Abstract
Simple Summary
The generation of free radical reactive oxygen species during freeze–thaw procedures is one of the major factors affecting the function and survival of sperm. Myoinositol is the most important natural form of inositol produced in the human body. Researchers have attempted to exploit the antioxidant nature of myoinositol to treat human infertility issues via the improvement of sperm quality traits and fertilization rates. We investigated the potential role of myoinositol neutralizing free radicals produced during the cryopreservation of dog semen. Myoinositol supplementation in the freezing medium resulted in improved quality-related parameters of dog semen including percentage motility, viability, plasma membrane integrity, and chromatin integrity. Improvement in post-thaw semen quality was confirmed by the expression of genes related to apoptosis, nuclear integrity, and reactive oxygen species generation.
Abstract
Oxidative stress during freeze–thaw procedures results in reduced semen fertility. A decrease in free radical levels can improve the post-thaw sperm quality. We examined the effects of myoinositol supplementation in freezing medium on the structure and function of cryopreserved dog sperm. Pooled ejaculates were diluted with buffer without or with myoinositol (1 or 2 mg/mL). Analysis of fresh semen revealed that the optimal concentration of myoinositol was 1 mg/mL, and this concentration was used in further experiments. Post-thaw semen quality in the myoinositol-supplemented group was superior (p < 0.05) compared with that in the control group in terms of motility (57.9 ± 0.4% vs. 47.8 ± 0.2%), sperm viability (57.5 ± 0.5% vs. 44.6 ± 0.6%), intact plasma membrane (56.6 ± 0.4% vs. 46.2 ± 0.6%), and acrosome membrane (59.3 ± 0.5% vs. 51.8 ± 0.5%). In addition, sperm in the myoinositol-supplemented group showed a significantly lower expression of pro-apoptotic (BAX) and mitochondrial reactive oxygen species (ROS) modulator (ROMO1) genes but higher expression of anti-apoptotic (BCL2), and protamine-related (PRM2 and PRM3) genes compared with that in the control group. Therefore, myoinositol supplementation before freezing can protect against oxidative stress and improve post-thaw dog sperm quality.
Keywords: myoinositol, dog sperm, cryo-survival, semen cryopreservation
1. Introduction
Since its domestication, the dog (Canis lupus familiaris) has emerged as one of the major companion animals, playing an imperative role in human society [1]. The increasing socio-economic value of dogs has resulted in an increasing demand for advances in assisted reproductive technologies (ART) [2], particularly artificial insemination using cryopreserved semen [3]. Semen cryopreservation permits the exchange of genetic material among populations and preserves the genes of valuable individuals over extended periods [4,5]. In dogs, semen cryopreservation can help to conserve threatened breeds, enhance the use of certain genetic traits [6], and facilitate the breeding of individuals that are unable to breed naturally [7].
Despite the extensive use of cryopreserved semen in ART, the process of cryopreservation causes severe damage to the sperm membranes, mitochondria, DNA, as well as alters the functioning and metabolism [8]. These adverse effects are manifested in the form of impaired motility [9], decreased adenosine triphosphate (ATP) production [10], and reduced fertility [11]. The factors responsible for these deleterious changes include ice crystals formation, osmotic pressure disturbances, oxidative stress, and cholesterol efflux [12], as well as the loss of structural organization of the plasma membrane [13]. Oxidative stress is the result of uncontrolled systemic generation of reactive oxygen species (ROS), accompanied by the failure of the enzymatic system to detoxify them [14]. The absence of cytoplasmic defense systems and the presence of polyunsaturated fatty acids-rich membrane renders sperm more prone to oxidative damage [15]. However, ROS should not be completely eliminated because a certain level of ROS is required for normal reproductive functions including sperm capacitation, acrosome reaction, and fertilization [16]. Therefore, to achieve the optimal outcome following semen cryopreservation, efforts should focus on creating a balance between ROS production and elimination.
Myo-inositol (Myo-Ins), the naturally existing most active form of inositol, belongs to group 1 of the vitamin B complex. In the human body, Myo-Ins is mainly synthesized from glucose-6-phosphate [15]. Myo-Ins regulates the intracellular calcium (Ca2+) level by acting as a precursor for secondary messengers in the cellular signal transduction system [17]. In addition, Myo-Ins plays a vital role in different cellular functions including morphogenesis, cytogenesis, membrane formation, growth, and lipid production [18]. Human testicles contain high concentrations of enzymes, including Myo-Ins monophosphatase-1 and myoinositol-1-phosphate synthase, which are involved in the synthesis of Myo-Ins [19]. Bull testicular sperm can synthesize lipids from inositol that have an important role in providing essential compounds necessary for sperm survival in epididymis [20]. Myo-Ins regulates sperm maturation, motility, capacitation, and acrosomal reaction [21], as well as osmo-regulates the seminal plasma [22]. Furthermore, it improves sperm mitochondrial functions via Ca2+ ion influx that stimulates oxidation and ATP generation, thereby condensing chromatin and preventing apoptosis [19].
Researchers have attempted to exploit the antioxidant nature of Myo-Ins for treating human infertility issues, via the improvement of sperm quality-related parameters and fertility [23,24]. In-vitro Myo-Ins supplementation has been reported to effectively improve sperm quality in patients with oligoteratoasthenozoospermic (OAT) [19,25] as well as fertilization rate and embryo quality [24]. Inositol protects the enzyme systems from cryo-damage, lipid peroxidation [26], and preserves the acrosomal integrity of post-thaw ram sperm [27]. The aim of the present study was to investigate the effects of Myo-Ins supplementation on the different quality-related parameters of post-thaw dog semen by neutralization of ROS.
2. Materials and Methods
All chemicals used were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated. Semen processing and evaluation was performed following the same protocol as previously explained [28].
2.1. Animals Used and Semen Collection
Four sexually mature and healthy male beagles, aged 2–4 years and weighing 8–11 kg, were used for semen collection. Separate indoor cages were used for each dog, furnished with all the necessary animal care facilities and procedures in accordance with the standards set up by the Committee for Accreditation of Laboratory Animals at Seoul National University. All experimental procedures were performed in compliance with the Guide for the Care and Use of Laboratory Animals at Seoul National University (approval no. SNU-180731-2). Semen was collected twice a week by digital manipulation. Ejaculates with a count of ≥ 100 × 106 sperm/mL, ≥ 70% motile sperm, and ≥ 80% of viable sperm with normal morphology were pooled before use.
2.2. Determination of Optimal Myo-Ins Concentration
In Experiment 1, Myo-Ins was dissolved in buffer 1 [Tris (hydroxymethyl) aminomethane 24 g/L, citric acid 14 g/L, fructose 8 g/L, and kanamycin sulfate 0.15 g/L in distilled water (pH 6.6, 290 mOsm)] to achieve the Myo-Ins concentrations of 0, 1, or 2 mg/mL. The selection of Myo-Ins concentrations was decided on the basis of previously published work (1 mg/mL Myo-Ins, according to Ramadan Saleh 2018 [29]; 2 mg/mL Myo-Ins, according to Condorelli, 2011 [19]). The pooled ejaculates were washed via centrifugation at 100× g for 1 min at room temperature (25–28 °C), ensuring that debris descended to the bottom of the tube, and sperm remained in the supernatant [30]. The supernatant was divided into three equal aliquots and centrifuged at 700× g for 5 min at room temperature (25–28 °C). The final concentration of 200 × 106 sperm/mL was achieved by separately resuspending each sperm pellet using buffer 1 with different Myo-Ins concentrations. The optimal Myo-Ins concentration was determined following the same procedure as described previously [31].
Sperm viability, motility, and kinematic parameters were assessed for each Myo-Ins concentration [30]. A drop of semen (10 µL) was placed onto a clean pre-warmed glass slide and mounted with a coverslip. Kinematic parameters were assessed by screening five different fields and tracking at least 200 motile sperm in each experiment using a sperm analysis imaging system (FSA2011 premium edition version 2011; Medical Supply, Gwangwon, Korea) [31,32].
Sperm viability was assessed using eosin-nigrosin staining. Briefly, sperm stain suspension was prepared by mixing a drop of semen (10 µL) in an equal volume of stain. Smears were made by spreading a drop of suspension on a clean glass slide followed by air drying. At least 200 sperm per slide were examined to assess membrane integrity; the membranes were classified as intact (white color) or damaged (pink color). The optimal Myo-Ins concentration was selected on the basis of outcomes of the analysis of fresh semen and was used for the remaining experiments.
2.3. Semen Cryopreservation and Thawing
Experiment 2 was performed to investigate the effects of Myo-Ins on sperm function and survival during cryopreservation. Pooled ejaculates were washed and diluted following the protocol described above. Sperm suspension was divided into two aliquots and diluted with buffer 2 [54% (v/v) buffer 1, 40% (v/v) egg yolk, and 6% (v/v) glycerol] supplemented with Myo-Ins; 0, or 1 mg/mL. Buffer 2 was added to the sperm suspension following a multistep dilution protocol (14%, 19%, 27% and 40% of total calculated volume, sequentially loaded at an interval 30 s) [28] until a final concentration of 100 × 106 sperm/mL was achieved. The diluted semen was filled into 0.5-mL semen straws (Minitube, Tiefenbach, Germany) and equilibrated at 4 °C for 45–60 min. Semen straws were placed horizontally 2 cm above the surface of liquid nitrogen (LN2) for 15 min and finally plunged into the LN2 for storage.
After 1 week of storage, frozen semen was thawed in a water bath at 37 °C for 30 s and diluted (1:5) with buffer 1 to 14%, 19%, 27%, and 40% of the total volume [32]. Progressive sperm motility, kinematic parameters, and viability were assessed after 5 min of thawing following the protocol described in ‘Section 2.2’.
2.4. Assessment of Sperm Plasma Membrane Integrity
Hypo-osmotic swelling (HOS) assay was performed to analyze sperm plasma membrane integrity [33]. A drop of semen (50 µL) from each group was mixed in 0.5 mL of HOS solution and incubated at 37 °C for 30 min. A 5-µL drop of the mixture was placed on a pre-warmed clean glass slide, and 200 sperm were examined within 5–10 min for their ability to swell using phase-contrast microscopy (Eclipse Ts2, Nikon, Minato-Ku, Tokyo, Japan). The swelling was indicated by the coiling of the sperm tail and such sperm were considered to possess an intact plasma membrane.
2.5. Assessment of Sperm Acrosome Membrane Integrity
Sperm acrosome membrane was analyzed using fluorescein isothiocyanate-conjugated peanut agglutinin (FITC-PNA) [34]. Post-thaw semen was smeared on glass slides and air-dried. Smears were fixed for 10 min at 20 °C–22 °C using absolute methanol, followed by air drying. Staining was performed by spreading 30-µL of FITC-PNA solution [100 µg/mL of phosphate buffer saline (PBS)] on the slides, followed by incubation at 37 °C for 30 min in the dark under humid conditions. Finally, the slides were rinsed with PBS, dried, and mounted with glycerol. The status of sperm acrosomes was analyzed using epifluorescence microscopy (Eclipse Ts2, Nikon) and classified as intact acrosome (strong green fluorescence), partially intact acrosome (dull green fluorescence), or non-intact acrosome (no fluorescence).
2.6. Mucus Penetration Test
Surrogate mucus (modified synthetic oviductal fluid) [28,30] was loaded into flat capillary tubes (80 ± 0.5 mm long, 1.25 ± 0.05 mm wide, Hilgenberg GMBH, Stutzerbach, Germany) sealed at one end. The capillary tubes were placed in the vertical position for 15 min to check the tightness of the seal and to remove bubbles following which the open end of the capillary tube was inserted into an Eppendorf tube containing 100 µL of semen suspension and placed horizontally for 2 h at room temperature (25–28 °C). Thereafter, the number of sperm that reached the 1 and 3 cm marks in the capillary tube was counted.
2.7. Protamine Deficiency Test
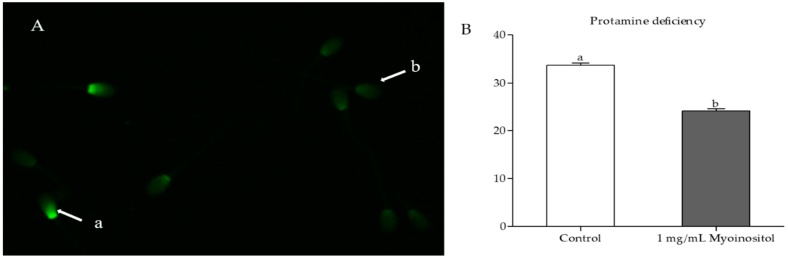
Chromomycin A3 (CMA3) staining was performed to examine the sperm chromatin packing [35]. Post-thaw sperm were washed using buffer 1 and smeared onto clean glass slides. Smears were fixed in methanol: Glacial acetic acid (3:1) for 5 min at 4 °C and treated with 100 µL of CMA3 solution (0.25 mg/mL of McI1vaine buffer supplemented with 10 mM MgCl2, pH 7.0) for 20 min. Smears were rinsed in McI1vaine buffer [30], mounted with buffered glycerol, and examined using epifluorescence microscopy (Eclipse Ts2, Nikon). CMA3-positive sperm with abnormal chromatin packing were indicated by their bright green fluorescent heads, whereas CMA3-negative sperm with normal chromatin packing were indicated by their dull green fluorescent heads (Figure 1A).
Figure 1.
(A) Chromomycin A3 (CMA3) staining of sperm chromatin to demonstrate protamine-deficient (a) and normal sperm (b). (B) Quantification of CMA3 in myoinositol-treated and untreated sperm (p < 0.05).
2.8. Assessment of Gene Expression
Quantitative polymerase chain reaction (qPCR) was used to analyze the messenger ribonucleic acid (mRNA) expression of genes related to apoptosis (B-cell lymphoma (BCL2), BCL2-associated X (BAX)), and protamine level measurement [protamine 2 (PRM2) and protamine 3 (PRM3)], as well as mitochondrial ROS modulator (ROS modulator 1 (ROMO1)). Briefly, RNA was extracted from post-thaw sperm both from the Myo-Ins-supplemented and control groups. Real-time qPCR (RT-qPCR) was used for assessing transcript abundance (primers listed in Table 1). Trizol reagent (Invitrogen, Carlsbad, CA, USA) was used for RNA extraction, following which complementary DNA synthesis was performed using the Compact cDNA synthesis kit (SJ BIOSCIENCE, Daejeon, Korea), according to the manufacturer’s instructions. Expression levels of RT-qPCR transcripts were analyzed using the Sybr Green Q-PCR Master Mix (SJ BIOSCIENCE, Daejeon, Korea) and the expression of each target gene was quantified relative to that of the internal gene β-actin (ACTB) using the equation, R = 2–[ΔCt sample − ΔCt control].
Table 1.
Primer sequences used for gene expression analysis.
| Gene | Primer Sequence (5′–3′) | Product Size (bp) | NCBI Accession No. |
|---|---|---|---|
| BACT | F: GAGGCATCCTGACTCTGA | 87 | XM_544346.3 |
| R: TCTGGCACCACACTTTCT | |||
| BCL2 | F: GACAGAGAGGATCATGCTGT | 141 | NM_001002949.1 |
| R: TGGCATGAGATGCAGGAAAT | |||
| BAX | F: CCAAGAAGCTGAGCGAATG | 123 | NM_001003011.1 |
| R: CTGCCACTCGGAAGAAGAC | |||
| PRM2 | F: CTCCAGAAGGGTCAGGAG | 169 | NM_001287148.1 |
| R: GGCTCCTTGCAAACTCAG | |||
| PRM3 | F: TCTGGAGAGGCAGCCAGA | 101 | XM_022420065.1 |
| R: AGGCCATGAGCTTCTTCA | |||
| ROMO1 | F: CTACGTGCTCCCGGAAGT | 100 | XM_534406.6 |
| R: TCGCTCAGTTCTACGTCTCAC |
F, forward; R, reverse; BCL2, B-cell lymphoma; BAX, BCL2-associated X; PRM2, protamine 2; PRM3, protamine 3; ROMO1, reactive oxygen species modulator 1.
2.9. Statistical Analysis
Data analysis was performed using SPSS 21.0 software (SPSS Inc., Chicago, IL, USA). All values are expressed as mean ± SEM, and p < 0.05 indicated statistical significance. Data related to the determination of optimal Myo-Ins concentration were analyzed using a one-way analysis of variance and Tukey’s multiple comparison test. The independent sample t-test was used to compare different quality-related parameters and gene expression between the post-thaw semen samples of the Myo-Ins-supplemented and control groups.
3. Results
3.1. Determination of Optimal Myo-Ins Concentration
The percentage motility of fresh sperm supplemented with 2 mg/mL Myo-Ins was significantly reduced (64.7% ± 0.7%) compared with that of the control and sperm supplemented with 1 mg/mL (73.8% ± 0.3% and 71.5% ± 0.4%, respectively; Table 2). The percentage linearity observed in both 1 and 2 mg/mL Myo-Ins-supplemented semen samples was similar (27.0% ± 0.5%, and 25.7% ± 0.4%) but higher than that in the control (24.1% ± 0.3%). The amplitude of lateral head displacement (ALH) was significantly lower in the 2 mg/mL Myo-Ins-supplemented semen samples (4.1% ± 0.1%) compared with that in the others (5.0% ± 0.1% for the control and 4.6% ± 0.1% for 1 mg/mL). Straightness (%) did not significantly differ among the groups.
Table 2.
Determination of optimal Myo-Ins concentration for dog semen cryopreservation.
| Group | Motility (%) | Linearity (%) | Straightness (%) | ALH (µm) | Live Sperm (%) |
|---|---|---|---|---|---|
| 0 mg/mL (control) | 73.8 ± 0.3 a | 24.1 ± 0.3 b | 48.4 ± 0.5 | 5.0 ± 0.1 a | 69.2 ± 0.2 a |
| 1 mg/mL | 71.5 ± 0.4 b | 25.7 ± 0.4 a,b | 49.5 ± 0.5 | 4.6 ± 0.1 a | 68.7 ± 0.2 a |
| 2 mg/mL | 64.7 ± 0.7 c | 27.0 ± 0.5 a | 49.5 ± 0.9 | 4.1 ± 0.1 b | 61.2 ± 0.3 b |
ALH, amplitude of lateral head displacement. a,b Values in columns with different superscript lowercase letters significantly differ (p < 0.05, n = 4).
The effects of Myo-Ins supplementation on fresh sperm viability were evaluated following the examination of four independent replicates. The difference in live sperm percentages was not significant between the control and 1 mg/mL Myo-Ins-supplemented semen samples (69.2% ± 0.2% and 68.7% ± 0.2%, respectively), but both of these differences were significantly higher than 2 mg/mL Myo-Ins-supplemented semen samples (61.2% ± 0.3%).
3.2. Effect of Myo-Ins on Post-Thaw Sperm Motility and Kinematic Parameters
The post-thaw analysis performed after 5 min of thawing revealed that the motile sperm percentage was significantly greater in Myo-Ins-supplemented sperm samples (57.9% ± 0.4%) compared with that in the control (47.8% ± 0.2%; Table 3). The kinematic parameters exhibited similar trends as observed for percentage motility among the samples of Myo-Ins-supplemented and control groups. The percentages of linearity, straightness, and ALH were significantly higher in Myo-Ins-supplemented sperm samples than those in the control (29.6% ± 1.1% vs. 23.9% ± 0.3%, 54.5% ± 0.6% vs. 50.8% ± 0.9%, and 3.3% ± 0.0% vs. 2.5% ± 0.0%; respectively), (Table 3).
Table 3.
Effect of Myo-Ins supplementation on post-thaw dog semen quality after 5 min of thawing.
| Group | Motility (%) | Linearity (%) | Straightness (%) | ALH (µm) | Live Sperm (%) | Membrane Integrity (%) |
|---|---|---|---|---|---|---|
| Control (0 mg/mL) | 47.8 ± 0.2 b | 23.9 ± 0.3 b | 50.8 ± 0.9 b | 2.5 ± 0.0 b | 44.6 ± 0.6 b | 46.2 ± 0.6 b |
| Treatment (1 mg/mL) | 57.9 ± 0.4 a | 29.6 ± 1.1 a | 54.4 ± 0.6 a | 3.3 ± 0.0 a | 57.5 ± 0.5 a | 56.6 ± 0.4 a |
ALH, amplitude of lateral head displacement. a,b Values in columns with different superscript lowercase letters significantly differ (p < 0.05, n = 4).
3.3. Effect of Myo-Ins on Sperm Viability and Plasma Membrane Integrity
Eosin-nigrosin staining of post-thaw sperm showed that the percentage of live sperm was significantly higher in Myo-Ins-supplemented samples than that in the control (57.5% ± 0.5% vs. 44.6% ± 0.6%, respectively; Table 3). Further investigations revealed that improvement in sperm plasma membrane integrity contributed to higher live sperm percentages. HOS assay showed Myo-Ins-supplemented sperm samples had a significantly higher percentage of intact plasma membrane (56.6% ± 0.4%) compared with the control (46.2% ± 0.6%).
3.4. Effect of Myo-Ins on Sperm Acrosome Integrity
FITC-PNA staining revealed that Myo-Ins supplementation resulted in a significantly higher percentage of sperm with intact acrosome compared with the control (59.3% ± 0.5% vs. 51.8% ± 0.5%; respectively; Table 4).
Table 4.
Effects of supplementing buffer 2 with Myo-Ins on post-thaw dog semen quality.
| Group | Acrosome Integrity (%) | Sperm Count at Mucus Penetration Distance | |
|---|---|---|---|
| 1 cm | 3 cm | ||
| Control | 51.8 ± 0.5 b | 139.6 ± 0.5 b | 48.1 ± 0.7 b |
| Treatment (1 mg/mL) | 59.3 ± 0.5 a | 150.4 ± 0.6 a | 57.0 ± 0.4 a |
a,b Values in columns with different superscript lowercase letters significantly differ (p < 0.05, n = 4).
3.5. Effect of Myo-Ins on Mucus Penetration
Mucus penetration test was performed to examine the effect of Myo-Ins supplementation on the ability of sperm to penetrate mucus. Post-thaw analysis showed that Myo-Ins-supplemented sperm penetrated the synthetic oviductal fluid more effectively. The sperm counts at both the 1 and 3 cm marks were significantly higher for Myo-Ins-supplemented sperm samples (150.4 ± 0.6 sperm and 57.0 ± 0.4 sperm, respectively) than those for the control group (139.6 ± 0.5 sperm and 48.1 ± 0.7 sperm, respectively; Table 4).
3.6. Effect of Myo-Ins on Chromatin Integrity
CMA3 staining was used to assess the quality of chromatin packing in post-thaw sperm. Samples that received 1 mg/mL Myo-Ins supplementation (group 1) during freezing showed a significantly lower proportion of sperm with protamine deficiency after thawing compared with the samples in the control group (24.1% ± 0.5% vs. 33.6% ± 0.5%; Figure 1B).
3.7. Effect of Myo-Ins on Gene Expression
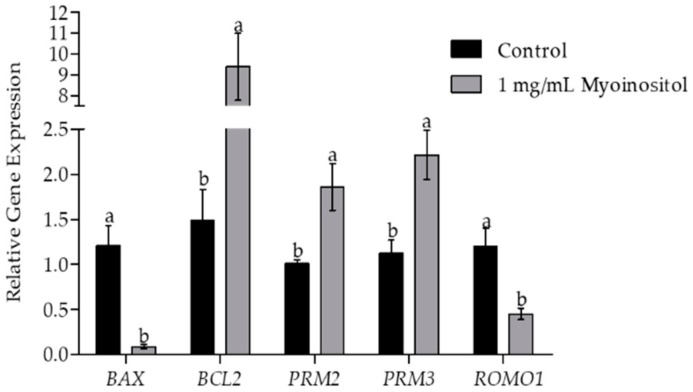
Analysis of expression levels of different genes in post-thaw semen samples was performed by RT-qPCR. The effects of the supplementation of buffer 2 with 1 mg/mL Myo-Ins on apoptosis-related genes were exhibited by the significantly enhanced expression levels of BCL2 and reduced expression of BAX compared with the control group (Figure 2). The expression levels of genes associated with protamine levels (PRM2 and PRM3) in post-thaw semen samples were significantly higher in Myo-Ins-supplemented semen samples than in the control group. Furthermore, the expression levels of the mitochondrial ROS modulator (ROMO1) were significantly reduced in Myo-Ins-supplemented sperm samples compared with the control.
Figure 2.
Expression of the pro-apoptotic gene BCL2-associated X, (BAX); the anti-apoptotic gene B-cell lymphoma (BCL2); the chromatin repair-related genes protamine-2 (PRM2) and protamine-3 (PRM3); and the mitochondrial ROS modulator gene (ROMO1) using RT-qPCR in myoinositol-supplemented and non-supplemented sperm samples. Values are presented as mean ± SEM. Different lowercase letters (a or b) represent a significant difference (p < 0.05).
4. Discussion
Semen cryopreservation is an essential part of ART. However, freeze–thaw procedures are associated with oxidative stress that causes reductions in sperm function [36], cryo-survival [37], and fertility [38,39,40]. Oxidative stress is a result of increased ROS generation and decreased antioxidant levels that render sperm more susceptible to lipid peroxidation [41]. To reduce the magnitude of oxidative damage during dog semen cryopreservation, we supplemented buffer 2 with Myo-Ins, a natural sugar produced in the human testicles that protects against ROS by improving membranal integrity, mitochondrial function, and chromatin compactness as well as preventing apoptosis [19]. To reduce the direct effects of Myo-Ins on metabolism in sperm, we determined the optimal Myo-Ins concentration using fresh dog semen. In the group supplemented with 2 mg/mL Myo-Ins, sperm motility, ALH, and viability were significantly reduced (Table 2), possibly due to a change in osmotic pressure or toxicity caused by the Myo-Ins. The optimal Myo-Ins concentration observed in this study was 1 mg/mL similar to that reported in for humans [29].
Results showed that supplementation of semen extender (buffer 2) with 1 mg/mL of Myo-Ins had beneficial effects on the post-thaw dog sperm motility (Table 3). In the middle piece of the sperm tail, the axosome and associated dense fibers are surrounded by mitochondria serving as a powerhouse for sperm by generating ATP [42]. It is well-known that ROS damage the axosome and mitochondria, resulting in sperm immobilization [43]. Sperm with disrupted membrane integrity exhibit high membrane permeability and are unable to control intracellular ion concentrations responsible for its motion [44]. The post-thaw results suggest that Myo-Ins supplementation significantly enhances dog sperm motility, membrane integrity (plasma and acrosome), and viability (Table 3 and Table 4) indicating the protective nature of Myo-Ins against cryo-damage due to oxidative stress. Myo-Ins improves mitochondrial function, affecting ATP generation in patients with altered semen quality [19]. Incubation of fresh human semen with Myo-Ins (2 mg/mL) resulted in a significant improvement of motility [25]. Similarly, the positive influence of Myo-Ins on motility of fresh and post-thaw human [15,45], and bovine [46,47].
Decreased levels of ROMO1 (a mitochondrial ROS modulator) in Myo-Ins-supplemented sperm indicate improved mitochondrial function. ROMO1 is the main gene involved in the generation of ROS from mitochondria [48], which is responsible for the programmed death of cells [49]. The decreased expression of the BAX (pro-apoptotic gene) along with increased expression of the BCL2 (anti-apoptotic gene) observed in this study indicate reduced apoptosis during sperm cryopreservation (Figure 2). Post-thaw analysis of Myo-Ins-supplemented semen samples confirmed significantly higher cryo-survival compared with the control (Table 3). Similarly, Myo-Ins supplementation (1 mg/mL) of the freezing medium reportedly results in a significant improvement in the survival rate of human sperm [29].
Sperm chromatin packing involves the replacement of the original histone protein with protamine [50,51]. The large segments of DNA have a stronger bond with protamine than with histone [52] and the resultant condensed strands of DNA (toroids) have better protection against the oxidative damage of chromatin [53]. The conservation of sperm protamine content during freeze–thaw indicates the integrity of sperm chromatin material. Post-thaw sperm analysis showed significantly lower percentages of protamine-deficient sperm in the Myo-Ins-supplemented group compared with those in the control (Figure 1). Transcript analysis of Myo-Ins supplemented semen samples showed a significantly higher expression of protamine genes (PRM2 and PRM3) compared with that in the control (Figure 2). Myo-Ins treatment has been shown to significantly enhance the integrity of human sperm chromatin by reducing DNA fragmentation [15]. Similarly, the supplementation of Myo-Ins prior to semen processing protects the integrity of bovine sperm DNA [47].
The mucus penetration test reflects the ability of sperm to pass through the cervical mucus barrier. It serves as a useful tool for measuring the migratory capability of sperm through the genital tract [54]. Additionally, the sperm’s ability to penetrate the cervical mucus surrogates is closely correlated with its ability to fuse with oocytes [55]. In the present study, a significantly higher number of sperm reached the 1 and 3 cm distance in the surrogate cervical mucus (synthetic oviductal fluids) in the Myo-Ins-supplemented sperm group. Improved mucus penetration ability and significant ALH increase indicate sperm hyperactivation, which can result in enhanced fertilization and pregnancy rates [56].
5. Conclusions
Myo-Ins (1 mg/mL) supplementation of buffer 2 (the extender) can provide protection to dog sperm during cryopreservation, improve the post-thaw sperm motility, kinematic parameters, membrane integrity, and reduce chromatin damage and apoptosis. However, further studies are required to determine the impact of Myo-Ins on in-vivo fertilization and pregnancy rates.
Acknowledgments
The authors would like to acknowledge the funding agency, Cooperative Research Program of RDA and the assistance provided by Feriel Yasmine Mahiddine and Ye Sol Yun. The authors would also like to extend their gratitude to Goo Jang at Seoul National University, for allowing the use of sperm analysis imaging system.
Author Contributions
Conceptualization, J.C. and A.Y.Q.; data curation, A.Y.Q. and M.J.K.; formal analysis, A.Y.Q. and M.J.K.; funding acquisition, J.C.; investigation, A.Y.Q., M.J.K., and X.F.; methodology, J.C., M.J.K., and A.Y.Q.; project administration, J.C. and M.J.K.; resources, J.C.; supervision, J.C. and M.J.K.; validation, M.J.K. and J.C.; visualization, M.J.K. and J.C.; writing—original draft, A.Y.Q.; writing—review and editing, A.Y.Q. and M.J.K. All authors read and approved the final manuscript.
Funding
This research was funded by the Cooperative Research Program of RDA (CCAR), grant numbers: PJ013954012019 and PJ013954022019.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jang G., Kim M.K., Lee B.C. Current status and applications of somatic cell nuclear transfer in dogs. Theriogenology. 2010;74:1311–1320. doi: 10.1016/j.theriogenology.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 2.Thomassen R., Farstad W. Artificial insemination in canids: A useful tool in breeding and conservation. Theriogenology. 2009;71:190–199. doi: 10.1016/j.theriogenology.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Khan J., Tahir M., Khalid A., Sattar A., Ahmad N. Effect of cholesterol-loaded cyclodextrins on cryosurvival of dog spermatozoa. Reprod. Domest. Anim. 2017;52:265–268. doi: 10.1111/rda.12893. [DOI] [PubMed] [Google Scholar]
- 4.Axner E., Lagerson E. Cryopreservation of dog semen in a tris extender with 1% or 2% soya bean lecithin as a replacement of egg yolk. Reprod. Domest. Anim. 2016;51:262–268. doi: 10.1111/rda.12675. [DOI] [PubMed] [Google Scholar]
- 5.Hong H.M., Sim G.Y., Park S.M., Lee E.J., Kim D.Y. Ameliorative effect of chitosan complex on miniature pig sperm cryopreservation. J. Anim. Reprod. Biotechnol. 2018;33:337–342. doi: 10.12750/JET.2018.33.4.337. [DOI] [Google Scholar]
- 6.Silva A.R., de Cassia Soares Cardoso R., Uchoa D.C., MacHado da Silva L.D. Effect of tris-buffer, egg yolk and glycerol on canine semen freezing. Vet. J. 2002;164:244–246. doi: 10.1053/tvjl.2002.0704. [DOI] [PubMed] [Google Scholar]
- 7.Michael A., Alexopoulos C., Pontiki E., Hadjipavlou-Litina D., Saratsis P., Boscos C. Effect of antioxidant supplementation on semen quality and reactive oxygen species of frozen-thawed canine spermatozoa. Theriogenology. 2007;68:204–212. doi: 10.1016/j.theriogenology.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 8.Johnston S.D., Satake N., Zee Y., Lopez-Fernandez C., Holt W.V., Gosalvez J. Osmotic stress and cryoinjury of koala sperm: An integrative study of the plasma membrane, chromatin stability and mitochondrial function. Reproduction. 2012;143:787–797. doi: 10.1530/REP-11-0436. [DOI] [PubMed] [Google Scholar]
- 9.Dejarkom S., Kunathikom S. Evaluation of cryo-injury of sperm chromatin according to computer controlled rate freezing method part 2. J. Med. Assoc. Thai. 2007;90:852–856. [PubMed] [Google Scholar]
- 10.O’Flaherty C., de Lamirande E., Gagnon C. Positive role of reactive oxygen species in mammalian sperm capacitation: Triggering and modulation of phosphorylation events. Free Radic. Biol. Med. 2006;41:528–540. doi: 10.1016/j.freeradbiomed.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 11.De Leeuw F.E., Chen H.C., Colenbrander B., Verkleij A.J. Cold-induced ultrastructural changes in bull and boar sperm plasma membranes. Cryobiology. 1990;27:171–183. doi: 10.1016/0011-2240(90)90009-S. [DOI] [PubMed] [Google Scholar]
- 12.John Morris G., Acton E., Murray B.J., Fonseca F. Freezing injury: The special case of the sperm cell. Cryobiology. 2012;64:71–80. doi: 10.1016/j.cryobiol.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Bailey J.L., Bilodeau J.F., Cormier N. Semen cryopreservation in domestic animals: A damaging and capacitating phenomenon. J. Androl. 2000;21:1–7. [PubMed] [Google Scholar]
- 14.Aitken R.J., Baker M.A., Nixon B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J. Androl. 2015;17:633. doi: 10.4103/1008-682X.153850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohammadi F., Varanloo N., Heydari Nasrabadi M., Vatannejad A., Amjadi F.S., Javedani Masroor M., Bajelan L., Mehdizadeh M., Aflatoonian R., Zandieh Z. Supplementation of sperm freezing medium with myoinositol improve human sperm parameters and protects it against DNA fragmentation and apoptosis. Cell Tissue Bank. 2019;20:77–86. doi: 10.1007/s10561-018-9731-0. [DOI] [PubMed] [Google Scholar]
- 16.Santamaria A., Giordano D., Corrado F., Pintaudi B., Interdonato M.L., Vieste G.D., Benedetto A.D., D’Anna R. One-year effects of myo-inositol supplementation in postmenopausal women with metabolic syndrome. Climacteric. 2012;15:490–495. doi: 10.3109/13697137.2011.631063. [DOI] [PubMed] [Google Scholar]
- 17.Foskett J.K. Inositol trisphosphate receptor Ca2+ release channels in neurological diseases. Pflügers Arch. 2010;460:481–494. doi: 10.1007/s00424-010-0826-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenberg F., Parthasarathy R. Methods in Enzymology. Volume 141. Academic Press; Amsterdam, The Netherlands: 1987. Measurement of biosynthesis of myo-inositol from glucose 6-phosphate; pp. 127–143. [DOI] [PubMed] [Google Scholar]
- 19.Condorelli R.A., La Vignera S., Di Bari F., Unfer V., Calogero A.E. Effects of myoinositol on sperm mitochondrial function in-vitro. Eur. Rev. Med. Pharmacol. Sci. 2011;15:129–134. [PubMed] [Google Scholar]
- 20.Voglmayr J., Amann R. The distribution of free myo-inositol in fluids, spermatozoa, and tissues of the bull genital tract and observations on its uptake by the rabbit epididymis. Biol. Reprod. 1973;8:504–513. doi: 10.1093/biolreprod/8.4.504. [DOI] [PubMed] [Google Scholar]
- 21.Bevilacqua A., Carlomagno G., Gerli S., Montanino Oliva M., Devroey P., Lanzone A., Soulange C., Facchinetti F., Carlo Di Renzo G., Bizzarri M., et al. Results from the International Consensus Conference on myo-inositol and D-chiro-inositol in Obstetrics and Gynecology-assisted reproduction technology. Gynecol. Endocrinol. 2015;31:441–446. doi: 10.3109/09513590.2015.1006616. [DOI] [PubMed] [Google Scholar]
- 22.Chauvin T.R., Griswold M.D. Characterization of the expression and regulation of genes necessary for myo-inositol biosynthesis and transport in the seminiferous epithelium. Biol. Reprod. 2004;70:744–751. doi: 10.1095/biolreprod.103.022731. [DOI] [PubMed] [Google Scholar]
- 23.Korosi T., Barta C., Rokob K., Torok T. Physiological Intra-Cytoplasmic Sperm Injection (PICSI) outcomes after oral pretreatment and semen incubation with myo-inositol in oligoasthenoteratozoospermic men: Results from a prospective, randomized controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2017;21:66–72. [PubMed] [Google Scholar]
- 24.Rubino P., Palini S., Chigioni S., Carlomagno G., Quagliariello A., De Stefani S., Baglioni A., Bulletti C. Improving fertilization rate in ICSI cycles by adding myoinositol to the semen preparation procedures: A prospective, bicentric, randomized trial on sibling oocytes. J. Assist. Reprod. Genet. 2015;32:387–394. doi: 10.1007/s10815-014-0401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Condorelli R.A., La Vignera S., Bellanca S., Vicari E., Calogero A.E. Myoinositol: Does it improve sperm mitochondrial function and sperm motility? Urology. 2012;79:1290–1295. doi: 10.1016/j.urology.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Carpenter J.F., Hand S.C., Crowe L.M., Crowe J.H. Cryoprotection of phosphofructokinase with organic solutes: Characterization of enhanced protection in the presence of divalent cations. Arch. Biochem. Biophys. 1986;250:505–512. doi: 10.1016/0003-9861(86)90755-1. [DOI] [PubMed] [Google Scholar]
- 27.Molinia F., Evans G., Maxwell W. Effect of polyols on the post-thawing motility of pellet-frozen ram spermatozoa. Theriogenology. 1994;42:15–23. doi: 10.1016/0093-691X(94)90658-6. [DOI] [PubMed] [Google Scholar]
- 28.Qamar A.Y., Fang X., Kim M.J., Cho J. Improved post-thaw quality of canine semen after treatment with exosomes from conditioned medium of adipose-derived mesenchymal stem cells. Animals. 2019;9:865. doi: 10.3390/ani9110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saleh R., Assaf H., El Maged A., Wafaa M., Elsuity M., Fawzy M. Increased cryo-survival rate in ejaculated human sperm from infertile men following pre-freeze in vitro myo-inositol supplementation. Clin. Exp. Reprod. Med. 2018;45:177–182. doi: 10.5653/cerm.2018.45.4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagashima J.B., Sylvester S.R., Nelson J.L., Cheong S.H., Mukai C., Lambo C., Flanders J.A., Meyers-Wallen V.N., Songsasen N., Travis A.J. Live births from domestic dog (Canis familiaris) embryos produced by In Vitro Fertilization. PLoS ONE. 2015;10:e0143930. doi: 10.1371/journal.pone.0143930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdillah D.A., Setyawan E.M.N., Oh H.J., Ra K., Lee S.H., Kim M.J., Lee B.C. Iodixanol supplementation during sperm cryopreservation improves protamine level and reduces reactive oxygen species of canine sperm. J. Vet. Sci. 2019;20:79–86. doi: 10.4142/jvs.2019.20.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setyawan E.M., Kim M.J., Oh H.J., Kim G.A., Jo Y.K., Lee S.H., Choi Y.B., Lee B.C. Maintaining canine sperm function and osmolyte content with multistep freezing protocol and different cryoprotective agents. Cryobiology. 2015;71:344–349. doi: 10.1016/j.cryobiol.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Root Kustritz M.V. The value of canine semen evaluation for practitioners. Theriogenology. 2007;68:329–337. doi: 10.1016/j.theriogenology.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Petrunkina A.M., Gropper B., Gunzel-Apel A.R., Topfer-Petersen E. Functional significance of the cell volume for detecting sperm membrane changes and predicting freezability in dog semen. Reproduction. 2004;128:829–842. doi: 10.1530/rep.1.00296. [DOI] [PubMed] [Google Scholar]
- 35.Iranpour F.G., Nasr-Esfahani M.H., Valojerdi M.R., al-Taraihi T.M. Chromomycin A3 staining as a useful tool for evaluation of male fertility. J. Assist. Reprod. Genet. 2000;17:60–66. doi: 10.1023/A:1009406231811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holt W.V. Alternative strategies for the long-term preservation of spermatozoa. Reprod. Fertil. Dev. 1997;9:309–319. doi: 10.1071/R96082. [DOI] [PubMed] [Google Scholar]
- 37.Miguel-Jiménez S., Mogas T., Peña A., Tamargo C., Hidalgo C., Muiño R., Rodríguez-Gil J., Morató R. Post-thaw changes in sperm membrane and ROS following cryopreservation of dairy bull semen using four different commercial extenders; Proceedings of the Physiology of Reproduction in Male and Semen Technology (Abstracts A191E to A205E): 32nd Meeting of the European Embryo Transfer Association (AETE); Barcelona, Spain. 9–10 August 2016; pp. 9–10. [Google Scholar]
- 38.Alvarez J.G., Storey B.T. Taurine, hypotaurine, epinephrine and albumin inhibit lipid peroxidation in rabbit spermatozoa and protect against loss of motility. Biol. Reprod. 1983;29:548–555. doi: 10.1095/biolreprod29.3.548. [DOI] [PubMed] [Google Scholar]
- 39.Sang-Hyoun Park I.-J.Y. Effect of antioxidant supplementation in freezing extender on porcine sperm viability, motility and reactive oxygen species. J. Anim. Reprod. Biotechnol. 2017;32:9–15. [Google Scholar]
- 40.Park S.H., Jeon Y., Yu I.J. Effects of antioxidants supplement in porcine sperm freezing on in vitro fertilization and the glutathione and reactive oxygen species level of presumptive zygotes. J. Anim. Reprod. Biotechnol. 2017;32:337–342. doi: 10.12750/JET.2017.32.4.337. [DOI] [Google Scholar]
- 41.Aitken R.J., Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reprod. Camb. 2001;122:497–506. doi: 10.1530/rep.0.1220497. [DOI] [PubMed] [Google Scholar]
- 42.Ghallab A.M., Shahat A.M., Fadl A.M., Ayoub M.M., Moawad A.R. Impact of supplementation of semen extender with antioxidants on the quality of chilled or cryopreserved Arabian stallion spermatozoa. Cryobiology. 2017;79:14–20. doi: 10.1016/j.cryobiol.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Aitken R.J., Clarkson J.S. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. Reproduction. 1987;81:459–469. doi: 10.1530/jrf.0.0810459. [DOI] [PubMed] [Google Scholar]
- 44.Baumber J., Ball B.A., Linfor J.J. Assessment of the cryopreservation of equine spermatozoa in the presence of enzyme scavengers and antioxidants. Am. J. Vet. Res. 2005;66:772–779. doi: 10.2460/ajvr.2005.66.772. [DOI] [PubMed] [Google Scholar]
- 45.Palmieri M., Papale P., Della Ragione A., Quaranta G., Russo G., Russo S. In vitro antioxidant treatment of semen samples in assisted reproductive technology: Effects of myo-inositol on nemaspermic parameters. Int. J. Endocrinol. 2016;2016:2839041. doi: 10.1155/2016/2839041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boni R., Gallo A., Cecchini S. Kinetic activity, membrane mitochondrial potential, lipid peroxidation, intracellular pH and calcium of frozen/thawed bovine spermatozoa treated with metabolic enhancers. Andrology. 2017;5:133–145. doi: 10.1111/andr.12259. [DOI] [PubMed] [Google Scholar]
- 47.Bucak M.N., Tuncer P.B., Sariozkan S., Baspinar N., Taspinar M., Coyan K., Bilgili A., Akalin P.P., Buyukleblebici S., Aydos S., et al. Effects of antioxidants on post-thawed bovine sperm and oxidative stress parameters: Antioxidants protect DNA integrity against cryodamage. Cryobiology. 2010;61:248–253. doi: 10.1016/j.cryobiol.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Shin J.A., Chung J.S., Cho S.-H., Kim H.J., Do Yoo Y. Romo1 expression contributes to oxidative stress-induced death of lung epithelial cells. Biochem. Biophys. Res. Commun. 2013;439:315–320. doi: 10.1016/j.bbrc.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 49.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brewer L., Corzett M., Balhorn R. Condensation of DNA by spermatid basic nuclear proteins. J. Biol. Chem. 2002;277:38895–38900. doi: 10.1074/jbc.M204755200. [DOI] [PubMed] [Google Scholar]
- 51.Boissonneault G. Chromatin remodeling during spermiogenesis: A possible role for the transition proteins in DNA strand break repair. FEBS Lett. 2002;514:111–114. doi: 10.1016/S0014-5793(02)02380-3. [DOI] [PubMed] [Google Scholar]
- 52.Yanagimachi R. The Sperm Cell: Production, Maturation, Fertilization, Regeneration. Cambridge University Press; Cambridge, UK: 2017. [Google Scholar]
- 53.Schulte R.T., Ohl D.A., Sigman M., Smith G.D. Sperm DNA damage in male infertility: Etiologies, assays, and outcomes. J. Assist. Reprod. Genet. 2010;27:3–12. doi: 10.1007/s10815-009-9359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cox J.F., Zavala A., Saravia F., Rivas C., Gallardo P., Alfaro V.C. Differences in sperm migration through cervical mucus in vitro relates to sperm colonization of the oviduct and fertilizing ability in goats. Theriogenology. 2002;58:9–18. doi: 10.1016/S0093-691X(02)00919-6. [DOI] [PubMed] [Google Scholar]
- 55.Aitken R.J., Bowie H., Buckingham D., Harkiss D., Richardson D.W., West K.M. Sperm penetration into a hyaluronic acid polymer as a means of monitoring functional competence. J. Androl. 1992;13:44–54. [PubMed] [Google Scholar]
- 56.Johnston R.C., Mbizvo M.T., Summerbell D., Kovacs G.T., Baker H.W. Relationship between stimulated hyperactivated motility of human spermatozoa and pregnancy rate in donor insemination: A preliminary report. Hum. Reprod. 1994;9:1684–1687. doi: 10.1093/oxfordjournals.humrep.a138774. [DOI] [PubMed] [Google Scholar]