Abstract
Objectives
To investigate the tissue-protective effects of Ajwa date fruits (a Prophetic medicinal remedy) against acute diclofenac toxicity.
Methods
Albino Sprague–Dawley rats were allocated to four experimental groups: a negative control group, an Ajwa-only group that received 2 g/kg of Ajwa date extract (ADE) orally, an acute diclofenac toxicity group that received 200 mg diclofenac once intraperitoneally, and a treatment group that received diclofenac and ADE after 4 h. Histological examinations of rat lung and liver tissues were performed.
Results
Acute diclofenac toxicity caused marked hepatic derangements, such as congested central veins, congested blood sinusoids, hyaline degeneration, and hepatocyte necrosis. Toxic diclofenac overdose resulted in markedly congested alveolar capillaries and alveolar haemorrhages, thick edematous alveolar walls, and edema fluid exudates in the alveoli. Upon treatment with ADE, significant reduction in diclofenac-induced hepatic and pulmonary derangements were observed.
Conclusion
ADE is a safe, tissue-protective nutritional agent that alleviates cellular and tissue-damaging effects due to acute diclofenac toxicity. ADE relieved hepatic and pulmonary changes induced by acute diclofenac toxicity. The use of ADE is recommended for the treatment of acute diclofenac toxicity.
Key words: Acute toxicity, Ajwa date extract, Diclofenac, Liver, Lung
الملخص
أهداف البحث
تهدف هذه الدراسة لفحص آثار حماية الأنسجة لثمار تمر العجوة (علاجات الطب النبوي) ضد التسمم الحاد بالديكلوفيناك.
طرق البحث
تم تقسيم فئران سبراغ داولي البيضاء إلى أربع مجموعات تجريبية: المجموعة السلبية، مجموعة العجوة فقط التي أخذت مستخلص تمر العجوة ٢ جرام⁄ كجم بالفم، ومجموعة التسمم الحاد بالديكلوفيناك التي أخذت ٢٠٠ مجم عبر الحقن البريتوني مرة واحدة، ومجموعة العلاج التي تناولت الديكلوفيناك متبوعا بعدها بأربع ساعات بمستخلص تمر العجوة. تم فحص أنسجة الرئة والكبد للفئران.
النتائج
أظهر التسمم الحاد بالديكلوفيناك اضطراب كبدي ملحوظ كاحتقان الوريد المركزي، واحتقان الجيوب الأنفية، وضمور زجاجي ونخر خلايا الكبد. كما تسبب التسمم الحاد بجرعة زائدة من الديكلوفيناك إلى احتقان بشكل ملحوظ في شعيرات الحويصلات الهوائية ونزيف الحويصلات الهوائية، وزيادة سمك جدار الحويصلات الهوائية وظهور استسقاء مائي داخلها، وظهور إفرازات واستسقاء مائي في الحويصلات الهوائية. وعند العلاج بمستخلص تمر العجوة، لوحظ تحسنا واضحا في التغييرات أثر التسمم الحاد بالديكلوفيناك على الكبد والرئة.
الاستنتاجات
مستخلص تمر العجوة هو علاج غذائي آمن حافظ للأنسجة ويخفف من الآثار الضارة التي تحدث من التسمم الحاد بالديكلوفيناك على الخلايا والأنسجة. ويخفف مستخلص تمر العجوة التغييرات الكبدية والرئوية التي تنشأ من التسمم الحاد بالديكلوفيناك. توصي هذه الدراسة باستخدام مستخلص تمر العجوة لعلاج التسمم الحاد بالديكلوفيناك.
الكلمات المفتاحية: ديكلوفيناك, التسمم الحاد, مستخلص تمر العجوة, الكبد, الرئة
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most commonly used analgesics worldwide.1 Diclofenac (phenylacetic acid) is a commonly used anti-inflammatory analgesic that is similar to morphine in terms of decreasing pain after coronary artery bypass graft surgery.2 Approximately 25% of adverse drug events in the United Kingdom and 21% in the United States are caused by NSAIDs, possibly due to self-administration without a medical prescription.3 Diclofenac is widely used as an analgesic and antipyretic to treat pain in rheumatoid arthritis, osteoarthritis, and other diseases. Diclofenac-mediated inhibition of the cyclooxygenase-2 (COX-2) pathway suppresses synthesis of prostaglandin from arachidonic acid. Diclofenac is absorbed from the gastrointestinal tract, leading to peak plasma concentrations 1.5–2 h after ingestion. Diclofenac is metabolised in the liver to hydroxydiclofenac, which undergoes conjugation to sulfate and glucuronic acid, facilitating excretion through the renal system.4
The therapeutic dose of diclofenac in children is 0.3–1 mg/kg5, while the maximum reported dose used to treat adult human rheumatoid arthritis is 225 mg/day (3.3 mg/kg). Diclofenac is usually sold as an over-the-counter drug. Chronic high doses of diclofenac administration have reportedly induced marked histopathological changes in the liver and kidneys.6 Diclofenac-induced toxicity mechanisms involve mitochondrial dysfunction and production of prooxidant radicals when it is metabolised by peroxidases.7
Drug-induced hepatic injury is a common complication of NSAIDs.8 To reduce NSAID-induced gastropathic bleeding, it is administered in combination with proton pump inhibitors.9 Moreover, NSAIDs induce acute kidney injury (e.g. structural renal damage, loss of kidney functions, decreased sodium excretion, decreased potassium excretion, and suppressed renal perfusion).10,11 Nephrotoxicity is indicated when serum creatinine increases by 0.5 mg/dl.12
Natural remedies and antioxidant therapies (e.g. prophetic medicine remedies) have been reported to exert tissue-protective effects against many toxins. Prophetic medicine is medical knowledge obtained from Prophetic Ahadith, spoken by the Prophet Mohammad, peace be upon him, regarding human health. Prophetic medicine is evidence-based, simple, inexpensive, easy to access, and practical.13 Prophetic medicine remedies may be tissue-protective against diclofenac-induced toxicity.
One remedy recommended by prophetic medicine is date fruits (Phoenix dactylifera L). Date fruit is a food, sweet, and medicine found in hot dry areas of the world (e.g. Arabian Peninsula). Date fruit is rich in sugars, proteins, fiber, trace elements, phenolics, flavonoids, carotenoids, minerals, amino acids, unsaturated fats, and organic acids.14 These ingredients confer many preventive and therapeutic effects on date fruits (e.g. hepatoprotective and nephroprotective effects).15 Interestingly, date fruit components have been confirmed to exert potent anti-inflammatory effects, encouraging their use to treat drug toxicity-induced inflammatory reactions. This prompted us to investigate Ajwa date extract (ADE) for effects that could combat acute diclofenac toxicity.
Materials and Methods
Study aims
The primary aim of this study is to investigate a possible role for ADE in treating diclofenac toxicity. Specifically, we aimed to investigate the pathological.effects on rat lungs and liver of acute diclofenac toxicity (overdose) after a single high dose (200 mg/kg) to simulate accidental diclofenac overdose. This study also aimed to assess a possible protective effect of Almadinah Almunawwarah ADE against tissue damage caused by a single toxic dose of diclofenac (simulating accidental acute diclofenac toxicity).
Preparation of Ajwa date extract (ADE)
Fresh dates (Phoenix dactylifera L.) (Ajwa) were purchased from a local store in Almadinah Almunawwarah, KSA. Ajwa flesh specimens (after removing nuclei) have been deposited at the department of Pharmacognosy and Pharmaceutical Chemistry, College of Pharmacy, Taibah University, KSA.
Ajwa dates were dried under shade (away from sunlight to preserve their active ingredients), cut into small pieces, and ground. Two batches of coarse powder (3000 g) of ground, lyophilised dates were each macerated in 3 L hydro-alcohol (80%), and incubated at room temperature for 72 h. The combined extracts were filtered using Whatman's filter paper on a Buchner funnel. Ethanol was eliminated in a rotary vacuum evaporator at 50 °C. Solvent-free plant extract (1800 g) was kept in a refrigerator at 4 °C for further tests and applications.
Experimental studies in animals
To our knowledge, this is the first report of potential tissue-damage from a single injected overdose of diclofenac (200 mg/kg). Sprague Dawley (S.D.) rats, an outbred multipurpose breed of albino rats were used for this work. 24 rats were assigned to four experimental groups (6 rats per group). Rats were maintained in the animal house of Taibah University. White albino rats (300–420 g) were obtained from the central animal facility of King Abdul-Aziz University, Jeddah, KSA. Animals were acclimatised for 2 weeks in the animal facility of Taibah University prior to experimentation. Animals were maintained in pathogen-free conditions at the central animal facility of Taibah University, KSA. Animal were maintained inside polypropylene cages at a temperature of 22 ± 3 °C, and a relative humidity of 65–86%. Animals were fed by mouth, using a standard laboratory pellet chow diet with ad libitum access to pathogen-free water throughout the study. Animals' weights were regularly checked prior to the experiment. The whole experimental procedure was conducted at Taibah University in Almadinah Almunawwarah.
The use of animals was approved by the ethics Committee of the Animal Research facility of Taibah University. Animals were dispersed to four different experimental groups:
-
•
1st group (untreated control = negative control): received no treatment.
-
•
2nd group (Ajwa treatment group): received ADE only (2 g/kg by oral gavage).
-
•
3rd group (Positive control for acute diclofenac toxicity): received diclofenac by intraperitoneal injection.
-
•
4th group (ADE treatment group): rats received diclofenac (intraperitoneal injection) followed 4 h later by oral administration of ADE.
After 4 days, all rats were sacrificed, and liver and lung tissues were examined for histological changes. Tissue analyses were performed by an expert histopathologist.
N.B. In a previous study (ref # 21), the dose of ADE used by the authors was 1 g/kg. For this study, the ADE dose was increased to 2 g/kg.
Tissue sample preparation
By the end of the previously determined experimental period (4 days of treatment), all rats in the study were anaesthetised with ether, and sacrificed. Ether anaesthesia was used for all animals in all 4 experimental groups. No changes were seen in negative control animals due to ether anaesthesia. Moreover, the time required for ether anaesthesia prior to sacrifice was short, and not expected to change any biological parameters.
Liver and lung specimens from each rat were cut into small pieces, fixed in 10% phosphate buffered formaldehyde, and embedded in paraffin. Then, 5-μm-thick sections were obtained using a microtome, and fixed onto glass slides for hematoxylin and eosin (H&E) staining. The slides were examined under a high-resolution microscope. Paraffinised histological liver and lungs specimens underwent further processing, with subsequent haematoxylin and eosin staining to evaluate general morphology.
Results
Acute diclofenac toxicity disturbs liver architecture and histology
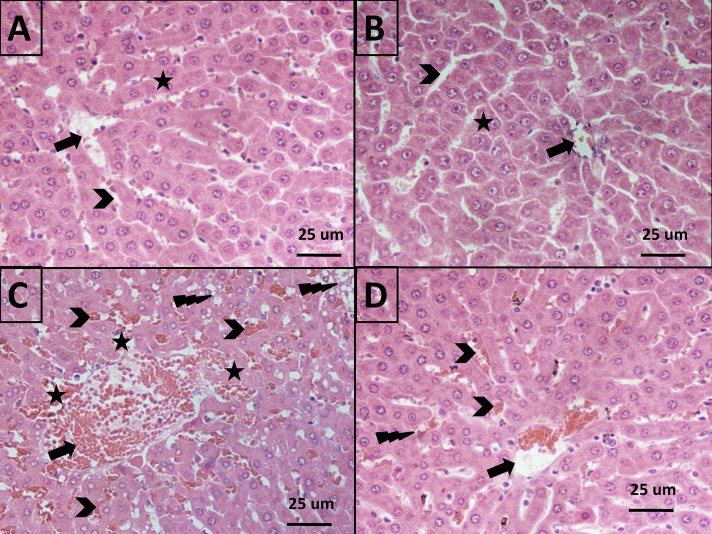
In negative control animals, liver sections exhibited normal histology, including a normal central vein (Black arrows), normal blood sinusoids (Chevron), and normal hepatocytes (star) (Figure 1A). The same findings were evident in the ADE treatment group (Figure 1B). Acute diclofenac toxicity caused congested central veins (Black arrows), congested blood sinusoids (Chevron), and hyaline degeneration and necrosis in hepatocytes (Star). There were excess fatty changes in liver cells (lightning bolt) and haemosiderin laden Kupffer cells (Figure 1C). Daily treatment of acute diclofenac toxicity with ADE for 4 consecutive days resulted in marked improvement and reversal of diclofenac-induced pathological liver changes (Figure 1D and Table 1). H&E staining, 400× magnification, and a scale bar of 25 μm were used.
Figure 1.
Effects of acute diclofenac toxicity and ADE treatment on liver histology: A: Group 1: Liver Sections showing normal liver histology: normal central vein (Black arrows), normal blood sinusoids (Chevron), and normal hepatocytes (star) in group1 control rats. B: Liver sections of group 2 rats, which received Ajwa alone (ADE 2 g/kg orally). C: Group 3: Acute diclofenac toxicity showing congested central veins (Black arrows), congested blood sinusoids (Chevron), hyaline degeneration, hepatocyte necrosis (Star), excess fatty changes in liver cells (lightning bolt), and haemosiderin laden Kupffer cells. D: Group 4: Treatment of acute diclofenac toxicity using ADE daily for 4 consecutive days showed much improvement in all diclofenac-induced pathological changes in the liver. H&E stained tissues, magnified 400×. Scale bar = 25 μm.
Table 1.
Semiquantitative assessment of pathological changes in liver tissue in all animal groups.
| Pathological features | Group 1 (Negative control) | Group 2 (ADE) | Group 3 (Acute diclofenac toxicity) | Group4 (Treatment of acute diclofenac toxicity with ADE) |
|---|---|---|---|---|
| Central vein congestion | _ | _ | +++ | ++ |
| Congested blood sinusoids | _ | _ | +++ | + |
| Hepatocyte necrosis | _ | _ | +++ | _ |
| Hydropic degeneration | _ | _ | +++ | + |
| Hepatocyte Fatty changes | _ | _ | +++ | + |
| Haemosiderin laden macrophages | _ | _ | +++ | ++ |
N.B. (−) means no change, (+) means mild changes, (++) means moderate changes and (+++) means severe changes.
Acute diclofenac toxicity disturbs lung architecture and histology
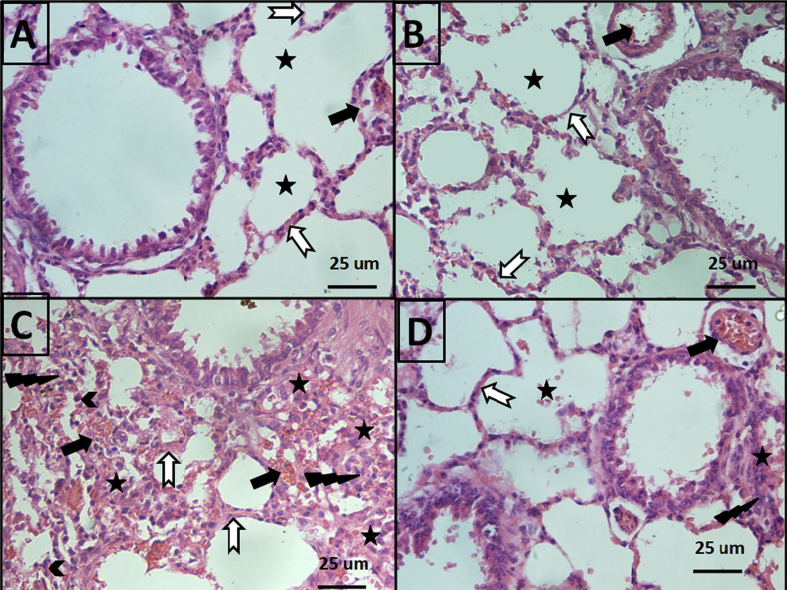
Sections from the lungs of the negative control group showed normal lung histology that was evident by light microscopy as normal alveolar capillaries (Black arrows), thin alveolar walls (White notched arrow), and clear alveoli (star) (Figure 2A). Ajwa alone (ADE 2 g/kg orally) exhibited the same normal histological characteristics (Figure 2B). Acute diclofenac toxicity (200 mg/kg intraperitoneally) caused marked histological lung damage. Acute diclofenac toxicity (200 mg/kg intraperitoneally) resulted in congested alveolar capillaries, and alveolar haemorrhages (Black arrows), thick edematous alveolar walls (White notched arrow), and edematous fluid exudates in alveoli (Star). Moreover, lung tissue was infiltrated by inflammatory cells (lightning bolt) and haemosedrine laden macrophages (Chevron) (Figure 2C and Table 2). ADE treatment (2 g/kg orally administered for 4 consecutive days) resulted in marked improvement in all diclofenac-induced pathological changes in the lung (Table 2). H&E stained, 400× magnification, and a scale bar of 25 μm.
Figure 2.
Effects of acute diclofenac toxicity (200 mg/kg intraperitoneally) and ADE (2 g/kg orally) on lung histology: A: Group 1 is the negative control. Lung sections showing normal histology (normal alveolar capillaries (Black arrows), thin alveolar walls (White notched arrow), and clear alveoli (star) in group 1. B: Group 2 received Ajwa alone (ADE 2 g/kg orally). C: Group 3 displays acute diclofenac toxicity, including congested alveolar capillaries and alveolar haemorrhage (Black arrows), thick edematous alveolar walls (White notched arrow), and edema fluid exudates in the alveoli (Star). There is lung tissue infiltration by inflammatory cells (lightning bolt) and haemosedrine laden macrophages (Chevron). D: Group 4: Treatment of acute diclofenac toxicity using ADE daily for 4 consecutive days significantly mitigated all diclofenac-induced pathological changes in the lung. H&E staining and 400× magnification. Scale bar = 25 μm.
Table 2.
Semiquantitative assessment of pathological changes in lung tissue in all groups.
| Pathological features | Group 1 (Negative control) | Group 2 (ADE) | Group 3 (Acute diclofenac toxicity) | Group4 (Treatment of acute diclofenac toxicity with ADE) |
|---|---|---|---|---|
| Alveolar vascular congestion | _ | _ | +++ | ++ |
| Thick edematous alveolar walls | _ | _ | +++ | + |
| Alveolar & interstitial haemorrhage | _ | _ | +++ | + |
| Alveolar fluid & cellular exudate | _ | _ | +++ | + |
| Inflammatory cells infiltrate | _ | _ | +++ | + |
| Haemosedrin laden macrophages | _ | _ | +++ | + |
N.B. (−) means no change, (+) means mild changes, (++) means moderate changes and (+++) means severe changes.
Discussion
Acute toxicity is an adverse effect of a single overdose or a few sequential large doses of diclofenac within a relatively short period of time. Acute diclofenac toxicity may be encountered when accidental overdose occurs during acute pain conditions (e.g. acute trauma, fractures, etc. in which patients facing agonising pain request multiple analgesic injections—particularly when emergency morphia is lacking). Acute drug toxicity may result in overt side effects, and mortality within days.16 Oxidative stress is the tissue damaging mechanism underlying diclofenac toxicity.7,17 Diclofenac-mediated cytotoxicity and its symptoms commonly occur through oxidative stress mechanisms. The reactive metabolite diclofenac acyl glucuronide can also mediate a significant proportion of diclofenac-induced toxicity incidents. This suggests possible curative and tissue-protective roles for antioxidants against diclofenac-induced tissue damage.6
Diclofenac use poses a significant cardiovascular health risk compared to non-use, paracetamol use, and use of other traditional non-steroidal anti-inflammatory drugs. The event rate associated with diclofenac initiators increased for each component of atrial fibrillation/flutter, 1.6 (1.3–2.0) for ischaemic stroke, 1.7 (1.4–2.0) for heart failure, 1.9 (1.6–2.2) for myocardial infarction, and 1.7 (1.4–2.1) for cardiac death, including for low dose users of diclofenac, compared with non-initiators. Concurrent Ajwa treatment for those using diclofenac is strongly recommended to minimize health risks attributed to diclofenac. Diclofenac causes more cardiovascular health risks compared with other non-steroidal anti-inflammatory drugs. Diclofenac use may increase atrial fibrillation/flutter, ischaemic stroke, heart failure, myocardial infarction, and cardiac death. Diclofenac increases the relative risk of major adverse cardiovascular events in diabetic patients. Diclofenac also increases the absolute risk for myocardial infarction, heart failure and upper gastrointestinal bleeding compared with ibuprofen or paracetamol. The risk increased to a similar extent with naproxen initiation.18
Hepatic injury due to diclofenac toxicity may be mild (raised liver enzymes), moderate (moderately raised serum levels of liver enzymes and bilirubin, with decreased albumin) or severe (evident jaundice with markedly raised serum levels of liver enzymes and bilirubin, and decreased albumin). Current treatment modalities for diclofenac poisoning and toxicity include preventing further exposure and giving antioxidants e.g. N-acetyl cysteine19 and spirulina6
Dates are a rich source of antioxidants owing to their high flavonoid and polyphenol content (e.g. ferulic, gallic, catechin, chlorogenic, caffeic, coumaric, resorcinol, and others).20 Interestingly, the main indication for use of Ajwa dates in prophetic medicine is to prevent intoxications and treat exposure to toxins and poisons. Due to their high levels of antioxidant phytoconstituents, dates are used to treat microbial infections, constipation, diabetes, and cancer.21 Numerous research studies have demonstrated preventive effects of dates against various environmental toxins in certain animal and human tissues. Dates have exhibited potent nephro-protective effects e.g. against ochratoxin-induced tissue damage.22
In this study, Ajwa proved to be a safe, nutritional treatment that did not itself exert any tissue-damaging effects (Figure 1A–B). Our data reveal that acute diclofenac toxicity can cause marked hepatic derangements (e.g. congested central veins, congested blood sinusoids, hyaline degeneration, and hepatocyte necrosis). Diclofenac toxicity induces excess fatty changes in liver cells, and infiltration by haemosiderin laden Kupffer cells (Figure 1C). Upon treatment with ADE, marked improvement of diclofenac-induced acute hepatic changes was evident (Figure 1D).
Our lung histology data confirms that Ajwa is a safe nutritional treatment that did not exert any lung-damaging effects (Figure 2A–B). Acute toxic overdose of diclofenac resulted in markedly congested alveolar capillaries, alveolar haemorrhages, thick edematous alveolar walls, and edema fluid exudates in the alveoli. We also observed infiltration of lung tissue by inflammatory cells and haemosedrine laden macrophages (Figure 2C). Treatment with ADE produced marked improvement of diclofenac-induced pulmonary changes (Figure 2D).
The findings of this study are in exact agreement with many previous reports in which ADE protected against tissue damage induced by many other toxins. ADE has been reported to exert potent antioxidant and tissue-protective effects sufficient to reverse the malignant phenotype induced by carcinogenic toxins e.g. diethylnitrosamine. Such tissue-protective effects have been attributed to the potent antioxidant capacity of Ajwa dates that can counter oxidative stress-induced mutagenesis and carcinogenesis.23 Our data are also in agreement with a report investigating lead intoxication. Lead toxicity induced oxidative damage in lung tissues of rabbits, producing marked changes in alveolar wall thickness, with wall destruction, capillary congestion, and marked alveolar infiltration by inflammatory cells. Marked histological improvements were reported in the Ajwa treated group.24 Ajwa conferred many preventive and therapeutic effects against lead-induced histopathological changes, with only mild congestion, and slight focal cellular degeneration via free radical scavenging and antioxidant properties.2,24 The ADE-induced tissue-protective effects against acute diclofenac toxicity are quite in agreement with the report by Zhang et al. who reported that Ajwa dates contain potent antioxidant and anti-inflammatory components.25
Ajwa dates are recommended in prophetic medicine to prevent and treat toxin injuries. In this way, Ajwa dates are superior to all other types of date available from farms and in the markets. Interestingly, Ajwa date prices are higher than all other types of date. Prophetic medicine quotes 'Aamir ibn Sa'eed as saying: I heard Saad (may Allah be pleased with him) saying: I heard the Messenger of Allah - peace be upon him - saying: «Whoever eats seven dates Ajwa in the early morning; he will not be harmed by poison or magic that day”.26
Further research studies regarding the use of ADE to treat drug toxicities are recommended. Development of ADE for pharmaceutical and food supplement use is strongly recommended.
Conclusion
Acute diclofenac toxicity is a clinical scenario that can severely damage histological structures in the liver and lungs, causing many life-threatening detrimental effects. ADE is quite safe and possesses tissue-protective properties that alleviate cellular and tissue-damaging effects due to acute diclofenac toxicity. ADE is strongly recommended to treat acute diclofenac toxicity.
Source of funding
The authors declare that they did not receive financial support from any source for this research article.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
The Ethical Committee of Taibah University, KSA (IRB Reg. No. 00008716) approved the protocol for this study.
Authors contributions
NA conceived and designed the study, conducted research, provided research materials, and collected and organized data. MAE analyzed and interpreted data. HME conducted research, provided research materials, and collected and organized data. AMAA, AMAO, SHF, BSA, SSA and OBA designed and conducted research, provided research materials, and collected and organized data. JMA shared in the experimental work, collected data and helped in drafting the article. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgment
The author is grateful to Taibah University, for providing a helpful environment to conduct this study. The authors are very grateful to Mr. A. Obaidi, Mr. R. Al-Raddadi, Mr. S Al-Hussini, Mr. M Abdel-samad, and Mr. W Barakat, College of Medicine, Taibah University for their technical help, and support for this work.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Ungprasert P., Cheungpasitporn W., Crowson C.S., Matteson E.L. Individual non-steroidal anti-inflammatory drugs and risk of acute kidney injury: a systematic review and meta-analysis of observational studies. Eur J Intern Med. 2015;26(4):285–291. doi: 10.1016/j.ejim.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Imantalab V., Mirmansouri A., Sedighinejad A., Naderi Nabi B., Farzi F., Atamanesh H., Nassiri N. Comparing the effects of morphine sulfate and diclofenac suppositories on postoperative pain in coronary artery bypass graft patients. Anesthesiol Pain Med. 2014 21;4(4) doi: 10.5812/aapm.19423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fries J.F., Williams C.A., Bloch D.A., Michel B.A. Nonsteroidal anti-inflammatory drug-associated gastropathy: incidence and risk factor models. Am J Med. 1991;91(3):213–222. doi: 10.1016/0002-9343(91)90118-h. [DOI] [PubMed] [Google Scholar]
- 4.Scialis R.J., Manautou J.E. Elucidation of the mechanisms through which the reactive metabolite diclofenac acyl glucuronide can mediate toxicity. J Pharmacol Exp Ther. 2016 Apr;357(1):167–176. doi: 10.1124/jpet.115.230755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H.F., Li Y.J., Li Y.Y., Huang C., Huang L.X., Bu S.J. Pharmacokinetics of diclofenac sodium injection in swine. Pol J Vet Sci. 2019 Jun;22(2):423–426. doi: 10.24425/pjvs.2019.129217. [DOI] [PubMed] [Google Scholar]
- 6.Peter S.J., Basha S.K., Giridharan R., Lavinya B.U., Sabina E.P. Suppressive effect of Spirulina fusiformis on diclofenac-induced hepato-renal injury and gastrointestinal ulcer in Wistar albino rats: a biochemical and histological approach. Biomed Pharmacother. 2017 Apr;88:11–18. doi: 10.1016/j.biopha.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Salimi A., Neshat M.R., Naserzadeh P., Pourahmad J. Mitochondrial permeability transition pore sealing agents and antioxidants protect oxidative stress and mitochondrial dysfunction induced by naproxen, diclofenac and celecoxib. Drug Res (Stuttg) 2019;69(11):598–605. doi: 10.1055/a-0866-9356. [DOI] [PubMed] [Google Scholar]
- 8.Jarrar Y.B., Jarrar Q., Abed A., Abu-Shalhoob M.3. Effects of nonsteroidal anti-inflammatory drugs on the expression of arachidonic acid metabolizing Cyp450 genes in mouse hearts, kidneys and livers. Prostaglandins Other Lipid Mediat. 2019;14:14–21. doi: 10.1016/j.prostaglandins.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Aycan İ.Ö., Elpek Ö., Akkaya B., Kıraç E., Tuzcu H., Kaya S., Coşkunfırat N., Aslan M. Diclofenac induced gastrointestinal and renal toxicity is alleviated by thymoquinone treatment. Food Chem Toxicol. 2018;118:795–804. doi: 10.1016/j.fct.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 10.Ulubay M., Yurt K.K., Kaplan A.A., Atilla M.K. The use of diclofenac sodium in urological practice: a structural and neurochemical based review. J Chem Neuroanat. 2018 Jan;87:32–36. doi: 10.1016/j.jchemneu.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Ungprasert P., Cheungpasitporn W., Crowson C.S., Matteson E.L. Individual non-steroidal anti-inflammatory drugs and risk of acute kidney injury: a systematic review and meta-analysis of observational studies. Eur J Intern Med. 2015 May;26(4):285–291. doi: 10.1016/j.ejim.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Amoghimath S., Majagi S. Drug induced kidney disease. J Toxicol. 2017;2(1) [Google Scholar]
- 13.Sheikh B.Y. The role of prophetic medicine in the management of diabetes mellitus: a review of literature. J Taibah Univ Med Sc. 2016;11(4):339–352. [Google Scholar]
- 14.Al Harthi S.S., Mavazhe A., Al Mahroqi H., Khan S.A. Quantification of phenolic compounds, evaluation of physicochemical properties and antioxidant activity of four date (Phoenix dactylifera L.) varieties of Oman. J Taibah Univ Med Sc. 2015;10(3):346–352. [Google Scholar]
- 15.El-Far A.H., Shaheen H.M., Abdel-Daim M.M. Date palm (phoenix dactylifera): protection and remedy food. Curr Trends Nutraceuticals. 2016;1:2. [Google Scholar]
- 16.Chung E.Y., Tat S.T. Nonsteroidal anti-inflammatory drug toxicity in children: a clinical review. Pediatr Emerg Care. 2016 Apr;32(4):250–253. doi: 10.1097/PEC.0000000000000768. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y.T., Lu C.C., Yen G.C. Phytochemicals enhance antioxidant enzyme expression to protect against NSAID-induced oxidative damage of the gastrointestinal mucosa. Mol Nutr Food Res. 2017 Jun;61(6) doi: 10.1002/mnfr.201600659. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M., Sørensen H.T., Pedersen L. Diclofenac use and cardiovascular risks: series of nationwide cohort studies. BMJ. 2018;362:k3426. doi: 10.1136/bmj.k3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nouri A., Heidarian E., Nikoukar M. Effects of N-acetyl cysteine on oxidative stress and TNF-α gene expression in diclofenac-induced hepatotoxicity in rats. Toxicol Mech Methods. 2017;27:561–567. doi: 10.1080/15376516.2017.1334732. [DOI] [PubMed] [Google Scholar]
- 20.Hamad I., Abdelgawad H., Al J.S., Zinta G., Asard H. Metabolic analysis of various date palm fruit (Phoenix dactylifera L.) cultivars from Saudi Arabia to assess their nutritional quality. Molecules. 2015;20:13620–13641. doi: 10.3390/molecules200813620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumaira Khalid S., Khalid N., Khan R.S., Haroon Ahmed H., Ahmad A. A review on chemistry and pharmacology of Ajwa date fruit and pit. Trends Food Sci Technol. May 2017;63:60–69. [Google Scholar]
- 22.Abdu S.B. Ameliorative influence of Ajwa dates on ochratoxin A-induced testis toxicity. J Microsc Ultrastruct. 2018 Jul-Sep;6(3):134–138. doi: 10.4103/JMAU.JMAU_14_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan F., Khan T.J., Kalamegam G., Pushparaj P.N., Chaudhary A., Abuzenadah A., Kumosani T., Barbour E., Al-Qahtani M. Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complement Altern Med. 2017;17(1):418. doi: 10.1186/s12906-017-1926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ragab A.R., Elkablawy M.A., Sheik B.Y., Baraka H.N. Antioxidant and tissue-protective studies on Ajwa extract: dates from Al-monwarah, saudia Arabia. J Environ Anal Toxicol. 2013;3(1):163. [Google Scholar]
- 25.Zhang C.R., Aldosari S.A., Vidyasagar P.S., Nair K.M., Nair M.G. Antioxidant and anti-inflammatory assays confirm bioactive compounds in Ajwa date fruit. J Agric Food Chem. 2013;61(24):5834–5840. doi: 10.1021/jf401371v. [DOI] [PubMed] [Google Scholar]
- 26.Saheeh al-Bukhaari (Explanation of Fatah al-Bari), Book: Medicine, Chapter: Medication by Agwa for Magic, (10/249), No. (5768).