Abstract
Background:
Adherence to the type 1 diabetes (T1D) regimen, while predictive of glycemic control, decreases during adolescence. For adolescents, attaining adequate sleep is an additional challenge. This study evaluates the impact of sleep on adherence in teens with T1D.
Subjects:
Forty-five adolescents aged 12 to 18 years, with T1D for at least 6 months while on insulin pump therapy.
Methods:
Adolescents logged their sleep on a written diary for two weeks. Corresponding insulin pump/glucometer downloads as well as sleep habit questionnaires were also obtained.
Results:
Data from 20 girls and 25 boys, with a mean age of 15 ±1.6 years and mean glycated hemoglobin of 8.7 ±1.1% (72mmol/mol) were analyzed. Overall, average sleep was 8.6 ± 0.9 hours per night. Sleep durations were compared to the next day’s frequency of self monitored blood glucose (SMBG) and total daily insulin bolus frequency. Associations were found between sleep duration and youths’ SMBG and insulin bolus frequencies (p<0.03 and p<0.001, respectively). Specifically, a 15 and 20-minute increase in sleep were associated with 1 additional SMBG check and 1 additional insulin bolus, respectively.
Conclusion:
Analyses suggest an associated increase in T1D self-management behaviors in youths with increased sleep duration. Given these results, diabetes care teams should consider counseling teens on the importance of adequate sleep.
Keywords: Type 1 Diabetes Mellitus, Sleep, Patient Adherence, Pediatrics, Adolescents
INTRODUCTION
Adolescents with type 1 diabetes (T1D) frequently fail to perform essential diabetes-related self-care behaviors, including accurate carbohydrate counting, self-monitoring of blood glucose (SMBG) and insulin bolusing for meals. Adherence to these self-care behaviors is predictive of glycemic control (1); therefore, glycemic control often declines during adolescence, partly as a result of suboptimal engagement in diabetes self-care tasks (2–4). Specifically, a loss of one blood glucose check per day has been associated with a 0.19–1.26 unit (%) increase in glycated hemoglobin (A1c) (1, 2, 5), while missing one mealtime insulin bolus each day has been related to a 1.5 unit (%) increase in A1c (5). In addition to problems with adherence, adolescents also can experience problems achieving adequate sleep at night. According to the American Academy of Pediatrics (AAP), only 20% of adolescents achieve the recommended amount of sleep (8.5–9.5 hours/night). While studies comparing sleep duration for youths with T1D versus youths without T1D have uncovered mixed findings (6–9), nearly all of the studies examining sleep in youths with T1D have identified some degree of disturbed sleep characterized by either a reduction in deep sleep (6) or an increase in overnight awakenings (8, 9). One study reported a relationship between adolescents’ sleep duration and their overall school performance (10). Specifically, they found that later bedtimes on non-school versus school nights was associated with lower grades in math, reading, and writing among youths, perhaps due to aggregate sleep loss and/or the lack of a consistent bedtime. Despite evidence suggesting an association between sleep duration and executive functioning as well as school performance in adolescents with T1D, the impact of sleep quality and sleep duration on adherence to diabetes self-care tasks remains unknown.
Whether sleep quality and sleep duration impact engagement with diabetes self-care tasks represents an important gap in knowledge. If sleep disturbances limit an adolescent’s ability to adhere to diabetes self-care tasks, strategies to minimize sleep disturbance may be of benefit. Evidence to that effect would suggest that interventions to improve sleep should be incorporated into routine diabetes education and clinic visits.
To begin to address this gap in knowledge, we recruited a sample of adolescents with T1D from a 13-site diabetes clinic network to participate in a prospective observational study assessing the relationship between sleep duration and adherence. We hypothesized that among adolescents with T1D, an increase in sleep duration would be positively associated with objective adherence measures, specifically frequency of SMBG, mealtime insulin BOLUS score (BOLUS), and total daily insulin bolus frequency.
METHODS
Participants
Adolescents between the ages of 12 and 18 years, with a physician-confirmed diagnosis of T1D based on American Diabetes Association (ADA) diagnostic criteria (3) for at least 6 months were recruited for the study. We elected to focus on adolescents because we expected that in this age group, most day-to-day adherence behaviors are performed by the youths themselves rather than their parent/caregiver, thus increasing the potential for an association between youths’ sleep duration and next day adherence. Only adolescents on continuous subcutaneous insulin infusion (CSII) therapy were included to allow calculation of total daily insulin bolus frequency and the mealtime insulin BOLUS score (5). However, this did not significantly reduce our available sample, as about 83% of the adolescent T1D clinic population at the study site utilizes CSII. To improve the likelihood that subjects could comply with the study procedures and study visits, an additional inclusion criterion for clinic attendance (attended ≥3 routine clinic visits in the 12 months prior to their enrollment) was applied to the sample. Youths who did not have T1D, those who anticipated significant changes to their daily routine during the observation period, or those who had sought referral/evaluation for known sleep problems were excluded. After reviewing the electronic health record, we identified 155 adolescents that met the inclusion criteria, of which 66 were reached by phone for recruitment. A total of 46 youths (70% of those contacted by phone) were consented for participation in the study. Enrollment was limited to the academic year, exclusive of holiday and summer breaks, so that subjects’ daily routine was as consistent as possible.
Procedure
All research protocols and procedures were approved by the Children’s Mercy – Kansas City Institutional Review Board and conducted in accordance with the Declaration of Helsinki. Adolescents eligible for the study were identified through systematic review of the electronic health record. Once eligibility was established, possible participants were contacted by phone approximately 3–4 weeks prior to their next T1D clinic visit. Subjects and their parent were informed that the study was evaluating the effects of sleep on diabetes self-care. Informed permission/assent was then obtained in two stages from all subjects prior to study participation. In the first stage, verbal permission and assent were obtained, and a standardized sleep diary was sent to the adolescent to complete 16 days ahead of his/her next routine T1D clinic appointment, when the study visit occurred. During the recording period, adolescents were asked not to alter their sleep habits, or the frequency of their self-management behaviors. On the day of their T1D clinic appointment, youths and parents completed the second stage of permission/assent by providing written informed consent for all further study-related procedures. Then, youths were asked to provide their completed sleep log, their glucometer and insulin pump for downloading, and to completed a battery of electronic questionnaires via iPad (11). Youths received $20 for their participation in the study.
Measures
Demographic Questionnaire: Youths and their parents reported demographic information including the youths’ age, date of diabetes diagnosis, race, ethnicity, past medical history, family socioeconomic status, and the number of family caregivers participating in the youths’ T1D management. Specific details regarding the subject’s current diabetes treatment regimen were collected from the adolescents and verified through the electronic health record. The health record was also used to obtain youths’ anthropometric data and the A1c level collected at the time of the study visit.
Sleep Diary: Adolescents completed a written study-specific sleep diary, adapted from Shapiro (1998). Adolescents were instructed to start the sleep diary 16 days prior to their next scheduled diabetes clinic appointment in order to ensure a record of 14 nights of sleep data followed by at least 14 full days of wake period. The sleep diary, a visual analogue of time, was oriented horizontally on the page, with each line beginning at 1800 hours and terminating the following day at 1800 hours. Each line was subdivided into 15 minute increments over the 24 hour period. Teens were instructed to draw a downward pointing arrow to indicate the time they went to bed, and an upward pointing arrow to indicate the time they arose. These arrows were repeated for overnight awakenings, with space provided to document the reason for the overnight awakening. An example line and instructions were included on the top of the diary for reference. The completed sleep diary was transcribed and managed using electronic data capture tools (11). Two independent raters reviewed each sleep log and recorded their findings into the database. Inter-class correlation coefficient between raters was 0.963 (p < 0.001) suggesting a very high rate of internal consistency in sleep time determinations. The total number of nights completed by study participants ranged from 5 to 14 (n = 628, mean 13.4 ±1.9 nights).
Sleep Disorders Scale for Children (SDSC) (12): Adolescents completed this 26-item measure of usual sleep habits, with particular attention to the previous two weeks. The SDSC measures six domains of sleep, including Disorders of Initiating and Maintaining Sleep, Sleep Disordered Breathing, Disorders of Arousal, Sleep-Wake Transition Disorders, Disorders of Excessive Somnolence and Sleep Hyperhydrosis.
Test for Diabetes Knowledge (TDK-5)(13): Adolescents completed this 41 item to measure their understanding of diabetes self-management. This survey was included in the study to control for youths’ diabetes understanding when modeling the impact of sleep on youths’ T1D adherence.
Adherence Assessment: Adolescents’ adherence to their T1D self-management was measured via daily frequency of SMBG checks, total daily insulin boluses, and the mealtime BOLUS score. The BOLUS score is a proxy measure of adherence, assigning one point for a meal-associated insulin bolus during each of the following time periods: 0600 to 1000, 1100 to 1500 and 1600 to 2200. A maximum score of 3 points may be attained each day. The average score over 14 days yields the final BOLUS score. Patton et al. found that this measure was superior to SMBG frequency in predicting youth’s A1c (5).
Analyses
Youths’ sleep duration, as determined by their sleep diary, was divided into quartiles and initially analyzed using one-way ANOVA with post-hoc comparisons. Simple and partial correlations were performed to assess differences in sleep duration, measured continuously, and youths’ A1c, SDSC scores and TDK-5 scores. Finally, generalized linear models and panel analysis were used to determine the association between youths’ nightly sleep and the following days’ frequencies of SMBG checks and insulin boluses.
RESULTS
Sample.
Youths included in the analysis were 20 girls and 25 boys with a mean age of 15 ± 1.6 years, T1D duration of 6.8 ± 3.9 years, and mean A1c of 8.7% (72 mmol/mol) ± 1.1% (Table 1). Our sample was generally representative of the population served by our T1D clinic as evidenced by comparison to a random sampling of 1195 youths included in a health outcomes repository (53% male, mean age 14.7 ± 3.5 years, T1D duration of 5.7 ± 3.5 years, and mean A1c of 8.8% [73 mmol/mol]). One subject was excluded from analysis, due to technical difficulties downloading insulin pump data. Diabetes knowledge at study enrollment was high, with average TDK-5 score of 93.7 ± 7.4%. A statistically significant negative correlation was identified with the percent correct on the TDK-5 and A1c (r = −0.3, p < 0.05). No patients reported previously diagnosed sleep disorders, and scores from the SDSC and all of its subscales were comparable between boys and girls, as well as between quartiles of sleep duration. There were no associations found between youths’ SDSC scores or A1c and either their daily adherence to SMBG, mealtime insulin BOLUS score, or total insulin bolus frequency.
Table 1:
Demographic, Questionnaire and Adherence Behaviors
| Demographics | Boys (n = 20) |
Girls (n = 25) |
Mean ± SD |
|---|---|---|---|
| Age (years) | 15.4 | 14.5 | 15 ± 1.6 (12.4–17.9) |
| Length of T1DM (yrs) | 7.2 | 6.3 | 6.8 ± 3.9 (0.8–15.2) |
| A1c (%) | 8.6 | 8.8 | 8.7 ± 1.1 (6.1–11.2) |
| Questionnaire Scores | |||
| TDK Score (%) | 93.1 | 94.4 | 93.7 ± 7.4 (61–100) |
| SDSC Score | 41.3 | 37.9 | 39.8 ± 8.3 (29–62) |
| Completed Days | 13.6 | 13.3 | 13.4 ± 1.8 (5–14) |
| Adherence Behaviors | |||
| SMBG/day | 4.9 | 4.7 | 4.8 ± 2.3 (0–9.8) |
| Bolus/day | 4.8 | 4.6 | 4.7 ± 1.8 (0–9) |
| Average Daily BG (mg/dL) | 207 | 209 | 208 ± 49 (111–373) |
| Sleep Duration (mins) | 509 | 520 | 514 ± 95 (195–960) |
Note. All p>0.05 for differences between boys and girls.
Sleep duration.
Study participants completed an average of 13.4 ± 1.8 night-day observations (Table 1), corresponding to a total of 605 nights with full next-day insulin pump and glucometer data to use for the analyses. Average sleep duration for the group was 514 ± 95 minutes (8.6 ± 1.6 hours) per night. Only 31% of recorded nights achieved the AAP’s recommendation of 8.5 to 9.5 hours of sleep, while nearly half (48%) of nights recorded less than 8.5 hours of sleep. In contrast, on recall, 44% of teens reported sleeping the recommended amount, suggesting a discrepancy between what teens report in near-real time versus retrospectively.
Primary (multivariable) analysis of sleep duration and adherence.
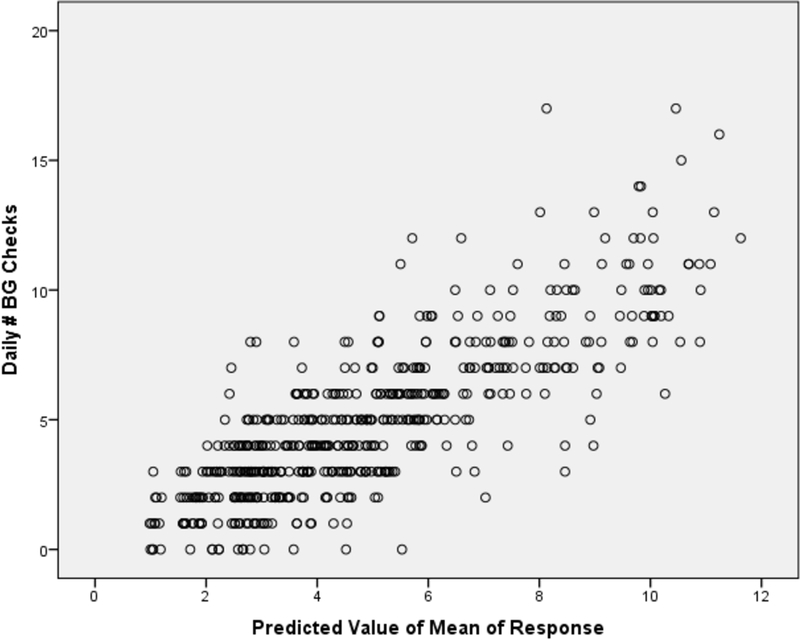
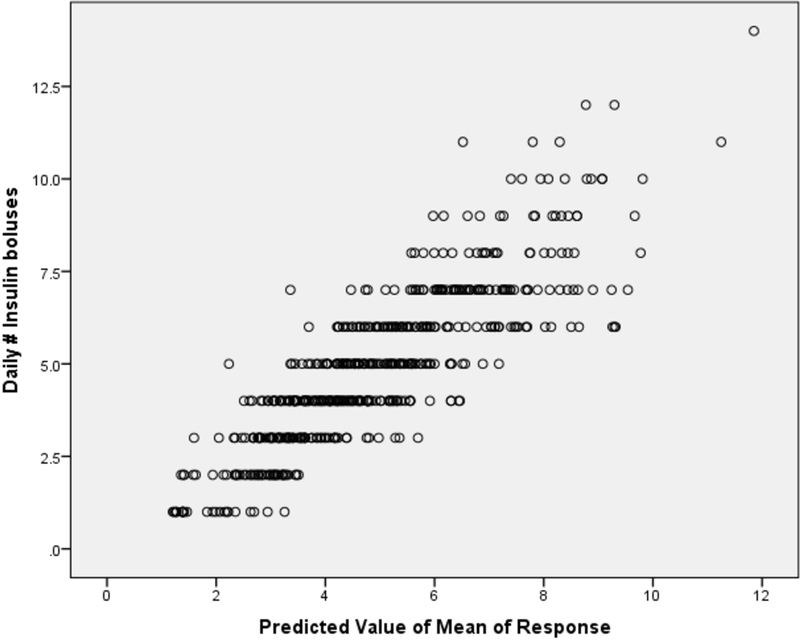
In multivariable analyses, statistically significant associations were found between youths’ mean sleep and the frequency of both their next-day SMBG checks and total daily insulin boluses. In contrast, no associations were found with mealtime BOLUS. Moreover, in a larger model, youths’ frequency of SMBG checks and total daily insulin boluses were found to be highly associated with youths’ mean sleep duration, changes in sleep duration, daily carbohydrate intake, and age at study visit. Examining the estimated coefficients revealed that every one minute increase (decrease) in average sleep was associated with a 1.4% increase (decrease) in the frequency of SMBG checks (p<0.03; Figure 2 displays the fitted versus actual). Similarly, every one minute increase (decrease) in average sleep was associated a 1.2% increase (decrease) in number of insulin boluses per day (p<0.001; Figure 3 displays the fitted versus actual). Therefore, in clinically relevant terms, 15- and 20-minute increases in sleep duration relate to a 1-event increase (decrease) in the average daily SMBG frequency and a 1-event increase (decrease) in the average daily insulin bolus frequency, respectively (Table 2).
Figure 2:
Daily SMBG compared to sleep duration
Figure 3:
Daily insulin boluses compared to sleep duration
Table 2:
Changes in Adherence Behaviors with Relation to Changes in Average Sleep Duration
| Sleep (minutes) | Sleep (hours) | Change in Average Sleep Time (minutes) | SMBG Frequency | Change to Average SMBG Frequencyb | Insulin Bolus Frequency | Change to Average Insulin Bolus Frequencyb |
|---|---|---|---|---|---|---|
| 479 | 7.98 | −35 | 3.0 | −1.9 | 3.1 | −1.6 |
| 484 | 8.07 | −30 | 3.2 | −1.7 | 3.3 | −1.4 |
| 489 | 8.15 | −25 | 3.4 | −1.4 | 3.5 | −1.2 |
| 494 | 8.23 | −20 | 3.7 | −1.2 | 3.7 | −1.0 |
| 499 | 8.32 | −15 | 3.9 | −0.9 | 3.9 | −0.8 |
| 504 | 8.4 | −10 | 4.2 | −0.6 | 4.2 | −0.5 |
| 509 | 8.48 | −5 | 4.5 | −0.3 | 4.4 | −0.3 |
| 514a | 8.57 | 0 | 4.8 | 0 | 4.7 | 0 |
| 519 | 8.65 | 5 | 5.2 | 0.4 | 5.0 | 0.3 |
| 524 | 8.73 | 10 | 5.6 | 0.7 | 5.3 | 0.6 |
| 529 | 8.82 | 15 | 6.0 | 1.1 | 5.6 | 0.9 |
| 534 | 8.9 | 20 | 6.4 | 1.6 | 6.0 | 1.3 |
| 539 | 8.98 | 25 | 6.9 | 2.0 | 6.3 | 1.6 |
| 544 | 9.07 | 30 | 7.4 | 2.5 | 6.7 | 2.0 |
Mean total sleep as reported by our cohort (514 minutes, 8.57 hours)
Changes in the average daily adherence behavior, when compared to mean total sleep time for the group
Of note, while a post-hoc one-way ANOVA confirmed a statistically significant difference in youths’ sleep duration (dependent variable) by night of the week (independent variable, p<0.001), when these night-of-the-week variables were included in the generalized linear models explaining the number of SMBG and the number of daily insulin boluses, these night-of-the-week variables were found to have statistically insignificant coefficients (p>0.05).
Bivariate analysis: ANOVA comparisons of sleep duration (by quartile) and adherence
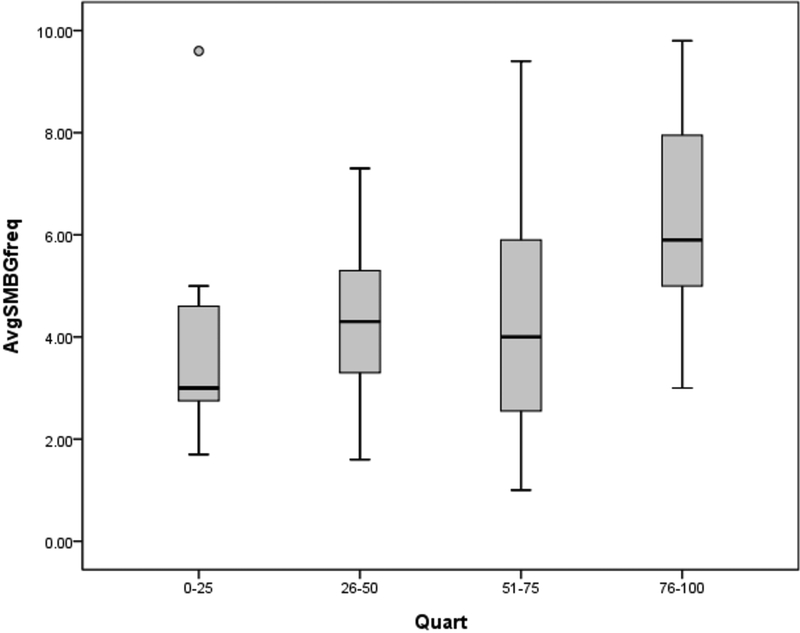
We first analyzed changes in adherence as a function of sleep duration (stratified by quartiles) across the sample. Mean daily SMBG frequency was 4.8 ± 2.3, with only 61% of days having ≥ 4 readings per day. Those whose average sleep duration was in the bottom quartile (< 477 minutes, < 8 hours) had statistically significantly lower average daily SMBG frequency than those in either of the two upper quartiles (525–545 minutes, 8.7–9.1 hours; > 545 minutes, > 9.1 hours) (3.9 ± 2.1 compared to 4.3± 2.5 and 6.4 ± 2.3 checks per day, respectively [Table 3, Figure 1]). Total daily insulin boluses (i.e., boluses given for either correction dosing or carbohydrate intake) averaged 4.7 ± 1.8 events per day. Interestingly, there were no statistically significant differences in total daily insulin boluses by quartile of sleep duration. The mean mealtime insulin BOLUS score was 2.1 (out of a maximum score of 3); again, no statistically significant differences were identified in the mealtime insulin bolus score across quartiles for sleep duration (Table 3).
Table 3:
Outcomes by Sleep Duration Quartiles
| Sleep Quartile (%ile) | Sleep Duration minutes (hours) | A1c | SMBG Frequency (avg/day) | Bolus Frequency (avg/day) | TDK5 Score | SDSC Score | BOLUS Score |
|---|---|---|---|---|---|---|---|
| 0–25 | < 477 (8h) | 8.8 ± 1.5 | 3.9 ± 2.1* | 3.9 ± 2.1 | 94.9 ± 3.1 | 40.8 ± 9.7 | 2 ± 0.68 |
| 26–50 | 478–525 (8–8.7h) | 8.1 ± 1 | 4.3 ± 1.7* | 5.2 ± 1.6 | 95.8 ± 4 | 42.4 ± 8.4 | 2.2 ± 0.76 |
| 51–75 | 525–545 (8.7–9.1h) | 9.1 ± 0.9 | 4.3 ± 2.5* | 4.8 ± 1.8 | 93.5 ± 7.8 | 39.5 ± 8.8 | 2.2 ± 0.6 |
| 76–100 | >545 (9.1h) | 8.7 ± 1.1 | 6.4 ± 2.3* | 4.8 ± 1.8 | 90.8 ± 11.3 | 36.7 ± 6.3 | 2.1 ± 0.65 |
Note.
p < 0.05 for differences in Mean SMBG Frequency by Sleep Quartile.
Figure 1: SMBG checks by sleep quartiles.
Statistically significant differences (p < 0.05) in average SMBG frequency were observed between the first and third, first and fourth, second and fourth, and third and fourth quartiles. AvgSMBGfreq, average daily frequency of blood glucose checks; Quart, quartiles of sleep duration.
DISCUSSION
In the present work, we examined the effects of sleep duration on adherence behaviors in youths with T1D through the use of self-report sleep diaries and insulin pump downloads, thereby quantifying an association between sleep duration and adherence that has not been previously described in pediatric diabetes literature. First, bivariate analyses identified associations between sleep duration and frequency of SMBG (but not frequency of total daily insulin boluses or mealtime insulin BOLUS scores). In contrast, multivariable analyses identified associations between sleep duration and both frequency of SMBG and frequency of total daily insulin boluses (but not mealtime insulin BOLUS score). We found that as little as a 15- and 20- minute increase in sleep duration could translate into a 1 event increase in average daily SMBG frequency and a 1 event increase in average daily insulin bolus frequency, respectively, suggesting adequate sleep may be an important variable to consider when counseling youths’ with T1D on their daily self-care. Interestingly, despite strong associations between sleep duration and adherence, we found no significant association between sleep duration and A1c. Specifically, there was no statistically significant difference in A1c between those participants with an average sleep duration within the recommended target (8.5–9.5 hours per night) compared to those outside of the target. Second, we analyzed for an association between night of week and adherence and found one in bivariate analyses, although the association disappeared in multivariable analyses. Logically, we interpret that this outcome occurred because the sleep variables we included in the main (multivariable) models were better estimators of the impact of sleep on adherence than a variable considering the night when the sleep occurred. This would explain why we did not find a separate night-of-the-week effect after controlling for the level of sleep, and potentially strengthens our conclusions about the influence of sleep on adherence. Adolescents reported generally lower-than-recommended adherence to SMBG and insulin use as measured by frequency of daily blood glucose checks, the mealtime insulin BOLUS score and frequency of total daily insulin boluses. Finally, youths’ sleep duration (based on daily sleep diary results) was typically well below the number of hours of sleep per night that is recommended by the AAP, despite 44% of youths recalling a nightly average sleep duration in compliance to the AAP recommendations.
Previous studies on sleep in type 1 diabetes have yielded mixed results. Hazen et al. (14) recently reported that increased sleep was associated with decreased adherence, specifically with declines in SMBG frequency, implying that adolescents were sleeping during times that diabetes cares were required. They also found that parental report of ‘sleeping more than most children’ was correlated with higher A1c (r = 0.24, p<0.05). However, limitations to that study include the use of a targeted sample of known poorly controlled adolescents with T1D, the lack of a prospectively collected near-real-time measure of sleep duration, and the reliance on secondary (parental) report of sleep quality/quantity in the adolescent subject. In contrast, our study measured total sleep time via a daily teen-recorded sleep diary and a composite tool for sleep quality, yielding very different results.
Notably missing from pediatric literature is an evaluation of sleep on long-term diabetes control, specifically A1c. Work by Borel et al. (15) in adults with T1D found that those sleeping less than 6.5 hours per night had higher A1c compared to those sleeping longer than 6.5 hours. Our analyses did not show a similar relationship to between sleep duration and A1c in adolescents. However, because we only collected 14 days of sleep data, this period may not have been long enough to measure adequate variability in youths’ sleep duration to find a potential relation with their A1c. For instance, the present study included only one adolescent who experienced an average sleep duration of less than 6.5 hours, which significantly limits the possibility of comparing the present results to those in the study by Borel. While the literature evaluating the impact of sleep on A1c is scare, an abundant literature describes the impact of adherence on A1c. A study by Rausch et al. (1) evaluated the adherence behaviors of early adolescents and found that for each one additional SMBG check per day, A1c decreased by 1.26 unit (%). This significant change in A1c from improvements in adherence becomes increasingly important as children enter adolescence, a time when diabetes self-care declines for a variety of reasons including ambivalence, feelings of invincibility and lack of concern and understanding of acute actions on chronic outcomes (16). Ziegler et al. (2) found that frequency of SMBG checks declined with age, such that children <6 years old averaged 6 checks/day, while adolescents aged 12–18 years had just 4.4 checks/day on average (p<0.001). In contrast to Rausch, after adjusting for confounding factors, Zeigler found that a change in 1 SMBG per day was associated with only a 0.2 unit (%) in decline in A1c; Zeigler also found no added benefit to A1c when SMBG frequency totaled more than 5 times per day. Patton et al. identified even strong associations between mealtime insulin BOLUS score and both present and future A1c (5). The present data, while indicating that those children who sleep more have an associated improvement in SMBG frequency and total daily insulin bolus frequency (but not mealtime insulin BOLUS frequency), did not show significant associations between sleep duration and A1c as described by Rausch or Zeigler. We hypothesize that we were unable to find significant differences in A1c due to our relatively small sample size and the short time period of observations in this study. As a result, the behavioral outcomes measured here, specifically daily SMBG and insulin bolus frequency, are more likely to be impacted by nightly changes in sleep duration than is A1c. We hypothesize that a longer observation period for future studies, with multiple A1c measures, may be able to more directly identify whether a relationship between sleep duration and A1c exists in adolescents.
The presence of a sleep deficiency is potentially harmful because it is known that such deficiencies may result in decreased attention span and diminished adaptive skills in children and adolescents (6, 7). Specific to T1D, one can hypothesize that an overall decline in sleep quality/quantity could lead to deficiencies in the focus, attention, and problem-solving skills that are integral to day-to-day diabetes self-care; we speculate that such deficiencies could explain the associations between sleep and objectively measured adherence behaviors. Previous research also suggests that overnight hypoglycemia and the diagnosis of diabetes itself are risk factors for less deep sleep and more disturbed sleep architecture (7–9). Reduced or disturbed sleep may independently effect lower daytime functioning (17), suggesting there may be a highly complex relationship between sleep, T1D adherence, and glycemic control in youths.
In puberty, youths experience a physiological change in melatonin secretion, offsetting the release of melatonin from the normal circadian rhythm and making it difficult for adolescents to fall asleep at an earlier time (18). As a result, sleep disorders and inadequate sleep in adolescents are, at least in part, related to a shift in their sleep/wake patterns. This results in later bedtimes during a period when school schedules require early morning awakenings, and consequent decreased total sleep time (18). Because disturbances in sleep duration/quality are well documented among adolescents (19, 20), the AAP has advocated for later school start times for all teens in order to better coincide with adolescents’ natural circadian rhythm and to improve their sleep durations (19). Whether such system-wide interventions could impact adherence and glycemic control among teens with T1D remains to be determined.
The present study should be interpreted in the context of certain limitations: 1) Given the relative rarity of diagnosed sleep disturbances in adolescents (20), the study’s small sample size may have been inadequately powered to identify a relationship between the presence of sleep disturbances and all measures of adherence in youths. 2) While the authors attempted to recruit youths who did not have a reported diagnosed sleep disorder in order to increase generalizability, one cannot rule out the possibility that a few adolescents with undetected or undiagnosed sleep disorders were included in the present cohort, since the investigators did not screen for sleep disorders as an inclusion criterion. 3) Because the investigators relied on SMBG values for information on glycemic control, the present study was also limited in its ability to assess day-to-day mean glycemia relative to adolescents’ sleep duration. 4) The authors’ decision to focus exclusively on adolescents limits the generalizability of the present findings to younger children. As such, it remains unknown if younger children who also experience sleep loss have an associated decrease in their adherence to SMBG or insulin use, which will have to be the focus of a future study. 5) Methodologically, the study team used near-real-time self-reported data to measure adolescents’ sleep duration which could be vulnerable to a Hawthorne effect. While the intention was for adolescents to record data daily, the authors cannot confirm whether adolescents did indeed record these data in real-time leading to the additional possibility of recall bias. However, one can infer that at least most data were recorded in near-real-time, since a post-hoc review of the sleep logs confirmed the dates to be correct, some degree of variability in the documented sleep times, and variation in whether the entries were completed in pen versus pencil. 6) While panel analysis (a combination of time series and cross-sectional analysis) was used to fit these relationships, we did not explore simultaneous equation models where adherence influences sleep and sleep influences adherence; however, we infer that adherences of the next day are much more responsive to sleep of the previous night as opposed to the adherences of a preceding day influencing the sleep of the next night. 7) Because sleep duration and adherence may be proxies for overall better health management, one cannot rule out the possibility of another variable mediating the observed relation between youths’ sleep duration and adherence. 8) The investigators did not include measures of mental health symptoms (i.e. anxiety and depression) and are thus unable to determine if depressive symptoms or anxiety may have mediated adolescents’ sleep loss and T1D adherence. Depressive symptoms and anxiety are common in youths with T1D and are clearly related to adherence (21), so these variables should be included in future studies relating sleep loss to adherence in adolescents. 9) Lastly, in order to determine accurate frequency and timing of insulin boluses, the study cohort was limited to those on CSII, which opens the possibility that the findings will not be generalize to youths using multiple daily injections (MDI). Finding a relation between sleep duration and adherence in youths using MDI will need to be the focus of a future study.
The present study is also characterized by some notable strengths: First, the data were collected prospectively. Second, the sample appears representative of the T1D population of a 13-site diabetes clinical network. Third, the study utilizes a near-real-time measure of sleep duration and objective measures of adherence. Lastly, the multivariable analysis in the present study controls for several variables which may influence adherence independent of sleep.
The authors conclude that sleep duration among adolescents relates to adherence to the T1D self-care regimen. Whether interventions designed to improve sleep hygiene and sleep duration, as well as decrease sleep disturbances, can improve adherence and glycemic control among teens with T1D remains to be determined. The most recent statement from the ADA on the Care of Adolescents with T1D discusses the need for behavioral and psychological screening, but does not specifically recommend counseling or screening for sleep disturbances (22). The present findings suggest that diabetes care teams should counsel teens on the possible impact of sleep on adherence and long-term diabetes complications, and that future studies assessing the relationship between sleep quality/duration and adherence and glycemic control among teens and pre-teens with T1D, and among parents of very young children with T1D, should be performed.
ACKNOWLEDGMENTS
We thank Ms. Jennifer Bedard and Ms. Lois Hester for their assistance in the recruitment of subjects for this study. We thank Ms. Teresa Lillis for her help in conceptualizing the design and selecting study measures. We thank Dr. Wayne V. Moore and the Division of Endocrinology &Diabetes, Department of Pediatrics, Children’s Mercy – Kansas City for the financial support for this study. We thank the adolescents and families who participated and gave of their time to help us better understand the challenges of living with type 1 diabetes.
Abbreviations:
- AAP
American Academy of Pediatrics
- ADA
American Diabetes Association
- BOLUS
Mealtime Insulin BOLUS score
- CSII
Continuous Subcutaneous Insulin Infusion
- SMBG
Self Monitored Blood Glucose
- T1D
Type 1 Diabetes Mellitus
REFERENCES
- 1.Rausch JR, Hood KK, Delamater A, et al. Changes in treatment adherence and glycemic control during the transition to adolescence in type 1 diabetes. Diabetes Care 2012; 35(6):1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler R, Heidtmann B, Hilgard D, et al. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatric Diabetes 2011; February;12(1):11–7. [DOI] [PubMed] [Google Scholar]
- 3.Silverstein J, Klingensmith G, Copeland K, et al. Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care 2005; 28(1):186–212. [DOI] [PubMed] [Google Scholar]
- 4.Borus JS, Laffel L. Adherence challenges in the management of type 1 diabetes in adolescents: prevention and intervention. Current Opinion in Pediatrics 2012; 22(4):405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patton SR, Clements MA, Fridlington A, Cohoon C, Turpin AL, DeLurgio SA. Frequency of mealtime insulin bolus as a proxy measure of adherence for children and youths with type 1 diabetes mellitus. Diabetes Technology &Therapeutics 2013; 15(2):124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perfect MM, Archbold K, Goodwin JL, Levine-Donnerstein D, Quan SF. Risk of behavioral and adaptive functioning difficulties in youth with previous and current sleep disordered breathing. Sleep 2013; 36(4):517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perfect MM, Patel PG, Scott RE, et al. Sleep, glucose, and daytime functioning in youth with type 1 diabetes. Sleep 2012; 35(1):81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillar G, Schuscheim G, Weiss R, et al. Interactions between hypoglycemia and sleep architecture in children with type 1 diabetes mellitus. The Journal of Pediatrics 2003; 142(2):163–8. [DOI] [PubMed] [Google Scholar]
- 9.Matyka KA, Crawford C, Wiggs L, Dunger DB, Stores G. Alterations in sleep physiology in young children with insulin-dependent diabetes mellitus: relationship to nocturnal hypoglycemia. The Journal of Pediatrics 2000; 137(2):233–8. [DOI] [PubMed] [Google Scholar]
- 10.Perfect MM. The relations of sleep and quality of life to school performance in yout with type 1 diabetes. Journal of Applied School Psychology 2014; 30(1):7–28 [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics 2009; 42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruni O, Ottaviano S, Guidetti V, et al. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. Journal of Sleep Research 1996;5(4):251–61. [DOI] [PubMed] [Google Scholar]
- 13.Johnson SB, Pollak RT, Silverstein JH, et al. Cognitive and behavioral knowledge about insulin-dependent diabetes among children and parents. Pediatrics 1982; 69(6):708–13. [PubMed] [Google Scholar]
- 14.Hazen RAFK, Fidler A, Cousino MK, Macleish SA, Gubitosi-Klug R. Sleep disruption in adolescents with Type 1 diabetes mellitus: relationships with adherence and diabetes control. Diabetes Management 2015;5(4):257–65. [Google Scholar]
- 15.Borel AL, Pepin JL, Nasse L, Baguet JP, Netter S, Benhamou PY. Short sleep duration measured by wrist actimetry is associated with deteriorated glycemic control in type 1 diabetes. Diabetes Care 2013; 36(10):2902–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu YP, Rausch J, Rohan JM, et al. Autonomy support and responsibility-sharing predict blood glucose monitoring frequency among youth with diabetes. Health Psychology 2014; 33(10):1224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caruso NC, Radovanovic B, Kennedy JD, et al. Sleep, executive functioning and behaviour in children and adolescents with type 1 diabetes. Sleep Medicine 2014;15(12): 1490–1499. [DOI] [PubMed] [Google Scholar]
- 18.Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: Implications for behavior. Annals of the New York Academy of Sciences 2004; 1021:276–91. [DOI] [PubMed] [Google Scholar]
- 19.Adolescent Sleep Working Group, Committee on Adolescent Sleep. School start times for adolescents. Pediatrics 2014; 134(3):642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meltzer LJ, Johnson C, Crosette J, Ramos M, Mindell JA. Prevalence of diagnosed sleep disorders in pediatric primary care practices. Pediatrics 2010; 125(6):e1410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ducat L, Rubenstein A, Philipson LH, Anderson BJ. A review of the mental health issues of diabetes conference. Diabetes Care 2015;38(2):333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang JL, Kirkman MS, Laffel LM, Peters AL. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 2014; 37(7):2034–54. [DOI] [PMC free article] [PubMed] [Google Scholar]