Abstract
Objectives
To analyse the correlation between periodontitis severity and disease activity, anti-double stranded DNA (anti-dsDNA) antibody, and interferon-gamma (IFN-γ) levels in patients with systemic lupus erythematous (SLE).
Methods
We selected 61 patients with SLE (age 18–55 years) selected from a hospital in Malang, Indonesia. Clinical examination and laboratory tests were performed to assess disease activity. The severity of SLE was measured using the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), while periodontitis severity was measured according to the Periodontal Index (PI) criteria. Levels of anti-dsDNA and IFN-γ were determined using an enzyme-linked immunosorbent assay. Optical density at 450 nm was measured using an automated plate reader.
Results
The mean age of the subjects with SLE was 29 years, and mean disease duration was 2.8 years. Fifty-four of 61 (88.53%) subjects with SLE had periodontitis according to the PI. SLE subjects exhibited other clinical manifestations such as lupus nephritis, vasculitis, arthritis, mucocutaneous manifestation, fatigue, fever, and/or leukopenia. SLE severity was assessed according to the average SLEDAI score (17.70 ± 12.70), and average anti-dsDNA (122.6 ± 81.01 U/mL), and IFN-γ (14.64 ± 11.17 pg/mL) levels. There was a significantly positive correlation between periodontitis score and SLEDAI score (r = 0.927; p ≤ 0.0001), anti-dsDNA antibody (r = 0.948; p ≤ 0.0001), and IFN-γ (r = 0.951; p ≤ 0.0001) levels.
Conclusion
Results of the present study demonstrated that periodontitis was associated with SLE disease activity, and was a biomarker of immune aging. Furthermore, this biomarker could be a reliable predictor of periodontal condition and prognosis of periodontitis and can also help in selecting the most appropriate treatment strategy for periodontitis in patients with SLE.
Keywords: Anti-dsDNA antibody, ELISA, IFN-γ, Periodontitis, SLE
الملخص
أهداف البحث
لتحليل توافق شدة التهاب اللثة مع نشاط المرض، والأجسام المضادة لـلحمض النووي الريبي المختزل مزدوج الضفيرة والمضاد الضوئي المناعي في مرضى الذئبة الحمامية المجموعية.
طرق البحث
في هذه الدراسة الرصدية المستعرضة، اخترنا ٦١ مريضا يعانون من مرض الذئبة الحمامية المجموعية (سن ١٨-٥٥ سنة) من مستشفى إندونيسي في مالانج. وأجري الفحص السريري والاختبارات المعملية لتقييم نشاط المرض. وتم قياس شدة مرض الذئبة الحمامية المجموعية باستخدام مؤشر نشاطها، وقيست شدة التهاب اللثة باستخدام معايير مؤشر اللثة. كما تم تحديد مستويات الأجسام المضادة لـلحمض النووي الريبي المختزل مزدوج الضفيرة والمضاد الضوئي المناعي باستخدام مقايسة الممتز المناعي المرتبط بالإنزيم (إلايسا). وتم قياس الكثافة البصرية على قارئ طبق آلي لـ ٤٥٠ نانومتر.
النتائج
كان متوسط عمر المصابين بمرض الذئبة الحمامية المجموعية ٢٩ سنة وكانت مدة المرض ٢.٨ سنة. ووجدنا أن ٥٤/٦١ (٨٨.٥٪) من المصابين بمرض الذئبة الحمامية المجموعية لديهم التهاب اللثة، بناءً على تقييم مؤشر اللثة. وكان لدى مرضى الذئبة الحمامية المجموعية مظاهر سريرية أخرى مثل التهاب الكلية الذئبي والتهاب الأوعية الدموية والتهاب المفاصل وأعراض الجلد والأغشية المخاطية والتعب والحمى ونقص الكريات البيضاء. تم تقييم شدة مرض الذئبة الحمامية المجموعية باستخدام مؤشر نشاط الذئبة الحمامية المجموعية بنتيجة متوسطة ١٧.٧ +/- ١٢.٧، ومتوسط نتيجة الأجسام المضادة لـلحمض النووي الريبي المختزل مزدوج الضفيرة ومستوى المضاد الضوئي المناعي ١٢٢.٦ +/- ٨١ وحدة / مل، و١٤.٦+/- ١١.١٧ بيكوجرام / مل. كان هناك توافق إيجابي كبير ذو قيمة إحصائية بين درجة التهاب اللثة ونتيجة مؤشر نشاط الذئبة الحمامية المجموعية (معامل التوافق: ٠.٩٢٧) ومضادات الحمض النووي الريبي المختزل مزدوج الضفيرة (معامل التوافق: ٠.٩٤٧) ومضاد ضوئي مناعي (معامل التوافق: ٠.٩٥١).
الاستنتاجات
أظهرت دراستنا أن التهاب اللثة مرتبط بنشاط مرض الذئبة الحمامية المجموعية، والمؤشر الحيوي للشيخوخة المناعية. علاوة على ذلك، يمكن أن يكون المؤشر الحيوي للشيخوخة أداة يُعتمد عليها في توقع حالات التهابات اللثة وأساليب تطورها، واختيار أفضل علاج لالتهاب اللثة في مرضى الذئبة الحمامية المجموعية.
الكلمات المفتاحية: الذئبة الحمامية المجموعية, التهاب اللثة, مقايسة الممتز المناعي المرتبط بالإنزيم (إلايسا), الأجسام المضادة لـلحمض النووي الريبي المختزل مزدوج الضفيرة, المضاد الضوئي المناعي
Introduction
Periodontitis is a chronic disease of the periodontal tissue, consisting of the gingiva, periodontal ligaments, cementum, and alveolar bone. Bacterial infections forming dental plaque can induce inflammation of the periodontal tissues. The clinical course of periodontitis may result in gingival atrophy, attachment loss of the teeth and bone, and mineral absorption of the teeth and periodontal tissues.1 Gingival inflammation can easily be found in the early stages of periodontitis and can further develop into periodontal inflammation when left untreated. As a result, periodontitis can damage the structure of the teeth and jaw, causing pain and severe disruption of the oral activity. Bacterial infections from periodontitis could continue to develop and cause systemic diseases such as cardiovascular disease.2
Periodontitis is common around the world, with a prevalence of approximately 20%–50% in the adult population.1 The prevalence of periodontitis in Malang, Indonesia, was reported to be 30.1%.3 Other studies performed in the United States reported that the overall percentage of tooth due to periodontal disease (50%) was higher than that for tooth extraction due to caries (36%) and other oral diseases (14.6%). Severe tooth-loss conditions cause a decrease in oral intake and nutrient absorption, which in turn can result in higher morbidity and mortality.4
Many studies have shown that periodontitis is multifactorial. Local factors, genetics, stress, and systemic disease have been associated with periodontitis. Systemic conditions reported to trigger periodontitis include diabetes mellitus, haematological diseases and, recently, autoimmune diseases such as systemic lupus erythematosus (SLE).
SLE is an autoimmune disease with many clinical manifestations. Hyperactivity of the immune response can have an impact on tissue and cause organ damage and contribute to the severity of periodontitis. SLE patients exhibit autoantibody activity that causes a decrease in the immune system's ability to fight bacteria; therefore, elimination of foreign antigens decreases and leads to susceptibility to infection. High autoantibody levels reflect hyperactivity of the immune system against the body's own cells, resulting in extensive tissue damage. One of the antibodies that play a major role in the course of SLE is anti-double stranded DNA (anti-dsDNA). SLE severity can be assessed using the SLE Disease Activity Index (SLEDAI).5
Loss of immune tolerance in SLE is characterised by dendritic cell hyperactivity, resulting in alterations to T and B cell lymphocytes. B cells induce plasma cell activity and trigger autoantibody production, including anti-dsDNA. Hyperactivity of T cells induce the secretion of tumour necrosis factor-alpha, and the cytokines interleukin (IL)-1, IL-6, and interferon-gamma (IFN-γ). It is believed that hyperactivity of the immune response in SLE triggers the secretion of inflammatory cytokines, especially IFN-γ. This condition causes continuous inflammation that triggers an increase in cell apoptosis and chronic inflammation in various tissues including the periodontal tissues.6,7 However, no study has definitively demonstrated whether there is an association between periodontitis and SLE severity using the SLEDAI and anti-dsDNA antibody levels. The aim of the present study, therefore, was to assess periodontitis in SLE patients, and determine whether there is a correlation between periodontitis, SLE severity, and anti-dsDNA antibody levels.
Materials and Methods
The present investigation was an observational analytical study with a cross-sectional design that included 61 patients with SLE. The study was performed from September 2017 to June 2018 at the Rheumatology Department of the Dr. Saiful Anwar General Hospital, Malang, Indonesia. Clinical examination of the oral cavity was performed in all SLE patients to assess the presence of periodontitis using the Periodontal Index (PI). Clinical examination and laboratory tests were performed to assess disease activity. The severity of SLE was measured using the SLEDAI, and anti-dsDNA antibody and IFN-γ levels were determined using an enzyme-linked immunosorbent assay (ELISA) method. Inclusion criteria were female subjects with a confirmed diagnosis of SLE, the ability to read and write, and full consciousness. Individuals who smoked, those who were pregnant, or had diabetes or other systemic disease(s) were excluded.
PI
PI assessment was performed using a mouth glass and a WHO periodontal examination probe. The PI is used to assess the entire surface of the teeth in the oral cavity and is scored as follows: 0 = neither overt inflammation in the investigated tissues nor loss of function due to destruction of supporting tissue; 1 = overt area of inflammation in free gingivae that does not circumscribe the tooth; 2 = inflammation completely circumscribes the tooth, but no apparent break in the epithelial attachment; 6 = epithelial attachment has been broken with a pocket (not merely a deepened gingival crevice due to swelling in the free gingivae). Additionally, there is no interference with normal masticatory function, the tooth is firm in its socket, and has not drifted; and, finally, 8 = tooth may be loose, may have drifted, may sound dull on percussion with a metallic instrument, or may be depressible in its socket. The score obtained is divided by the number of teeth in the oral cavity.8
SLEDAI score
The SLEDAI is an index used to determine SLE severity, using MEX-SLEDAI and scores are obtained from the results of clinical examination. The final score is summed from all clinical appearances in SLE patients. All patients involved in the present study were assisted by researchers accompanied by a specialist in rheumatoid medicine. Patients with scores <2 were classified as mild SLE or inactive disease; scores between 2 and 5 were categorised as moderate or active; and scores > 5 were categorised as severe SLE. The MEX-SLEDAI (the Mexican version of the SLEDAI) demonstrated a sensitivity of 85.7% and a specificity of 100%.9
Anti-dsDNA antibody and IFN-γ levels using ELISA
The levels of anti-dsDNA and IFNγ were determined using commercially available sandwich ELISA kits (anti-dsDNA AccuDiag ELISA Catalog number: 2553-1 and Human IFNγ Elisa Max™ Deluxe Set, Biolegend, Catalog no: 430106, respectively). Briefly, after coating plates with non-labelling antibodies and blocking, 100 μl of undiluted serum and biotinylated anti-dsDNA, reacting with a different epitope from that recognised by the coating antibody, were added to each microplate well. After a 2 h incubation at room temperature, the plates were washed five times and avidin-peroxidase was added. After 30 min incubation at room temperature, the plate was washed five times and o-phenylenediamine (OPD) was added. After another 30 min incubation at room temperature, the reaction was terminated with 2.5 m H2SO4. The optical density of each well at 450 nm was measured using an automated plate reader (3550 UV Microplate Reader, BioRad, Hercules, CA). The levels of anti-dsDNA were determined by comparison with a standard curve obtained in U/mL and IFNγ in pg/mL.10
Data analysis
The collected data were analysed using SPSS version 25 (IBM Corporation, Armonk, NY, USA), which was performed by the Medical Public Health Department Brawijaya University. The data were tested for normality using the Kolmogorov–Smirnov test, and differences in the severity of SLE in patients with periodontitis were analysed using Spearman's/Pearson's correlation test and the Mann–Whitney test for comparisons.11
Results
A total of 61 SLE patients were included in this study. As shown in Table 1, the mean age of the subjects was 29 years and mean disease duration was 2.8 years. Fifty-four of 61 (88.53%) subjects with SLE had periodontitis based on PI assessment. SLE subjects also had other clinical manifestations such as lupus nephritis, vasculitis, arthritis, mucocutaneous manifestations, fatigue, fever, and leukopenia.
Table 1.
Patient Characteristics, including Age and Disease Duration (years), and Clinical Manifestation Frequency, total and percentage, in Subjects with Systemic Lupus Erythematosus.
| Variable (n = 61) | Mean (±SD) | Range (Min-Max) |
|---|---|---|
| Age (years) | 29.47 ± 9.62 | 34 (17–51) |
| Disease duration (years) |
2.8 ± 1.20 |
4.30 (2.0–6.3) |
| Clinical Manifestation |
Total, n |
Percentage |
| Lupus Nephritis | 26 | 42.6 |
| Vasculitis | 14 | 23.0 |
| Arthritis | 18 | 29.5 |
| Mucocutaneous manifestation | 28 | 45.9 |
| Fatigue | 35 | 57.3 |
| Fever | 28 | 45.9 |
| Leukopenia | 15 | 24.6 |
| Periodontitis | 54 | 88.5 |
As shown by the data presented in Table 2, SLE severity was assessed according to the average SLEDAI score (17.70 ± 12.70) and average anti-dsDNA and IFN-γ levels (122.6 ± 81.01 U/mL and 14.64 ± 11.17 pg/mL, respectively).
Table 2.
Systemic Lupus Erythematosus (SLE) Severity Assessed according to SLEDAI score, anti-double stranded DNA and Interferon-gamma (IFN-γ) Levels in the Serum of SLE subjects.
| No. | SLE subjects (n = 61) | Mean ± SD | Range (Min-Max) |
|---|---|---|---|
| 1. | SLEDAI score | 17.70 ± 12.70 | 42 (0–42) |
| 2. | Anti-dsDNA level (U/mL) | 122.6 ± 81.01 | 279.10 (8,30–278.40) |
| 3. | IFN-γ (μg/ml) | 6.07 ± 4.26 | 14.90 (0.1–15.00) |
SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; Min, minimum; Max, maximum.
Patients were classified into three groups based on the severity of periodontitis according to the PI, divided by normal periodontium (PI, 0–0.6), mild periodontitis (PI, 0.7–1.9), and severe periodontitis (PI, 2.0–5.0). SLE subjects with severe periodontitis exhibited higher SLEDAI scores and anti-dsDNA levels than those with mild periodontitis and normal periodontal condition (Table 3). Analysis of periodontal status in SLE subjects revealed a significant difference in SLEDAI score (p < 0.0001) and anti-dsDNA levels (p < 0.001) among the three groups. Based on periodontal status, periodontitis risk factor (shown with OR) was 9.6 times for SLEDAI score and 4.3 times in SLE subjects.
Table 3.
Systemic Lupus Erythematosus (SLE) Severity and Anti-double stranded (ds) DNA Levels Based on Periodontitis Status.
| No. | Parameter | Normal Periodontal (PI 0–0.6) (n = 7) | Mild Periodontitis (PI 0.7–1.9) (n = 11) | Severe Periodontitis (PI 2.0–5.0) (n = 43) | p | OR |
|---|---|---|---|---|---|---|
| 1. | SLEDAI score | 1.66 ± 0.81 | 5.09 ± 1.86 | 23.48 ± 10.42 | <0.001 | 9.6 |
| 2. | Anti-dsDNA level (U/ml) | 26.06 ± 8.03 | 40.76 ± 7.57 | 159.16 ± 68.62 | <0.0001 | 4.3 |
| 3. | IFN-γ (μg/ml) | 0.3 ± 1.14 | 0.86 ± 0.24 | 8.34 ± 2.84 | <0.0001 |
Periodontitis status was assessed according to Periodontal Index divided into three categories, normal periodontal, mild periodontitis and severe periodontitis. P was significant difference and OR was risk factor calculated in SPSS version 25.0 (IBM Corporation, Armonk, NY, USA).
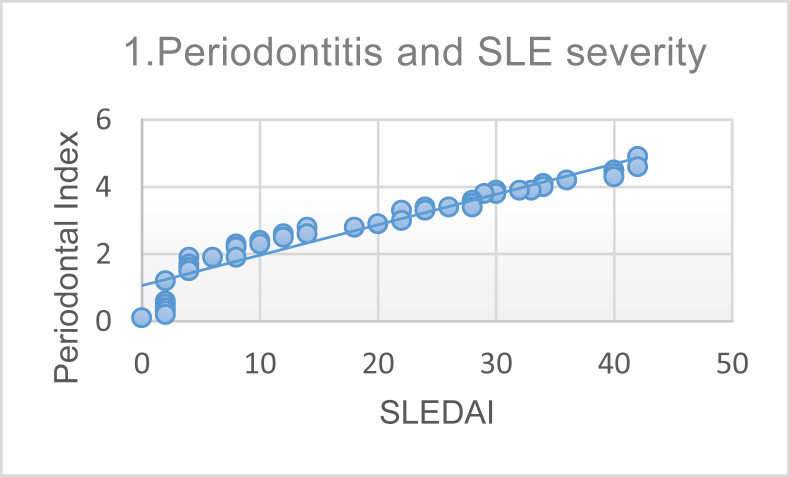
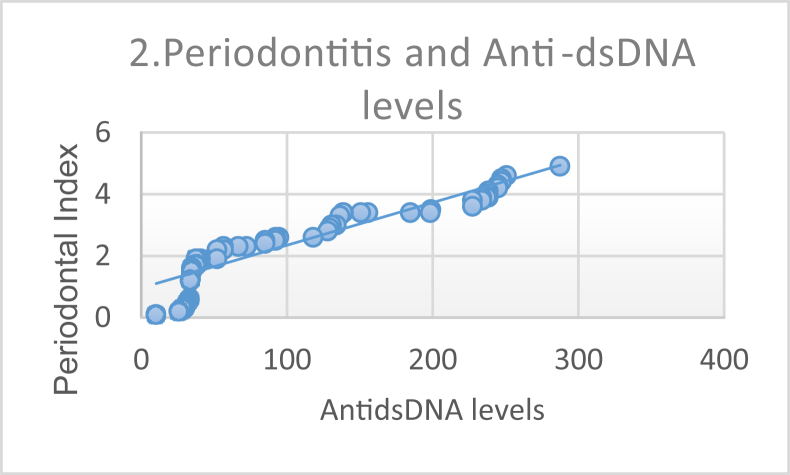
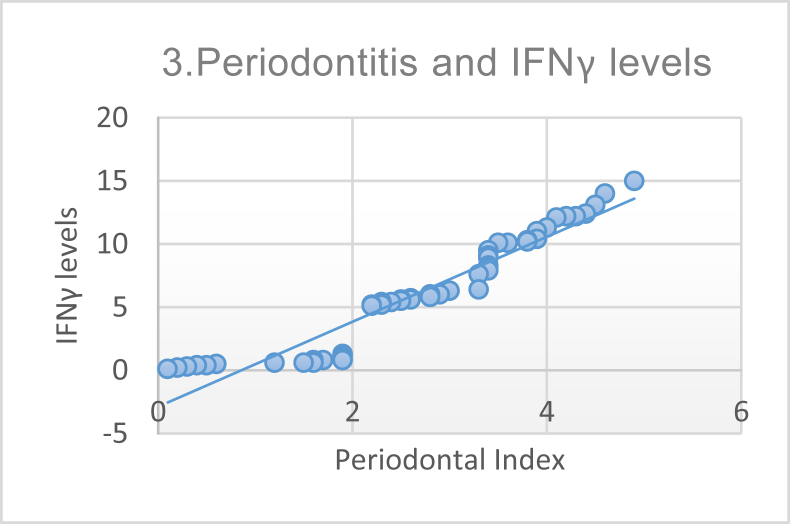
Furthermore, we performed a correlation analysis to investigate the association of SLE severity (using the SLEDAI score), anti-dsDNA levels, and IFN-γ levels with periodontitis; results are shown in Table 4. Pearson correlation analysis demonstrated a significant and strong correlation between periodontitis and SLE severity, as can be seen in Figure 1. The correlation between periodontitis and anti-dsDNA levels is shown in Figure 2, and that between periodontitis and IFN-γ levels in Figure 3.
Table 4.
Correlation between Periodontitis and SLE parameters, including SLE severity, Anti-dsDNA and IFN-γ levels assessed using Pearson's correlation analysis.
| Parameter | p (2-tailed) | r (correlation) |
|---|---|---|
| SLEDAI score | <0.0001 | 0.948 |
| Anti-dsDNA level | <0.0001 | 0.927 |
| IFN-γ level | <0.0001 | 0.951 |
SLE, systemic lupus erythematosus; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; ds, double stranded; IFN, interferon.
Figure 1.
Correlation between Periodontal index and Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score demonstrating a strong positive correlation (p ≤ 0.0001, r = 0.948).
Figure 2.
Correlation between Periodontal Index and anti-double stranded DNA level demonstrating a strong positive correlation (p ≤ 0.0001, r = 0.927).
Figure 3.
Correlation between Periodontal Index and interferon-gamma (IFN-γ) levels demonstrating a strong and positive correlation (p ≤ 0.0001, r = 0.951).
Discussion
Recently, there has been increasing interest in the relationship between periodontitis and autoimmune diseases such as SLE. Individuals with severe SLE exhibit abnormalities in the immune response, causing an increasing prevalence and severity of periodontitis.12 The increase in the incidence of periodontitis in patients with SLE is also evidenced by in vivo studies involving non-human primates and rodents.13
The SLEDAI is an index that reflects SLE disease activity. The SLEDAI is quite important because it can assess disease activity and determine therapy administered to SLE patients. In this study, analysis investigating the relationship between SLEDAI score and periodontitis (p < 0.0001 and r = 0.948) revealed a strong and significant correlation. These results suggest that the severity of periodontitis is a reflection of SLE disease activity. The SLEDAI can be used as a standard to assess SLE disease activity and has good reliability for clinical and laboratory applications, which, in this study, also reflected patient periodontal status.14
Studies have shown that an increase in anti-dsDNA levels is one of several important characteristics of SLE. Antibodies to dsDNA are a pathognomonic criterion and are found in 60%–70% of SLE cases.15 The relationship between the levels of anti-dsDNA and PI (p < 0.0001 and r = 0.927) illustrates a strong and significant correlation between the two. Anti-dsDNA is a more specific examination than anti-nuclear antibodies, because anti-dsDNA is found to be positive in SLE cases and not in healthy individuals or those with other rheumatological diseases. These antibodies are also important for monitoring SLE disease activity because they tend to have high levels in active SLE conditions. This suggests that patients with active SLE tend to have poor periodontal conditions.16 Inflammation due to B cell hyperactivity activates plasma cells and binds to toll-like receptor (TLR) 9 to form dsDNA antibodies; as such, there is an increase in anti-dsDNA antibodies in patients with SLE. These antibodies lead to the formation of immune complexes that are deposited in the tissues and cause chronic inflammation and play a direct role in causing tissue damage.17
Anti-dsDNA antibodies are also a prognostic factor for mortality in patients with SLE. Studies have shown that SLE patients with positive anti-dsDNA antibodies are 2.94 times more likely to die sooner compared with SLE patients negative for these antibodies. Anti-dsDNA antibodies are often associated with inflammation and tissue damage. The antibodies bind to nucleosomes, which consist of DNA enveloped by histone proteins (H2A, H2B, H3, and H4). H1 histone proteins function to excrete nucleosomes during apoptosis. Failure of uptake of cells undergoing apoptosis into macrophages in SLE patients will lead to the production of anti-dsDNA antibodies. The pathogenicity of these antibodies depends on their ability to produce inflammatory cells via the immunoglobulin G receptor and complement activation. Anti-dsDNA antibodies can bind to proteins in various organs and play a role in mortality and, in this case, periodontitis.16
SLE patients have abnormalities that have been described for adaptive immune B and T cells. An immune aging effect on periodontal tissues has been suggested based on molecular changes in the cellular array and inflammation condition of the periodontium. It affects differentiation and bone processes (osteoblasts, osteoclasts), changing microbial conditions, and the environment and systemic conditions related to the host due to cytokines.14 The regulation of expression of enzymes and proteins can affect the periodontal inflammatory response, including stimulation, migration, and termination of the immune cells, and also in the exacerbation and resolution of the inflammation process. This response functions through complement, cytokines, and other biomarker molecules. Cytokines such as IFN-γ may have important roles during different human physiological and pathological processes.16
The correlation between IFN-γ level and PI was strong and significant (p < 0.0001 and r = 0.951). These results are supported by other studies that in the gingival sulcus fluid, the level of IFN-γ represents the presence of periodontal disease manifestations. IFN-γ is a cytokine that plays a role in increasing expression of TLRs; increases antigen presentation by major histocompatibility complex class I and II; triggers chemokine secretion, phagocytosis, and activation of CD8-positive T cells; and causes severe inflammation.17
The effects of IFN in the gingival and salivary crevicular fluid of patients with periodontitis indicate that IFN-γ, which is a cytokine, plays an important role in periodontal disease. Stem cells in the periodontal ligament and stem cells from other tissues have an immunomodulatory capacity regulated by IFN-γ. IFN-γ plays a role in systemic disease through its receptors consisting of two polypeptide chains, known as IFNGR1 and IFNGR2.18 IFN-γ can increase T cell production and induce B cells to produce autoantibodies through the neutrophil extracellular traps pathway. The increase in IFN-γ causes activation of neutrophils, forming antibodies to DNA consisting of an immune complex, resulting in severe tissue inflammation.19
In vitro and in vivo studies in mouse models of SLE have demonstrated a high rate of periodontal disease and that IFN-γ contributed to the onset and progression of periodontitis. IFN-γ can activate the ubiquitin-proteasome pathway in osteoclasts so that TRAF6 degradation occurs and RANKL signalling increases, thereby accelerating bone resorption in the T cell response. When the IFN-γ levels are high, the RANKL barrier function is reduced leading to induction of osteoclastogenesis and increased bone resorption.20
Conclusion
Periodontitis demonstrated an association with the severity of clinical manifestation of SLE according to the SLEDAI and anti-dsDNA antibody levels. Disease severity in SLE patients is reflected by a hyperactive immune response; additionally, IFN-γ plays a role in periodontitis. Further research examining B and T lymphocytes to support the theory of pathogenesis of SLE and periodontitis with cytokines in the oral cavity is warranted.
Recommendations
Results of this study are anticipated to raise awareness of the frequency of periodontitis, as well as its relationship with SLE. They can also be used as a reference to determine treatment plans in patients with SLE and periodontitis.
Source of funding
This work was supported by Universitas Brawijaya and Ministry of Research, Technology and Education of the Republic of Indonesia.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
The work was approved by the Ethics Committees from Dr. Saiful Anwar General Hospital Malang, Indonesia (No. 400/120/K.3/302/2017).
Authors contributions
All authors have made substantive contribution to this study and/or manuscript, and all have reviewed the final paper prior to its submission. NRPG performed the experiment, analysis, and interpretation and wrote the manuscript, NN performed the experiments, contributed to conception and data design, and critically revised the manuscript, KH and HK designed the study and critically revised the manuscript. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgment
The authors are grateful for all support provided for this study.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Nazir M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci. 2017;1(2):72–80. [PMC free article] [PubMed] [Google Scholar]
- 2.Lang N.P., Bartold P.M. Periodontal health. J Clin Periodontol. 2018;45:S9–S16. doi: 10.1111/jcpe.12936. [DOI] [PubMed] [Google Scholar]
- 3.Diah Widodorini T., Nugreheni N.E. Difference gingivitis total incidence between pre-puberty and puberty age in Malang. E-Prodenta Journal of Dentistry. 2018;2(1):108–115. 2018. [Google Scholar]
- 4.Rutter-Locher Z., Fuggle N., Orlandi M. Periodontal disease and autoimmunity: what we have learned from microbiome in rheumatology. J intech. 2017;6:127–129. [Google Scholar]
- 5.Salama R.Mahito S., Matthew H., Masashi N. Cellular senescence and its effector programs. Genes Dev. 2014;28:99–114. doi: 10.1101/gad.235184.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajishengallis G. Too old to fight? Aging and its toll on innate immunity. Molecular Oral Microbiology. 2010;25:25–37. doi: 10.1111/j.2041-1014.2009.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulop T., Witkowsk J.M., Pawelec G., Alan C., Larbi A. On the immunological theory of aging. Aging: Facts and Theories. 2014;39:163–176. doi: 10.1159/000358904. [DOI] [PubMed] [Google Scholar]
- 8.Beltrán-Aguilar E.D., Eke P.I., Thornton-Evans G., Petersen P.E. Recording and surveillance systems for periodontal diseases. Periodontology 2000. 2012;60(1):40–53. doi: 10.1111/j.1600-0757.2012.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etchegaray-Morales I., Méndez-Martínez S., Jiménez-Hernández C., Mendoza-Pinto C., Alonso-García N.E., Montiel-Jarquín A. Factors associated with health-related quality of life in Mexican lupus patients using the LupusQol. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gan S.D., Patel K.R. Enzyme immunoassay and enzyme-linked immunosorbent assay. J Investig Dermatol. 2013;133(12):54–59. doi: 10.1038/jid.2013.287. [DOI] [PubMed] [Google Scholar]
- 11.Mukaka M. A guide to appropriate use of Correlation coefficient in medical research. Malawi Med J: The Journal of Medical Association of Malawi. 2012;24(3):69–71. [PMC free article] [PubMed] [Google Scholar]
- 12.Hajishengallis G. Aging and its impact on innate immunity and inflammation: implications for periodontitis. J Oral Biosci. 2014;56(1):30–37. doi: 10.1016/j.job.2013.09.001. February 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandruvada S.N., Gonzalez O.A., Kirakodu S., Gudhimella S., Stromberg A.J., Ebersole J.L., Orraca L., Gonzalez-Martinez J., Novak M.J., Huja S.S. Bone biology-related gingival transcriptome in ageing and periodontitis in non-human primates. J Clin Periodontol. 2016;43:408–417. doi: 10.1111/jcpe.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saleh A.M., Kurniati N., dan Syarif B.H. Assesment of systemic lupus erytematous using SLEDAI score in pediatric department RSCM jakarta Indonesia. Sari Pediatri. 2016;16(4):292–298. [Google Scholar]
- 15.Hiraki L.T., Benseler S.M., Tyrrell P.N. Clinical and laboratory characteristics and long-term outcome of pediatric systemic lupus erythematosus: a longitudinal study. J Pediatr. 2008;152(4):550–556. doi: 10.1016/j.jpeds.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Isenberg D.A., Manson J.J., Ehrenstein M.R. Fifty years of anti-dsDNA antibodies: are we approaching journey's end? Rheumatology. 2007;46(7):1052–1056. doi: 10.1093/rheumatology/kem112. [DOI] [PubMed] [Google Scholar]
- 17.Nainar D.A., Alamelu S., Arun K.V., dan Rajasekaran M. Immunological profile of periapical and periodontal lesions: current concept in the role of T cells. J Oper Dent Endod. 2016;1(2):70–75. [Google Scholar]
- 18.Lemos J.C., Gurgel B.C.D.V., dan Freitas R.D.A. Th2 cells and the IFN-γ R1 subunit in early and advanced experimental periodontitis in rats; an immunohistochemical study. Braz J Oral Sci. 2015;14(2):106–111. [Google Scholar]
- 19.Isaza-Guzmán D., Cardona-Vélez N., Gaviria-Correa D. Association study between salivary levels of interferon (IFN)-gamma, interleukin (IL)-17, IL-21, and IL-22 with chronic periodontitis. Arch Oral Biol. 2015;60(1):91–99. doi: 10.1016/j.archoralbio.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Mizraji Cell reports porphyromonas gingivalis promotes unrestrained type I interferon production by dysregulating TAM signaling via MYD88 degradation. Cell Rep. 2017;18:419–431. doi: 10.1016/j.celrep.2016.12.047. [DOI] [PubMed] [Google Scholar]