Abstract
The complement cascade is part of the innate immune system whose actions protect hosts from pathogens. Recent research shows complement involvement in a wide spectrum of renal disease pathogenesis including antibody-related glomerulopathies and non-antibody-mediated kidney diseases, such as C3 glomerular disease, atypical hemolytic uremic syndrome, and focal segmental glomerulosclerosis. A pivotal role in renal pathogenesis makes targeting complement activation an attractive therapeutic strategy. Over the last decade, a growing number of anti-complement agents have been developed; some are approved for clinical use and many others are in the pipeline. Herein, we review the pathways of complement activation and regulation, illustrate its role instigating or amplifying glomerular injury, and discuss the most promising novel complement-targeting therapies.
Keywords: complement, alternative complement pathway, complement-targeting therapies, C3 glomerulopathy, hemolytic uremic syndrome, focal segmental glomerulosclerosis
1. Complement Cascade
The complement system consists of soluble or membrane-bound molecules, mostly zymogens, activated through a tightly regulated proteolytic cascade [1,2]. Current understanding of complement immune mechanisms include (1) functioning as opsonins; (2) producing chemoattractants to recruit immune cells thereby enhancing site specific angiogenesis, vasodilation and coagulation cascade regulator; and (3) functioning as an enhancing bridge to adaptive T and B lymphocyte responses [3]. Current research suggests that abnormal complement activation plays a role in autoimmune inflammatory diseases and particularly in those targeting the kidney.
2. Complement Cascade Activation and Regulation
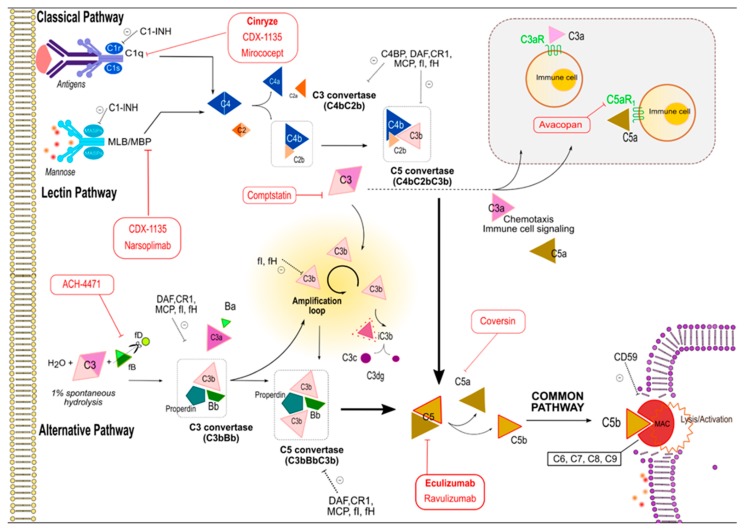
Complement activation proceeds via three pathways: the classical, alternative, and mannitol-binding lectin (MBL). The pathways have both unique and overlapping proteins, activated by a proteolytic cascade, that respond to pathogenic insults [3,4] (Figure 1). The classical and MBL pathways are initiated by antibodies and bacterial mannose motifs binding to C1q and mannose-associated serine proteases (MASPs), respectively [5,6]. Conversely, spontaneous hydrolysis of C3 on cell surfaces produces constitutive alternative pathway (AP) activation [7], and is tightly controlled by a number of regulators [8]. Decay accelerating factor (DAF) and membrane cofactor protein (MCP) (CD46, murine homolog Crry) are cell surface-expressed complement regulators that accelerate the decay of surface-assembled C3 convertases, thereby limiting amplification of the downstream cascade. DAF restrains convertase-mediated C3 cleavage; MCP and factor H (fH) also have a cofactor activity: together with soluble factor I (fI), they irreversibly cleave C3b into iC3b, thereby preventing reformation of the C3 convertase.
Figure 1.
Overview of the complement cascade and principal complement targeting molecules. Three pathways can initiate complement cascade: (1) The classical, (2) the mannose-binding lectin (MBL), and (3) the alternative pathway. They all converge on C3 convertases formation which continuously cleave C3; after they are activated, the C3 convertase from alternative pathway dominates within an amplification loop that sustains the production of C3b (circular arrow). The three C3 convertases associate with an additional C3b to form the C5 convertases, which cleave C5 into C5a + C5b. C5b fragments recruits C6, C7, C8, and multiple C9 molecules to generate the terminal membrane attack complex (MAC); MAC inserts pores into cell membranes to induce cell lysis or activation. Anaphilotoxins C3a and C5a through their G protein-coupled receptors C3aR and C5aR, respectively, can promote signaling, inflammation, chemotaxis of leukocytes, vasodilation, cytokine and chemokine release, and activation of adaptive immunity. Dotted black arrows: inhibitor function of a complement effector on its target. Red arrows emerging from balloons: inhibitor function of anti complement drug on its target (bold drugs’ names are the FDA approved ones). Full arrow: consequential interaction between complement fractions leads to the subsequent cascade step. Full bold arrow: final convergence of the three complement pathways on same final target. MBL: Mannose binding lectin; MASP: Mannose-binding lectin-associated serine protease; C4BP: C4 binding protein; C1-INH: C1 inhibitor; DAF: Decay accelerating factor; CR1: Surface complement receptor 1; MCP: Membrane cofactor protein; CD59: Protectin; fD: Factor D; fB: Factor B; fI: Factor I; fH: Factor H. Red balloons highlights complement target drugs’ points of action (see Table 1).
The three activation pathways converge in C3 convertases that continuously cleave C3 into C3a and C3b. C3a signals on cell surface G protein-coupled receptor (GPCR) C3aR, while C3b forms additional alternative pathways C3 convertases (even if initiated via the other pathways), as well as C5 convertases. C5 convertases produce the split products C5a and C5b. While C5a functions similarly to C3a (but signals via C5aR) C5b, in conjunction with C6–C9, forms the membrane attack complex (MAC) leading to cell lysis/activation [9]. Countering distal complement MAC formation is CD59, a circulating complement inhibitor.
3. Effector Functions
Complement proteins promote inflammation and immune cell activity in multiple ways. C3a and C5a ligate their transmembrane-spanning receptors, C3aR and C5aR, on immune cells, leading to the production of proinflammatory cytokines and chemokines and promoting vasodilation. They also mediate neutrophil and macrophage chemoattraction, activate macrophages to promote intracellular killing of engulfed organisms, and contribute to T-cell and antigen-presenting cell (APC) activation, expansion, and survival [10,11,12,13]. The more distal complement proteins (C5b-9) form MAC complexes on cell membranes, promoting lysis of non-nucleated cells, such as red blood cells, or pathogens lacking cell surface complement inhibitors, such as bacteria. MAC insertion into nucleated eukaryotic cells generally does not result in lysis, but rather induces immune activation [14] and/or promotes tissue injury [15]. C3b and other bound cleavage products function as opsonins binding to specific surface-expressed receptors (complement receptors CR1, CR2, CR3, and CR4).
4. Complement in Glomerular Diseases
4.1. Diseases with Antibody-Mediated Complement Activation
The majority of circulating, or fluid-phase, complement components are produced by the liver. Liver produced complement components are involved in auto-antibody initiated glomerulonephritis (GN) through the classical and/or MBL pathways. Inadequate regulation of alternative pathway activity, due to inherited and/or acquired abnormalities of complement regulators, can result in glomerular injury from persistent C3 convertase activity with consequent excessive MAC activity. Complement can also be produced by parenchymal (e.g., tubular cells in the kidney [16] and resident/infiltrating immune cells, (e.g., T cells and APCs) [17,18,19]. The relative contributions of systemic or locally produced complement in GN pathogenesis remains unclear.
4.2. IgA Nephropathy
IgA nephropathy (IgAN) is the most common form of GN worldwide [20,21]; its clinical presentation varies, but it often includes proteinuria and hematuria. The disease is associated with aberrant O-glycosylation of mucosal IgA1 with galactose-deficient IgA1 (Gd-IgA1) which plays a pivotal role in the progression of IgAN [22]. They deposit in glomeruli and, subsequently, development of circulating/in situ immunoglobulins (IgA or IgG) targeting glycosylated IgA takes place [23,24]. Mesangial IgA and immune complexes deposits are often observed and initiate glomerular injury.
In vitro and in vivo studies showed that polymeric mucosal IgA activates the complement system through the alternative or MBL pathway [25], and glomerular MBL correlates with greater disease severity and a worse prognosis [26]. Although C3 levels in plasma are usually normal, glomerular C3 deposits can be detected in approximately 85% of biopsies, with C5b-9 also often present along with C3 during infections [27]. MAC generated from complement activation attack on mesangial cells inducing them to proliferate and over-produce oxidants, proteases, cytokines, growth factors (e.g., transforming growth factor β and platelet-derived growth factor) and extracellular matrix material that together result in the typical focal proliferative GN with mesangial matrix expansion characteristic of IgA nephropathy [28].
A genomic wide association study (GWAS) of IgAN in a cohort of 3144 cases of Chinese and European ancestry, linked allele deletion polymorphisms of complement factor H-related proteins one and three (CFHR1 and CFHR3) with less severe IgA nephropathy [29,30]. Since CFHR proteins interfere with factor H complement regulation (Figure 1) their deficiency might reduce complement activation and lessen IgAN. CFHR1, CFHR3, and CFHR5 especially have been studied for their role in IgAN [29,31]; another study of 1126 Chinese patients concluded that circulating CFHR5 levels are an independent risk factor for the disease: levels were higher in IgAN subjects compared to heathy controls, and correlated directly with worst Oxford MEST pathology score, a lower glomerular filtration rate (GFR), and hypertension [32].
4.3. Membranous Nephropathy
Membranous nephropathy (MN) is the second most commonly diagnosed GN, and the most common cause for nephrotic syndrome in adults (20–50%) [33]. Disease progression varies greatly with ~1/3 of patients undergoing spontaneous remission, ~1/3 developing progressive renal insufficiency, and ~1/3 maintaining normal GFR despite persistent proteinuria. MN is characterized by presence of anti-podocyte antibodies in the subepithelial space of glomerular capillary loops and granular deposits of IgG4 and C3. M-type phospholipase A2 receptor (PLA2R) has been found as the main target podocyte antigen for autoantibodies in 70–80% of patients with primary MN, while antibodies against thrombospondin type 1 domain-containing 7A (THSD7A) are detected in a minority of patients [34]. Although antibodies from IgG4 subclass are poor classical complement pathway activators, deposition of C3 and breakdown of C4b products are detectable in almost all patients with a primary form of MN [27]. Mannose binding lectin and MBL-associated serine protease expression (MASP-1, MASP-2) are detected in PLA2R positive patients’ glomeruli, suggesting that complement activation proceeds through this pathway [35]. Hypogalactosylated IgG (including IgG4) binds MBL and activates the complement providing a possible explanation to the IgG4 conundrum [36]. Animal studies also indicate a role for MAC insertion into podocytes; blocking their formation prevents disease [37]. Sublytic activation alters podocyte cytoskeletal structure crucial for slit diaphragm integrity and function, leading to proteinuria [38,39].
4.4. Post Infectious Glomerulonephritis
Post infectious GN is a common cause of nephritic syndrome that develops after self-limited bacterial infections (most commonly from streptococcal or staphylococcal species). It occurs mainly in childhood, but can also be seen in adults. It is characterized by hypercellularity within the capillary loops (caused by neutrophils infiltration and endothelial proliferation) and strong C3 staining, usually in addition to IgG. Post infectious GN occurs due to passive glomerular trapping of circulating immune complexes composed of nephritogenic bacterial antigens and IgG, complement activation, and attraction of neutrophils responsible for glomerular injury [28]. However, levels of C1q and C4 deposition are lacking or low in most of the cases [40,41], suggesting contributions from lectin and alternative pathway. This is eventually triggered from specific pathogens’ components; for example, streptococcal pyrogenic exotoxin B is a possible alternative pathway activation [42]. Autoantibodies with C3 nephritic factor (C3nef), activity that binds to and stabilizes C3 convertases, has also been reported in post-infectious GN and may be associated with an enhanced cleavage of C3 [28]. In some patients underlying genetic defects in the regulation of the alternative pathway, including mutations in complement regulators (fH or CFHR5) and presence of C3Nef, lead to persistent glomerular deposition of complement factors within the glomeruli and inflammatory infiltrates that resemble features of a persistent proliferative glomerulonephritis [43]. Interestingly, in few cases, post infectious GN evolved into C3 glomerulopathy (C3G) [44]: recent reports document repeat biopsies demonstrating transformation of post infectious GN to C3G, including identical appearing early lesions of C3G and initiation of C3G by streptococcal infection. Sethi et al. [43] described that most of the cases with biopsy-proven persistent post-infectious GN had underlying genetic mutations and/or auto-antibodies affecting regulation of the alternative complement pathway. These findings indicate that glomerular injuries initiated by infection may transfer to C3G by imbalanced alternative complement pathway activation: C3G is initiated by heterogeneous insults, leading to a final common pathway of alternative complement dysregulation.
4.5. Immune Complex-Mediated Membranoproliferative Glomerulonephritis (MPGN)
Membranoproliferative glomerulonephritis (MPGN) is a histopathological pattern of glomerular injury characterized by mesangial hypercellularity, capillary wall changes (i.e., “tram-tracking”), and endocapillary proliferation found in 7–10% of biopsy-diagnosed glomerulonephritis [45]. MPGN classification was based on electron micrograph ultrastructural findings but advances in our understanding of underlying pathomechanisms produced a rethinking of MPGN and a classification schema based on immunofluorescence findings; MPGN is caused by immune complex deposition, C3 dysregulation, or thrombotic microangiopathy (TMA) [45]. Immune complex-mediated MPGN is caused by immune complex deposition in the subendothelial space activating complement classical pathways and causing glomerular injury. When not linked to a systemic disease, it is termed ‘idiopathic’ but secondary forms more commonly occur in association with infections (e.g., hepatitis B, C, or tuberculosis), autoimmune diseases (e.g., Sjogren’s Syndrome or systemic lupus erythematosus SLE), or monoclonal gammopathy. Clinical evidence of classical complement activation in immune complex-mediated MPGN includes preferential consumption of plasma C4 (although C3 is often low as well) and detection of C1q and terminal C5b-9 complex in glomeruli. This phase is followed by an influx of leukocytes, promoted by formation of the C3a and C5a anaphylatoxins, leading to capillary damage and proteinuria [46]. Activation of classical pathway through immunoglobulins is the most prominent pathogenic process, but heterozygous mutations in alternative pathway complement regulators and the presence of circulating C3nef factor are also identified in some patients with immune complex-mediated MPGN, suggesting additional contributions from the alternative pathway [47]. These findings raise the possibility that in individuals with genetic or acquired complement alternative pathway dysregulation, immune complex deposition initially triggers injury through the classical pathway but chronic kidney injury is sustained through the enhanced alternative pathway [46]. The complement also features prominently in the two other dominant etiologies of MPGN: C3 glomerulopathies and TMA from atypical Hemolytic Uremic Syndrome (aHUS), and these are discussed in detail later in this paper.
4.6. Anti-GBM Glomerulonephritis
Anti-glomerular basement membrane (GBM) is a rare life-threatening autoimmune disease, caused by IgG autoantibodies against alpha 3 NC1 domain of collagen IV of the GBM. Antibody binding to the GBM leads to injury characterized by strong complement activation, leukocyte infiltration, and proteinuria; leading to crescent formation, scarring, and, frequently, end-stage renal disease (ESRD). Evidence of complement pathogenic role comes from detection of complement components MBL, C1q, factor B (fB), properdin, C3d/C4d, and C5b-9 in GBM, and circulating MAC levels that correlate with kidney injury severity [48]. Local complement activation produces C3a- and C5a-mediated inflammation, as well as MAC-dependent sublytic activation of glomerular cells, which together enhance inflammation and extracellular matrix formation [49]. Pathways of complement activation in anti-GBM disease have been studied in murine models by injection of heterologous antibodies against GBM, where C3 and C4 deficiency prevented full manifestation of renal disease [46,50,51]. This evidence supports involvement of, at least, both classical and alternative pathways in anti-GBM disease.
4.7. ANCA Induced Renal Vasculitis
Antineutrophil cytoplasmic antibody (ANCA) associated vasculitides commonly target the kidney, with abundant complement component deposition in vessels and glomeruli without immunoglobulin (pauci-immune). Current research supports that vascular injury is due to cytokine-primed neutrophils displaying surface ANCA-binding antigens (myeloperoxidase and proteinase-3) that undergo degranulation while simultaneously activating alternative complement pathways which potentiates neutrophil recruitment via C5a [8]. C5a generation functions as an amplification loop for ANCA-mediated neutrophil activation, eventually culminating in the severe necrotizing inflammation of the vessel walls. Studies in animal models have also shown complement contributes to pathogenesis, and agents blocking C5 cleavage/C5a signaling or C5 and fB deficiency themselves are protective; conversely, preventing MAC formation is ineffective, supporting the importance of C5a in ANCA-mediated pathogenesis [52,53]. A recent trial of patients with ANCA vasculitis compared the use of rituximab/cyclophosphamide in addition to either placebo and high-dose steroids, avacopan (C5aR antagonist) plus reduced dose steroids, or avacopan and no steroids. Regimens with low or absent steroids were non-inferior to traditional regimens; this illustrates complement’s essential role in ANCA vasculitis and suggests C5aR antagonism as a feasible alternative in patients where steroids are contraindicated [54].
4.8. Lupus Nephritis
Systemic lupus erythematosus (SLE) is an autoimmune disorder characterized by antibodies to self-antigens (e.g., anti-nuclear) leading to the formation of immune complexes that deposit in target organs. Lupus nephritis (LN) is one severe complication of SLE. Up to 50% of patients with SLE have clinically evident kidney disease at presentation, and up to 75% develop it during the course of the disease [55]. The hallmark of renal pathology is simultaneous glomerular deposition of IgG, IgM, IgA, C4, and C3, referring to the poker hand ranking, the “full house pattern” [55]. Complement deposition is not merely a biomarker of LN, as it mediates direct glomerular injury: Immune complex-mediated classical pathway plays a key pathogenic role through both intra capillary generation of neutrophil and macrophage chemotactic factors (class II–IV) and formation of MAC (class V) [56]. Circulating C3 and C4 levels are reduced in more than 90% of patients with diffuse proliferative LN and their decline often reflects a worsening in disease activity [57]. Extensive data from animal models also indicate a significant role for alternative pathway activation: Deletion of regulators (i.e., fH) or activators (i.e., fB and fD) worsen or ameliorate, respectively, experimental LN [58,59,60]. In humans, plasma Bb levels (but not C3) are associated with LN outcome and strongly correlated with MAC levels. In addition, Bb co-localized with MAC in the glomeruli with LN, overall supporting the concept that activation of MAC in LN reflects alternative pathway activation [57]. Experimental LN can be prevented by blockade of all complement pathways through the administration of CR2-Crry fusion protein [61]. Data also show that the disease severity can be ameliorated by C5aR blockade [62] or anti-C5 mAb [63], which suggests the potential clinical relevance of complement pathway intervention. A phase 1 human trial with eculizumab (anti-C5) suggested preliminary efficacy, but the treatment period was too short to draw definitive conclusions [64]. The complement system seems to have a paradoxical role in SLE: genetically determined complement deficiencies or development of anti-complement antibodies involving components of the classical pathway (anti-C1q or C1-INH) [65], are strong risk factors. Susceptibility is likely due to a defective clearance of nuclear antigens released by injured and apoptotic cells since experimental studies have shown that such deficiencies lead to autoantibody production and glomerular injury [66].
5. Disease with Complement Activation in the Absence of Detectable Serum Antibodies
5.1. Atypical Hemolytic Uremic Syndrome
Hemolytic uremic syndrome (HUS) is defined by the triad of mechanical hemolytic anemia, thrombocytopenia and acute kidney injury. Renal pathology shows typically diffuse fibrin thrombi, endothelial swelling and capillary lumens narrowed/collapsed (acute features), or reduplication of GMB, mesangiolysis, and vessels recanalization (chronic phase). Typical forms of HUS are related to infection by Shiga toxin (Stx) producing Escherichia coli (STEC), while aHUS) is a condition due to defects in alternative complement activation. It has been associated with a predisposing genotype, usually an inherited heterozygous mutation [67,68], rather than an acquired mutation or loss of complement proteins (e.g., fI, fH, MCP).
The first identified mutant gene encodes for fH [28], the most important alternative pathway regulator in plasma and on cell surfaces. Subsequently, over 100 mutations were identified and most commonly lead to normal levels of a protein that is unable to bind and regulate complement components on endothelial cells [69].
Most complement genes mutations associated with aHUS result in an altered cell surface regulation: MCP mutation and fI mutation prevent effective degradation of C3 convertase. Although rarer, factors C3/C3b or fB gain of function mutations have been described [28]. Formation of blocking antibodies direct against fH is also another possible pathogenic mechanism [46,68]. 3–5% of patients with aHUS also carry heterozygous mutation of thrombomodulin (THBD), a molecule that normally enhances fI function [70]. As discussed below, complement targeting therapies have been extremely effective in treating this condition.
5.2. C3 Nephropathy
C3 nephropathy (C3N) is a rare nephritic disease, with a poor long-term outcome. The membranoproliferative pattern is the most common (not unique) histological presentation of C3 nephropathy and is further divided in the two entities of: Dense deposit disease (DDD), with typical ultrastructural evidence of intramembranous highly electron-dense osmophilic deposits with or without IgG and C3 on immunofluorescence, and C3 glomerulonephritis (C3GN) diagnosed with C3 positive on IF while all others immunoglobulins are negative [71,72]. Low serum C3 and glomerular deposits of C3 are emblematic of alternative pathway dysregulation.
A major subset of C3 glomerulopathies arises from C3Nef autoantibodies (present in 40% of C3GN and 80% of DDD) stabilizing C3 convertases against complement regulatory proteins (CRegs), or from other antibodies (former anti-fB, anti-fH) targeting directly CRegs [47]. C3 glomerulopathy can be due to genetic missense or non-sense mutations, affecting genes that encode for complement components or regulators [46,73]. The most important seems to involve fH, fI, and CFHR proteins with loss of function [74]; C3 mutation with gain of function and resistance to fH is uncommon [75] and when C5 genes are affected a less severe form of GN occurs [76]. fH/fI deficiency/resistance play a critical role in developing the disease because the GBM does not express CRegs and therefore relies on circulating ones (i.e., fH/fI) to prevent excessive local fluid phase AP activation.
Several cases of familiar C3 glomerulopathy have also been described when mutation of CFHR gene cluster occurs; recently an autosomal dominant inheritance among some Cypriote families has been described. In this nephropathy, CFHR5 has reduced affinity for surface-bound complement [77], the glomeruli present with C3GN features but C3 levels in the serum tend to be normal suggesting that improper complement activation occurs in the glomerulus rather than plasma [46,78,79,80,81].
6. Other Glomerular Diseases
Focal Segmental Glomerulosclerosis
Focal segmental glomerulosclerosis (FSGS), is one of the leading causes of nephrotic syndrome in adults. Patients with non-nephrotic proteinuria have good prognosis and about 15% progress to ESRD over the course of 10 years, whereas 50% of patients with nephrotic-range proteinuria progress to ESRD over 5–10 years [82]. In patients with massive proteinuria (10–14 g/d), the course is malignant, resulting in ESRD by 2–3 years on average [82]. It is characterized by focal and segmental obliteration of glomerular capillary tufts, with an increase in matrix. The sources of podocyte damage are varied and not altogether known, but include circulating factors, genetic abnormalities, viral infection, and medications that produce common deleterious effects on podocytes. C3 and IgM glomerular deposition is typical, suggesting complement activation contributes to FSGS pathogenesis [83]. Sclerotic lesions are significantly higher in patients with C3 deposition combined with IgM; they have worse renal dysfunction and limited response to therapy [84]. Murine models of FSGS clarified that glomerular IgM deposits activate complement, suggesting that glomerular injury simultaneously increases classical pathway activation by natural IgM, which binds to injury associated epitopes, while also decreasing glomerular alternative complement regulating abilities [85]. Consistent with a pathogenic role for IgM, B cell absence in murine FSGS models prevents IgM deposition and albuminuria [85]. In humans, mutations in fH and C3 have been described in literature cases of biopsy documented FSGS [86] and FSGS patient urine and plasma are enriched with complement fragments C3a, C3b, Ba, Bb, C4a, sC5b-9 compared to samples from patients with other renal diseases [87].
7. Complement Inhibitory Drugs in Kidney Diseases
Identification of complement component contributions to renal pathogenesis recently spurred pharmaceutical industry efforts to therapeutically target complement
Many available agents target terminal complement molecules and more proximal roles, such as opsonization, remains preserved. Even so, since these agents are immunosuppressants, they convey increased infection risk. More importantly, relevant to kidney diseases, many upstream elements (e.g., C3a, C5a, and C3b) contribute to pathogenesis and are not effectively targeted by available compounds. Currently, Food and Drug Administration (FDA) approved complement inhibitors include the monoclonal anti-C5 antibody Eculizumab, and Cynrize an inhibitor of fragment C1 (C1-INH) [88,89,90].
7.1. Eculizumab
Eculizumab is a humanized murine monoclonal antibody to complement C5 that acts on the terminal complement cascade preventing the formation of C5a, C5b, and C5b-9. The drug use has been approved by European Medicines Agency’s (EMA) and FDA for treatment of paroxysmal nocturnal hemoglobinuria (PNH) and aHUS [91]. The major side effect is predisposition to infections, especially from gram-negative bacteria; as such, all patients are advised to be immunized for meningococcus before receiving eculizumab. Eculizumab approval for aHUS treatment was given based on results from two prospective trials: one involving 17 aHUS patients with thrombocytopenia and the other with 20 aHUS patients requiring persistent plasma exchange (PE) [92]. Whole patients from these cohorts no longer required PE and 88% reached normal hematological values after median of 63 weeks of Eculizumab treatment. This medication has dramatically improved renal morbidity, with consistent decreases in ESRD risk, but its clinical use is still limited by uncertainty over patient selection, timing, and duration of treatment. An ongoing multi center single-arm trial is now testing the safety Eculizumab discontinuation in patients with aHUS (NCT02574403). Eculizumab has also been successfully used to prevent or treat recurrence of aHUS after kidney transplant [93,94,95], but appears ineffective in preventing delayed graft function (NCT01919346) [96] in sensitized kidney transplant recipients (NCT00670774, NCT01095887, NCT01327573) [97,98].
Eculizumab has successfully treated patients with DDD and C3GN highlighting its potential with these rare diseases. Treatment of six patients (three C3GN and three DDD) resulted in complete to partial remission in four patients at one year of follow-up [99]. This positive effect was limited to patients with crescentic rapidly progressive C3 glomerulopathy as opposed to a more insidious C3GN suggesting that it is specific to disease pathogenesis. Moreover, advantages are not seen so far in C3GN recurrence after kidney transplantation [100]. Eculizumab use in patients with glomerular diseases other than aHUS or C3GN/DDD is limited to case reports and of uncertain efficacy. The price of eculizumab is a factor that limits its use. Competitors have in clinical development both similar agents targeting C5 (e.g., Ravulizumab), as well as agents that affect complement component C5a (i.e., Avacoban).
7.2. C1 Inhibitor
Despite the potential advantages of terminal complement inhibition, these approaches may not be sufficient in conditions stemming from more proximal complement activation. Classical complement pathway activation occurs when C1q binds the Fc portion of antigen bound immunoglobulin. Preventing this process is C1-esterase inhibitor and its absence or mutated function produces the condition hereditary angioedema. Cinryze, a human serum derived C1 inhibitor (C1-INH) is FDA approved for treatment of hereditary angioedema. C1-INH does not have approved indications for kidney disease, but a phase I/II study was conducted on highly sensitized renal transplant recipients randomized to C1INH or placebo. Antibody mediated rejection was prevented in all 10 patients receiving C1INH group and 9/10 only receiving placebo. Efficacy and safety of C1INH for treatment of acute antibody mediated rejection in kidney transplantation is being evaluated in a randomized double-blind study of donor-sensitized kidney transplants recipients (NCT02052141, NCT02547220) [101]. Results may broaden its use to patients with antibody mediated rejection.
8. Conclusions
The complement system is a complex network of proteins that augment immune system function and, in many cases, contribute to kidney disease pathogenesis. Increasing research suggests that selective interventions to stop cascade activation can halt or even reverse renal disease. Ongoing research, both translational and in animal models, will help delineate which pathway(s), and at what level, intervention could be effective. Although infrequently a primary insult, common mutations affecting complement regulation synergize with other pathological features perpetuating inflammation and, ultimately, nephron loss. The advent of selective complement-targeting therapeutics offers the opportunity for new treatment strategies for renal disease, an area in desperate need of new options.
Table 1.
Summary of complement blocking agents.
| Name. | Class | Pharmacodinamics | Disease | Status | Additional Info |
|---|---|---|---|---|---|
| Eculizumab | Humanized monoclonal antibody | Binds C5 preventing MAC generation | aHUS, DDD, C3GN | Available for use in PHN and aHUS | First USA FDA-approved among anti-complement drugs |
|
Ravulizumab
ALXN1210 |
Humanized monoclonal antibody | Binds C5 preventing MAC generation | aHUS | Phase III for PHN | Induces prolonged decease of C5 plasmatic levels allowing longer dosing intervals compared to Eculizumab |
| Coversin | Small dimension recombinant protein | Prevents cleavage of C5 into C5a/C5b by C5 convertase | aHUS | Phase II for PHN | Valid alternative for patients bearer of C5 molecule polymorphisms which interferes with correct binding of Eculizumab |
|
Avacopan
CCX168 |
Small dimension anti-inflammatory molecule | Inhibits selectively C5aR | aHUS ANCA-vasculitides |
Phanse III for ANCA vasculitides. Phase II for aHUS | Effective replacing high-dose glucocorticoids in treating vasculitis |
| CDX-1135 | C1R-based molecule | Inhibits CR1 | DDD | Phase I for DDD | |
|
Mirococept
APT070 |
CR1-based molecule | Inhibits CR1 | IRI in Tx | Phase I for DDD, C3GN | |
| Cinryze | C1 estarase | Inhibits CR1 | Antibody-mediated rejection in renal transplant | Available for use in HAE. Phase III for prevention of DGF in cadaveric allograft | FDA approved for hereditary angioedema |
|
Narsoplimab
OMS721 |
Humanized monoclonal antibody | Binds the mannan-binding lectin-associated serinprotease-2 | aHUS, TTP IgAN |
Phase II for aHUS, IgA, LES, MN, C3G | Multi-dose administration is needed |
| ACH-4471 | Small dimension molecule |
Inhibits factor D | aHUS | Phase II for IC-MPGN, DDD, C3GN | Oral assumption with delivery advantage over intravenously infused agents |
aHUS: Atypical hemolytic uremic syndrome, DDD: Dense deposit disease, C3GN: C3 glomerular disease, MAC: Membrane attack complex, ANCA: Antineutrophilic cystoplasmic antibody, IRI: Ischemia riper fusion injury, TTP: Thrombotic thrombocytopenia, IgAN: IgA Nephropathy, HAE: Hereditary angioedema, DGF: Delayed graft function.
Author Contributions
S.A. and J.L. searched the literature and wrote the manuscript. G.Z. and P.C. contributed to the literature search and literature analysis. P.C. revised the manuscript. All authors read and approved the final manuscript.
Funding
Paolo Cravedi is funded by the NIH NIIDDK grant R01DK119431-01.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Walport M.J. Complement−First of Two Parts. N. Engl. J. Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 2.Walport M.J. Complement: Second of two parts: Complement at the interface between innate and adaptive immunity. N. Engl. J. Med. 2001;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 3.Mizuno M., Suzuki Y., Ito Y. Complement regulation and kidney diseases: Recent knowledge of the double-edged roles of complement activation in nephrology. Clin. Exp. Nephrol. 2018;22:3–14. doi: 10.1007/s10157-017-1405-x. [DOI] [PubMed] [Google Scholar]
- 4.Hourcade D.E., Spitzer D., Mitchell L.M., Atkinson J.P. Properdin Can Initiate Complement Activation by Binding Specific Target Surfaces and Providing a Platform for De Novo. J. Immunol. Ref. 2007;179:2600–2608. doi: 10.4049/jimmunol.179.4.2600. [DOI] [PubMed] [Google Scholar]
- 5.Holmskov U., Thiel S., Jensenius J.C. Collectin and Ficolins: Humoral Lectins of the Innate Immune Defense. Annu. Rev. Immunol. 2003;21:547–578. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 6.Endo Y., Matsushita M., Fujita T. The role of ficolins in the lectin pathway of innate immunity. Int. J. Biochem. Cell Biol. 2011;43:705–712. doi: 10.1016/j.biocel.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Forneris F., Ricklin D., Wu J., Tzekou A., Wallace R.S., Lambris J.D., Gros P. Structures of C3b in Complex with Factors B and D Give Insight into Complement Convertase Formation. Science. 2010;330:1816–1820. doi: 10.1126/science.1195821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathern D.R., Heeger P.S. Molecules Great and Small: The Complement System. Clin. J. Am. Soc. Nephrol. 2015;10:1636–1650. doi: 10.2215/CJN.06230614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement-a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo R.-F., Ward P.A. Role of C5a in inflamatory response. Annu. Rev. Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 11.Kwan W.H., van der Touw W., Paz-Artal E., Li M.O., Heeger P.S. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J. Exp. Med. 2013;210:257–268. doi: 10.1084/jem.20121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Der Touw W., Cravedi P., Kwan W.-H., Paz-Artal E., Merad M., Heeger P.S. Receptors for C3a and C5a modulate stability of alloantigen-reactive induced regulatory T cells. J. Immunol. 2013;190:5921–5925. doi: 10.4049/jimmunol.1300847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klos A., Tenner A.J., Johswich K.-O., Ager R.R., Reis E.S., Köhl J. The Role of the Anaphylatoxins in Health and Disease. Mol. Immunol. 2009;46:2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jane-wit D., Manes T.D., Yi T., Qin L., Clark P., Kirkiles-Smith N.C., Abrahimi P., Devalliere J., Moeckel G., Kulkarni S., et al. Alloantibody and Complement Promote T Cell-Mediated Cardiac Allograft Vasculopathy through Non-Canonical NF-κB Signaling in Endothelial Cells. Circulation. 2013;128:2504–2516. doi: 10.1161/CIRCULATIONAHA.113.002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler S., Baker P.J., Johnson R.J., Ochi R.F., Pritzl P., Couser W.G. Complement Membrane Attack Complex. Stimulates Production of Reactive Oxygen Metabolites by Cultured Rat Mesangial Cells. J. Clin. Investig. 1996;77:762–767. doi: 10.1172/JCI112372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peake P.W., O’Grady S., Pussell B.A., Charlesworth J.A. C3a is made by proximal tubular HK-2 cells and activates them via the C3a receptor. Kidney Int. 1999;56:1729–1736. doi: 10.1046/j.1523-1755.1999.00722.x. [DOI] [PubMed] [Google Scholar]
- 17.Lalli P.N., Strainic M.G., Yang M., Lin F., Medof M.E., Heeger P.S. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;11:1759–1766. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strainic M.G., Liu J., Huang D., An F., Lalli P.N., Muqim N., Shapiro V.S., Dubyak G.R., Heeger P.S., Medof M.E. Locally Produced Complement Fragments C5a and C3a Provide Both Costimulatory and Survival Signals to Naive CD4 + T. Cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heeger P.S., Lalli P.N., Lin F., Valujskikh A., Liu J., Muqim N., Xu Y., Medof M.E. Decay-accelerating factor modulates induction of T cell immunity. J. Exp. Med. 2005;201:1523–1530. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanko J.B., Mullan R.N., O’rourke D.M., Mcnamee P.T., Maxwell A.P., Courtney A.E. The changing pattern of adult primary glomerular disease. Nephrol. Dial. Transpl. 2009;24:3050–3054. doi: 10.1093/ndt/gfp254. [DOI] [PubMed] [Google Scholar]
- 21.Nair R., Walker P.D. Is IgA nephropathy the commonest primary glomerulopathy among young adults in the USA? Kidney Int. 2006;69:1455–1458. doi: 10.1038/sj.ki.5000292. [DOI] [PubMed] [Google Scholar]
- 22.Wada Id Y., Matsumoto K., Suzuki T., Saito T., Kanazawa N., Tachibanaid S., Iseri K., Sugiyama M., Iyoda M., Shibata T. Clinical significance of serum and mesangial galactose-deficient IgA1 in patients with IgA nephropathy. PLoS ONE. 2018;13:e0206865. doi: 10.1371/journal.pone.0206865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mestecky J., Raska M., Julian B.A., Gharavi A.G., Renfrow M.B., Moldoveanu Z., Novak L., Matousovic K., Novak J. IgA Nephropathy: Molecular Mechanisms of the Disease. Annu. Rev. Pathol: Mech. Dis. 2012;8:217–240. doi: 10.1146/annurev-pathol-011110-130216. [DOI] [PubMed] [Google Scholar]
- 24.Lafayette R.A., Kelepouris E., Lafayette R. Immunoglobulin A Nephropathy: Advances in Understanding of Pathogenesis and Treatment. Rev. Artic. Am. J. Nephrol. 2018;47:43–52. doi: 10.1159/000481636. [DOI] [PubMed] [Google Scholar]
- 25.Oortwijn B.D., Eijgenraam J.W., Rastaldi M.P., Roos A., Daha M.R., van Kooten C. The Role of Secretory IgA and Complement in IgA Nephropathy. Semin. Nephrol. 2008;28:58–65. doi: 10.1016/j.semnephrol.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Roos A., Rastaldi M.P., Calvaresi N., Oortwijn B.D., Schlagwein N., Van Gijlswijk-Janssen D.J., Stahl G.L., Matsushita M., Fujita T., Van Kooten C., et al. Glomerular Activation of the Lectin Pathway of Complement in IgA Nephropathy Is Associated with More Severe Renal Disease. J. Am. Soc. Nephrol. 2006;17:1724–1734. doi: 10.1681/ASN.2005090923. [DOI] [PubMed] [Google Scholar]
- 27.Thurman J.M. Complement in Kidney Disease: Core Curriculum. Am. J. Kidney Dis. 2015;65:156–168. doi: 10.1053/j.ajkd.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couser W.G. Pathogenesis and treatment of glomerulonephritis-an update. J. Bras. Nefrol. 2016;38:107–122. doi: 10.5935/0101-2800.20160016. [DOI] [PubMed] [Google Scholar]
- 29.Gharavi A.G., Kiryluk K., Choi M., Li Y., Hou P., Xie J., Sanna-Cherchi S., Men C.J., Julian B.A., Wyatt R.J., et al. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat. Genet. 2011;43:321–329. doi: 10.1038/ng.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie J., Kiryluk K., Li Y., Mladkova N., Zhu L., Hou P., Ren H., Wang W., Zhang H., Chen N., et al. Fine Mapping Implicates a Deletion of CFHR1 and CFHR3 in Protection from IgA Nephropathy in Han Chinese. J. Am. Soc. Nephrol. 2016;27:3187–3194. doi: 10.1681/ASN.2015111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medjeral-Thomas N.R., Lomax-Browne H.J., Beckwith H., Willicombe M., McLean A.G., Brookes P., Pusey C.D., Falchi M., Cook H.T., Pickering M.C. Circulating complement factor H–related proteins 1 and 5 correlate with disease activity in IgA nephropathy. Kidney Int. 2017;92:942–952. doi: 10.1016/j.kint.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu L., Guo W.Y., Shi S.F., Liu L.J., Lv J.C., Medjeral-Thomas N.R., Lomax-Browne H.J., Pickering M.C., Zhang H. Circulating complement factor H–related protein 5 levels contribute to development and progression of IgA nephropathy. Kidney Int. 2018;94:150–158. doi: 10.1016/j.kint.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 33.Akiyama S.I., Imai E., Maruyama S. Immunology of membranous nephropathy. F1000 Res. 2019;8 doi: 10.12688/f1000research.17589.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z., Wen L., Dou Y., Zhao Z. Human anti-thrombospondin type 1 domain-containing 7A antibodies induce membranous nephropathy through activation of lectin complement pathway. Biosci. Rep. 2018:38. doi: 10.1042/BSR20180131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y., Wang C., Jin L., He F., Li C., Gao Q., Chen G., He Z., Song M., Zhou Z., et al. IgG4 anti-phospholipase A2 receptor might activate lectin and alternative complement pathway meanwhile in idiopathic membranous nephropathy: An inspiration from a cross-sectional study. Immunol. Res. 2016;64:919–930. doi: 10.1007/s12026-016-8790-1. [DOI] [PubMed] [Google Scholar]
- 36.Ma H., Sandor D.G., Beck L.H., Jr. The role of complement inmembranous nephropathy. Semin. Nephrol. 2013;33:531–542. doi: 10.1016/j.semnephrol.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker P.J., Ochi R.F., Schulze M., Johnson R.J., Campbell C., Couser W.G. Depletion of C6 Prevents Development of Proteinuria in Experimental Membranous Nephropathy in Rats. Am. J. Pathol. 1989;135:185–194. [PMC free article] [PubMed] [Google Scholar]
- 38.Saran A.M., Yuan H., Takeuchi E., McLaughlin M., Salant D.J. Complement mediates nephrin redistribution and actin dissociation in experimental membranous nephropathy. Kidney Int. 2003;64:2072–2078. doi: 10.1046/j.1523-1755.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- 39.Yuan H., Takeuchi E., Taylor G.A., Mclaughlin M., Brown D., Salant D.J. Nephrin Dissociates from Actin, and Its Expression Is Reduced in Early Experimental Membranous Nephropathy. J. Am. Soc. Nephrol. 2002;13:946–956. doi: 10.1681/ASN.V134946. [DOI] [PubMed] [Google Scholar]
- 40.Morel-Maroger L., Leathem A., Richet G. Glomerular abnormalities in nonsystemic diseases. Relationship between findings by light microscopy and immunofluorescence in 433 renal biopsy specimens. Am. J. Med. 1972;53:170–184. doi: 10.1016/0002-9343(72)90127-1. [DOI] [PubMed] [Google Scholar]
- 41.Verroust P.J., Wilson C.B., Cooper N.R., Edgington T.S., Dixon F.J. Glomerular Complement Components in Human Glomerulonephritis. J. Clin. Investig. 1974;53:77–84. doi: 10.1172/JCI107562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hisano S., Matsushita M., Fujita T., Takeshita M., Iwasaki H. Activation of the lectin complement pathway in post-streptococcal acute glomerulonephritis. Pathol. Int. 2007;57:351–357. doi: 10.1111/j.1440-1827.2007.02107.x. [DOI] [PubMed] [Google Scholar]
- 43.Sethi S., Fervenza F.C., Zhang Y., Zand L., Meyer N.C., Borsa N., Nasr S.H., Smith R.J.H. Atypical post-infectious glomerulonephritis is associated with abnormalities in the alternative pathway of complement. Kidney Int. 2013;83:293–299. doi: 10.1038/ki.2012.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito N., Ohashi R., Nagata M. C3 glomerulopathy and current dilemmas. Clin. Exp. Nephrol. 2017;21:541–551. doi: 10.1007/s10157-016-1358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masani N., Jhaveri K.D., Fishbane S. Update on membranoproliferative GN. Clin. J. Am. Soc. Nephrol. 2014;9:600–608. doi: 10.2215/CJN.06410613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noris M., Remuzzi G. Glomerular Diseases Dependent on Complement Activation, Including Atypical Hemolytic Uremic Syndrome, Membranoproliferative Glomerulonephritis, and C3 Glomerulopathy: Core Curriculum 2015. Am. J. Kidney Dis. 2015;66:359–375. doi: 10.1053/j.ajkd.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Servais A., Ne Noël L.-H., Roumenina L.T., Le Quintrec M., Ngo S., -Agnès Dragon-Durey M., Macher M.-A., Zuber J., Karras A., Provot F., et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454–464. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- 48.Ma R., Cui Z., Hu S.-Y., Jia X.-Y., Yang R. The Alternative Pathway of Complement Activation May Be Involved in the Renal Damage of Human Anti-Glomerular Basement Membrane Disease. PLoS ONE. 2014;9:91250. doi: 10.1371/journal.pone.0091250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minto A.W., Kalluri R., Togawa M., Bergijk E.C., Killen P.D., Salant D.J. Augmented expression of glomerular basement membrane specific type IV collagen isoforms (alpha3-alpha5) in experimental membranous nephropathy. Proc. Assoc. Am. Physicians. 1998;110:207–217. [PubMed] [Google Scholar]
- 50.Sheerin N.S., Springall T., Carroll M.C., Hartley B., Sacks S.H. Protection against anti-glomerular basement membrane (GBM)-mediated nephritis in C3-and C4-deficient mice. Clin. Exp. Immunol. 1997;110:403–409. doi: 10.1046/j.1365-2249.1997.4261438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fischer E.G., Lager D.J. Anti-glomerular basement membrane glomerulonephritis: A morphologic study of 80 cases. Am. J. Clin. Pathol. 2006;125:445–450. doi: 10.1309/NPTP4UKV7JU3ELMQ. [DOI] [PubMed] [Google Scholar]
- 52.Xiao H., Dairaghi D.J., Powers J.P., Ertl L.S., Baumgart T., Wang Y., Seitz L.C., Penfold M.E., Gan L., Hu P., et al. C5a Receptor (CD88) Blockade Protects against MPO-ANCA GN Necrotizing and crescentic GN (NCGN) and vasculitis are associated with ANCA. J. Am. Soc. Nephrol. 2014;25:225–231. doi: 10.1681/ASN.2013020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao H., Schreiber A., Heeringa P., Falk R.J., Jennette J.C. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am. J. Pathol. 2007;170:52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jayne D.R.W., Bruchfeld A.N., Harper L., Schaier M., Venning M.C., Hamilton P., Burst V., Grundmann F., Jadoul M., Szombati I., et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J. Am. Soc. Nephrol. 2017;28:2756–2767. doi: 10.1681/ASN.2016111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Markowitz G.S., D’Agati V.D. Classification of lupus nephritis. Curr. Opin. Nephrol. Hypertens. 2009;18:220–225. doi: 10.1097/MNH.0b013e328327b379. [DOI] [PubMed] [Google Scholar]
- 56.Couser W.G. Basic and Translational Concepts of Immune-Mediated Glomerular Diseases. J. Am. Soc. Nephrol. 2012;23:381–399. doi: 10.1681/ASN.2011030304. [DOI] [PubMed] [Google Scholar]
- 57.Song D., Guo W.Y., Wang F.M., Li Y.Z., Song Y., Yu F., Zhao M.H. Complement Alternative Pathway’s Activation in Patients with Lupus Nephritis. Am. J. Med. Sci. 2017;353:247–257. doi: 10.1016/j.amjms.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Pickering M.C., Botto M. Are anti-C1q antibodies different from other SLE autoantibodies? Nat. Publ. Gr. 2010;6:490–493. doi: 10.1038/nrrheum.2010.56. [DOI] [PubMed] [Google Scholar]
- 59.Bao L., Haas M., Quigg R.J. Complement Factor H Deficiency Accelerates Development of Lupus Nephritis. J. Am. Soc. Nephrol. 2011;22:285–295. doi: 10.1681/ASN.2010060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bao L., Quigg R.J. Complement in Lupus Nephritis: The Good, the Bad, and the Unknown. Semin. Nephrol. 2007;27:69–80. doi: 10.1016/j.semnephrol.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Tomlinson S., Atkinson C., Qiao F., Song H., Gilkeson G.S. Mice lpr MRL/ Manifestations of Autoimmune Disease in Protects against Renal Disease and Other Low-Dose Targeted Complement Inhibition. J. Immunol. Ref. 2019;180:1231–1238. doi: 10.4049/jimmunol.180.2.1231. [DOI] [PubMed] [Google Scholar]
- 62.Bao L., Osawe I., Puri T., Lambris J.D., Haas M., Quigg R.J. C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur. J. Immunol. 2005;35:2496–2506. doi: 10.1002/eji.200526327. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y., Hu Q., MADRIt J.A., Rollins S.A., Chodera A., Matis L.A., Talmage D.W. Amelioration of lupus-like autoimmune disease in NZB/W F1 mice after treatment with a blocking monoclonal antibody specific for complement component C5. Proc. Natl. Acad. Sci. USA. 1996;93:8563–8568. doi: 10.1073/pnas.93.16.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murdaca G., Colombo B.M., Puppo F. Emerging biological drugs: A new therapeutic approach for Systemic Lupus Erythematosus. An update upon efficacy and adverse events. Autoimmun. Rev. 2011;11:56–60. doi: 10.1016/j.autrev.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 65.Mészáros T., Füst G., Farkas H., Jakab L., Temesszentandrási G., Nagy G., Kiss E., Gergely P., Zeher M., Griger Z., et al. C1-inhibitor autoantibodies in SLE. Lupus. 2010;19:634–638. doi: 10.1177/0961203309357059. [DOI] [PubMed] [Google Scholar]
- 66.Al-Mayouf S.M., Abanomi H., Eldali A. Impact of C1q deficiency on the severity and outcome of childhood systemic lupus erythematosus. Int. J. Rheum. Dis. 2011;14:81–85. doi: 10.1111/j.1756-185X.2010.01574.x. [DOI] [PubMed] [Google Scholar]
- 67.Heurich M., Martínez-Barricarte R., Francis N.J., Roberts D.L., Rodríguez De Córdoba S., Morgan B.P., Harris C.L. Common polymorphisms in C3, factor B, and factor H collaborate to determine systemic complement activity and disease risk. Proc. Natl. Acad. Sci. USA. 2011;108:8761–8766. doi: 10.1073/pnas.1019338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Noris M., Caprioli J., Bresin E., Mossali C., Pianetti G., Gamba S., Daina E., Fenili C., Castelletti F., Sorosina A., et al. Relative Role of Genetic Complement Abnormalities in Sporadic and Familial aHUS and Their Impact on Clinical Phenotype. Clin. J. Am. Soc. Nephrol. 2010;5:1844–1859. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Manuelian T., Hellwage J., Meri S., Caprioli J., Noris M., Heinen S., Jozsi M., Neumann H.P.H., Remuzzi G., Zipfel P.F. Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J. Clin. Invest. 2003;111:1181–1190. doi: 10.1172/JCI16651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caprioli J., Noris M., Brioschi S., Pianetti G., Castelletti F., Bettinaglio P., Mele C., Bresin E., Cassis L., Gamba S., et al. Genetics of HUS: The impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267–1279. doi: 10.1182/blood-2005-10-007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sethi S., Haas M., Markowitz G.S., D’agati V.D., Rennke H.G., Jennette J.C., Bajema I.M., Alpers C.E., Chang A., Cornell L.D., et al. Mayo Clinic/Renal Pathology Society Consensus Report on Pathologic Classification, Diagnosis, and Reporting of GN. J. Am. Soc. Nephrol. 2016;27:1278–1287. doi: 10.1681/ASN.2015060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sethi S., Fervenza F.C. Pathology of Renal Diseases Associated with Dysfunction of the Alternative Pathway of Complement: C3 Glomerulopathy and Atypical Hemolytic Uremic Syndrome (aHUS) Semin. Thromb. Hemost. 2014;40:416–421. doi: 10.1055/s-0034-1375701. [DOI] [PubMed] [Google Scholar]
- 73.Łukawska E., Polcyn-Adamczak M., Niemir Z.I. The role of the alternative pathway of complement activation in glomerular diseases. Clin. Exp. Med. 2018;18:297–318. doi: 10.1007/s10238-018-0491-8. [DOI] [PubMed] [Google Scholar]
- 74.Barbour T.D., Pickering M.C., Cook H.T. Recent insights into C3 glomerulopathy. Nephrol. Dial. Transpl. 2013;28:1685–1693. doi: 10.1093/ndt/gfs430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schramm E.C., Roumenina L.T., Rybkine T., Chauvet S., Vieira-Martins P., Hue C., Maga T., Valoti E., Wilson V., Jokiranta S., et al. Mapping interactions between complement C3 and regulators using mutations in atypical hemolytic uremic syndrome. Blood. 2015;125:2359–2369. doi: 10.1182/blood-2014-10-609073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pickering M.C., Warren J., Rose K.L., Carlucci F., Wang Y., Walport M.J., Cook H.T., Botto M. Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc. Natl. Acad. Sci. USA. 2006;103:9649–9654. doi: 10.1073/pnas.0601094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gale D.P., De Jorge E.G., Cook H.T., Martinez-Barricarte R., Hadjisavvas A., McLean A.G., Pusey C.D., Pierides A., Kyriacou K., Athanasiou Y., et al. Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet. 2010;376:794–801. doi: 10.1016/S0140-6736(10)60670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiao X., Ghossein C., Tortajadam A., Zhang Y., Meyer N., Jones M., Borsa N.G., Nester C.M., Thomas C.P., de Córdoba S.R., et al. Familial C3 glomerulonephritis caused by a novel CFHR5-CFHR2 fusion gene. Mol. Immunol. 2016;77:89–96. doi: 10.1016/j.molimm.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 79.Chen Q., Wiesener M., Eberhardt H.U., Hartmann A., Uzonyi B., Kirschfink M., Amann K., Buettner M., Goodship T., Hugo C., et al. Complement factor H-related hybrid protein deregulates complement in dense deposit disease. J. Clin. Investig. 2014;124:145–155. doi: 10.1172/JCI71866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kościelska-Kasprzak K., Bartoszek D., Myszka M., Zabińska M., Klinger M. The Complement Cascade and Renal Disease. Arch. Immunol. Ther. Exp. 2014;62:47–57. doi: 10.1007/s00005-013-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim M.-K., Maeng Y.-I., Lee S.-J., Lee I.H., Bae J., Kang Y.-N., Park B.-T., Park K.-K. Pathogenesis and significance of glomerular C4d deposition in lupus nephritis: Activation of classical and lectin pathways. Int. J. Clin. Exp. Pathol. 2013;6:2157–2167. [PMC free article] [PubMed] [Google Scholar]
- 82.Korbet S.M. Treatment of Primary FSGS in Adults. J. Am. Soc. Nephrol. 2012;23:1769–1776. doi: 10.1681/ASN.2012040389. [DOI] [PubMed] [Google Scholar]
- 83.D’Agati V.D., Fogo A.B., Bruijn J.A., Jennette J.C. Pathologic Classification of Focal Segmental Glomerulosclerosis: A Working Proposal. Am. J. Kidney Dis. 2004;43:368–382. doi: 10.1053/j.ajkd.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y., Gu Q., Huang J., Qu Z., Wang X., Meng L., Wang F., Liu G., Cui Z., Zhao M. Article Clinical Significance of IgM and C3 Glomerular Deposition in Primary Focal Segmental Glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2016;11:1582–1589. doi: 10.2215/CJN.01190216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Strassheim D., Renner B., Panzer S., Fuquay R., Kulik L., Ljubanovi D., Holers V.M., Thurman J.M. IgM Contributes to Glomerular Injury in FSGS. J. Am. Soc. Nephrol. 2013;24:393–406. doi: 10.1681/ASN.2012020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sethi S., Fervenza F.C., Zhang Y., Smith R.J. Secondary Focal and Segmental Glomerulosclerosis Associated with Single-Nucleotide Polymorphisms in the Genes Encoding Complement Factor H and C3 HHS Public Access. Am. J. Kidney Dis. 2012;60:316–321. doi: 10.1053/j.ajkd.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thurman J.M., Wong M., Renner B., Frazer-Abel A., Giclas P.C., Joy M.S., Jalal D., Radeva M.K., Gassman J., Gipson D.S., et al. Complement Activation in Patients with Focal Segmental Glomerulosclerosis. PLoS ONE. 2015;10:e0136558. doi: 10.1371/journal.pone.0136558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tomlinson S., Thurman J.M. Tissue-targeted complement therapeutics. Mol. Immunol. 2018;102:120–128. doi: 10.1016/j.molimm.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Horiuchi T., Tsukamoto H. Complement-targeted therapy: Development of C5-and C5a-targeted inhibition. Inflamm. Regen. 2016;36:11. doi: 10.1186/s41232-016-0013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cicardi M., Aberer W., Banerji A., Bas M., Bernstein J.A., Bork K., Caballero T., Farkas H., Grumach A., Kaplan A.P., et al. Classification, diagnosis, and approach to treatment for angioedema: Consensus report from the Hereditary Angioedema International Working Group. Allergy. 2014;69:602–616. doi: 10.1111/all.12380. [DOI] [PubMed] [Google Scholar]
- 91.Rother R.P., Rollins S.A., Mojcik C.F., Brodsky R.A., Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat. Biotechnol. 2007;25:1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 92.Legendre C.M., Licht C., Muus P., Greenbaum L.A., Babu S., Bedrosian C., Bingham C., Cohen D.J., Delmas Y., Douglas K., et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 93.Chatelet V., Frémeaux V., Frémeaux-Bacchi F., Lobbedez T., Ficheux M., Hurault De Ligny B. Safety and Long-Term Efficacy of Eculizumab in a Renal Transplant Patient with Recurrent Atypical Hemolytic-Uremic Syndrome. Am. J. Transpl. 2009;9:2644–2645. doi: 10.1111/j.1600-6143.2009.02817.x. [DOI] [PubMed] [Google Scholar]
- 94.Wong E.K.S., Goodship T.H.J., Kavanagh D. Complement therapy in atypical haemolytic uraemic syndrome (aHUS) Mol. Immunol. 2013;56:199–212. doi: 10.1016/j.molimm.2013.05.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaabak M., Babenko N., Shapiro R., Zokoyev A. A prospective randomized, controlled trial of eculizumab to prevent ischemia-reperfusion injury in pediatric kidney transplantation. Pediatr. Transpl. 2018;22 doi: 10.1111/petr.13129. [DOI] [PubMed] [Google Scholar]
- 96.Marks W.H., Mamode N., Montgomery R.A., Stegall M.D., Ratner L.E., Cornell L.D., Rowshani A.T., Colvin R.B., Dain B., Boice J.A., et al. Safety and efficacy of eculizumab in the prevention of antibody-mediated rejection in living-donor kidney transplant recipients requiring desensitization therapy: A randomized trial. Am. J. Transpl. 2019:1–13. doi: 10.1111/ajt.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Glotz D., Russ G., Rostaing L., Legendre C., Tufveson G., Chadban S., Grinyó J., Mamode N., Rigotti P., Couzi L., et al. Safety and efficacy of eculizumab for the prevention of antibody-mediated rejection after deceased-donor kidney transplantation in patients with preformed donor-specific antibodies. Am. J. Transpl. 2019;19:2865–2875. doi: 10.1111/ajt.15397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bomback A.S., Smith R.J., Barile G.R., Zhang Y., Heher E.C., Herlitz L., Stokes B.M., Markowitz G.S., D’agati V.D., Canetta P.A., et al. Article Eculizumab for Dense Deposit Disease and C3 Glomerulonephritis. Clin. J. Am. Soc. Nephrol. 2012;7:748–756. doi: 10.2215/CJN.12901211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Radhakrishnan S., Lunn A., Kirschfink M., Thorner P., Hebert D., Langlois V., Pluthero F., Licht C. Eculizumab and refractory membranoproliferative glomerulonephritis. N. Engl. J. Med. 2012;366:1165–1166. doi: 10.1056/NEJMc1106619. [DOI] [PubMed] [Google Scholar]
- 100.Mccaughan J.A., O’rourke D.M., Courtney A.E. Recurrent Dense Deposit Disease After Renal Transplantation: An Emerging Role for Complementary Therapies. Am. J. Transpl. 2012;12:1046–1051. doi: 10.1111/j.1600-6143.2011.03923.x. [DOI] [PubMed] [Google Scholar]
- 101.Thurman J.M., Le Quintrec M. Targeting the complement cascade: Novel treatments coming down the pike. Kidney Int. 2016;90:746–752. doi: 10.1016/j.kint.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]