Abstract
BACKGROUND: Diabetes mellitus is unfavorably associated with cancer risk. The purpose of this multidisciplinary project was to evaluate a possible association of diabetes mellitus and other comorbidities and their treatment with progression of colorectal cancer. PATIENTS AND METHODS: We investigated the correlation between pathological characteristics and clinical course, including comorbidities in 1004 Czech patients diagnosed and surgically treated for colorectal adenocarcinoma (CRC) between 1999 and 2016. RESULTS: In our data set, CRC patients treated with metformin due to coexisting diabetes mellitus type 2 (T2DM) developed fewer distant metastases which clinically correlates with slower CRC progression. Survival in metformin subgroup was longer, particularly in men with CRC. Osteoporosis may be a negative factor of survival in CRC patients. CONCLUSIONS: Our findings also indicate that aging, higher tumor grade and TNM stage, coexistence of selected endocrine disorders, and metabolic abnormalities may change the tumor microenvironment and impact survival in colorectal cancer, although mechanism of these observations yet to be explained. Patients with diabetes mellitus type 2 treated with metformin may represent the altered microenvironment with specifically tuned metabolic molecular responses and with various epigenetic characteristics. More awareness and increased understanding of the mechanisms underlying the positive effect of metformin on patients' survival could offer insight into new treatment methods and permit more individualized treatment plans.
Introduction
Colorectal cancer (CRC) is frequently diagnosed malignancy worldwide and continues to be a serious cause of morbidity and mortality despite ongoing screening efforts [1]. According to NIH National Cancer Institute statistics, approximately 1 in 22 men and 1 in 24 women will be diagnosed with CRC in their lifetime. Five-year survival 2008–2014 in the United States was 64.5%. CRC affects both sexes most commonly in the 6th decade of life but the proportion of cases diagnosed in individuals younger than 50 years of age increased to 11% in 2013 [2]. In Czech Republic, the incidence follows the worldwide trend [3].
It is known that CRC can develop as a result of accumulated genetic mutations over time similar to other malignancies or silencing tumor suppressor genes in Li-Fraumeni syndrome, although most of CRC cases are the consequence of chromosomal and microsatellite instability and their course and aggressiveness are consequent to epigenetic characteristics [4]. Diabetes mellitus together with obesity and increased level of adipocytokines, fasting hyperglycemia, insulin resistance and hyperinsulinemia, chronic inflammation, and recurrent increased level carcinoembryonic antigen are all thought to play a role in the increased risk for CRC progression and death. All of these characteristics are considered to be negative prognostic factors [[5], [6], [7], [8]].
A number of retrospective studies dealing with CRC in patients with diabetes have been published but were clearly underpowered to detect possible subtle interrelationships between treatment details and patients' survival. It has been proposed that glycemic load adverse effects correlate with recurrence and survival of CRC stage III in recent observational prospective studies [9,10]. Publications based on metaanalyses of large data sets showed up to 44% overall survival benefit in metformin-treated CRC patients with stages I–III [[11], [12], [13]].
Phosphorylated insulin receptors together with increased blood level of IGF1 possibly play a role in development of metastases in CRC which correlates with poor prognosis. The nuclear location of IGF-1R has been observed in chemotherapy resistance [14,15].
The course of CRC and survival may be affected not only by the pathophysiological systemic effects of T2DM but adversely by action of peroral antidiabetics (PAD). Biguanides (metformin, phenformin, and buformin) are derived from the herb Galega officinalis. Metformin was developed as therapy for T2DM in the 1950s. It works via inhibition of hepatic gluconeogenesis and increased uptake of glucose in muscle. In malignant cells, metformin action includes LKB1-mediated activation of AMPK, inhibiting mTORC1 signaling, and affects liver homeostasis and metastatic development [[16], [17], [18]]. It has been published that glucose level impacts various cellular morphoproteomic changes (via Sirt1, PIAS1, STAT1, MRP1, mTOR) with adequate consequences for tumor growth as well as metastatic progression [19].
United Kingdom prospective prevention diabetes study suggests that metformin also works by mechanism of insulin resistance in liver, by bile acids metabolism, incretins release, and decrease deposit of amyloid in metformin users [20]. Some studies observed a 15–30% increased survival of CRC patients treated with metformin compared with patients on other antidiabetic agents [21,22]. Other studies which attempted to avoid the potential confounders of adjuvant therapy, focused more on stratified patients by TNM stage, did not show any effect of metformin on the course of CRC. Recent prospective studies suggested at least a 15% decrease in risk of all-cause mortality in CRC patients with T2DM treated with metformin compared with patients receiving insulin [[23], [24], [25], [26]].
Thyroid hormone substitution in patients with clinical hypothyroidism seems to have protective effects concerning CRC progression [27] when assessing circulating levels of thyroid hormones or receptor THβ1 expression in patients with abnormalities of the thyroid gland [28].
Materials and Methods
Study Population
We identified 1004 Caucasian patients diagnosed with colon and rectal adenocarcinoma in four Czech hospitals between January 1, 1999, and June 30, 2016. In 770 cases, comprehensive clinical data were available. 234 patients were excluded (due to missing lab or clinical data e.g. oncology treatment details, if a statistically insufficient number of CRC patients with specific comorbidity was identified e.g. with neurodegenerative diseases, and because of insulin therapy exclusion criteria). 189 patients were lost to follow-up in one year, and 548 patients were lost to follow-up within five years after CRC diagnosis. The participating institutions were two major hospitals in Prague (General University Hospital, Na Bulovce Hospital) and two regional hospitals, one in the most northern part of the country (Jablonec nad Nisou) and the other in the most southern part of the country (Jindrichuv Hradec). The pathological and clinical data were carefully collected by hand from online records of the healthcare institutions and analyzed retrospectively.
Compliance with Ethical Standards
The study was approved by the Ethics Committee of Na Bulovce Hospital (EK NNB 30.6.2014/7248/EK-Z on September 4, 2014), Hospital Jablonec nad Nisou (LEK 9/2016/St on November 15, 2016), General University Hospital, Prague (158/15 S-IV on March 19, 2015), and Hospital Jindrichuv Hradec (approval was not numbered on February 3, 2015). All procedures involving human participants were in accordance with the ethical standards of the institutional Ethical Committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Definitions
Cases of adenocarcinoma histology C18.0–C20.9 were included. Primary tumors were located in all parts of large intestine: left colon, transversum (including lienal and hepatic flexures), right colon, and rectum; we also recorded rare locations in appendix and Meckel's diverticulum. Five cases of ulcerative colitis and seven cases of Lynch syndrome were found in the data set. The day of surgical resection was considered the day of diagnosis although the disease was always verified by colonoscopy and biopsy a short time prior to resection. The resected specimens were routinely described macroscopically, including tumor location and size. The fat surrounding the large intestine was left overnight in Carnoy's solution to identify all lymphatic nodes. The resection margin was examined. Hematoxylin–eosin staining was used for histologic study and the slides were evaluated using light microscopy by board-certified pathologists. Histopathology evaluation consisted of tumor type, grade, and TNM stage [29].
Collected Data
We recorded the following: a) Age and sex of the patient; b) Location and size of the tumor; c) Histopathology: tumor type; grade; growth pattern; stroma type (desmoplastic, mucinous, fibroproductive, with chronic inflammatory cells); d) Pathological TNM staging combined with clinical data about distant metastases; e) Local recurrence and distant metastases; f) Chemotherapy and radiation data; g) Laboratory values including glucose, creatinine, liver enzymes; h) Last follow-up; i) Death (number of months after diagnosis and the cause related or unrelated to CRC); j) T2DM subgroups according to treatment regimens: diet, insulin, metformin, other oral antidiabetics (gliclazide, glimepiride, glibenclamide); k) Hypothyreosis; l) Other endocrine disorders: struma nodosa and status poststrumectomy, hyperparathyroidism and hyperaldosteronism, adenoma of hypophysis, thyroid, parathyroid, and subrenal glands, adrenal hyperplasia, status postovarectomy, and hormone replacement therapy (HRT) in women, status postorchiectomy and prostatectomy, mastopathy in men, abnormal glucose tolerance; m) Autoimmune diseases: chronic atrophic thyroiditis, hepatitis and gastritis, rheumatoid arthritis, psoriasis, and sarcoidosis; n) Metabolic syndrome: obesity, steatohepatitis, hyperlipidemia; o) Osteoporosis (age >65 and clinical statement).
The strengths of this study are consistent data collection that was performed by the same person in all four centers, and multidisciplinary approach. The access to correlating CRC specimens (paraffin blocks) can be advantageous for morphoproteomic studies in the future. On the other hand, some data were not available as some patients were operated on in the university surgical center but they continued to have oncology treatment and follow-up in smaller hospitals out of our reach. Despite our effort, some subgroups of patients are small (including with metformin treatment) and for that reason not all results are statistically significant, which needs to be taken into account when making clinical and scientific judgment on outcomes of this study.
Statistical Methods
The main aim of the study was to explore the impact of different disorders on the overall survival of patients with CRC. Data preprocessing, including cleaning, was applied before analysis. Continuous variable (age) was characterized by the average, minimum, and maximum. Categorical variables were described as frequencies and the relative frequencies of individual variants. In univariate analysis was to compare the characteristics of subjects with and without T2DM, respectively, with and without metformin use, for characteristics measured on the nominal scale, the Yates chi-squared test was used. The Cox proportional hazards (PH) regression model was used to evaluate the effect of different clinical and pathologic parameters on survival and generate hazard ratios for death. The estimated parameters with the standard error of these parameters, hazard ratios, and corresponding confidence intervals and p-values are listed.
In 770 evaluated cases with CRC there were 99 deaths from progression of colorectal carcinoma. Figures display the Kaplan–Meier curves for the compared groups and the p-values of the log-rank tests. Kaplan–Meier curves do not go all the way down to zero when the largest observed time (which is around 15 years) is censored. P-values less than 0.05 indicate that there is statistical significant difference between the populations (compared groups) in the probability of a death by colorectal carcinoma at any time point.
Results
Our data set consists of data on 483 (62.7%) men and 287 (37.3%) women. The age range was 31–95 years, the average age 68 years. The pathological and clinical findings are summarized in Table 1.
Table 1.
Characteristics of CRC Patients with Coexisting T2DM. Statistical Significance of Differences (Univariate Analysis) Is Shown in Bold.
| Characteristics | All Patients (n = 770) | With T2DM (n = 118) | Without T2DM (n = 652) | Chi-square Test (p) |
|---|---|---|---|---|
| Total Known Deaths | 178 (23.1%) | 31 (26.3%) | 147 (22.5%) | 0.445 |
| Gender | ||||
| Men | 483 (62.7%) | 81 (68.6%) | 402 (61.7%) | 0.178 |
| Women | 287 (37.3%) | 37 (31.4%) | 250 (38.3%) | |
| Age | ||||
| <60 | 167 (21.7%) | 8 (6.8%) | 159 (24.4%) | <0.001 |
| >60 | 603 (78.3%) | 110 (93.2%) | 493 (75.6%) | |
| Primary Tumor Location | ||||
| Left colon | 356 (46.2%) | 52 (44.1%) | 304 (46.6%) | 0.371 |
| Right colon | 207 (26.9%) | 31 (26.3%) | 176 (27%) | |
| Rectum | 177 (23%) | 27 (22.9%) | 150 (23%) | |
| Transversum colon | 30 (3.9%) | 8 (6.8%) | 22 (3.4%) | |
| Histopathology Grade | ||||
| Well differentiated | 173 (22.5%) | 29 (24.6%) | 144 (22.1%) | 0.076 |
| Moderately differentiated | 503 (65.3%) | 82 (69.5%) | 421 (64.6%) | |
| Poorly differentiated | 94 (12.2%) | 7 (5.9%) | 87 (13.3%) | |
| Morphology | ||||
| Adenocarcinoma | 625 (81.2%) | 106 (89.8%) | 519 (79.6%) | 0.026 |
| Adenocarcinoma with mucinous stroma | 137 (17.8%) | 12 (10.2%) | 125 (19.2%) | |
| Signet ring cell adenocarcinoma | 8 (1%) | – | 8 (1.2%) | |
| pTNM Stage | ||||
| In situ | 5 (0.6%) | 1 (0.8%) | 4 (0.6%) | 0.724 |
| I | 36 (4.7%) | 7 (5.9%) | 29 (4.4%) | |
| II | 156 (20.3%) | 21 (17.8%) | 135 (20.7%) | |
| III | 474 (61.6%) | 77 (65.3%) | 397 (60.9%) | |
| IV | 99 (12.9%) | 12 (10.2%) | 87 (13.3%) | |
| Lymphatic Nodes at Time of Resection | ||||
| With metastases | 331 (43%) | 49 (41.5%) | 282 (43.3%) | 0.805 |
| Without metastases | 439 (57%) | 69 (58.5%) | 370 (56.7%) | |
| Distant Metastases | ||||
| Without metastases | 578 (75.1%) | 87 (73.7%) | 491 (75.3%) | 0.861 |
| At least one (at resection time) | 99 (12.9%) | 17 (14.4%) | 82 (12.6%) | |
| At least one (later) | 93 (12.1%) | 14 (11.9%) | 79 (12.1%) | |
| Type of CRC Comorbidities | ||||
| Osteoporosis | 339 (44%) | 69 (58.5%) | 270 (41.4%) | 0.001 |
| Metabolic syndrome | 131 (17%) | 31 (26.3%) | 100 (15.3%) | 0.006 |
| Hypothyreosis | 70 (9.1%) | 13 (11%) | 57 (8.7%) | 0.537 |
| Endocrinopathy | 52 (6.8%) | 5 (4.2%) | 47 (7.2%) | 0.325 |
| Autoimmune diseases | 27 (3.5%) | 3 (2.5%) | 24 (3.7%) | 0.729 |
Survival analyses in Table 2.
Table 2.
Fitted Stochastic Cox PH Model for Overall Survival Time of CRC Patients (770 Patients, 99 Deaths for CRC).
| Predictors | Coef | SE (Coef) | HR∗ | 95% CI for HR | p |
|---|---|---|---|---|---|
| Age | 0.022 | 0.010 | 1.02 | (1.00; 1.04) | 0.039 |
| pTNM Stage | |||||
| T = In situ, I, II, III; baseline | – | – | – | – | – |
| T = IV | 0.681 | 0.242 | 1.98 | (1.23; 3.18) | 0.005 |
| Histopathology Grade | |||||
| Grade I (well differentiated; baseline) | – | – | – | – | – |
| Grade II (moderately differentiated) | 0.333 | 0.330 | 1.40 | (0.73; 2.67) | 0.313 |
| Grade III (poorly differentiated) | 0.983 | 0.390 | 2.67 | (1.25; 5.73) | 0.012 |
| Metastases in Lymphatic Nodes | |||||
| Without metastases; baseline | – | – | – | – | – |
| With metastases | 1.145 | 0.248 | 3.14 | (1.93; 5.11) | <0.001 |
| Distant Metastases | |||||
| Without metastases; baseline | – | – | – | – | – |
| At least one metastasis at resection time | 1.764 | 0.267 | 5.84 | (3.46; 9.85) | <0.001 |
| At least one metastasis later | 1.215 | 0.256 | 3.37 | (2.04; 5.57) | <0.001 |
Score (log-rank overall) test: p < 0.001.
SE, standard error; CI, confidence interval; HR, hazard ratio.
A positive (negative) coefficient estimate in the Cox PH model corresponds to a higher (lower) risk of death and thus on average a shorter (longer) OS time.
A survival model for patients with diabetes mellitus was designed to determine the possible effect of metformin use on the survival probability. The estimated parameters with the standard error of these parameters, hazard ratios, and corresponding confidence intervals and p-values are shown in Table 4. The estimated hazard rates of dying (statistically significant different from constant 1) are listed as well. The goodness-of-fit for the chosen model was performed by the score log-rank test with significant p-value (less than 0.001).
Table 4.
Fitted Stochastic Cox PH Model for Overall Survival Time of Patients with T2DM and CRC (118 Patients, 14 Deaths for CRC).
| Predictors | Coef | SE (Coef) | HR∗ | 95% CI for HR | p |
|---|---|---|---|---|---|
| Age | 0.086 | 0.041 | 1.09 | (1.01; 1.18) | 0.033 |
| Metformin | |||||
| Diet or other PAD | – | – | – | – | – |
| Metformin | −2.367 | 0.841 | −10.64 | (−2.02; −55.55) | 0.005 |
| Metastases in Lymphatic Nodes | |||||
| Without metastases; baseline | – | – | – | – | – |
| With metastases | 1.501 | 0.714 | 4.49 | (1.11; 18.17) | 0.036 |
| Distant Metastases | |||||
| Without metastases; baseline | – | – | – | – | – |
| At least one metastasis at resection time | 2.357 | 0.715 | 10.56 | (2.60; 42.90) | 0.001 |
| At least one metastases later | 0.153 | 0.860 | 1.16 | (0.22; 6.29) | 0.859 |
Score (log-rank overall) test: p < 0.001.
SE, standard error; CI, confidence interval; HR, hazard ratio.
A positive (negative) coefficient estimate in the Cox PH model corresponds to a higher (lower) risk of death and thus on average a shorter (longer) OS time.
In 118 patients with CRC and T2DM, there were 14 deaths because of progression of colorectal carcinoma. Figures display the Kaplan–Meier curves for the compared groups and the p-values of the log-rank tests. Kaplan–Meier curves do not go all the way down to zero when the largest observed time (which is around 11 years for metformin users and around 12 years for patients with diet or other PAD) is censored. P-values less than 0.05 indicate that there is statistical significant difference between the populations (compared groups) in the probability of a death by colorectal carcinoma at any time point.
Survival analyses in Table 4.
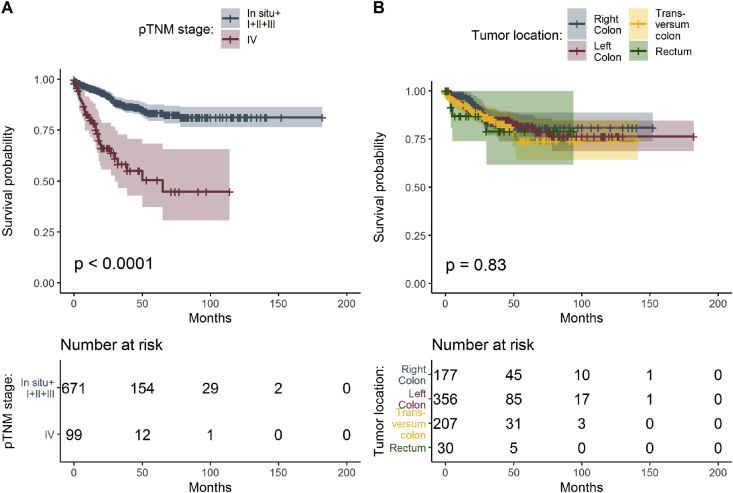
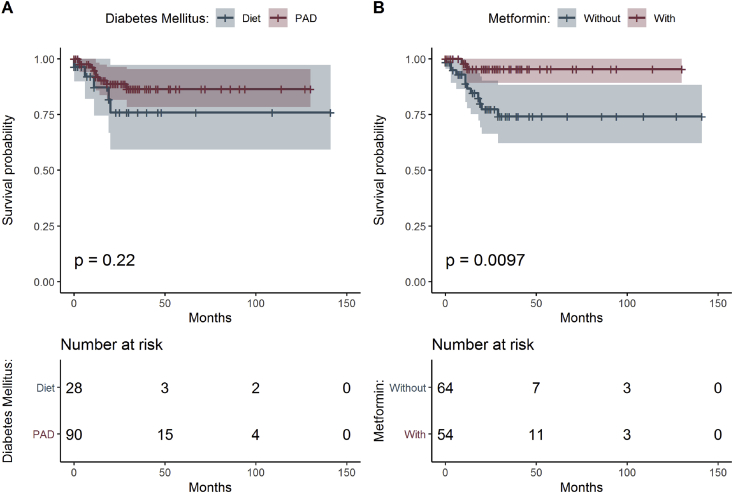
In concordance with current pathological and clinical experience, survival reflected most significantly aging, higher tumor grade, and TNM stage (Table 1 and Figure 2A). The most frequent comorbidity in CRC in our data set was diabetes mellitus type 2 with prevalence of 16.8% in men and 12.9% in women. T2DM was treated with diet, other PAD, and metformin (Table 3, Figure 3A and B).
Figure 2.
Estimated survival functions for different pTNM classification (A) and carcinoma locations in colon (B), the p-value of log-rank test comparing survival of all groups and table of frequencies of all patients.
Table 3.
Characteristics of Patients with T2DM Depending on Coexisting Metformin Treatment. Statistical Significance of Differences (Univariate Analysis) Is Shown in Bold.
| Characteristics | Patients with DM2T (n = 118) | Patients with Metformin Treatment (n = 54) | Patients without Metformin Treatment (n = 64) | Chi-square Test (p) |
|---|---|---|---|---|
| Total Known Deaths by CRC | 14 (11.9%) | 2 (3.7%) | 12 (18.8%) | 0.026 |
| Gender | ||||
| Men | 81 (68.6%) | 36 (66.7%) | 45 (70.3%) | 0.821 |
| Women | 37 (31.4%) | 18 (33.3%) | 19 (29.7%) | |
| Age | ||||
| <60 | 8 (6.8%) | 2 (3.7%) | 6 (9.4%) | 0.393 |
| >60 | 110 (93.2%) | 52 (96.3%) | 58 (90.6%) | |
| Primary Tumor Location | ||||
| Left colon | 52 (44.1%) | 23 (42.6%) | 29 (45.3%) | 0.621 |
| Right colon | 31 (26.3%) | 12 (22.2%) | 19 (29.7%) | |
| Rectum | 27 (22.9%) | 15 (27.8%) | 12 (18.8%) | |
| Transversum colon | 8 (6.8%) | 4 (7.4%) | 4 (6.3%) | |
| Histopathology Grade | ||||
| Well differentiated | 29 (24.6%) | 14 (25.9%) | 15 (23.4%) | 0.632 |
| Moderately differentiated | 82 (69.5%) | 38 (70.4%) | 44 (68.8%) | |
| Poorly differentiated | 7 (5.9%) | 2 (3.7%) | 5 (7.8%) | |
| Morphology | ||||
| Adenocarcinoma | 106 (89.8%) | 49 (90.7%) | 57 (89.1%) | >0.999 |
| Adenocarcinoma with mucinous stroma | 12 (10.2%) | 5 (9.3%) | 7 (10.9%) | |
| Signet ring cell adenocarcinoma | – | – | – | |
| pTNM Stage | ||||
| In situ | 1 (0.8%) | 1 (1.9%) | – | 0.405 |
| I | 7 (5.9%) | 3 (5.6%) | 4 (6.3%) | |
| II | 21 (17.8%) | 12 (22.2%) | 9 (14.1%) | |
| III | 77 (65.3%) | 31 (57.4%) | 46 (71.9%) | |
| IV | 12 (10.2%) | 7 (13%) | 5 (7.8%) | |
| Lymphatic Nodes at Resection | ||||
| With metastases | 49 (41.5%) | 21 (38.9%) | 28 (43.8%) | 0.729 |
| Without metastases | 69 (58.5%) | 33 (61.1%) | 36 (56.3%) | |
| Distant Metastases | ||||
| Without metastases | 87 (73.7%) | 45 (83.3%) | 42 (65.6%) | 0.067 |
| At least one (at resection time) | 17 (14.4%) | 6 (11.1%) | 11 (17.2%) | |
| At least one (later) | 14 (11.9%) | 3 (5.6%) | 11 (17.2%) | |
| Type of CRC Comorbidities | ||||
| Osteoporosis | 69 (58.5%) | 30 (55.6%) | 39 (60.9%) | 0.687 |
| Metabolic syndrome | 31 (26.3%) | 14 (25.9%) | 17 (26.6%) | >0.999 |
| Hypothyreosis | 13 (11.0%) | 6 (11.1%) | 7 (10.9%) | >0.999 |
| Endocrinopathy | 5 (4.2%) | 3 (5.6%) | 2 (3.1%) | 0.846 |
| Autoimmune diseases | 3 (2.5%) | 2 (3.7%) | 1 (1.6%) | 0.881 |
Figure 3.
Estimated survival functions for men and women, the p-value of log-rank test comparing survival and table of frequencies of all patients treated by diet and other PAD (A) and patients treated with metformin and without (B).
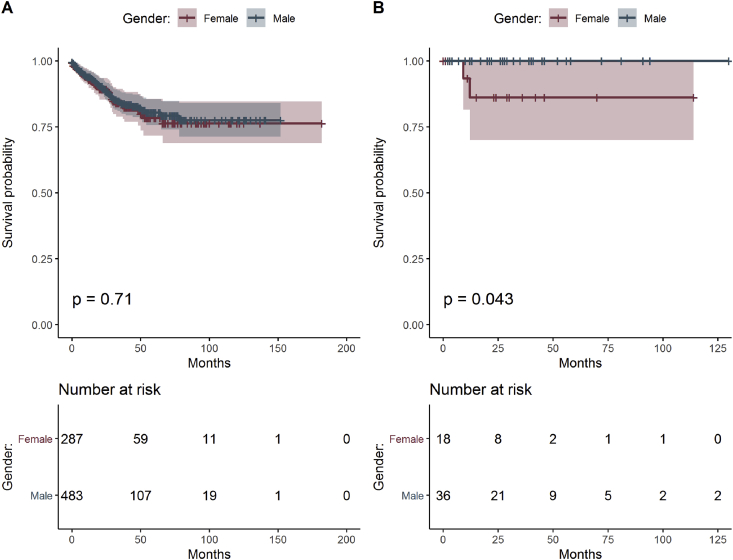
The survival analysis showed no difference between men and women with CRC in total pool of data (Figure 1A). Better survival, important for prognosis, is seen in men with CRC, particularly in the subgroup treated with metformin due to T2DM (Figure 1B). Patients treated with metformin also developed fewer distant metastases (5.6%) if CRC progressed later compared with subgroup of CRC patients without metformin therapy (17.2%) and these results were statistically significant (Table 3).
Figure 1.
Estimated survival functions for men and women, the p-value of log-rank test comparing survival of both groups and table of frequencies of all patients (A) and patients with T2DM treated with metformin (B).
CRC is a highly heterogenous carcinoma and therapies targeting different cellular pathways are expected to have various final effects. Some publications promote rectal adenocarcinoma as a separate category from colonic adenocarcinoma pathogenetically and by progression [30]. Although generally the tumors develop in right and left colon equally, our data suggest most CRC primary tumors were located in left colon regardless diabetic status. The exception was colon transversum where CRC primary tumor was found in 6.8% with T2DM versus 3.4% in nondiabetic population (Table 1). Right-sided tumors showed longest survival regardless any comorbidities–gender–age compared with CRC in left colon, rectum, and colon transversum (Figure 2B). Here anatomy conditions and embryology specifications, blood supply factors, and related biochemical and molecular alterations could make the difference and these factors are worth further investigating.
Adenocarcinoma as most frequent histological type was found in >80%, followed in frequency by adenocarcinoma with mucinous type of stroma with content <50% of mucin in histology (Table 1). Signet ring cell tumor representing intracellular mucin involvement was diagnosed in patients in nondiabetic patients in 1.2% vs. none patients with T2DM (Table 1). Poorly differentiated primary tumors were least frequent in CRC patients without T2DM; however, this group of patients was small (Table 1) and it is difficult to comment on any possible correlation on overall CRC survival in diabetic and nondiabetic population based on tumor morphology features.
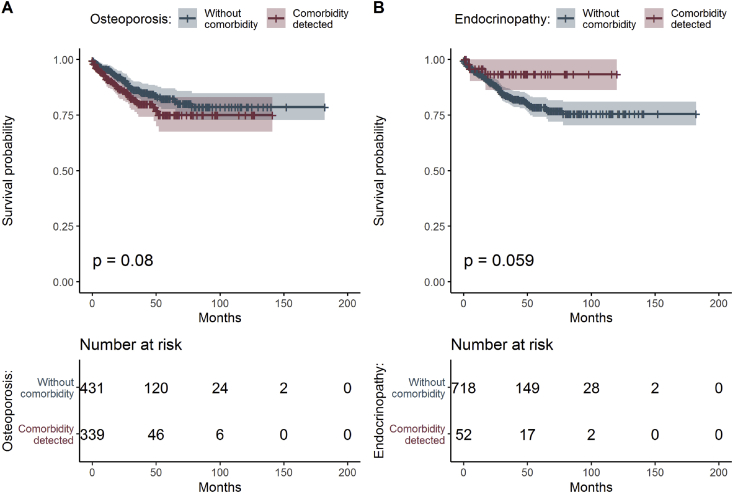
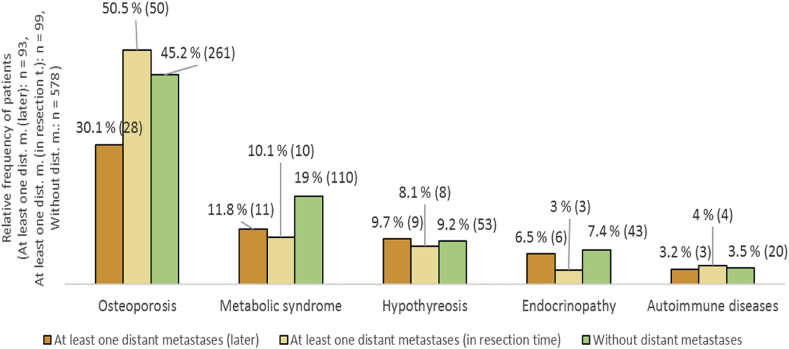
Overall survival in CRC patients with osteoporosis was shorter and coexisting other endocrinopathies (Materials and methods/Collected data and Table 1) correlated with longer survival in CRC patients (Figure 4A and B), although the subgroup of patients with these disorders was small and affected the statistical significance. Coexisting osteoporosis was present in 50% of CRC cases with one distant metastasis at time diagnosis and in 30% of CRC cases with at least one metastasis developed later (Figure 5).
Figure 4.
Estimated survival functions for different osteoporosis (A) and other endocrinopathies (B), p-value of log-rank test comparing survival for all groups and table of frequencies of all patients.
Figure 5.
The incidence of endocrine comorbidities among CRC patients according to the distant metastases development.
Discussion
It is known that clinical outcomes in CRC patients are affected by some comorbidities. The better outcome in patients younger than 60 years of age could be result of a lower incidence of comorbidities compared with older patients, a better physical condition, the ability to adhere to treatment more consistently, and perhaps stronger support of peers and family. Diabetes mellitus increases the risk for development of cancer [31]. Patients with a self-reported history of T2DM should be also considered at higher risk for cancer [32]. With the increasing worldwide prevalence of diabetes mellitus, a higher incidence of CRC can be expected, along with a less favorable prognosis for those patients on diet only for diabetes, compared with CRC patients without diabetes and treated with PAD, with special attention to subgroup treated exclusively with metformin (Figure 3B).
Diabetes and CRC share some risk factors related to lifestyle, such as lack of exercise and higher body mass index, represented by metabolic syndrome in our data set (obesity, steatohepatitis, and hyperlipidemia). The fact that metabolic syndrome did not worsen survival could result by high level of lipids in blood as well as in liver controlled by therapy for it in most patients in our study. There is evidence in preclinical studies that a high fat diet and low insulin levels are correlated with the inhibition of tumor growth and proliferation in CRC.
Although the pathophysiological mechanism to explain the negative prognostic effect of diabetes has not been elucidated, clinical and epidemiological investigations have suggested that abnormal glucose and lipid metabolism, hyperglycemia, hyperinsulinemia, insulin resistance in peripheral tissues, adipocytokines, long-term chronic inflammation with IL-6, CRP and other cytokines, as well as antiinflammatory chemokines are factors associated with the increased risk of CRC development [[33], [34], [35], [36]].
A poorer survival of CRC patients with T2DM was observed with poorer glycaemia controls correlating with HbA1c ≥ 7.5% and when exogenous insulin was part of diabetic treatment, diabetes developed at younger age and CRC was more advanced and in the right colon [37]. In literature, high blood glucose level was associated with higher risk of colon cancer in men [38]. Our results indicate longer survival in men with CRC treated with metformin (Figure 1B), although it is not statistically significant due to small number of patients in this subgroup. That could correlate with proper control of blood glucose level combined with metformin or an action of this medication in the microenvironment. Evaluating these findings in the context of gender, genetic predisposition, physical activity, nutrition, and hormones might prove a rewarding area for future investigation.
It is known that fibrocytic stromal transition is associated with local invasiveness of CRC but it might be the mucinous mixture in stroma responsible for molecular transition or interactions with tumor microenvironment that makes diseases to progress and contributes to development of distant organ metastases. Stromal transformation [39,40] and locally hypoxic tumor environment are both possible factors underlying CRC progression at the tissue and cellular level [41].
We identified numerous patients with CRC and with history of long-term treatment for T2DM with specific peroral andiabetic medication, including metformin. The majority of our patients were on metformin for more than one year. The effect of metformin may vary in CRC depending on duration of T2DM. The daily dose of metformin in most patients was 850 mg which could suggest that such a dose is sufficient to improve survival. In vivo testing in rats has shown that metformin accumulates in tissues. It is not known if an accumulation of medication could result in a more pronounced effect. Laboratory models show antineoplastic activity of metformin, but metformin concentrations used in many experiments exceed those achieved with conventional doses used for diabetes treatment. The pharmacological effect of metformin includes respiration reduction of cells ATP related [42] and mimics caloric restriction both in cells and systemically, affecting production of metabolic hormones [43]. Decreased blood glucose levels in metformin patients, and possibly a de novo synthesis of cholesterol through the intestinal MAPK dependent pathway documented during in vitro studies, may lead to tumor cell mitochondrial dysfunction and could explain the systemic protective effect and improved survival in patients with well-controlled diabetes, particularly in the metformin group. Some studies found metformin to potentially promote the immune system response against cancer [44]. Adjuvant therapy with metformin has been observed having positive impact in pancreatic, lung, and liver malignancies, but not in breast and prostate cancer [45,46]. The systematic analysis at biochemical markers and morphology characteristics in correlated CRC specimens (proliferative index, stroma type) might be useful to assess.
Estrogen alone has the proinflammatory effect in colon and can have various effects at tumorigenesis in vivo [47]. It has been shown that female hormones, estrogen and medroxyprogesterone acetate, reduced the number of colon cancer in postmenopausal women by 37% compare with placebo in 5-year interval [48,49]. Osteoporosis not only contributes to more advanced local disease in CRC but also negatively advances CRC progression. The impact of osteoporosis in the course of CRC can also relate to estrogen and testosterone. The blood levels of these hormones are known to be reduced by metformin. Recent clinical data showed that hormone replacement therapy (HRT) in women might reduce CRC risk, suggesting possible opportunities for translational research [50]. Growth hormone (GH) therapy and autonomous hyperproduction of GH together with increased level of IGF-1 are associated with a higher risk for CRC, and close follow-up for these patients is highly advised [51]. Hypothyroidism did not correlate with survival in CRC patients, possibly because of long-term thyroid hormone replacement in all patients and two-thirds of CRC patients being men in whom thyroiditis is much less frequent.
Conclusion
We provide evidence in this large retrospective study from Central Europe that, while diabetes is associated with a higher risk for CRC impacting the incidence, T2DM as comorbidity does not significantly impact survival among CRC patients and contrary, peroral treatment due to T2DM, particularly metformin, demonstrates improved survival in CRC. We emphasize that evaluation of location of primary tumor and histopathologic characteristics is essential for assessment of prognosis for patients with CRC, and it is also of great value to consider the complete clinical profile of each patient, including the medication history, as this may impact the cancer environment. Improved understanding of the effect of metabolic disorders coexisting with cancer and their pharmacologic targeting could lead to better screening methods and updated treatment guidelines to improve outcomes for patients with colorectal cancer.
Funding
The research of Marta Powell was supported by Charles University Grant Agency project GAUK No. 235215. The research of Michal Pesta was supported by the Czech Science Foundation project GACR No. 18-01781Y. Statistical analyses of Martina Litschmannova and Katerina Janurova were supported by the internal grant agency of VSB Technical University of Ostrava, Faculty of Electrical Engineering and Computer Science, Czech Republic, under the project no. SP2019/16 and by The Ministry of Education, Youth and Sports from the National Programme of Sustainability (NPS II) project “IT4Innovations excellence in science—LQ1602.” The research of Pavel Dundr was supported by Ministry of Health, Czech Republic (Conceptual development of research organization 64165, General University Hospital in Prague), and by Charles University (Project Progress Q28/LF1).
Contributions
MKP and PH initiated the study and wrote the first draft of the paper. MKP collected data and completed writing this manuscript. MP, ML and KJ completed the statistical analysis. DC and PD provided the access to most of pathological data and also valuable comments in manuscript. REB contributed to manuscript final review. TG, FT and JG commented on the correlating clinical findings. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing financial interests.
Acknowledgments
The authors thank the laboratories and offices personnel of Pathology Departments of each involved Czech healthcare institutions for the cooperation in the course long-term data collection.
References
- 1.Bella F., Minicozzi P., Giacomin A., Crocetti E., Federico M., Ponz de Leon M., Fusco M., Tumino R., Mangone L., Giuliani O. Impact of diabetes on overall and cancer-specific mortality in colorectal cancer patients. Cancer Res Clin Oncol. 2013;139:1303–1310. doi: 10.1007/s00432-013-1439-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NIH National Cancer Institute. https://seer.cancer.gov/ [accessed on 12.12.18].
- 3.International Agency on Research on Cancer. http://gco.iarc.fr/ [accessed on 12.12.18].
- 4.Müller M.F., Ibrahim A., Arends M. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016;469:125–134. doi: 10.1007/s00428-016-1956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yunusova A.V., Kondakova A.V., Kolomiets L.A., Afanas'ev S.G., Kishkina A.Y., Spirina L.V. The role of metabolic syndrome variant in the malignant tumors progression. Diabetes Metab Syndr. 2018;12(5):807–812. doi: 10.1016/j.dsx.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 6.Park H., Cho S., Woo H., Park S.K., Shin H.R., Chang S.H., Yoo K.Y., Shin A. Fasting glucose and risk of colorectal cancer in the Korean Multi-center Cancer Cohort. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0188465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peeters P.J., Bazellier M.T., Leufkens H.G., de Vries F., De Bruin M.L. The risk of colorectal cancer in patients with type 2 diabetes: associations with treatment stage and obesity. Diabetes Care. 2015;38(3):495–502. doi: 10.2337/dc14-1175. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher E.J., LeRoith D. Diabetes, antihyperglycemic medications and cancer risk: smoke or fire? Curr Opin Endocrinol Diabetes Obes. 2013;20(5):485–494. doi: 10.1097/01.med.0000433065.16918.83. [DOI] [PubMed] [Google Scholar]
- 9.Meyerhardt J.A., Sato K., Niedzwiecki D., Ye C., Saltz L.B., Mayer R.J., Mowat R.B., Whittom R., Hantel A., Benson A. Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Natl Cancer Inst. 2012;104(22):1702–1711. doi: 10.1093/jnci/djs399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mei Z.B., Zhang Z.J., Liu C.Y., Liu Y., Cui A., Liang Z.L., Wang G.H., Cui L. Survival benefits of metformin for colorectal cancer patients with diabetes: asystematic review and meta-analysis. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0091818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrett C.R., Hassabo H.M., Bhamdkamkar N.A., Wen S., Baladandayuthapani V., Kee B.K., Eng C., Hassan M.M. Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. Br J Cancer. 2012;106:1374–1378. doi: 10.1038/bjc.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spillane S., Bennett K., Sharp L., Barron T.I. A cohort study of metformin exposure and survival in patients with stage I–III colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(8):1364–1373. doi: 10.1158/1055-9965.EPI-13-0347. [DOI] [PubMed] [Google Scholar]
- 13.Weber M.M., Fottner C.H., Liu S.B., Jung M.C., Engelhardt D., Baretton G.B. Overexpression of the insulin-like growth factor I receptor in human colon carcinomas. Cancer. 2002;95(10):2086–2095. doi: 10.1002/cncr.10945. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y., Yakar S., Zhao L., Henninghausen L., LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth. Cancer Res. 2002;62:1030–1035. [PubMed] [Google Scholar]
- 15.Codony-Servat J., Cuatrecasas M., Asensio E., Montironi C., Martínez-Cardús A., Marín-Aguilera M., Horndler C., Martínez-Balibrea E., Rubini M., Jares P. Nuclear IGF-1R predicts chemotherapy and targeted therapy resistence in metastatic colorectal cancer. Br J Cancer. 2017;17:1777–1786. doi: 10.1038/bjc.2017.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowling R.J.O., Goodwin P.L., Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011;9:33–39. doi: 10.1186/1741-7015-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howel J., Hellberg K., Shaw R.J., Manning B.D. Mechanism and consequences of hepatic regulation of mTORC1 by metformin. Cancer Metab. 2014;2(1):P28. [Google Scholar]
- 18.Pernicova I., Korbonits M. Metformin-mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143–156. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 19.Schroll M.M., Liu X., Herzog S.K., Skube S.B., Hummon A.B. Nutrient restriction of glucose or serum results in similar proteomic expression changes in 3D colon cancer cell cultures. Nutr Res. 2016;36(10):1068–1080. doi: 10.1016/j.nutres.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans J.M.M., Donnelly L.A., Emslie-Smith A.M., Alessi D.R., Morris A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(4):1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramjeesingh R., Orr C., Bricks C.S., Hopman W.M., Hammad N. A retrospective study on the role of diabetes and metformin in colorectal cancer disease survival. Curr Oncol. 2016;23(2):116–122. doi: 10.3747/co.23.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh B.Y., Park Y.A., Huh J.W., Cho Y.B., Yun S.H., Lee W.Y., Park H.C., Choi D.H., Park Y.S., Kim H.C. Metformin enhances the response too radiotherapy in diabetic patients with rectal cancer. J Cancer Res Clin Oncol. 2016;142(6):1377–1385. doi: 10.1007/s00432-016-2148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiao E.Y., Preethi P.V., Naik A.D. The impact of diabetes process and outcome quality measures on overall survival in patients with co-morbid colorectal cancer. J Cancer Surviv. 2010;4:381–387. doi: 10.1007/s11764-010-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prieto I., Del Puerto-Nevado L., Gonzalez N., Portal-Nuñez S., Zazo S., Corton M., Minguez P., Gomez-Guerrero C., Arce J.M., Sanz A.B. Colon cancer modulation by a diabetic environment : a single institutional experience. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0172300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMenamin U.C., Murray L.J., Hughes C.M., Cardwell C.R. Metformin use and survival after colorectal cancer: a population-based cohort study. Int J Cancer. 2016;138:369–379. doi: 10.1002/ijc.29720. [DOI] [PubMed] [Google Scholar]
- 26.Fransgaard T., Thygesen L.C., Gögenur I. Association between metformin use after surgery for colorectal cancer and oncological outcomes: a nationwide register-based Study. Int J Cancer. 2018;143:63–72. doi: 10.1002/ijc.31305. [DOI] [PubMed] [Google Scholar]
- 27.Boursi B., Haynes K., Mamtani R., Yang Y.X. Thyroid dysfunction, thyroid hormone replacement, and colorectal cancer risk. J Natl Cancer Inst. 2015;107(6):1–7. doi: 10.1093/jnci/djv084. Print 2015 Jun 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown A.R., Simmen R.C.M., Simmen F.A. The role of thyroid hormone signalling in the prevention of digestive systém cancers. Int J Mol Sci. 2013;14:16240–16257. doi: 10.3390/ijms140816240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.International Classification of Diseases, 9th revision based on the World Health Organization's Ninth Revision, International Classification of Diseases (ICD-9), https://www.cdc.gov/nchs/icd/icd9cm.htm.
- 30.Tamas K., Walenkamp A.M.E., de Vries E.G.E., van Vugt M.A.T.M., Beets-Tan R.G., van Etten B., de Groot D.J.A., Hospers G.A.P. Rectal and colon cancer: not just a different anatomic site. Cancer Treat Rev. 2015;41:671–679. doi: 10.1016/j.ctrv.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Orgel E., Mittelman S.D. The links between insulin resistance, diabetes, and cancer. Curr Diab Rep. 2013;13:213–222. doi: 10.1007/s11892-012-0356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itatani Y., Kawada K., Inamoto S., Yamamoto T., Ogawa R., Taketo M.M., Sakai Y. The role of chemokines in promoting colorectal cancer invasion/metastasis. Int J Mol Sci. 2016;17(5) doi: 10.3390/ijms17050643. E643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Dyke A.L., Lang Kuhs K.A., Shiels M.S., Koshiol J., Trabert B., Loftfield E., Purdue M.P., Wentzensen N., Pfeiffer R.M., Katki H.A. Associations between self-reported diabetes and 78 circulating markers of inflammation, immunity, and metabolism among adults in the United States. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0182359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coghill A.E., Newcomb P.A., Poole E.M., Hutter C.M., Makar K.W., Duggan D., Potter J.D., Ulrich C.M. Genetic variation in inflammatory pathways is related to colorectal cancer survival. Clin Cancer Res. 2011;17(22):7139–7147. doi: 10.1158/1078-0432.CCR-11-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriquez A.J., Mastronardi C., Paz-Filho G. Leptin as a risk factor for development of colorectal cancer. Transl Gastrointest Res. 2013;2(4):211–222. [Google Scholar]
- 36.Tsujimoto T., Kajio H., Sugiyama T. Association between hyperinsulinemia and increased risk of cancer death in nonobese and obese people: a population-based observational study. Int J Cancer. 2017;141:102–111. doi: 10.1002/ijc.30729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siddiqui A.A., Spechler S.J., Huerta S., Dredar S., Little B.B., Cryer B. Elevated HbA1c is an independent predictor of aggressive clinical behavior in patients with colorectal cancer: a case control study. Dig Dis Sci. 2008;53:2486–2494. doi: 10.1007/s10620-008-0264-4. [DOI] [PubMed] [Google Scholar]
- 38.Vulcan A., Manjer J., Ohlsson B. High blood glucose levels are associated with higher risk of colon cancer in men: a cohort study. BMC Cancer. 2017;17:842–849. doi: 10.1186/s12885-017-3874-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conti J., Thomas G. The role of tumour stroma in colorectal cancer invasion and metastasis. Cancers. 2011;3:2160–2168. doi: 10.3390/cancers3022160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Pelt G.W., Sandberg T.P., Morreau H., Gelderblom H., van Krieken J.H.J.M., Tollenaar R.A.E.M., Mesker W.E. The tumor–stroma ratio in colon cancer: the biological role and its prognostic impact. Histopathology. 2018;73:197–206. doi: 10.1111/his.13489. [DOI] [PubMed] [Google Scholar]
- 41.Andrzejewski S., Gravel S.P., Polak M., St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014 Aug 28;2(12) doi: 10.1186/2049-3002-2-12. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hursting S.D., Dunlap S.M., Ford N.A., Hursting M.J., Lashinger L.M. Calorie restriction and cancer prevention:a mechanistic perspective. Cancer Metab. 2013 Mar 7;1(1):10. doi: 10.1186/2049-3002-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heckman-Stoddard B.M., DeCensi A., Sahasrabuddhe V.V., Ford L.G. Repurposing metformin for the prevention and cancer recurrence. Diabetologia. 2017;60:1639–1647. doi: 10.1007/s00125-017-4372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cameron A.R., Morrison D.L., Levin D., Mohan M., Forteath C., Beall C., McNeilly A.D., Balfour D.J., Savinko T., Wong A.K. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ Res. 2016;119:652–665. doi: 10.1161/CIRCRESAHA.116.308445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li D., Yeung S.C., Hassan M.M., Konopleva M., Abbruzzese J.L. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Currie C.J., Poole C.D., Gale E.A. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetetologia. 2009;52(9):1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 47.Heijmans J., Wielenga M.C., Rosekrans S.L., van Lidth de Jeude J.F., Roelofs J., Groothuis P., Ederveen A., de Jonge-Muller E.S., Biemond I., Hardwick J.C. Oestrogens promote tumorigenesis in a mouse model for colitis-associated cancer. Gut. 2014;63:310–316. doi: 10.1136/gutjnl-2012-304216. [DOI] [PubMed] [Google Scholar]
- 48.Anderson G.L., Limacher M., Assaf A.R., Bassford T., Beresford S.A., Black H., Bonds D., Brunner R., Brzyski R., Caan B. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. J Am Med Assoc. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 49.Simon M.S., Chlebowski R.T., Wactawski-Wende J., Johnson K.C., Muskovitz A., Kato I., Young A., Hubbell F.A., Prentice R.L. Estrogen plus progestin and colorectal cancer incidence and mortality. J Clin Oncol. 2012;30:3983–3990. doi: 10.1200/JCO.2012.42.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Symer M.M., Wong N.Z., Abelson J.S., Milsom J.W., Yeo H.L. Hormone replacement therapy and colorectal cancer incidence and mortality in the prostate, lung, colorectal, and ovarian cancer screening trial. Clin Colorectal Cancer. 2018;17(2):e281–e289. doi: 10.1016/j.clcc.2018.01.003. Epub 2018 Jan 12. [DOI] [PubMed] [Google Scholar]
- 51.Vilar L., Naves L.A., Caldato C., Caldato M. Acromegaly and colorectal cancer. Transl Gastrointest Cancer. 2015;4(1):28–38. [Google Scholar]