Abstract
We report a case of phaeohyphomycosis that affected the leg of a 45-year-old Brazilian man, car mechanic and renal transplanted. The direct mycological examination evidenced dematiaceous septated hyphae. The pathogenic fungal species was identified as Exophiala xenobiotica. Antifungal activity in vitro revealed terbinafine as the best antifungal. For treatment, it was chosen surgical excision of the entire lesion and used systemic itraconazole. Phaeohyphomycosis caused by Exophiala xenobiotica is extremely rare and is closely related to transplant patients.
Keywords: Exophiala xenobiotica, Phaeohyphomycosis, Immunosuppressed, Renal transplantation
1. Introduction
Dematiaceous fungi are found in nature and one of their characteristics is the presence of melanin, responsible for the dark pigmentation of their spores and conidia and that seems to behave as a virulence factor. More than a hundred species and sixty genera of these fungi are connected to a broad spectrum of human infections [1]. The main clinical forms of presentation of the infections through these dematiaceous fungi include phaeohyphomycoses (cutaneous, subcutaneous and systemic), that affect both immunocompetent and immunocompromised individuals [2].
Phaeohyphomycosis is a term used to denominate opportunistic, cutaneous and systemic infections [3]. The mainly genus are Wangiella, Alternaria and Exophiala. There are more than 100 species of fungi associated with phaeohyphomycosis [4]. The most prevalent species are E. jeanselmei and E. dermatitidis. Exophiala xenobiotica is a rare species. This disease usually affects rural and tropical populations in Central and South America [5,6]. The clinical polymorphism presents in phaeohyphomycosis is exacerbated by iatrogenic immunosuppression, making the diagnosis extremely difficult. The treatment is not defined, and it is usually carried out empirically [7].
2. Case
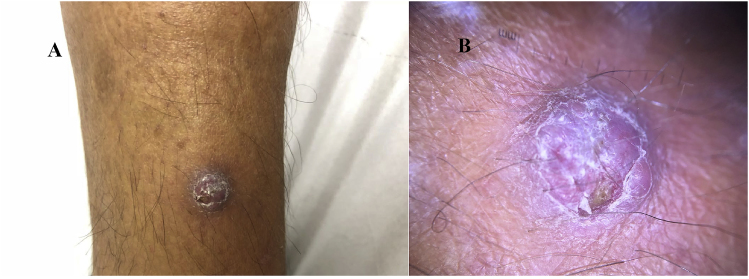
A 45-year-old white man, resident in Porto Alegre, car mechanic, hypertensive, with hepatitis C and renal transplanted two years ago, using mycophenolic acid, tacrolimus, prednisone, sulfamethoxazole-trimethoprim, sofosbuvir, daclatasvir and anti-hypertensive drugs, was attended in the department of Dermatology of the Hospital Santa Casa de Porto Alegre. He referred pruritic and fibrotic nodule on the right leg three months ago, with no secretion (Fig. 1A and B).
Fig. 1.
(A) Fibrotic nodule in the right leg. (B) Fibrotic nodule - photo taken with dermlite 3gen DL4.
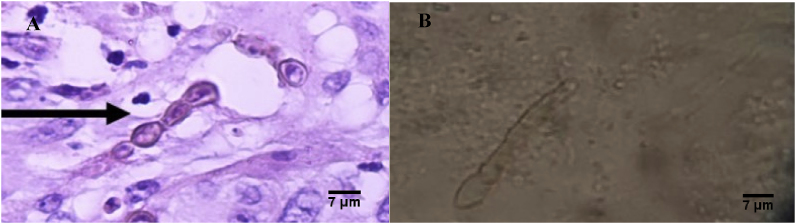
An incisional biopsy was performed, in the first day that he consulted (day 0), with histopathologic features of leveduriform cells and brownish hyphae within a dermal infiltrate which was compatible with phaeohyphomycosis (Fig. 2A and B).
Fig. 2.
(A) Hematoxylin Eosin Stain. Leveduriform cells and brownish hyphae within a dermal infiltrate (400X magnification). (B) Direct mycological examination - dematiaceous septated hyphae (400X magnification).
On the same day of the biopsy (day 0), direct and culture microscopic examinations were performed. The fungal isolate was grown in Sabouraud agar medium in 30 °C for 14 days. Mycelium was taken and the genomic DNA was extracted (day14) using Power Soil DNA isolation kit (Mobio, USA). Polymerase chain reaction was performed (day 14) targeting the ITS1-5.8S rDNA-ITS2 region using the ITS1 and ITS4 universal primers and the polymerase chain reaction (PCR) was performed under the following conditions: denaturation step at 94 °C for 5 min, 30 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min, extension at 72 °C for 2 min, and final extension at 72 °C for 10 min [8].
The PCR product was purified using ExoSap-IT (Affumetrix, USA) and sequenced in the ABI-PRISM 300 Genetic Analyzer (Applied Biosystems) following the manufacturer's instruction. The obtained sequence was analyzed using Staden Package [9] and compared with sequence of type strain available in the GenBank database using the BLAST tool [10]. The sequence obtained had a coverage of 92% and 100% of identity with Exophiala xenobiotica (CBS118157) type strain. The sequence was deposited at GenBank database under the identification number MK411560. The strain susceptibility assay was performed (day 14) in triplicates against amphotericin B, itraconazole, ketoconazole, voriconazole, posaconazole and terbinafine in the final concentration range of 0.03–16 μg/mL, all drugs were from Sigma-Aldrich. Microdilution technique from M27-A3 standardized by the Clinical and Laboratory Standards Institute (CLSI) was followed [11]. The Exophiala xenobiotica was grown for 14 days at 30 °C and the inoculum was prepared with a 0.85% of sterile saline solution, filtered and the conidial-only presence was verified and counted in Neubauer chamber by microscopy, to a final concentration in the wells were 2.5 × 104 CFU/mL. The microplates were incubated in a temperature of 35 °C for up to 5 days, after, the minimal inhibitory concentrations (MICs) were determined by visualization of 100% of inhibition comparing the wells with the growth control. Antifungal activity in vitro revealed terbinafine as the best antifungal. In vitro antifungal activity MIC were: amphotericin B (16 μg/mL), itraconazole (16 μg/mL), ketoconazole (1 μg/mL), voriconazole (2 μg/mL), posaconazole (0,125 μg/mL) and terbinafine (>0,5 μg/mL).
The patient returned 30 days after the biopsy with the renal transplant medical team that decided to treat the patient with itraconazole (day 30), that is available in the public health system. Itraconazol was given at a dose of 100 mg every 12 hours for three months (day 120). The patient could not do the treatment with terbinafine because it is not available in the public health system and he was not able to pay for medication. The dermatology team also chose to do a surgical excision of the entire lesion (day 33). The patient is in follow-up with no signs of relapse.
3. Discussion
We report a case of cutaneous phaeohyphomycosis caused by E. xenobiotica in a renal transplant patient treated with systemic itraconazole and surgery. Phaeohyphomycosis is an opportunistic disease. The incidence of phaeohyphomycosis in transplanted patients is increasing in the last decades due to the increased number of transplanted patients and the use of imunosuppressants, the majority of cases occur two years after transplantat. Calcineurin inhibitors appear to further increase susceptibility to fungal infections when compared to other immunosuppressants [5,12].
Surgical excision is the first choice for localized and well delimited lesions, it is preferred to use antifungal drugs before and after surgery, but there is no consensus about which drug to choose or how long the treatment will last. It is also recommended in severe or disseminated cases to reduce the doses of immunosuppressants [[13], [14], [15], [16]]. Most melanized fungi are susceptible to azoles, which makes itraconazole and voriconazole the main drugs used, followed by amphotericin B [17].
The early clinical suspicion of pheohypomycosis in transplant patients is extremely important, because immunosuppression is the main risk factor for infection and responsible for the systemic spread of the disease, culminating in severe and fatal conditions. In transplant patients, the therapeutic is challenging due to the antifungals high doses required, besides the possibility of drug interactions. Based on our case and in the literature, it is extremely important to use the molecular identification to discover the causative agent, as well as the evaluation of in vitro antifungal activity to use the best treatment for the patient [18].
Declaration of competing interest
There are none.
References
- 1.Revankar S.G. Dematiaceous fungi. Mycoses. 2007;50:91–101. doi: 10.1111/j.1439-0507.2006.01331.x. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa H., Taniguchi Y. Phaeohyphomycosis due to Exophiala oligosperma in an immunocompromised host. J. Rheumatol. 2019;46:6. doi: 10.3899/jrheum.180774. [DOI] [PubMed] [Google Scholar]
- 3.Jinkala S.R., Basu D., Neelaiah S., Stephen N., Hanuman S.B., Singh R. Subcutaneous phaeohyphomycosis: a clinical mimic of skin and soft tissue neoplasms—a descriptive study from India. World J. Surg. 2018;42:3861–3866. doi: 10.1007/s00268-018-4745-0. [DOI] [PubMed] [Google Scholar]
- 4.Silveira F., Nucci M. Emergence of Black moulds in fungal disease: epidemiology and therapy. Curr. Opin. Infect. Dis. 2001;14(6):679–684. doi: 10.1097/00001432-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira W.R.P., Borsato M.F.L., Festa Neto C., Rocha L.A., Nunes R.S. Feohifomicose em transplante renal: relato de dois casos. An. Bras. Dermatol. 2016;91(1):93–96. [Google Scholar]
- 6.Rossetto A.L., Pérsio R.A., Cruz R.C.B., Dellatorre G., Romeiro J.C.M. Feo-hifomicose subcutânea por Exophialajeanselmei localizada na bolsa escrotal - Relato de caso. An. Bras. Dermatol. 2010;85(4):517–520. doi: 10.1590/s0365-05962010000400013. [DOI] [PubMed] [Google Scholar]
- 7.Singal A., Pandhi D., Bhattacharya S.N., Das S., Aggarwal S., Mishra K. Pheohy- phomycosis causedby Exophiala spinifera: arareoccurrence. Int. J. Dermatol. 2008;47:44–47. doi: 10.1111/j.1365-4632.2007.03430.x. [DOI] [PubMed] [Google Scholar]
- 8.Heidrich D., González G.M., Pagani D.M., Ramírez-Castrillón M., Scroferneker M.L. Chromoblastomicosis caused by Rhinocladiella similis: case report. Med. Mycol. Case Rep. 2017;16:25–27. doi: 10.1016/j.mmcr.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staden R. The staden sequence analysis package. Mol. Biotechnol. 1996;5:233–241. doi: 10.1007/BF02900361. [DOI] [PubMed] [Google Scholar]
- 10.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 11.CLSI . Clinical and Laboratory Standards Institute (CLSI), Approved Standard M27- A3. third ed. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Wayne, PA. [Google Scholar]
- 12.Hoffmann C.C., Danuclov I.P., Purim K.S.M., Queiroz-Telles F. Infecções causadas por fungos demácios e suas correlações anátomo-clinicas. An. Bras. Dermatol. 2011;86(1):138–141. doi: 10.1590/s0365-05962011000100021. [DOI] [PubMed] [Google Scholar]
- 13.Ocampo M.A., Kanitakis J., Bienvenu A.L., Chauvet C., Euvrard S. Phaeohyphomycosis caused by Pyrenochaetaromeroi mimicking a plantar wart in a kidney transplant recipient. Transpl. Infect. Dis. 2012;14:E173–E174. doi: 10.1111/tid.12018. [DOI] [PubMed] [Google Scholar]
- 14.Vermeire S.E., de Jonge H., Lagrou K., Kuypers D.R. Cutaneous phaeohyphomycosis in renal allograft recipients: report of 2 cases and review of the literature. Diagn. Microbiol. Infect. Dis. 2010;68:177–180. doi: 10.1016/j.diagmicrobio.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Cunha D., Amaro C., Vieira M.R., Martins Mda L., Maduro A.P., Inácio J. Phaeohyphomycosis caused by Alternariainfectoria presenting as multiple vegetating lesions in a renal transplant patient. Rev. Iberoam. De. Micol. 2012;29:44–46. doi: 10.1016/j.riam.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes N.C., Nacif D., Akiti T., Cuzzi T. Subcutaneous phaeohyphomycosis caused by Cladophialophora sp.: a case report. Rev. do Inst. Med. Trop. São Paulo. Mar./Apr. 2007;49(2) doi: 10.1590/s0036-46652007000200008. São Paulo. [DOI] [PubMed] [Google Scholar]
- 17.Isa-Isa R., García C., Isa M., Arenas R. Subcutaneous phaeohyphomycosis (mycotic cyst) Clin. Dermatol. 2012;30:425–431. doi: 10.1016/j.clindermatol.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Daboit T.C., Duquia R.P., Magagnin C.M., Mendes S.D.C., Ramírez-Castrillón M., Steglich R. A case of Exophialaspinifera infection in Southern Brazil: molecular identification andantifungalsusceptibility. Med. Mycol. Case Rep. 2012;1:72–75. doi: 10.1016/j.mmcr.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]