Abstract
Background
Late gadolinium enhancement cardiovascular magnetic resonance imaging (LGE CMR) is more sensitive than echocardiography for the detection of intracardiac thrombus due to its unique ability to identify thrombus based on tissue characteristics related to avascularity. The long-term prognostic significance of left ventricular (LV) thrombus detected by LGE CMR is unknown.
Methods
We performed a matched cohort study of consecutive adult patients with LV thrombus detected by LGE CMR who were matched on the date of CMR, age, and LV ejection fraction to up to three patients without LV thrombus. We investigated the long-term incidence of a composite of embolic events: stroke, transient ischemic attack, or extracranial systemic arterial embolism. We also compared outcomes among patients with LV thrombus detected by LGE CMR stratified by whether the LV thrombus was also detected by echocardiography or not.
Results
Of 157 LV thrombus patients, 155 were matched to 400 non-LV thrombus patients. Over a median follow-up of 3.3 years, the cumulative incidence of embolism was significantly higher in LV thrombus patients compared with the matched non-LV thrombus patients (p<0.001), with annualized rates of 3.7% and 0.8% for LV thrombus and matched non-LV thrombus patients respectively. LV thrombus was the only independent predictor of the composite embolic endpoint (hazard ratio 3.99; 95% confidence interval 1.54–10.35; p=0.004). The cumulative incidence of embolism was not different in patients with LV thrombus that was also detected by echocardiography versus patients with LV thrombus not detected by echocardiography (p=0.25).
Conclusions
Despite contemporary antithrombotic treatment, LV thrombus detected by LGE CMR is associated with a four-fold higher long-term incidence of embolism compared with matched non-LV thrombus patients. LV thrombus detected by LGE CMR but not by echocardiography is associated with a similar risk of embolism as that detected by both LGE CMR and echocardiography.
Keywords: Left ventricular thrombus, cardiovascular magnetic resonance, embolism, prognosis, echocardiography
INTRODUCTION
Patients with cardiomyopathy and heart failure are at increased risk for thromboembolism, which contributes to mortality and morbidity1–4. One of the mechanisms responsible for thromboembolism in heart failure is left ventricular (LV) thrombosis4. LV thrombus can be detected by cardiovascular magnetic resonance (CMR) imaging with the late gadolinium enhancement (LGE) technique, based on tissue characteristics related to its avascular nature5. Prior work has validated LV thrombus detection by LGE CMR using comparisons with pathology findings and clinical embolism at 6 months5. LGE CMR is superior to echocardiography6, 7 and is currently considered the most accurate imaging modality for the detection of LV thrombus8. It is indicated for the evaluation of suspected LV thrombus in the American College of Cardiology Foundation/American College of Radiology/American Heart Association/North American Society for Cardiovascular Imaging/Society for Cardiovascular Magnetic Resonance 2010 Expert Consensus Document on CMR9.
While it is generally believed that LV thrombus provides a substrate for embolism, studies of patients with LV thrombus detected by LGE CMR have noted few embolic events5, 10–14. It is unclear whether this lack of association is related to small sample sizes, limited duration of follow-up, or a true lack of association between LV thrombus detected by LGE CMR and embolism. Whether LV thrombus detected by LGE CMR is associated with embolism is important to know because the standard recommended treatment of LV thrombus – oral anticoagulation – is well known to be associated with an increased risk of bleeding complications14. Therefore, this knowledge has the potential to inform therapeutic decision-making and clinical follow-up of patients with LV thrombus detected by LGE CMR. The prognostic significance of LV thrombus detected by LGE CMR but not by echocardiography is also unknown. This knowledge could guide the selection of the optimal imaging modality for the screening of patients at high risk for LV thrombus.
Accordingly, the aims of this study were 2-fold: 1) to determine long-term embolic outcomes in patients with LV thrombus detected by LGE CMR, and 2) to compare the outcomes in patients with LV thrombus also detected by echocardiography with those in patients with LV thrombus not detected by echocardiography.
METHODS
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Patient selection and study design
Study patients were identified from the University of Minnesota Cardiovascular Magnetic Resonance Registry, an ongoing observational registry including all patients that undergo CMR at the University of Minnesota15–19. At our institution, all patients undergo long inversion time (TI) imaging – LGE CMR with a TI of 600 ms5, 20 – for dedicated thrombus assessment routinely as part of the protocol when LGE CMR is performed for any indication.
We used a matched cohort design with LV thrombus patients matched to non-LV thrombus patients. Consecutive adult patients referred for a clinical CMR between 2006 and 2017 who had LV thrombus detected by long TI imaging formed the LV thrombus group. From the remaining patients without LV thrombus, a group of matched patients (up to 3 per LV thrombus patient) was assembled using individual matching. First, the non-LV thrombus patients had to have had a CMR within 180 days of the LV thrombus patient’s CMR. Next, we matched patients based on age (± 5 years) and LV ejection fraction (LVEF; ± 5%), the 2 key confounders of the association between LV thrombus and embolism. While up to 3 non-LV thrombus patients could be matched to each LV thrombus patient, non-LV thrombus patients could only be matched to 1 LV thrombus patient.
To compare outcomes in patients with LV thrombus detected by echocardiography with those in patients with LV thrombus not detected by echocardiography, echocardiograms were analyzed for all patients with LV thrombus on CMR who had an echocardiogram within 10 days. Echocardiograms were performed as part of routine clinical care at the discretion of the treating physicians.
Detailed demographic, clinical, medication, and outcomes data were collected by review of the electronic medical record. The etiology of cardiomyopathy was classified as ischemic or nonischemic based on the presence or absence of angiographically significant coronary artery disease (≥70% stenosis of a major epicardial artery or ≥50% of the left main artery), in conjunction with the presence or absence of ischemic and/or non-ischemic fibrosis21. Patients with normal LVEF (>55%) and no fibrosis were deemed to have no cardiomyopathy. The study was approved by University of Minnesota’s Institutional Review Board with a waiver of signed informed consent.
CMR protocol
CMR was performed using 1.5T scanners (Siemens Sonata, Avanto, or Aera, Siemens, Malvern, Pennsylvania) with phased-array coil systems. All patients underwent a CMR protocol consisting of: 1) cine CMR for anatomical and functional assessment using a steady-state free-precession sequence (typical repetition time of 3.0 to 3.5 ms; echo time of 1.2 to 1.5 ms; in-plane spatial resolution of 1.8 x 1.4 mm; temporal resolution of 35 to 40 ms; in short-axis from base to apex, and 2-, 3-, and 4-chamber long-axis views); 2) long TI LGE CMR sequence for detection of intracardiac thrombus5, 20 [single-shot inversion recovery, steady-state free-precession, multiple slices in short axis, 2-chamber long axis and 4-chamber long axis orientations obtained 5 to 10 min after gadolinium (0.15 mmol/kg) administration; using a TI set at 600 ms to selectively null avascular tissue such as thrombus; in-plane spatial resolution of 2.1 × 1.6 mm; slice thickness of 4 mm); and 3) LGE CMR for tissue characterization performed 10 to 15 min after gadolinium administration, using a segmented inversion-recovery sequence (set to null viable myocardium using a TI of 250 to 350 ms; in-plane spatial resolution of 1.8 x 1.5 mm; temporal resolution of 180 to 200 ms; slice thickness of 6 mm, in short-axis from base to apex, and 2-, 3-, and 4-chamber long-axis views).
Echocardiography protocol
Echocardiograms were performed by experienced sonographers using commercial equipment. Images were acquired in standard orientations according to recommendations from the American Society of Echocardiography22. Echocardiography contrast agents (perflutren lipid [Definity, Lantheus Medical Imaging, North Billerica, Massachusetts] or protein [Optison, GE Healthcare, Waukesha, Wisconsin] microspheres) were injected for cavity opacification, endocardial border delineation, and identification of LV thrombus, if deemed clinically necessary.
CMR analyses
CMR analyses were performed for this study by an experienced CMR cardiologist blinded to clinical and echocardiography data. An LV thrombus was detected on long TI LGE CMR images based on its location within the LV cavity and appearance with no signal intensity (i.e., homogeneously black) due to the absence of vascularity, surrounded by structures with contrast uptake (LV cavity and myocardium). An LV thrombus was categorized as protuberant if its borders were distinct from the adjacent endocardium and it protruded into the LV cavity, or as mural, if its borders were contiguous with the adjacent endocardium, as previously described7, 23. If >1 thrombus was present, the morphology was characterized as protuberant if any thrombus protruded into the LV cavity. An LV thrombus was categorized as mobile if it was noted to be independently mobile on cine CMR imaging. If an LV thrombus could not be distinctly detected on cine CMR, it was categorized as not mobile. LV thrombus volume was quantified by planimetry. Thrombus location was scored based on adjacent myocardial segments using the standard American Heart Association 17-segment LV model.
Left and right ventricular volumes were quantified by planimetry of the end-diastolic and -systolic endocardial borders on a stack of short-axis cine CMR images acquired from base to apex, which were used to calculate the respective end-diastolic and -systolic volumes and LVEFs. The presence of an LV aneurysm was assessed on cine CMR images. An LV aneurysm was defined as a discrete akinetic or dyskinetic protrusion interrupting the normal LV contour during both diastole and systole5, 24.
The extent of LGE was semi-quantitatively assessed on a 17-segmental basis based on the area of hyperenhanced myocardium on LGE CMR images on a 5-point scale as previously described5, 25: 0 = no hyperenhancement; 1 = 1% to 25%; 2 = 26% to 50%; 3 = 51% to 75%; 4 = 76% to 100%. The global LGE extent was assessed as a percentage of the LV myocardium by adding the segmental scores weighted by the mid-point of the range of hypereenhancement and dividing by 17 for the total number of segments5, 25. This method of LGE quantification has been shown to have similar reproducibility as automated quantification methods26.
Echocardiography analyses
Echocardiography analyses were performed for this study by an experienced echocardiography cardiologist (different from the cardiologist that performed the CMR analyses) blinded to clinical and CMR data. To reduce the potential for observer bias, echocardiograms from non-LV thrombus patients were also included in the analyses. LV thrombus was detected using established anatomic criteria; it was defined as a mass within the LV cavity with margins distinct from the endocardium and distinguishable from papillary muscles, chordae, trabeculations, or technical artifacts27.
Assessment of clinical outcomes
Clinical outcomes were assessed from the electronic medical record by an investigator blinded to CMR data and verified by a second independent investigator. At our institution, patients with LV thrombus without a contraindication to antithrombotic therapy are treated with anticoagulation for 3 months28–30, unless there is a secondary reason (e.g., atrial fibrillation) to continue it indefinitely. The study endpoint was a composite of stroke, transient ischemic attack (TIA) or extracranial systemic arterial embolism after the CMR. Stroke was defined as a rapid onset of a documented focal neurologic deficit lasting 24 hours or until death, or if < 24 hours, there was a clinically relevant lesion on brain imaging. Patients with focal neurologic deficits secondary to brain trauma, tumor, infection, or other non-vascular cause were excluded31, 32. TIA was defined as a documented episode(s) of focal neurologic deficit lasting 30 seconds to 24 hours and without brain imaging suggesting stroke32. Extracranial systemic arterial embolism was defined as a documented extracranial arterial embolism manifesting as a sudden loss of perfusion of a limb or organ. It includes upper and lower extremity, renal, mesenteric, and abdominal aortic systems33.
We also assessed for major bleeding as defined by the Bleeding Academic Research Consortium Definition for Bleeding34, and the following censoring events: death, orthotopic heart transplantation, and left ventricular assist device implantation. Mortality status and death dates were cross-verified with records from the Minnesota Department of Health’s Office of Vital Records and the US Social Security Death Index.
Statistical analyses
Statistical analyses were performed using R version 3.6.0 (2019–04-26). Continuous variables were expressed as means and standard deviations, or medians and interquartile ranges (IQR) for data that were not normally distributed. The sensitivity of echocardiography to detect LV thrombus was calculated with LGE CMR as the reference standard. The Kaplan-Meier method was used to estimate the cumulative incidence of the composite embolic endpoint. Death, orthotopic heart transplantation, and left ventricular assist device implantation were considered censoring events. Follow-up was limited to 8 years after the CMR. Univariable and multivariable Cox proportional hazard regression analyses were used to assess variables associated with the composite embolic endpoint. The multivariate model included variables that were a priori deemed to be confounders of the relationship between LV thrombus as an exposure and embolism as an outcome: CHA2DS2-VASc score, LVEF on CMR, and anticoagulation after CMR. Since the number of outcome events was anticipated to be modest, the CHA2DS2-VASc score was included as an integrated marker of the risk of thromboembolism related and unrelated to atrial fibrillation. The CHA2DS2-VASc score is a simple clinical risk score used to estimate the risk of thromboembolism in patients with atrial fibrillation, and includes points for congestive heart failure or LV dysfunction, hypertension, age ≥75 years (doubled), diabetes, stroke/TIA/thromboembolism (doubled), vascular disease (prior myocardial infarction [MI], peripheral artery disease, or aortic plaque), age 65–74 years, and female sex, with 10 possible points and higher scores indicating higher risk35. The score is associated with the risk of ischemic stroke, thromboembolism, and death among patients with incident heart failure with or without atrial fibrillation36. “Time 0” for the time-to-event analyses was the time of CMR. Follow-up time was defined as the time between the date of CMR and the date of an embolic event, censoring event, or end of follow-up, whichever came first. After fitting the Cox regression model, we tested the proportional hazards assumption by analysis of Schoenfeld residuals for the global test and the scaled Schoenfeld residuals for individual covariates. To evaluate the incremental prognostic value of LV thrombus, the final model was compared with a model in which LV thrombus was not included, using the likelihood ratio chi-square test. All statistical comparisons were 2 tailed, and a p value of <0.05 was considered as indicating statistical significance.
RESULTS
Of 7,492 consecutive unique adult patients that underwent CMR with contrast between January 1, 2006 and December 31, 2017, 157 were found to have LV thrombus on long TI LGE CMR. Half of these patients were referred after an LV thrombus was suspected on other imaging, while the rest were referred for evaluation of cardiomyopathy. The remaining 7,335 patients were used for the selection of the matched non-LV thrombus patients. Of the 157 LV thrombus patients, 110 were matched to 3 non-LV thrombus patients each, 25 were matched to 2 matched non-LV thrombus patients each, 20 were matched to 1 matched non-LV thrombus patients each, and 2 had no matches. Thus, for the matched comparisons, there were 155 LV thrombus patients and 400 matched non-LV thrombus patients. All 157 LV thrombus patients were included in analyses not involving comparisons with the non-LV thrombus patients.
Patient characteristics
Clinical and LV characteristics on CMR of the LV thrombus and the matched non-LV thrombus groups are shown in Table 1. The 2 groups were well matched for age and sex. The prevalence of coronary artery disease, MI, and ischemic cardiomyopathy were significantly higher in the LV thrombus patients compared with the matched non-LV thrombus patients, consistent with prior literature5. LV thrombus patients had a higher prevalence of cerebrovascular disease before the CMR. The prevalence of atrial fibrillation was not significantly different between the 2 groups. The median CHA2DS2-VASc score was significantly higher in the LV thrombus group compared with the non-LV thrombus group. The use of anticoagulants at the time of CMR did not differ between the 2 groups. On CMR, the mean LVEF was significantly lower at 27.3% for the LV thrombus patients compared with 32.5% for the matched non-LV thrombus patients. In concordance with the higher prevalence of ischemic cardiomyopathy, there were significantly higher prevalence of LV aneurysms, any LGE, ischemic LGE, and global LGE extent in the LV thrombus group.
Table 1.
Baseline characteristics of patients with LV thrombus (n = 155) and matched non-LV thrombus patients (n = 400)
| Characteristics | LV thrombus patients | Matched non-LV thrombus patients | P value |
|---|---|---|---|
| Age, years ± SD | 57.8 ± 14.8 | 59.0 ± 13.8 | 0.36 |
| Male, % | 110 (71) | 273 (68.3) | 0.53 |
| Body mass index, kg/m2 ± SD | 28.1 ± 6.3 | 29.6 ± 6.5 | 0.010 |
| Body surface area, m2 ± SD | 2.1 ± 0.3 | 2.2 ± 0.3 | 0.026 |
| Hypertension, % | 96 (61.9) | 266 (66.5) | 0.31 |
| Dyslipidemia, % | 97 (62.6) | 235 (58.8) | 0.41 |
| Diabetes mellitus, % | 30 (19.4) | 110 (27.5) | 0.048 |
| Tobacco use, % | 94 (60.6) | 233 (58.2) | 0.61 |
| Atrial fibrillation or flutter, % | 20 (12.9) | 55 (13.8) | 0.79 |
| Known coronary artery disease, % | 101(65.2) | 136 (34.0) | <0.001 |
| Myocardial infarction, % | 78 (50.3) | 86 (21.5) | <0.001 |
| Recent myocardial infarction, % | 39 (25.2) | 11 (2.8) | <0.001 |
| Percutaneous coronary intervention, % | 40 (25.8) | 73 (18.3) | 0.047 |
| Coronary artery bypass graft surgery, % | 10 (6.5) | 33 (8.3) | 0.47 |
| Cerebrovascular disease, % | 33 (21.3) | 33 (8.3) | <0.001 |
| Peripheral vascular disease, % | 14 (9.0) | 31 (7.8) | 0.62 |
| CHA2DS2-VASc score, (IQR) | 4 (2, 5) | 3 (2, 4) | 0.010 |
| Medications at the time of CMR | |||
| Aspirin, % | 61 (39.4) | 193 (48.3) | 0.059 |
| Thienopyridine, % | 17 (11.0) | 40 (10.0) | 0.74 |
| Warfarin, % | 15 (9.7) | 47 (11.8) | 0.49 |
| Other novel anticoagulants, % | 2 (1.3) | 12 (3.0) | 0.25 |
| Beta-blocker, % | 80 (51.6) | 216 (54.0) | 0.61 |
| ACE-inhibitor or ARB, % | 77 (49.7) | 204 (51.0) | 0.78 |
| Statin, % | 69 (44.5) | 175 (43.8) | 0.87 |
| Loop diuretic, % | 36 (23.2) | 100 (25.0) | 0.66 |
| Spironolactone, % | 15 (9.7) | 25 (6.3) | 0.16 |
| Digoxin, % | 3 (1.9) | 8 (2.0) | 0.96 |
| LV characteristics on CMR | |||
| LVEF, % ± SD | 27.3 ± 13.1 | 32.5 ± 12.9 | <0.001 |
| LVEDV, ml (IQR) | 222.0 (161.3 – 276.0) | 201.0 (145.7 – 265.4) | 0.08 |
| LVEDVI, ml/m2 (IQR) | 101.4 (74.3 – 129.7) | 62.9 (39.7 – 88.6) | 0.007 |
| LVESV, ml (IQR) | 155.3 (109.0 – 220.5) | 137.8 (85.9 – 195.6) | 0.008 |
| LVESVI, ml/m2 (IQR) | 72.9 (50.1 – 101.9) | 62.9 (39.7 – 88.6) | <0.001 |
| LV aneurysm, % | 52 (33.5) | 36 (9.0) | <0.001 |
| Any LGE, % | 141 (91.0) | 220 (55.0) | <0.001 |
| Ischemic LGE, % | 123 (79.4) | 168 (42.0) | <0.001 |
| Non-ischemic LGE, % | 23 (14.8) | 59 (14.8) | 0.98 |
| Global LGE extent, % | 22.0 (12.0 – 32.5) | 2.0 (0.0 – 21.2) | <0.001 |
| Etiology of cardiomyopathy | |||
| Ischemic, % | 115 (74.2) | 159 (39.8) | <0.001 |
| Non-ischemic, % | 40 (25.8) | 208 (52.0) | |
| No cardiomyopathy, % | – | 33 (8.3) |
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CMR = cardiovascular magnetic resonance; IQR = interquartile range; LGE = late gadolinium enhancement; LV = left ventricle; LVEDV = left ventricular end-diastolic volume; LVEDVI = left ventricular end-diastolic volume index; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; LVESVI = left ventricular end-systolic volume index; SD = standard deviation
Table 2 lists the LV thrombus characteristics. Eighty-one percent of patients had 1 LV thrombus. Sixty-nine percent of patients had protuberant LV thrombus. Ninety percent of patients had apical LV thrombus. Fifteen percent had mobile LV thrombus.
Table 2.
LV thrombus characteristics (n = 157)
| Number, % | |
|---|---|
| 1 | 127 (80.9) |
| 2 | 25 (15.9) |
| 3 | 5 (3.2) |
| Morphology | |
| Protuberant, % | 108 (68.8) |
| Mural, % | 49 (31.2) |
| Volume, cm3 (IQR) | 2.1 (1.1 – 4.8) |
| Apical location, % | 142 (90.4) |
| Mobile, % | 24 (15.3) |
| Adjacent to LGE, % | 134 (85.4) |
| Additional intracardiac thrombi, % | 21 (13.4) |
| Detected by echocardiography, % | 53/110 (48.2) |
| Detected by non-contrast echocardiography, % | 15/40 (37.5) |
| Detected by contrast echocardiography, % | 38/70 (54.3) |
IQR = interquartile range; LGE = late gadolinium enhancement; LV = left ventricle
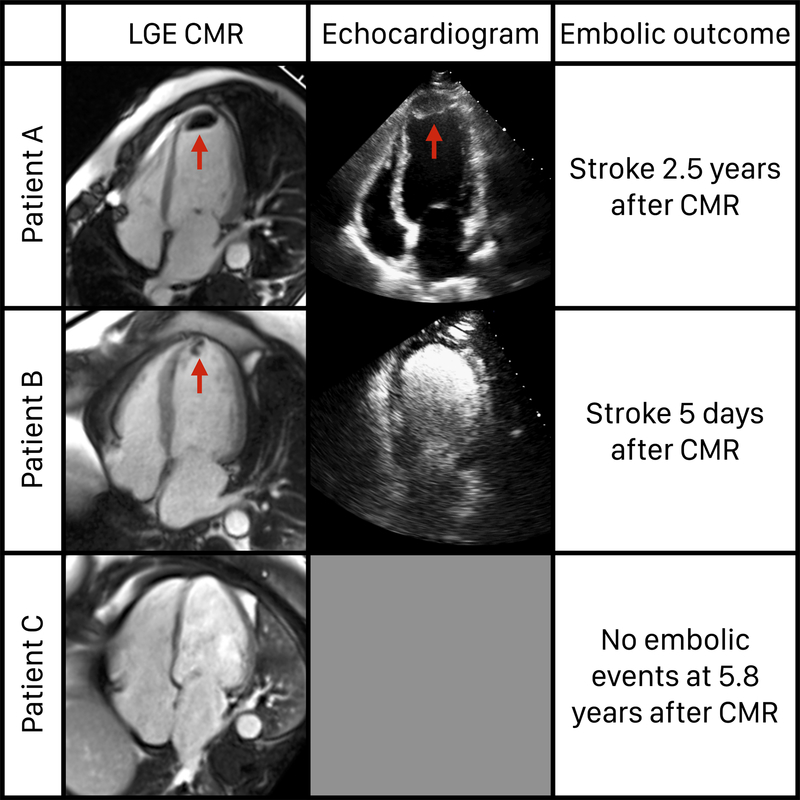
Of the 157 LV thrombus patients, 110 (70%) had echocardiography performed within 10 days of the CMR. The median time between CMR and echocardiography was 2 days (IQR 1–3 days). Among the 110 patients, LV thrombus was detected by echocardiography in 48%. The sensitivity of non-contrast echocardiography was 37% and that of contrast echocardiography was 54%. Examples of study patients are shown in Figure 1.
Figure 1. Examples of study patients.
Patient A had LV thrombus detected on LGE CMR and echocardiography and had an embolic outcome. Patient B had LV thrombus detected on LGE CMR but not echocardiography, and also had an embolic outcome. Patient C had no LV thrombus detected on LGE CMR and had no embolic outcome.
Management of LV thrombus after CMR
After detection of LV thrombus by LGE CMR, 111 (71%) patients were started or continued on aspirin, 50 (32%) patients were started or continued on a thienopyridine, and 139 (89%) patients were started or continued on an anticoagulant. Of those on an anticoagulant, 125 (90%) were on warfarin, 3 (2%) were on low-molecular-weight heparin, and 11 (8%) were on direct oral anticoagulants. Thirty (22%) were also on aspirin and a thienopyridine (triple antithrombotic therapy), 66 (48%) were also on aspirin without a thienopyridine, and 10 (7%) were also on a thienopyridine without aspirin. Thus, only 32 (23%) were on an anticoagulant without concomitant aspirin or a thienopyridine.
Among the 18 patients not treated with anticoagulation, reasons for not starting anticoagulation were: high bleeding risk (n = 9), patient refusal (n = 1), anticipated non-compliance (n = 1), and could not be ascertained from a review of the medical records (n = 7). Of these, 9 (50%) were on aspirin and a thienopyridine, 5 (28%) were on aspirin only, and 1 (6%) was on a thienopyridine only.
Two patients underwent surgical removal of LV thrombus for large thrombus burden: 1 was a 22-year-old male with a hypercoagulable state due to myelodysplastic syndrome and a large protuberant LV thrombus, and the other was a 57-year-old male with a basal inferior aneurysm and a large LV pseudoaneurysm, both filled with thrombus, who underwent repair of the pseudoaneurysm.
Embolism in LV thrombus versus matched non-LV thrombus patients
Over a median follow-up of 3.3 years (IQR 1.7–5.5 years), 31 patients reached the embolic endpoint: 19 in the LV thrombus group and 12 in the matched non-LV thrombus group. The annualized rate of embolism was 3.7% for LV thrombus patients and 0.8% for matched non-LV thrombus patients.
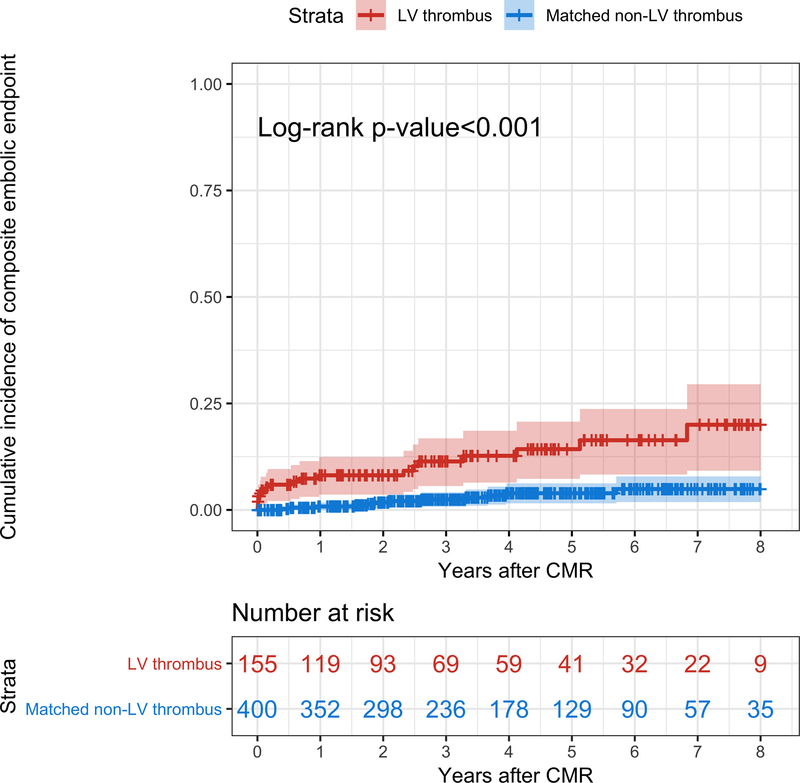
On Kaplan-Meier analyses, the cumulative incidence of embolism was significantly higher in LV thrombus patients than in matched non-LV thrombus patients (p<0.001) (Figure 2). Censoring events included death in 143, orthotopic heart transplantation in 18, and left ventricular assist device implantation in 17 patients.
Figure 2. Incidence of embolism in LV thrombus patients compared with matched non-LV thrombus patients.
Kaplan-Meier curves demonstrate the cumulative incidence of the composite embolic endpoint in the LV thrombus (in red) and in the matched non-LV thrombus (in blue) groups. Note the significant difference in the cumulative incidence of embolic events between the 2 groups.
On Cox univariable analyses (Table 3), variables associated with the embolic endpoint were known coronary artery disease, peripheral vascular disease, LV thrombus, LV aneurysm, ischemic LGE, global LGE extent, and anticoagulation. On Cox multivariable analyses (Table 4), LV thrombus was the only independent predictor of the composite embolic endpoint with a hazard ratio of 3.99 (95% CI 1.54–10.35; p = 0.004) after adjustment for the CHA2DS2-VASc score, LVEF, and anticoagulation after CMR.
Table 3.
Univariable associations of the composite embolic endpoint among LV thrombus (n = 155) and matched non-LV thrombus patients (n = 400)
| Hazard ratio (95% CI) | P value | |
|---|---|---|
| Age | 0.99 (0.96 – 1.01) | 0.35 |
| Male | 0.66 (0.32 – 1.36) | 0.26 |
| Body mass index | 0.96 (0.90 – 1.02) | 0.18 |
| Body surface area | 0.37 (0.11 – 1.23) | 0.11 |
| Hypertension | 0.85 (0.41 – 1.76) | 0.67 |
| Dyslipidemia | 1.78 (0.80 – 3.98) | 0.16 |
| Diabetes mellitus | 1.95 (0.94 – 4.02) | 0.07 |
| Tobacco use | 1.04 (0.50 – 2.14) | 0.92 |
| Atrial fibrillation or flutter | 0.47 (0.11 – 1.95) | 0.30 |
| Known coronary artery disease | 2.40 (1.15 – 5.02) | 0.020 |
| Recent myocardial infarction | 2.23 (0.86 – 5.82) | 0.10 |
| Cerebrovascular disease | 1.95 (0.80 – 4.76) | 0.14 |
| Peripheral vascular disease | 4.04 (1.74 – 9.41) | 0.001 |
| CHA2DS2-VASc score | 1.15 (0.93 – 1.43) | 0.19 |
| LV thrombus on CMR | 4.67 (2.26 – 9.62) | <0.001 |
| LVEF on CMR | 0.98 (0.95 – 1.00) | 0.10 |
| LVEDVI on CMR | 1.00 (0.99 –1.01) | 0.94 |
| LVESVI on CMR | 1.00 (0.99 – 1.01) | 0.68 |
| LV aneurysm on CMR | 3.45 (1.67 – 7.11) | <0.001 |
| Ischemic LGE on CMR | 2.57 (1.15 – 5.75) | 0.021 |
| Non-ischemic LGE on CMR | 0.62 (0.19 – 2.05) | 0.43 |
| Global LGE extent on CMR | 1.03 (1.00 – 1.05) | 0.019 |
| Aspirin after CMR | 2.12 (0.95 – 4.75) | 0.07 |
| Thienopyridine after CMR | 1.92 (0.90 – 4.07) | 0.09 |
| Anticoagulation after CMR | 2.84 (1.36 – 5.92) | 0.005 |
| Ischemic cardiomyopathy | 0.89 (0.50 – 1.59) | 0.70 |
CI = confidence interval; CMR = cardiovascular magnetic resonance; LGE = late gadolinium enhancement; LV = left ventricle; LVEDVI = left ventricular end-diastolic volume index; LVEF = left ventricular ejection fraction; LVESVI = left ventricular end-systolic volume index
Table 4.
Multivariable associations of the composite embolic endpoint among LV thrombus (n = 155) and matched non-LV thrombus patients (n = 400)
| Hazard ratio (95% CI) | P value | |
|---|---|---|
| CHA2DS2-VASc score | 1.12 (0.90 – 1.38) | 0.30 |
| LVEF on CMR | 0.98 (0.95 – 1.01) | 0.25 |
| Anticoagulation after CMR | 1.10 (0.42 – 2.88) | 0.85 |
| LV thrombus | 3.99 (1.54 – 10.35) | 0.004 |
CI = confidence interval; CMR = cardiovascular magnetic resonance; LV = left ventricle; LVEF = left ventricular ejection fraction
The proportional hazards assumption was valid for all covariates individually and the model overall (p = 0.36). The addition of LV thrombus to a Cox model that included the CHA2DS2-VASc score, LVEF, and anticoagulation after CMR resulted in a significantly improved model fit as assessed with the likelihood ratio test (p = 0.003), suggesting an incremental prognostic value for the composite embolic endpoint.
Embolism in patients with LV thrombus on CMR also detected by echocardiography versus not
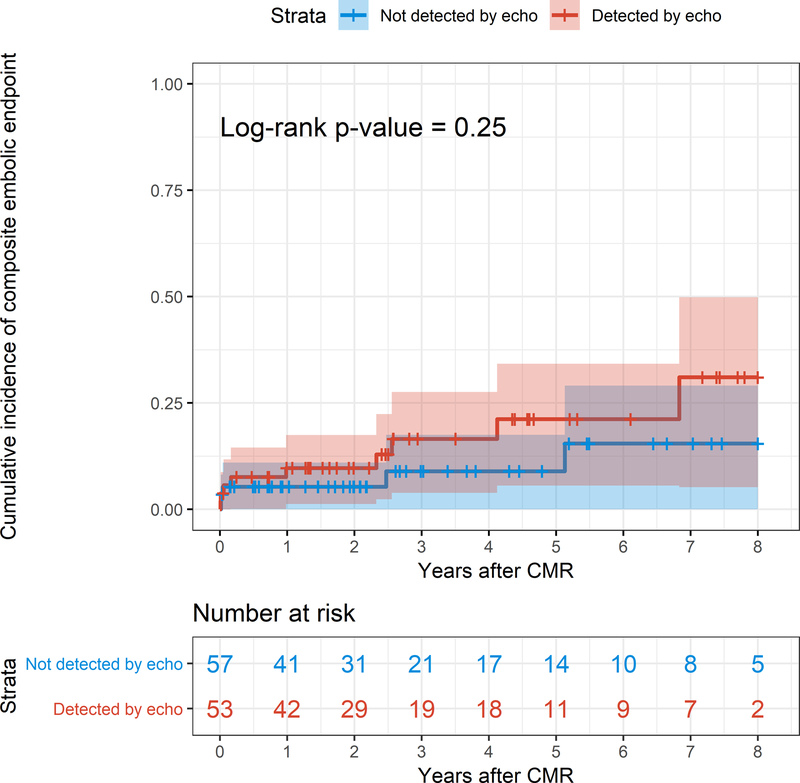
Kaplan-Meier analysis comparing patients with LV thrombus on CMR that was also detected by echocardiography (n = 53) versus not (n = 57) showed no significant difference in the incidence of the composite embolic endpoint (p = 0.25) (Figure 3).
Figure 3. Incidence of embolism in patients with LV thrombus detected by echocardiography compared with patients with LV thrombus not detected by echocardiography.
Kaplan-Meier curves demonstrate the cumulative incidence of the composite embolic endpoint in patients with LV thrombus detected by echocardiography (in red) and in patients with LV thrombus not detected by echocardiography (in blue). Note the lack of a significant difference in the cumulative incidence of embolic events between the 2 groups.
Embolism in patients with LV thrombus characteristics associated with detection by echocardiography versus not
Since only 110 (70%) of LV thrombus patients had echocardiography data, we also analyzed all 157 LV thrombus patients by stratifying them according to characteristics associated with detection by echocardiography. LV thrombus on CMR that was also detected by echocardiography was more likely to be protuberant in morphology, larger in volume, and mobile (Supplemental Table 1) compared with LV thrombus not detected on echocardiography.
Kaplan-Meier analysis comparing LV thrombus patients stratified by protuberant versus mural morphologies showed no significant difference in the incidence of the composite embolic endpoint (p = 0.39) (Supplemental Figure 1). Similarly, an analysis comparing LV thrombus patients stratified by volume median versus < median showed no significant difference in the incidence of the composite embolic endpoint (p = 0.50) (Supplemental Figure 2). Finally, an analysis comparing LV thrombus patients stratified by mobility showed no significant difference in the incidence of the composite embolic endpoint (p = 0.12) (Supplemental Figure 3). Thus, the risks of embolism associated with a mural, smaller, and immobile thrombus were not different from those associated with a protuberant, larger, and mobile thrombus respectively.
Major bleeding
Major bleeding occurred in 26 patients: 13 (8.4%) in the LV thrombus group and 13 (3.2%) in the matched non-LV thrombus group. LV thrombus patients had a significantly higher rate of major bleeding compared with matched non-LV thrombus patients (p = 0.010).
DISCUSSION
In a large cohort of patients with LV thrombus detected by LGE CMR, there was an annualized incidence of embolism of 3.7%, despite contemporary antithrombotic treatment with anticoagulants in 89% of patients. This was significantly higher than the 0.8% annualized incidence of embolism in matched non-LV thrombus patients. Next, among patients with LV thrombus detected by LGE CMR, those not detected by echocardiography had a similar rate of embolism as patients with LV thrombus detected by echocardiography. Finally, when patients with LV thrombus were stratified by thrombus characteristics associated with detection by echocardiography – morphology, size, and mobility – there were no significant differences in the incidence of embolism between the groups with and without the characteristics.
Prior studies of LV thrombus detected by LGE CMR have included few patients with LV thrombus and/or had a relatively short-term follow-up, leading to very few or no patients with embolism (Table 5)5, 10–13, 37, 38. In comparison with the prior studies, ours includes the largest number of patients with LV thrombus detected by LGE CMR, almost 3 times more than the next largest study by Weinsaft et al. with 55 LV thrombus patients5. At a median follow-up of 3.3 years (mean 194 weeks), our follow-up is the longest reported thus far, which together with a large number of LV thrombus patients, provided us with more than twice as many embolic events as the total number of events in all prior studies combined. Additionally, our cohort includes consecutive patients with LV thrombus, regardless of the etiology of the cardiomyopathy. Thus, compared with the findings of most prior studies that were limited to acute MI patients11–13, 37, ours are more generalizable to all-comer patients with LV thrombus detected by LGE CMR.
Table 5.
Embolism in published studies of LV thrombus detected by LGE CMR
| Study | Number of patients with LV thrombus | Duration of follow up | Number of embolic events | Type(s) of embolic events |
|---|---|---|---|---|
| Weinsaft et al.5 | 55 | 6 months | 3 | 2 strokes, 1 TIA |
| Weir et al.10 | 15 | 6 months | 0 | |
| Delewi et al.11 | 17 | Up to 24 months | 0 | |
| Meurin et al.35 | 19 | 270 days (median) | 1 | Extracranial systemic arterial embolism |
| Poss et al.12 | 26 | 12 months | 1 | Stroke |
| Cambronero-Cortinas et al.13 | 27 | 181 weeks (mean) | 0 | |
| Merkler et al.36 | 33 | In-hospital | 3 | Ischemic strokes |
TIA = transient ischemic attack
Compared with LGE CMR as the reference standard, echocardiography has been shown to have a sensitivity of 24–37% when contrast is not used13, 39, 40, with a higher sensitivity of up to 64% when contrast is used6, 40. While LGE CMR identifies twice as many LV thrombi as echocardiography, whether the additional thrombi detected by LGE CMR but not by echocardiography carry the same prognostic significance as those detected by echocardiography has been unknown. This question is particularly of interest since thrombi detected by LGE CMR but not by echocardiography tend to be small and mural6; these are intuitively not as worrisome as the large and protuberant thrombi detected by echocardiography.
We demonstrate that LV thrombus detected by LGE CMR but not by echocardiography is associated with a similar rate of embolism as that detected by both LGE CMR and echocardiography. We also used a second approach to study this topic; we assessed embolism in LV thrombus patients stratified by 3 characteristics significantly associated with detection by echocardiography: protuberant morphology, large size, and mobility. This allowed us to compare rates of embolism within the entire group of LV thrombus patients, rather than only the subgroup with echocardiograms. Again, we found no differences in the rates of embolism in all 3 subgroup analyses. This validates our finding that LV thrombus detected by LGE CMR but not by echocardiography carries a similar risk of embolism as LV thrombus detected by both LGE CMR and echocardiography.
Implications
Our study shows that patients with LV thrombus detected by LGE CMR have a high long-term risk for embolism despite contemporary antithrombotic treatment. These findings highlight the need for research on the appropriate management of LV thrombus focusing on the types, doses, and duration of antithrombotic treatments. For patients with LV thrombus in the setting of an acute MI, guidelines typically recommend 3 months of treatment28–30. This recommendation is based on studies showing that the risk of stroke after an acute MI is highest during the first 1–2 weeks with a subsequent decline over 3 months41–43. There are no recommendations for the treatment of LV thrombus in a non-MI setting. We show that in all-comers with LV thrombus detected by LGE CMR, the risk of embolism persists well beyond 3 months.
Since patients with LV thrombus detected by LGE CMR but not by echocardiography have a similar rate of embolism as those with LV thrombus detected by both LGE and echocardiography, there may be a role for the routine use of LGE CMR in place of echocardiography for the detection of LV thrombus in high-risk patients. Such patients include those with severe cardiomyopathy, high myocardial scar burden, apical wall motion abnormalities after an acute MI40, an acute cardioembolic event44, or a left ventricular aneurysm45.
Limitations
Our study was limited by its single-center observational design, in which participants were patients clinically referred for CMR. Therefore, referral bias is inevitable. The causes of death were not known in many cases, and it is possible that instances of embolism resulting in death were missed. The subgroup of patients that were not anticoagulated was too small to study the impact of anticoagulation on clinical outcomes. Due to the retrospective nature of the study, it was not feasible to obtain reliable measures of the efficacy of anticoagulation such as the time in therapeutic range in all LV thrombus patients. Similarly, our study design limits our ability to determine the optimal duration of anticoagulation after the detection of LV thrombus. For comparisons between CMR and echocardiography, only patients with echocardiograms performed within 10 days of the CMR were included, which may be a biased subgroup. Echocardiographic image quality was not assessed.
Conclusions
Despite contemporary antithrombotic treatment, patients with LV thrombus detected by LGE CMR have a significantly higher long-term incidence of embolism compared with matched non-LV thrombus patients. Patients with LV thrombus detected by LGE CMR but not by echocardiography have a similar rate of embolism as those with LV thrombus detected by both LGE CMR and echocardiography.
Supplementary Material
SHORT COMMENTARY.
Late gadolinium enhancement cardiovascular magnetic resonance imaging (LGE CMR) is more sensitive than echocardiography for the detection of intracardiac thrombus. However, two important questions have remained unanswered: 1) What is the long-term prognostic significance of left ventricular (LV) thrombus detected by LGE CMR? 2) What is the prognostic significance of LV thrombus detected by LGE CMR but not by echocardiography? To investigate these, we performed a large matched cohort study of consecutive adult patients with LV thrombus detected by LGE CMR who were matched to patients without LV thrombus. We investigated the long-term incidence of a composite of embolic events: stroke, transient ischemic attack, or extracranial systemic arterial embolism. We then compared outcomes among patients with LV thrombus detected by LGE CMR stratified by whether the LV thrombus was also detected by echocardiography or not. We found that despite antithrombotic treatment, patients with LV thrombus detected by LGE CMR have a significantly higher long-term incidence of embolism compared with matched non-LV thrombus patients. These findings highlight the need for research on the appropriate management of LV thrombus focusing on the types, doses, and duration of antithrombotic treatments. We also found that patients with LV thrombus detected by LGE CMR but not by echocardiography have a similar rate of embolism as those with LV thrombus detected by both LGE CMR and echocardiography. These findings indicate that there may be a role for the routine use of LGE CMR in place of echocardiography for the detection of LV thrombus in high-risk patients.
Acknowledgments
SOURCES OF FUNDING
Christopher Choo was supported by The Melvin P. Nelson & Esther L. Nelson Heart Scholarship from the American Heart Association Midwest Affiliate for this research project. Mehmet Akçakaya was supported by NIH grant R00HL111410. Jonathan W. Weinsaft was supported by NIH grant R01HL128278. Chetan Shenoy was supported by NIH grant K23HL132011, University of Minnesota Clinical and Translational Science Institute KL2 Scholars Career Development Program Award (NIH grant KL2TR000113-05), and NIH grant UL1TR000114.
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Kamel H and Healey JS. Cardioembolic Stroke. Circ Res. 2017;120:514–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lip GY, Ponikowski P, Andreotti F, Anker SD, Filippatos G, Homma S, Morais J, Pullicino P, Rasmussen LH, Marin F, et al. Thrombo-embolism and antithrombotic therapy for heart failure in sinus rhythm. A joint consensus document from the ESC Heart Failure Association and the ESC Working Group on Thrombosis. Eur J Heart Fail. 2012;14:681–95. [DOI] [PubMed] [Google Scholar]
- 3.Loh E, Sutton MS, Wun CC, Rouleau JL, Flaker GC, Gottlieb SS, Lamas GA, Moye LA, Goldhaber SZ and Pfeffer MA. Ventricular dysfunction and the risk of stroke after myocardial infarction. N Engl J Med. 1997;336:251–7. [DOI] [PubMed] [Google Scholar]
- 4.Bettari L, Fiuzat M, Becker R, Felker GM, Metra M and O’Connor CM. Thromboembolism and antithrombotic therapy in patients with heart failure in sinus rhythm: current status and future directions. Circ Heart Fail. 2011;4:361–8. [DOI] [PubMed] [Google Scholar]
- 5.Weinsaft JW, Kim HW, Shah DJ, Klem I, Crowley AL, Brosnan R, James OG, Patel MR, Heitner J, Parker M, et al. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol. 2008;52:148–57. [DOI] [PubMed] [Google Scholar]
- 6.Weinsaft JW, Kim RJ, Ross M, Krauser D, Manoushagian S, LaBounty TM, Cham MD, Min JK, Healy K, Wang Y, et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc Imaging. 2009;2:969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinsaft JW, Kim HW, Crowley AL, Klem I, Shenoy C, Van Assche L, Brosnan R, Shah DJ, Velazquez EJ, Parker M, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging. 2011;4:702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy CP, Vaduganathan M, McCarthy KJ, Januzzi JL Jr., Bhatt DL and McEvoy JW. Left Ventricular Thrombus After Acute Myocardial Infarction: Screening, Prevention, and Treatment. JAMA Cardiol. 2018;3:642–649. [DOI] [PubMed] [Google Scholar]
- 9.American College of Cardiology Foundation Task Force on Expert Consensus D, Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, Ho VB, Jerosch-Herold M, Kramer CM, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;121:2462–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weir RA, Martin TN, Petrie CJ, Murphy A, Clements S, Steedman T, Wagner GS, McMurray JJ and Dargie HJ. Cardiac and extracardiac abnormalities detected by cardiac magnetic resonance in a post-myocardial infarction cohort. Cardiology. 2009;113:1–8. [DOI] [PubMed] [Google Scholar]
- 11.Delewi R, Nijveldt R, Hirsch A, Marcu CB, Robbers L, Hassell ME, de Bruin RH, Vleugels J, van der Laan AM, Bouma BJ, et al. Left ventricular thrombus formation after acute myocardial infarction as assessed by cardiovascular magnetic resonance imaging. Eur J Radiol. 2012;81:3900–4. [DOI] [PubMed] [Google Scholar]
- 12.Poss J, Desch S, Eitel C, de Waha S, Thiele H and Eitel I. Left Ventricular Thrombus Formation After ST-Segment-Elevation Myocardial Infarction: Insights From a Cardiac Magnetic Resonance Multicenter Study. Circ Cardiovasc Imaging. 2015;8:e003417. [DOI] [PubMed] [Google Scholar]
- 13.Cambronero-Cortinas E, Bonanad C, Monmeneu JV, Lopez-Lereu MP, Gavara J, de Dios E, Rios C, Perez N, Racugno P, Paya A, et al. Incidence, Outcomes, and Predictors of Ventricular Thrombus after Reperfused ST-Segment-Elevation Myocardial Infarction by Using Sequential Cardiac MR Imaging. Radiology. 2017;284:372–380. [DOI] [PubMed] [Google Scholar]
- 14.Bulluck H, Chan MHH, Paradies V, Yellon RL, Ho HH, Chan MY, Chin CWL, Tan JW and Hausenloy DJ. Incidence and predictors of left ventricular thrombus by cardiovascular magnetic resonance in acute ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention: a meta-analysis. J Cardiovasc Magn Reson. 2018;20:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H, Nijjar PS, Misialek JR, Blaes A, Derrico NP, Kazmirczak F, Klem I, Farzaneh-Far A and Shenoy C. Accuracy of left ventricular ejection fraction by contemporary multiple gated acquisition scanning in patients with cancer: comparison with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2017;19:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin LQ, Kazmirczak F, Chen KA, Okasha O, Nijjar PS, Martin CM, Akcakaya M, Farzaneh-Far A and Shenoy C. Impact of Cardiovascular Magnetic Resonance Imaging on Identifying the Etiology of Cardiomyopathy in Patients Undergoing Cardiac Transplantation. Sci Rep. 2018;8:16212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazmirczak F, Nijjar PS, Zhang L, Hughes A, Chen KA, Okasha O, Martin CM, Akcakaya M, Farzaneh-Far A and Shenoy C. Safety and prognostic value of regadenoson stress cardiovascular magnetic resonance imaging in heart transplant recipients. J Cardiovasc Magn Reson. 2019;21:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazmirczak F, Chen KA, Adabag S, von Wald L, Roukoz H, Benditt DG, Okasha O, Farzaneh-Far A, Markowitz J, Nijjar PS, et al. Assessment of the 2017 AHA/ACC/HRS Guideline Recommendations for Implantable Cardioverter-Defibrillator Implantation in Cardiac Sarcoidosis. Circ Arrhythm Electrophysiol. 2019;12:e007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes A, Okasha O, Farzaneh-Far A, Kazmirczak F, Nijjar PS, Velangi P, Akcakaya M, Martin CM and Shenoy C. Myocardial Fibrosis and Prognosis in Heart Transplant Recipients. Circ Cardiovasc Imaging. 2019;12:e009060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitkungvan D, Nabi F, Ghosn MG, Dave AS, Quinones M, Zoghbi WA, Valderrabano M and Shah DJ. Detection of LA and LAA Thrombus by CMR in Patients Referred for Pulmonary Vein Isolation. JACC Cardiovasc Imaging. 2016;9:809–818. [DOI] [PubMed] [Google Scholar]
- 21.Assomull RG, Shakespeare C, Kalra PR, Lloyd G, Gulati A, Strange J, Bradlow WM, Lyne J, Keegan J, Poole-Wilson P, et al. Role of cardiovascular magnetic resonance as a gatekeeper to invasive coronary angiography in patients presenting with heart failure of unknown etiology. Circulation. 2011;124:1351–60. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyankin KO, Palma RA and Velazquez EJ. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32:1–64. [DOI] [PubMed] [Google Scholar]
- 23.Domenicucci S, Chiarella F, Bellotti P, Bellone P, Lupi G and Vecchio C. Long-term prospective assessment of left ventricular thrombus in anterior wall acute myocardial infarction and implications for a rational approach to embolic risk. Am J Cardiol. 1999;83:519–24. [DOI] [PubMed] [Google Scholar]
- 24.Stratton JR, Lighty GW Jr., Pearlman AS and Ritchie JL. Detection of left ventricular thrombus by two-dimensional echocardiography: sensitivity, specificity, and causes of uncertainty. Circulation. 1982;66:156–66. [DOI] [PubMed] [Google Scholar]
- 25.Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ and Judd RM. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374–9. [DOI] [PubMed] [Google Scholar]
- 26.Klem I, Heiberg E, Van Assche L, Parker MA, Kim HW, Grizzard JD, Arheden H and Kim RJ. Sources of variability in quantification of cardiovascular magnetic resonance infarct size - reproducibility among three core laboratories. J Cardiovasc Magn Reson. 2017;19:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asinger RW, Mikell FL, Elsperger J and Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med. 1981;305:297–302. [DOI] [PubMed] [Google Scholar]
- 28.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–236. [DOI] [PubMed] [Google Scholar]
- 29.O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr., Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–425. [DOI] [PubMed] [Google Scholar]
- 30.Vandvik PO, Lincoff AM, Gore JM, Gutterman DD, Sonnenberg FA, Alonso-Coello P, Akl EA, Lansberg MG, Guyatt GH and Spencer FA. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e637S–e668S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polak JF, Sacco RL, Post WS, Vaidya D, Arnan MK and O’Leary DH. Incident stroke is associated with common carotid artery diameter and not common carotid artery intima-media thickness. Stroke. 2014;45:1442–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). Circulation. 2015;132:302–61. [DOI] [PubMed] [Google Scholar]
- 33.Bekwelem W, Connolly SJ, Halperin JL, Adabag S, Duval S, Chrolavicius S, Pogue J, Ezekowitz MD, Eikelboom JW, Wallentin LG, et al. Extracranial Systemic Embolic Events in Patients With Nonvalvular Atrial Fibrillation: Incidence, Risk Factors, and Outcomes. Circulation. 2015;132:796–803. [DOI] [PubMed] [Google Scholar]
- 34.Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47. [DOI] [PubMed] [Google Scholar]
- 35.Lip GY, Nieuwlaat R, Pisters R, Lane DA and Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–72. [DOI] [PubMed] [Google Scholar]
- 36.Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB and Lip GY. Assessment of the CHA2DS2-VASc Score in Predicting Ischemic Stroke, Thromboembolism, and Death in Patients With Heart Failure With and Without Atrial Fibrillation. JAMA. 2015;314:1030–8. [DOI] [PubMed] [Google Scholar]
- 37.Meurin P, Brandao Carreira V, Dumaine R, Shqueir A, Milleron O, Safar B, Perna S, Smadja C, Genest M, Garot J, et al. Incidence, diagnostic methods, and evolution of left ventricular thrombus in patients with anterior myocardial infarction and low left ventricular ejection fraction: a prospective multicenter study. Am Heart J. 2015;170:256–62. [DOI] [PubMed] [Google Scholar]
- 38.Merkler AE, Alakbarli J, Gialdini G, Navi BB, Murthy SB, Goyal P, Kim J, Devereux RB, Safford MM, Iadecola C, et al. Short-Term Risk of Ischemic Stroke After Detection of Left Ventricular Thrombus on Cardiac Magnetic Resonance Imaging. J Stroke Cerebrovasc Dis. 2019;28:1027–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roifman I, Connelly KA, Wright GA and Wijeysundera HC. Echocardiography vs. Cardiac Magnetic Resonance Imaging for the Diagnosis of Left Ventricular Thrombus: A Systematic Review. Can J Cardiol. 2015;31:785–91. [DOI] [PubMed] [Google Scholar]
- 40.Weinsaft JW, Kim J, Medicherla CB, Ma CL, Codella NC, Kukar N, Alaref S, Kim RJ and Devereux RB. Echocardiographic Algorithm for Post-Myocardial Infarction LV Thrombus: A Gatekeeper for Thrombus Evaluation by Delayed Enhancement CMR. JACC Cardiovasc Imaging. 2016;9:505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keating EC, Gross SA, Schlamowitz RA, Glassman J, Mazur JH, Pitt WA and Miller D. Mural thrombi in myocardial infarctions. Prospective evaluation by two-dimensional echocardiography. Am J Med. 1983;74:989–95. [DOI] [PubMed] [Google Scholar]
- 42.Komrad MS, Coffey CE, Coffey KS, McKinnis R, Massey EW and Califf RM. Myocardial infarction and stroke. Neurology. 1984;34:1403–9. [DOI] [PubMed] [Google Scholar]
- 43.Mooe T, Eriksson P and Stegmayr B. Ischemic stroke after acute myocardial infarction. A population-based study. Stroke. 1997;28:762–7. [DOI] [PubMed] [Google Scholar]
- 44.Takasugi J, Yamagami H, Noguchi T, Morita Y, Tanaka T, Okuno Y, Yasuda S, Toyoda K, Gon Y, Todo K, et al. Detection of Left Ventricular Thrombus by Cardiac Magnetic Resonance in Embolic Stroke of Undetermined Source. Stroke. 2017;48:2434–2440. [DOI] [PubMed] [Google Scholar]
- 45.Lee GY, Song YB, Hahn JY, Choi SH, Choi JH, Jeon ES, Park SJ, Lee SC, Park SW and Gwon HC. Anticoagulation in ischemic left ventricular aneurysm. Mayo Clin Proc. 2015;90:441–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.