus mosquito vector Aedes aegypti at high elevation in México. Am. J. Trop. Med. Hyg. 2012;87:902–909. doi: 10.4269/ajtmh.2012.12-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina J.P., Brady O.J., Pigott D.M., Golding N., Kraemer M.U.G., Scott T.W., Wint G.R.W., Smith D.L., Hay S.I. The many projected futures of dengue. Nat. Rev. Microbiol. 2015;13:230–239. doi: 10.1038/nrmicro3430. [DOI] [PubMed] [Google Scholar]
- Ministerio de Asuntos Exteriores y de Cooperación . 2017. República de colombia. [Google Scholar]
- Nelson M.J. 1986. Aedes Aegypti: Biologia Y Ecologia. Py2.1. [Google Scholar]
- Ortega-Huerta M. a, Peterson A.T. Modeling ecological niches and predicting geographic distributions: a test of six presence-only methods. Rev. Mex. Biodivers. 2008;79:205–216. [Google Scholar]
- Osborne P.E., Suarez-Seoane S. Should data be partioned spatially before building large distribution models? Ecol. Model. 2002;157:249–259. [Google Scholar]
- Palomino R., Córdoba-Machado S., Esteban-Parra M.J. Evaluación de modelos climáticos globales del CMIP5 sobre el noroeste de América del Sur. Rev. Biodivers. Neotrop. 2015;5(1):16–22. ISSN 2027-8918, ISSN-e 2256-5426, 2015 (Ejemplar Dedic. a Rev. Biodivers. Neotrop. [Google Scholar]
- Pearson R.G. Species’ distribution modeling for conservation educators and practitioners. Lessons Conserv. 2010;3:54–89. [Google Scholar]
- Pearson R.G., Dawson T.P. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003:361–371. [Google Scholar]
- Pearson R.G., Raxworthy C.J., Nakamura M., Townsend Peterson A. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J. Biogeogr. 2007;34:102–117. [Google Scholar]
- Peterson A.T., Martínez-Campos C., Nakazawa Y., Martínez-Meyer E. Time-specific ecological niche modeling predicts spatial dynamics of vector insects and human dengue cases. Trans. R. Soc. Trop. Med. Hyg. 2005;99:647–655. doi: 10.1016/j.trstmh.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Phillips S., Anderson R., Schapire R. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006;190:231–259. [Google Scholar]
- Phillips S. A brief tutorial on maxent. Am. Museum Nat. Hist. 2010 [Google Scholar]
- Phillips S.J., Dudík M. Modeling of species distribution with Maxent: new extensions and a comprehensive evalutation. Ecograpy. 2008;31:161–175. [Google Scholar]
- Phillips S.J., Dudik M., Elith J., Graham C.H., Leathwick J., Ferrier S., Applications S.E., Jan N., Phillips S.J., Dud M., Elith J., Graham C.H., Lehmann A., Leathwick J., Ferrier S. Vol. 19. 2016. pp. 181–197. (Sample Selection Bias and Presence-Only Distribution Models : Implications for Background and Pseudo-absence Data). [DOI] [PubMed] [Google Scholar]
- Radosavljevic A., Anderson R.P. Making better Maxent models of species distributions: complexity, overfitting and evaluation. J. Biogeogr. 2014;41:629–643. [Google Scholar]
- Rodríguez A. Evaluación de las simulaciones de precipitación y temperatura de los modelos climáticos globales del proyecto CMIP5 con el clima presente en Colombia. Ideam-Meteo. 2012;34 [Google Scholar]
- Rogers D.J., Suk J.E., Semenza J.C. Using global maps to predict the risk of dengue in Europe. Acta Trop. 2014;129:1–14. doi: 10.1016/j.actatropica.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Rückert C., Weger-Lucarelli J., Garcia-Luna S.M., Young M.C., Byas A.D., Murrieta R.A., Fauver J.R., Ebel G.D. Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat. Commun. 2017;8:15412. doi: 10.1038/ncomms15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda L.M., Patel K.J., Axtell R.C., Stinner R.E. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 1990;27:892–898. doi: 10.1093/jmedent/27.5.892. [DOI] [PubMed] [Google Scholar]
- Samy A.M., Elaagip A.H., Kenawy M.A., Ayres C.F.J., Peterson A.T., Soliman D.E. Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus, vector of West Nile virus and lymphatic filariasis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J., Meneses B.M. An integrated approach for the assessment of the Aedes aegypti and Aedes albopictus global spatial distribution, and determination of the zones susceptible to the development of Zika virus. Acta Trop. 2017;168:80–90. doi: 10.1016/j.actatropica.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Sillero N. What does ecological modelling model? A proposed classification of ecological niche models based on their underlying methods. Ecol. Model. 2011;222:1343–1346. [Google Scholar]
- Suárez M.F., Nelson M.J. Registro de altitud del Aedes aegypti en Colombia. Biomédica. 1981;1:225. [Google Scholar]
- Tjaden N.B., Suk J.E., Fischer D., Thomas S.M., Beierkuhnlein C., Semenza J.C. Modelling the effects of global climate change on Chikungunya transmission in the 21st century. Scientific Reports. 2017;7:3813. doi: 10.1038/s41598-017-03566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuiller W., Lafourcade B., Engler R., Araújo M.B. BIOMOD - a platform for ensemble forecasting of species distributions. Ecography (Cop.). 2009;32:369–373. [Google Scholar]
- Tognelli M., Roig-Junent S., Marvaldi A., Flores G., Lobo J. An evaluation of methods for modelling distribution of Patagonian insects. Rev. Chil. Hist. Nat. 2009;82:347–360. [Google Scholar]
- VanDerWal J., Shoo L.P., Graham C., Williams S.E. Selecting pseudo-absence data for presence-only distribution modeling: how far should you stray from what you know? Ecol. Model. 2009;220:589–594. [Google Scholar]
- Veloz S.D. Spatially autocorrelated sampling falsely inflates measures of accuracy for presence-only niche models. J. Biogeogr. 2009;36:2290–2299. [Google Scholar]
- Watts D.M., Burke D.S., Harrison B.A., Whitmire R.E., Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am. J. Trop. Med. Hyg. 1987;36:143–152. doi: 10.4269/ajtmh.1987.36.143. [DOI] [PubMed] [Google Scholar]
- Weaver S.C., Osorio J.E., Livengood J.A., Chen R., Stinchcomb D.T. Chikungunya virus and prospects for a vaccine. Expert Rev. Vaccines. 2012;11:1087–1101. doi: 10.1586/erv.12.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilby R.L., Dessai S. Robust adaptation to climate change. Weather. 2010;65:180–185. [Google Scholar]
- Wisz M.S., Hijmans R.J., Li J., Peterson A.T., Graham C.H., Guisan A., Elith J., Dudík M., Ferrier S., Huettmann F., Leathwick J.R., Lehmann A., Lohmann L., Loiselle B.A., Manion G., Moritz C., Nakamura M., Nakazawa Y., Overton J.M.C., Phillips S.J., Richardson K.S., Scachetti-Pereira R., Schapire R.E., Soberón J., Williams S.E., Zimmermann N.E. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008;14:763–773. [Google Scholar]
- Wiwanitkit V. An observation on correlation between rainfall and the prevalence of clinical cases of dengue in Thailand. J. Vector Borne Dis. 2006;43:73–76. [PubMed] [Google Scholar]
- Yang X.Q., Kushwaha S.P.S., Saran S., Xu J., Roy P.S. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol. Eng. 2013;51:83–87. [Google Scholar]
- Zeng Y., Low B.W., Yeo D.C.J. Novel methods to select environmental variables in MaxEnt: a case study using invasive crayfish. Ecol. Model. 2016;341:5–13. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.