Abstract
Breast cancer is the most common noncutaneous malignancy and the second most lethal form of cancer among women in the United States. It currently affects more than one in ten women worldwide. The chance for a female to be diagnosed with breast cancer during her lifetime has significantly increased from 1 in 11 women in 1975 to 1 in 8 women (Altekruse, SEER Cancer Statistics Review, 1975–2007. National Cancer Institute, Bethesda, 2010). This chance for a female of being diagnosed with cancer generally increases with age (Howlader et al, SEER Cancer Statistics Review, 1975–2010. National Cancer Institute, Bethesda, 2013). Fortunately, the mortality rate from breast cancer has decreased in recent years due to increased emphasis on early detection and more effective treatments in the White population. Although the mortality rates have declined in some ethnic populations, the overall cancer incidence among African American and Hispanic population has continued to grow. The goal of the work presented in this book chapter is to highlight similarities and differences in breast cancer morbidity and mortality rates among non-Hispanic white and non-Hispanic black populations. This book chapter also provides an overview of breast cancer, racial/ethnic disparities in breast cancer, breast cancer incidence and mortality rate linked to hereditary, major risk factors of breast cancer among minority population, breast cancer treatment, and health disparity. A considerable amount of breast cancer treatment research have been conducted, but with limited success for African Americans compared to other ethnic groups. Therefore, new strategies and approaches are needed to promote breast cancer prevention, improve survived rates, reduce breast cancer mortality, and ultimately improve the health outcomes of racial/ethnic minorities. In addition, it is vital that leaders and medical professionals from minority population groups be represented in decision-making in research so that racial disparity in breast cancer can be well-studied, fully addressed, and ultimately eliminated in breast cancer.
Keywords: Breast cancer, Racial disparity, Black women, White women, other ethnic groups
3.1. Introduction
Apart from skin cancer, breast cancer is the most common form of cancer affecting women in the U.S. It is also the most prevalent cancer affecting women of every ethnic group in the United States. Breast cancer currently affects more than one in ten women worldwide [3]. The rate of getting and dying from breast cancer differs among ethnic groups [4–6]. Recent studies showed that new cases of breast cancer are about the same for Black and White women. However, the incidence rate of breast cancer before age 45 is higher among Black women than White women, whereas between the ages of 60 and 84, breast cancer incidence rates are strikingly higher in White women than in Black women. Yet, Black women are more likely to die from breast cancer at every age [7, 8]. Meanwhile, incidence and death rates for breast cancer are lower among women of other racial and ethnic groups than among non-Hispanic White and Black women. Asian/Pacific Islander women have the lowest incidence and death rates [7, 8].
While racial and ethnic disparities in cancer survival remain, studies have identified potential reasons for this disparity and possible ways of reducing racial disparity in breast cancer outcome in our populations. Different subtypes of breast cancer have been identified; the ER+ and HER2/neu-positive subtype, the ER+ and HER2/neu-negative subtype, and the basal-like breast cancer also known as triple negative tumors which are high-grade tumors and the most aggressive subtype. The incidence of this subtype in Black women especially, younger ones is twice the incidence observed in White women. Studies have now shown that pregnancy and higher parity increase the risk of basal-like breast cancer but reduces the risk of ER+/PR+ breast cancer. However, breastfeeding was found to eliminate that increased risk of triple-negative cancer [9]. It is also observed that Black women have more children especially at a younger age and lower rate of breastfeeding than White women. These factors could account for the racial disparity in breast cancer. Other studies have identified possible differences in biological properties between Black women and White women, especially in the plasma levels of growth factors and hormones [10], reproductive factors [11, 12], susceptibility loci [13, 14], and primary tumor characteristics, including the presence and expression of steroid and growth factor receptors [12, 15–17], cell cycle proteins [18–20], tumor suppressor genes [21, 22], and chromosomal abnormalities [23]. These possible differences in biological properties between Black women and White women have the potential to influence breast cancer screening and treatment outcomes between the two ethnic groups. Since the early 1990s, several strategies, including early detection and diagnosis, reduction of tobacco smoking, widespread breast cancer screening, and improvement of breast cancer therapies, have been developed to improve the health of patients with breast cancer [24, 25].
Despite medical improvements in early detection, diagnosis and screening, many Black women are less likely to obtain adequate treatment compared with White women [26, 27]. Given all the research work that has been conducted for breast cancer treatment with limited success for African Americans; new strategies and approaches are needed to promote breast cancer prevention, improve survival rates, reduce breast cancer mortality, and improve the health outcomes of racial/ethnic minorities. In addition, it is vital that leaders and medical professionals from minority population groups be represented in decision-making in research studies so that racial disparity in breast cancer can be well-studied, fully addressed, and ultimately eliminated in breast cancer.
3.2. Racial and Ethnic Disparities in Breast Cancer
Racial and ethnicity disparities in breast cancer incidence and mortality rates remain largely unknown, but the possible risk factors include socioeconomic status, late stage of breast cancer at diagnosis, biologic and genetic differences in tumors, differential access to health care, and disease-related molecular mechanistic differences [28]. Traditionally, breast cancer incidence has been lower among Black women compared to White women [29]. Even though the incidence of breast cancer was initially lower in Black women than in White women, breast cancer rates for these two ethnic groups have converged in 2012. This indicated a slow and constant increase in the incidence of breast cancer in Black women while its rate remains stable in White women [30]. Hispanic women show an overall incidence of breast cancer lower than in non-Hispanic white women. However, their breast cancers are often diagnosed at a later stage and they generally present larger tumors than White women. Breast cancer remains the most common cancer (and the leading cause of cancer death) among this ethnic group as well [30]. Meanwhile, the mortality rate of breast cancer remains significantly higher among Black compared to White women and other ethnic groups [30, 31]. Black women tend to be diagnosed with breast cancer at a younger age than White women [32]. For example, the median age at diagnosis for Black women is 59, compared to 63 for White women [32]. Records show an increase in the incidence of breast cancer of 0.4% per year among Black women since 1975 and an increase of 1.5% per year among Asian/Pacific Islanders women since 1992. In contrast, the incidence remained stable among non-Hispanic Whites, Hispanics, or American Indians/Alaska Native women.
Asian-Americans who have recently immigrated to the U.S. show lower rates of breast cancer than those who have lived in the U.S. for many years. However, for Asian American women born in the U.S., the risk is about the same as that of White women [30]. The breast cancer 5-year relative survival rate has increased significantly for both Black and white Women in the last 40 years. Still, substantial racial gap remains. A 5-year survival rate was observed to be 81% for Black women and 92% for White women in recent years [8].
Chinese and Japanese women have the highest breast cancer survival rates whereas Black women have the lowest survival rate of any racial or ethnic group [30]. Overall, breast cancer mortality rate is still higher among Black women compared to White women and other ethnic groups [33]. The gap in breast cancer mortality rate among Black women continues to increase. For example, a report between 2000 and 2010 indicated that breast cancer mortality increased from 30.3% to 41.8% among African American women and that at the advanced stage, 5% of breast cancers are detected among White women compared to 8% of breast cancers among Black women [34].
3.3. Racial and Ethnic Variations in Breast Cancer Incidence and Mortality
Breast cancer does not strike all racial and ethnic groups equally. It varies by race and there is a troubling reality about survival rates for women with breast cancer. White women are more likely to be diagnosed with breast cancer, but Black women are more likely to die from the disease (Table 3.1). Table 3.1 below shows that in 2014, White women had the highest rate of getting breast cancer, followed by Black, Asian/Pacific Islander, Hispanic, and American Indian/Alaska Native (AI/AN) women with the lower incidence rate [29]. It also shows that in 2014, Black women were more likely to die of breast cancer than any other group, followed by White, Hispanic, Asian/Pacific Islander, and American Indian/Alaska Native women with the lower death rate [29].
Table 3.1.
Racial or ethnic variations in female breast cancer incidence and mortality per 100,000 people in the United States in 2014 [29]
| White | Black | Asian/Pacific Islander | Hispanic | American Indian/Alaska Native | |
|---|---|---|---|---|---|
| Incidence rates | 127.7 | 125.1 | 98.5 | 93.1 | 82.2 |
| Death rates | 20.6 | 29.2 | 11.3 | 14.4 | 10.8 |
As seen on Table 3.1, the gap of breast cancer incidence is quite close between Black women and White women in the United State, but Black women are 42% more likely to die from this disease. Breast cancer also varies between states and different countries. Breast cancer incidence rates around the world vary significantly with approximately 80% in North America, Japan, and Sweden to about 60% in middle-income countries and below 40% in poor countries [35]. In 2012, it was estimated that more than 1.7 million new cases of breast cancer occurred among women worldwide [32].
3.4. Socioeconomic Disparities in Breast Cancer
Breast cancer incidence, survival, mortality rates as well as its risk factors vary not only between race and ethnic groups but also with socioeconomic status [36, 37]. Studies have suggested that racial disparities in breast cancer are reduced compared to the disparity observed when social and economic factors are examined alone. When socioeconomic status is considered, certain studies suggest that racial disparities in breast carcinoma are smaller than when social and economic factors are examined alone, but these disparities still persist [38, 39, 40]. Socioeconomic determinants affecting disparity in breast cancer mortality involve poverty, culture, and social injustice [41].
Poverty is a critical social player driving health disparity [42]. Low income women have significantly lower rates of breast cancer screening, greater probability for late-stage diagnosis, and very often receive inadequate and disparate treatment, resulting in higher mortality from breast cancer [37]. Poverty is associated with poorer breast cancer outcomes for all Americans, regardless of race; however, because a larger proportion of Black than Whites live in poverty [43]. Black are more likely to have the higher mortality rate due to breast cancer [44]. Low income women do not have a regular healthcare provider resulting in lower rates of mammography screening and greater probability for late-stage diagnosis [45, 46]. Living in disadvantaged areas with lack of infrastructures is another challenge that economically deprived women have to face to have access to primary care clinics and physicians for diagnosis, treatment, or follow up [47]. Moreover, health care providers available in underserved communities are not always equipped and trained to provide the adequate information or treatment to the population that they serve [47–50]. Lack or inadequate health insurance is another factor driving breast cancer disparity among women. Studies have shown that Black women are twice as likely to be uninsured and to depend on public insurance as White women [51, 52]. Low income Black women are not always able to take the time off from their job for preventative care due to their very limited financial resources and other competing survival priorities [44, 53]. The prevalence of comorbidity (obesity, diabetes, hypertension, cardiovascular disease, respiratory disease) is higher in low income women and particularly in Black women limiting their treatment options [37, 54, 55].
Poverty is also linked to less education and lack of information on breast cancer prevention and the importance of early detection leading late-stage diagnosis of breast cancer and lower survival rate [47, 56]. Other breast cancer risk factors associated with poverty are tobacco use, poor nutrition, physical inactivity, and obesity. Poor and minority communities are often targets of tobacco companies for marketing. Those populations often have limited access to fresh foods and healthy nutrition, and have fewer opportunities for safe recreational physical activity [45, 57–59]. Those factors result in greater body mass index and abdominal obesity which are associated with poorer breast carcinoma prognosis [60, 61]. Black women are more likely to have a diet high fat diet, low in fruits and vegetables, and are less likely to exercise regularly, and are more likely to be obese than White women [62–64]. Collectively, poverty and its associated factors including lack of primary care physician, geographical location, comorbidity, lack or limited health insurance, poor lifestyle, lack of information and lower education as well as other challenges contribute to breast cancer disparity among women. These conditions are mostly observed in the Black women population [43].
Cultural factors such as spirituality, misconception on the susceptibility of breast cancer, cultural beliefs and views as well as medical mistrust are more prominent in Black women when deciding about breast cancer screening, diagnosis, and treatment options. Spirituality has a strong influence on how many Black women manage their health condition [65, 66]. Black women are more likely than White women to rely on divine intervention alone for treatment rather than seeking appropriate medical treatment which can be detrimental for their survival [67]. However, other studies have suggested that spirituality could be beneficial in the life of some Black women as it can also promote early breast cancer screening and proper treatment [68, 69]. Some Black women tend to believe that they have lower risks of developing breast cancer than White women [67, 70], regardless of their family history of breast cancer [71, 72]. This view contributes to a decrease in mammography screening and inadequate actions to address breast issues [67]. Beliefs and attitudes towards breast cancer differ between White and Black women as well. Some Black women believe that any breast trauma or big breast is risk factors for breast cancer [73, 74], More likely than White women, Black women would consider any swelling or lump in the breast that is not painful as non-cancerous and would not seek immediate care [75]. Overall, factors such as poverty, culture, and social injustice contribute directly and indirectly to breast cancer disparity among women. Black women are more likely to be affected by those determinants than White women. These factors often lead to lower breast cancer survival rates among Black women as compared to White women.
3.5. Majors Risk Factors in Breast Cancer Affecting Minority Populations
All women are at risk for developing breast cancer; however, there are several factors that alter the degree of risk for individual women. These factors include sex, age, genetic factors, family history, poor diet, personal health history, lack of physical activity and obesity [76]. They may belong to one of the three categories: genetic/family, environmental, and lifestyle.
3.5.1. Age and Sex Risk Factors in Breast Cancer Disparities
Age and sex are considered important risk factors in breast cancer incidence rates and mortality. Breast cancer incidence rates are higher among Blacks than Whites for women under age 45. It is rarely diagnosed in women younger than 25 years of age. The median age a woman is diagnosed with breast cancer is 61 years. The median age of diagnosis for black women is 58 years and 62 years for White women. The median age at breast cancer death is 68 years for all races; 62 years for Black women and 69 years for White women [77]. Approximately 252,710 women and 2470 men are estimated to be diagnosed with breast cancer in 2017. Men have a 1 in 1000 risk of developing breast cancer over his lifetime whereas approximately 1 in 8 women will develop breast cancer in her lifetime. Siegel and colleagues estimated that about 41,070 people (40,610 women and 460 men) will die from breast cancer in 2017 [78].
3.5.2. Family History and Genetic Mutations Risk Factors in Breast Cancer Disparities
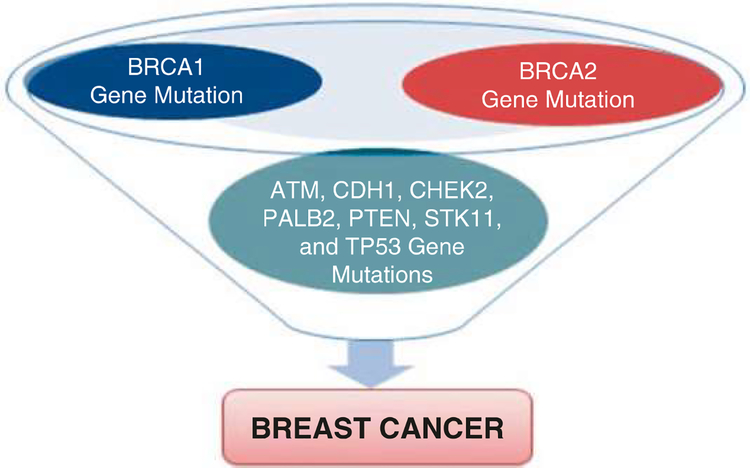
One of the most widely recognized breast cancer risk factors is family history. Family history of breast cancer is a heterogeneous risk factor that depends on the number of family members affected, the age at diagnosis, and the number of unaffected women in the pedigree. A woman’s breast cancer risk is increased if she has a first-degree relative with breast cancer at a young age or if she has multiple relatives with breast cancer [79–81]. Approximately 5–10% of breast cancers are thought to be hereditary [82], The BRCA1 (breast cancer gene 1) and BRCA2 (breast cancer gene 2) gene mutations located on chromosomes 17 and 13, respectively, account for most of the autosomal dominant inherited breast cancers. BRCA1 and BRCA2 are human genes that produce tumor suppressor proteins. These proteins help repair damaged DNA and play a role in ensuring the stability of the cell’s genetic material. When these genes are mutated, altered, or do not function property, DNA damage is repaired. Thus, cells are more likely to generate additional genetic alterations that can lead to cancer development. Prevalence rates of these mutations vary by ethnicity and race. For instance, BRCA1 mutations, the highest rates occur among Ashkenazi Jewish women (8.3%), followed by Hispanic women (3.5%), non-Hispanic white women (2.2%), Black women (1.3%), and Asian women (0.5%) [83, 84]. Approximately 55–65% of women who inherit a harmful BRCA1 mutation and about 45% of women who inherit a harmful BRCA 2 mutation will develop breast cancer by the age of 70. Moreover, 39% of women who inherit a harmful BRCA1 mutation and 11–17% of women who inherit a harmful BRCA2 mutation will develop ovarian cancer by the age of 70 [85, 86]. Women who have been diagnosed with breast cancer with harmful BRCA1 or BRCA2 mutations are more likely to develop a second cancer in with the ipsilateral breast or the contralateral breast than women who do not carry these mutations. Breast cancers in women with a harmful BRCA1 mutation are also more likely to be triple-negative cancers, which have poorer prognosis than other breast cancers. BRCA2 is a risk factor for male breast cancer [87]. Therefore, doctors recommend that women with early-onset breast cancer and women with a family history consistent with a mutation in BRCA1 and BRCA2 genes have genetic testing when breast cancer is diagnosed.
Although harmful mutations in BRCA 1 and BRCA 2 are responsible for breast cancer in almost 50% of families with multiple cases of breast cancer, a number of mutations in other genes have been associated with increased risks of breast cancer [88, 89]. Rare mutations include PTEN, TP53, MLH1, MLH2, and STK11 genes, as well as ATM, BRIP1, CDH1, CHEK2, MRE11A, NBN, PALB2, RAD50, RAD51C, and SEC23B [90]. The majority of the mutations in these other genes are linked with smaller increases in breast cancer risk than are seen with mutations in BRCA1 and BRCA2. However, mutations in the PALB2 gene are associated with a risk of breast cancer almost as high as the risk associated with inherited BRCA1 and BRCA2 mutations. PALB2 is a tumor suppressor gene. The PALB2 protein interacts with the BRCA1 and BRCA 2 proteins to help repair breaks in DNA. Approximately 33% of women with a harmful mutation in the PALB2 gene will develop breast cancer by age 70. The risk is even higher at 58% for women who have a family history of breast cancer and the harmful PALB2 mutation [91].
The differences in the genetics and biology of breast cancer incidence among Black women compared with White women are well-documented in the literature. A breast cancer study that evaluated 4885 White patients, 1016 Black patients, and 777 Hispanic patients reported significant differences in 5-year survival rates [92]. Findings from this research reported a 5-year survival rate of 75% ± 1% for White patients, 70% ± 2% for Hispanic patients, and 65% ± 2% for Black patients [92]. Despite most of the breast cancers not being of hereditary origin, lifestyle and environmental factors, such as diet, obesity, smoking, alcohol consumption, infectious diseases, and radiation have a profound influence on cancer development [93]. Although the hereditary factors cannot be modified, some lifestyle and environmental factors are modifiable and can be prevented. We recently reviewed and listed possible genes mutations that are associated with breast cancer development (Fig. 3.1) [94]. As seen in Fig. 3.1, BRCA1 and BRCA2 mutations increase breast cancer risk for breast cancer development. Other possible genes mutations that are linked to a smaller increase in breast cancer risk include ATM, CDH1, CHEK2, PALB2, PTEN, TP53, and STK11 genes.
Fig. 3.1.
Possible genes mutations associated with breast cancer development
3.5.3. Lack of Physical Activity Risk Factors in Breast Cancer Disparities
Many studies have demonstrated that women who are physically active have a lower risk of breast cancer than inactive women. This reduced risk of breast cancer has been seen in both premenopausal and postmenopausal women; however, the evidence for the association is stronger for postmenopausal breast cancer [95–98]. Moreover, postmenopausal women who increase physical activity may result in a lower risk of breast cancer than women who do not exercise after menopause [95, 96]. A retrospective study in 1994 reported that women who were 40 years old and younger who engaged in 4 or more hours of physical activity per week lowered their breast cancer risk by more than 50% when compared with less active women of the same age [99]. In a 2013 meta-analysis study, breast cancer risk was reduced by an average of 12% from physical activity [98]. A report from the International Agency for Research on Cancer estimated that approximately one fourth to one third of cancer cases are associated with elevated body weight and inadequate physical activity [100]. African Americans are often overweight, obese, and have higher BMI and waist-to-hip ratios compared to Caucasians [101, 102], Scientific data indicated that over 50% of Black women aged 40 years or older are obese and are over 80% overweight [103]. The lack of regular physical exercise among African American may explain why they have higher rates of obesity, a major risk factor of breast cancer [104].
A 2014 study by the Carolina Breast Cancer found racial differences in physical activity among breast cancer survivors revealed that African American women, compared to Caucasian women, are significantly less likely to meet national physical activity guidelines after diagnosis [105]. The lack or limited physical exercises have some implications for breast cancer care. Sisters Network Inc. suggests that only 47% of African American breast cancer survivors may be meeting these physical activity guidelines. Another study by the Northeast Ohio Breast Cancer Survivors found a gradual decline in physical activity levels after high school completion in African American compared to White women and revealed that only 12.3% of African American breast cancer survivors were meeting exercise guidelines [106]. Exercise lowers the levels of estrogen and other growth factors that have been associated with breast cancer development [107]. Exercise also controls blood sugar and regulates blood levels of insulin growth factor, a hormone linked to the growth and function of breast cells. People who are physically active tend to be healthier and are more likely to maintain a healthy body weight compared to people who do not exercise. A proposed breast cancer care model recommended that breast cancer patients should be educated about the importance of physical exercise at the point of breast cancer diagnosis, and provide them with the necessary support to stay active during the stage of breast cancer diagnosis-treatment and beyond [108].
3.5.4. Poor Diet and Obesity Risk Factors in Breast Cancer Disparities
Diet is a major contributor to health disparity in breast cancer and other chronic diseases. A person’s diet can increase or decrease his or her risk for cancer. The American Cancer Society recommends eating a diet composed of mostly fruits, vegetables and whole grains. They urge people to consume less red beef, pork, lamb, bacon, sausage, luncheon meats, hot dogs, and fewer sweets. Nutritional factors including, dietary fat, meat, fiber, and vitamin D have been investigated as either promoting or inhibiting breast cancer development and survival [109]. Dietary fat intake is associated with breast cancer outcomes. The Women’s Intervention Nutrition Study (WINS) concluded that modest weight loss associated with randomization to a low-fat diet improved relapse-free survival in early-stage breast cancer; however, the significance of these associations was not maintained in the long-term follow-up [110]. Furthermore, the intervention that included fat reduction and a diet high in vegetables, fruits, and fiber but did not lead to weight in the Women’s Healthy Eating and Living (WHEL) randomized trial reported no association with recurrence or better prognosis in women with breast cancer [111]. These contradicting results may be a result of weight loss in the WINS and not the WHEL trial [109]. Several studies have demonstrated that a diet rich in vegetables, fruit, poultry, fish, and low-fat dairy products has been associated with a reduced risk of breast cancer. However, it is unclear if specific vegetables, fruits, or other foods can decrease breast cancer risk [109]. For example, the HEAL study recruited African American and Hispanic women from California, Washington, and New Mexico and reported that women with early-stage breast cancer who consumed a diet low in calories, added sugar, alcohol and saturated fat (quality diet) had a 60% reduced risk of all-cause mortality and an 88% lower risk of breast cancer-related mortality [112]. Another report from the HEAL study revealed that a quality diet was correlated with reduced levels of circulating inflammatory markers [113]. The HEAL study is unique since it is one of only a few large studies that is comprised of an ethnically diverse patient population recruited from different geographic locations that investigated an association between diet quality and breast cancer prognosis. Ethnically and racially diverse breast cancer survivors differ in levels of long-term adherence to dietary interventions, levels of physical activity and rates of obesity [114, 115]. Overall, the role of several dietary factors in breast cancer risk is inconclusive; however, there is much evidence from epidemiologic studies that indicate that diet may be linked with promotion or inhibition of the development of breast cancer, which may be due to a woman’s food intake. Higher consumption of dietary fiber and vitamin D along with lower intake of saturated fat and red meat may reduce breast cancer risk. Further studies such as, well designed epidemiological and laboratory studies are warranted to investigate the association between diet and breast cancer risk [109].
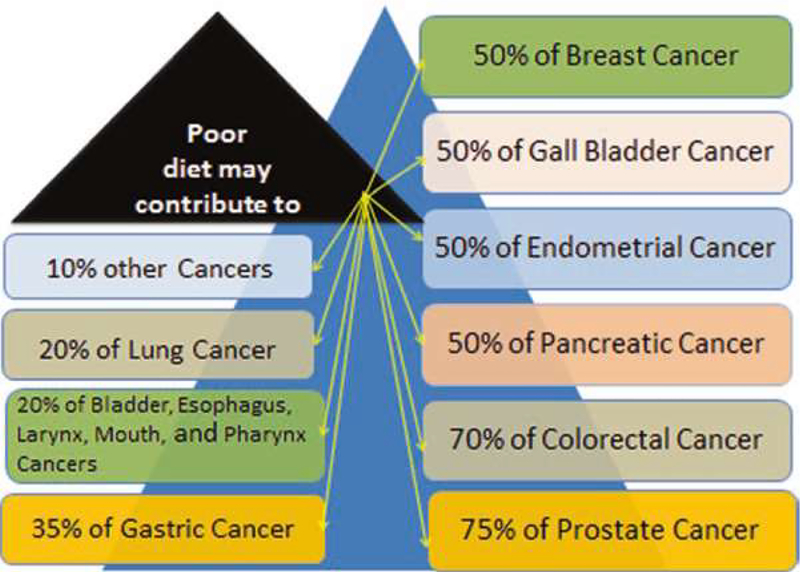
Obesity has been a significant public health issue. Obesity, cardiovascular disease, and diabetes are more prevalent in Hispanics [116]. Type II diabetes is more prevalent among Native Americans [117]. African Americans have hypertension at an earlier age and tend to develop severe high blood pressure, but they are less likely to receive better treatment [118]. All these disorders are associated with cancer development. Diet-related disparity is a major contributor to differences in breast cancer incidence and treatment outcomes in racial/ethnic minorities compared to Whites. Therefore, in order to address and eliminate breast cancer health disparities, it is important to understand how diet contributes to these disparities. We recently reviewed the impact of poor diet on different types of cancers. We observed that approximately 10–75% of cancer related-deaths are attributed to poor diet (Fig. 3.2) [94]. As seen on Fig. 3.2, if a woman is eating a diet rich in vegetables and fruits, she can reduce her risk of getting breast cancer by 50%. For a man who is eating a diet rich in vegetable and fruit can reduce his risk of getting prostate cancer by 75%.
Fig. 3.2.
Cancer deaths express in percentage that are associated with poor diet
3.6. Breast Cancer Prevention, Treatment, and Health Disparity
The majority of breast cancer cases are prevented if chemoprevention is applied in appropriate at-risk populations and the major modifiable risk factors such as maintaining a healthy weight, exercising regularly, and reducing alcohol intake are instituted [119]. Lack of insurance, fear of testing, delay in seeking care, barriers to early detection and screening, more advanced stages of disease at diagnosis among minorities, and unequal access to improvements in breast cancer treatment may explain the differences in survival rates between African American and White women [120–122]. Breast cancer tumors among Black and Hispanic women are more likely to be greater than 2 cm in diameter at diagnosis, are more likely to be estrogen-receptor and progesterone-receptor negative, and are more likely to have characteristics of poor differentiation, with nuclear atypia and higher S phase fraction. Furthermore, the prevalence of estrogen receptor-positive breast tumors is lower in African Americans and Hispanics than in Whites [123, 124], which might account for racial/ethnic differences in the use of tamoxifen. Scientific evidence suggests that, because of the increased risk of stroke, pulmonary embolism, and deep vein thrombosis associated with tamoxifen, African Americans, who already have a higher prevalence of risk factors for these conditions, may receive less overall benefit from tamoxifen [125]. Physician behaviors contribute to disparities in breast cancer mortality. For instance, a survey from York State hospitals revealed that physicians have more negative perceptions towards African Americans and people of low or middle socioeconomic status (SES) than of Whites and people of high socioeconomic status [126]. This finding and lack of information on how physician attitudes toward patients affect their care need further research, particularly with regard to how such negative perceptions might contribute to racial/ethnic disparities in breast cancer treatment.
A 2006 report from the NCI-supported research showed that aggressive forms of breast cancers are common in younger African American/Black and Hispanic/Latino women living in low SES areas. These aggressive forms of breast cancer such as triple negative breast cancer are less responsive to standard cancer treatments and are associated with poorer survival [122]. Triple-negative breast cancer is a heterogeneous disease in which tumors are defined by lack of expression of the estrogen receptor, the progesterone receptor, and the human epidermal growth factor receptor 2. It account for about 10–20% of invasive breast cancers and this subtype carries a poorer prognosis than the luminal tumors [22–24]. There are no targeted therapies currently available for the treatment of triple-negative breast cancer.
Low vitamin D levels have been associated with more aggressive triple-negative tumors [127]. Black women generally have much lower levels of vitamin D than White women because of the rich content of their skin in melanin that limits vitamin D absorption from the sun. Collectively, parity at a younger age, multiple parities, low rate of breastfeeding, in addition to lower levels of vitamin D could significantly contribute to the breast cancer disparity between Black women and White women [128]. Socioeconomic factors are additional determinants of breast cancer disparity among women. Moreover Black women rely more that White women on breast self-examination as effective method for breast cancer detection therefore reducing their rate of mammography screening [129]. Behavior and beliefs also vary between White and Black women in the way they approach screening practices. Black women are more likely to avoid mammography screening by fear of pain, discomfort, embarrassment and radiation [130–132]. In addition, anxiety about the screening outcome is a tangible factor reducing mammography screening in Black women [133].
Misconception about surgery in breast cancer treatment is more prevalent in Black women than in White women [74]. Black women are less likely to seek surgery compared to White women, therefore limiting their treatment options leading to lower breast cancer survival rate [134]. A history of experimentation and abuse endured by Black in general has led to the development of medical mistrust. This factor has been suggested to contribute to the way Black women manage their overall health care and could account for breast cancer disparities between Black and White women [135, 136]. Racial bias may also account for the differences in mammography referrals between Black and White women. Studies have found that found that Black women were more likely than White women to mention lack of recommendation of mammography screening by their physician as a reason for not having undergone breast cancer screening [137]. Another report from the 2000 National Health Interview Study indicated that a 41% of Black women versus 28% of White women stated that their doctor had never suggested mammography [138].
3.7. Conclusions
In this book chapter we sought to describe racial breast cancer disparity primarily in the United State. The mortality rates among breast cancer patients are significantly higher among minority African American women compared to White women and other ethnic group in the United States [139, 140]. There are strong evidences showing that major disparities exist in breast cancer. Scientific data show that breast cancer incidence among Black/non-Hispanic Black women is slightly close to White/non-Hispanic White women [141]. However, Black women have more aggressive breast cancers developing at earlier ages and lower survival rates compared to White women [30, 31]. For example, the percentage of breast cancer mortality among Black women is about 42% higher compared to White women [29]. In addition, breast cancer survival rate has remained lower among White women and has increased over time in Black women [141]. The high mortality and low survival rates in breast cancer among Black women compared to ethnic groups can be attributed to late stage of breast cancer at diagnosis, barriers to health care access, biologic and genetic differences in tumors, and prevalence of risk factors [31, 142]. Other possible reasons for low survival rate among Black women include barriers to early detection and screening, lack of medical coverage, and unequal access to improvements in cancer treatment [121, 122, 124]. The continued growth of the Black-White breast cancer mortality gap suggests that the current approaches to preventing or eliminating racial/ethnic disparities in breast cancer are not sufficient. Therefore, new strategies and approaches are needed to promote breast cancer prevention, improve survival rates, reduce breast cancer mortality, and improve the health outcomes of racial/ethnic minorities. In addition, it is vital that leaders and medical professionals from minority population groups be represented in decision-making in research studies so that racial disparity in breast cancer can be well-studied, fully addressed, and ultimately eliminated in breast cancer.
Acknowledgments
This research was supported in part by the National Institutes of Health (NIH) grant #G12MD007581 through the RCMI-Center for Environmental Health and in part by the NIH grant #P20MD006899 through the Center for Minority Health and Health Disparities at Jackson State University. Research reported in this publication was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number P20MD006899. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest The authors declare no conflict of interest.
Contributor Information
Clement G. Yedjou, Natural Chemotherapeutics Research Laboratory, NIH/NIMHD RCMI-Center for Environmental Health, College of Science, Engineering and Technology, Jackson State University, Jackson, MS, USA
Jennifer N. Sims, Department of Epidemiology and Biostatistics, College of Public Service, Jackson State University, Jackson Medical Mall - Thad Cochran Center, Jackson, MS, USA
Lucio Miele, LSU Health Sciences Center, School of Medicine, Department of Genetics, New Orleans, LA, USA.
Felicite Noubissi, Natural Chemotherapeutics Research Laboratory, NIH/NIMHD RCMI-Center for Environmental Health, College of Science, Engineering and Technology, Jackson State University, Jackson, MS, USA.
Leroy Lowe, Getting to Know Cancer (NGO), Truro, NS, Canada.
Duber D. Fonseca, Natural Chemotherapeutics Research Laboratory, NIH/NIMHD RCMI-Center for Environmental Health, College of Science, Engineering and Technology, Jackson State University, Jackson, MS, USA
Richard A. Alo, Natural Chemotherapeutics Research Laboratory, NIH/NIMHD RCMI-Center for Environmental Health, College of Science, Engineering and Technology, Jackson State University, Jackson, MS, USA
Marinelle Payton, Department of Epidemiology and Biostatistics, College of Public Service, Jackson State University, Jackson Medical Mall - Thad Cochran Center, Jackson, MS, USA.
Paul B. Tchounwou, Natural Chemotherapeutics Research Laboratory, NIH/NIMHD RCMI-Center for Environmental Health, College of Science, Engineering and Technology, Jackson State University, Jackson, MS, USA
References
- 1.Altekruse S et al. (2010) SEER Cancer statistics review, 1975–2007. National Cancer Institute, Bethesda [Google Scholar]
- 2.Howlader et al. (2013) SEER Cancer statistics review, 1975–2010. National Cancer Institute, Bethesda [Google Scholar]
- 3.Torre LA et al. (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108 [DOI] [PubMed] [Google Scholar]
- 4.Ward E et al. (2004) Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 54(2):78–93 [DOI] [PubMed] [Google Scholar]
- 5.Ries (1998) SEER Cancer statistics review, 1973–1994. National Cancer Institute, Bethesda; 1997 NIH Pub. No. 97–2789 [Google Scholar]
- 6.Dalaker et al. (1999) Bureau of the Census, current population report, series P60–210 Poverty in the United States; 1997. U.S. Government Printing Office, Washington, DC [Google Scholar]
- 7.Copeland et al. (2015) Cancer in North America: 2008–2012 Volume one: combined Cancer incidence for the United States, Canada and North America. North American Association of Central Cancer Registries, Springfield [Google Scholar]
- 8.Howlader et al. (2015) SEER Cancer statistics review, 1975–2012. National Cancer Institute, Bethesda [Google Scholar]
- 9.Palmer JR et al. (2011) Parity and lactation in relation to estrogen receptor negative breast cancer in African American women. Cancer Epidemiol Biomark Prev 20(9):1883–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinheiro SP et al. (2005) Racial differences in premenopausal endogenous hormones. Cancer Epidemiol Biomark Prev 14(9):2147–2153 [DOI] [PubMed] [Google Scholar]
- 11.Hall IJ et al. (2005) Comparative analysis of breast cancer risk factors among African-American women and white women. Am J Epidemiol 161(1):40–51 [DOI] [PubMed] [Google Scholar]
- 12.Chlebowski RT et al. (2005) Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst 97(6):439–48 [DOI] [PubMed] [Google Scholar]
- 13.Haiman CA et al. (2011) A common variant at the TERT-CLPTM1L locus is associated with estrogen receptor-negative breast cancer. Nat Genet 43(12):1210–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato I et al. (2009) African American-preponderant single nucleotide polymorphisms (SNPs) and risk of breast cancer. Cancer Epidemiol 33(1):24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray M, Polite BN (2010) Triple-negative breast cancers: a view from 10,000 feet. Cancer J 16(1):17–22 [DOI] [PubMed] [Google Scholar]
- 16.Setiawan VW et al. (2009) Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol 169(10):1251–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunnwald LK, Rossing MA, Li CI (2007) Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 9(1):R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter PL et al. (2004) Racial differences in the expression of cell cycle-regulatory proteins in breast carcinoma. Cancer 100(12):2533–2542 [DOI] [PubMed] [Google Scholar]
- 19.Martin DN et al. (2009) Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One 4(2):e4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gukas ID et al. (2008) A comparison of clinicopathological features and molecular markers in british and nigerian women with breast cancer. Clin Med Oncol 2:347–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehrotra J et al. (2004) Estrogen receptor/progesterone receptor-negative breast cancers of young African-American women have a higher frequency of methylation of multiple genes than those of Caucasian women. Clin Cancer Res 10(6):2052–2057 [DOI] [PubMed] [Google Scholar]
- 22.Dookeran KA et al. (2010) p53 as a marker of prognosis in African-American women with breast cancer. Ann Surg Oncol 17(5):1398–1405 [DOI] [PubMed] [Google Scholar]
- 23.Loo LW et al. (2011) Genome-wide copy number alterations in subtypes of invasive breast cancers in young white and African American women. Breast Cancer Res Treat 127(1):297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yip CH et al. (2008) Guideline implementation for breast healthcare in low- and middle-income countries: early detection resource allocation. Cancer 113(8 Suppl):2244–2256 [DOI] [PubMed] [Google Scholar]
- 25.Ferlay J et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386 [DOI] [PubMed] [Google Scholar]
- 26.Tammemagi CM (2007) Racial/ethnic disparities in breast and gynecologic cancer treatment and outcomes. Curr Opin Obstet Gynecol 19(1):31–36 [DOI] [PubMed] [Google Scholar]
- 27.Hirschman J, Whitman S, Ansell D (2007) The black:white disparity in breast cancer mortality: the example of Chicago. Cancer Causes Control 18(3):323–333 [DOI] [PubMed] [Google Scholar]
- 28.Ademuyiwa FO et al. (2011) Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer 117(18):4132–4140 [DOI] [PubMed] [Google Scholar]
- 29.Howlader et al. (2017) SEER Cancer statistics review, 1975–2014. National Cancer Institute, Bethesda [Google Scholar]
- 30.ACS, American Cancer Society (2015) Breast cancer facts & figures, 2015–2016. 2015. American Cancer Society, Atlanta [Google Scholar]
- 31.ACS, American Cancer Society (2016) Breast cancer facts & figures, 2015–2016. 2016. American Cancer Society, Atlanta [Google Scholar]
- 32.Howlader et al. (2016) SEER Cancer statistics review, 1975–2013. National Cancer Institute, Bethesda [Google Scholar]
- 33.Connor CS et al. (2000) Local recurrence following breast conservation therapy in African-American women with invasive breast cancer. Am J Surg 179(1):22–26 [DOI] [PubMed] [Google Scholar]
- 34.ACS, American Cancer Society (2013) Cancer facts & figures for African Americans 2013–2014. American Cancer Society, Atlanta, 2013 [Google Scholar]
- 35.Coleman MP et al. (2008) Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol 9(8):730–756 [DOI] [PubMed] [Google Scholar]
- 36.Newman LA, Martin IK (2007) Disparities in breast cancer. Curr Probl Cancer 31(3):134–156 [DOI] [PubMed] [Google Scholar]
- 37.Bigby J, Holmes MD (2005) Disparities across the breast cancer continuum. Cancer Causes Control 16(1):35–44 [DOI] [PubMed] [Google Scholar]
- 38.Hunter CP et al. (1993) Breast cancer: factors associated with stage at diagnosis in black and white women. Black/white Cancer survival study group. J Natl Cancer Inst 85(14):1129–1137 [DOI] [PubMed] [Google Scholar]
- 39.Michalski TA, Nattinger AB (1997) The influence of black race and socioeconomic status on the use of breast-conserving surgery for Medicare beneficiaries. Cancer 79(2):314–319 [PubMed] [Google Scholar]
- 40.Yood MU et al. (1999) Race and differences in breast cancer survival in a managed care population. J Natl Cancer Inst 91(17):1487–1491 [DOI] [PubMed] [Google Scholar]
- 41.Freeman HP, Chu KC (2005) Determinants of cancer disparities: barriers to cancer screening, diagnosis, and treatment. Surg Oncol Clin N Am 14(4):655–669, v [DOI] [PubMed] [Google Scholar]
- 42.Freeman HP (2004) Poverty, culture, and social injustice: determinants of cancer disparities. CA Cancer J Clin 54(2):72–77 [DOI] [PubMed] [Google Scholar]
- 43.Census (2006) The statistical abstract. 2006, U.S. Census Bureau [Google Scholar]
- 44.Gerend MA, Pai M (2008) Social determinants of black-white disparities in breast cancer mortality: a review. Cancer Epidemiol Biomark Prev 17(11):2913–2923 [DOI] [PubMed] [Google Scholar]
- 45.Jones BA et al. (1997) Severe obesity as an explanatory factor for the black/white difference in stage at diagnosis of breast cancer. Am J Epidemiol 146(5):394–404 [DOI] [PubMed] [Google Scholar]
- 46.O’Malley AS, Forrest CB, Mandelblatt J (2002) Adherence of low-income women to cancer screening recommendations. J Gen Intern Med 17(2):144–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lacey L et al. (1993) Referral adherence in an inner city breast and cervical cancer screening program. Cancer 72(3):950–955 [DOI] [PubMed] [Google Scholar]
- 48.Williams DR, Jackson PB (2005) Social sources of racial disparities in health. Health Aff (Millwood) 24(2):325–334 [DOI] [PubMed] [Google Scholar]
- 49.Tamblyn R et al. (2002) Association between licensure examination scores and practice in primary care. JAMA 288(23):3019–3026 [DOI] [PubMed] [Google Scholar]
- 50.Bach PB et al. (2004) Primary care physicians who treat blacks and whites. N Engl J Med 351(6):575–584 [DOI] [PubMed] [Google Scholar]
- 51.Ni et al. (2004) Trends in health insurance coverage by race/ethnicity among persons under 65 years of age: United States 1997–2001. National Center for Health Statistics, Hyattsville: 2004 [Google Scholar]
- 52.Thomasson M (2006) Racial differences in health insurance coverage and medical expenditures in the United States: a historical perspective. Soc Sci Hist 30:529–550 [Google Scholar]
- 53.Underwood SM et al. (1994) Obstacles to cancer care: focus on the economically disadvantaged. Oncol Nurs Forum 21(1):47–52 [PubMed] [Google Scholar]
- 54.Bickell NA et al. (2006) Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol 24(9):1357–1362 [DOI] [PubMed] [Google Scholar]
- 55.Tammemagi CM et al. (2005) Comorbidity and survival disparities among black and white patients with breast cancer. JAMA 294(14):1765–1772 [DOI] [PubMed] [Google Scholar]
- 56.Gazmararian JA et al. (1999) Health literacy among Medicare enrollees in a managed care organization. JAMA 281(6):545–551 [DOI] [PubMed] [Google Scholar]
- 57.Coates RJ et al. (1990) Race, nutritional status, and survival from breast cancer. J Natl Cancer Inst 82(21):1684–1692 [DOI] [PubMed] [Google Scholar]
- 58.Forshee RA, Storey ML, Ritenbaugh C (2003) Breast cancer risk and lifestyle differences among premenopausal and postmenopausal African-American women and white women. Cancer 97(1 Suppl):280–288 [DOI] [PubMed] [Google Scholar]
- 59.Bernstein L et al. (2003) Ethnicity-related variation in breast cancer risk factors. Cancer 97(1 Suppl):222–229 [DOI] [PubMed] [Google Scholar]
- 60.Long E (1993) Breast cancer in African-American women. Review of the literature. Cancer Nurs 16(1):1–24 [PubMed] [Google Scholar]
- 61.Van Loon AJ, Goldbohm RA, Van den Brandt PA (1994) Socioeconomic status and breast cancer incidence: a prospective cohort study. Int J Epidemiol 23(5):899–905 [DOI] [PubMed] [Google Scholar]
- 62.Crespo CJ et al. (1996) Leisure-time physical activity among US adults. Results from the Third National Health and Nutrition Examination Survey. Arch Intern Med 156(1):93–98 [PubMed] [Google Scholar]
- 63.Stoll BA (1998) Western diet, early puberty, and breast cancer risk. Breast Cancer Res Treat 49(3):187–193 [DOI] [PubMed] [Google Scholar]
- 64.Ogden CL et al. (2006) Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295(13):1549–1555 [DOI] [PubMed] [Google Scholar]
- 65.Johnson KS, Elbert-Avila KI, Tulsky JA (2005) The influence of spiritual beliefs and practices on the treatment preferences of African Americans: a review of the literature. J Am Geriatr Soc 53(4):711–719 [DOI] [PubMed] [Google Scholar]
- 66.Ellison et al. (1996) Turning to prayer: social and situational antecedents of religious coping among African Americans. Rev Relig Res 38:111–130 [Google Scholar]
- 67.Lannin DR et al. (2002) Impacting cultural attitudes in African-American women to decrease breast cancer mortality. Am J Surg 184(5):418–423 [DOI] [PubMed] [Google Scholar]
- 68.Mansfield CJ, Mitchell J, King DE (2002) The doctor as God’s mechanic? Beliefs in the Southeastern United States. Soc Sci Med 54(3):399–409 [DOI] [PubMed] [Google Scholar]
- 69.Potts RG (1996) Spirituality and the experience of cancer in an African American community: implications for psychosocial oncology. J Psychosoc Oncol 14 [Google Scholar]
- 70.Olsen SJ, Frank-Stromborg M (1994) Cancer prevention and screening activities reported by African-American nurses. Oncol Nurs Forum 21(3):487–494 [PubMed] [Google Scholar]
- 71.Hughes C, Lerman C, Lustbader E (1996) Ethnic differences in risk perception among women at increased risk for breast cancer. Breast Cancer Res Treat 40(1):25–35 [DOI] [PubMed] [Google Scholar]
- 72.Royak-Schaler R et al. (1995) Breast cancer in African-American families. Risk perception, cancer worry, and screening practices of first-degree relatives. Ann N Y Acad Sci 768:281–285 [DOI] [PubMed] [Google Scholar]
- 73.Carter J et al. (2002) Cancer knowledge, attitudes, beliefs, and practices (KABP) of disadvantaged women in the South Bronx. J Cancer Educ 17(3):142–149 [DOI] [PubMed] [Google Scholar]
- 74.Skinner CS, Arfken CL, Sykes RK (1998) Knowledge, perceptions, and mammography stage of adoption among older urban women. Am J Prev Med 14(1):54–63 [DOI] [PubMed] [Google Scholar]
- 75.Winstead-Fry P et al. (1999) The relationship of rural persons’ multidimensional health locus of control to knowledge of cancer, cancer myths, and cancer danger signs. Cancer Nurs 22(6):456–462 [DOI] [PubMed] [Google Scholar]
- 76.Parkin DM (2011) 1. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer 105(Suppl 2):S2–S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeSantis CE et al. (2016) Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin 66(1):31–42 [DOI] [PubMed] [Google Scholar]
- 78.Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30 [DOI] [PubMed] [Google Scholar]
- 79.Claus EB, Risch NJ, Thompson WD (1990) Age at onset as an indicator of familial risk of breast cancer. Am J Epidemiol 131(6):961–972 [DOI] [PubMed] [Google Scholar]
- 80.Collaborative Group on Hormonal Factors in Breast, C (2001) Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet 358(9291):1389–1399 [DOI] [PubMed] [Google Scholar]
- 81.Metcalfe KA et al. (2009) Breast cancer risks in women with a family history of breast or ovarian cancer who have tested negative for a BRCA1 or BRCA2 mutation. Br J Cancer 100(2):421–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bogdanova N, Helbig S, Dork T (2013) Hereditary breast cancer: ever more pieces to the polygenic puzzle. Hered Cancer Clin Pract 11(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.John EM et al. (2007) Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA 298(24):2869–2876 [DOI] [PubMed] [Google Scholar]
- 84.Malone KE et al. (2006) Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res 66(16):8297–8308 [DOI] [PubMed] [Google Scholar]
- 85.Antoniou A et al. (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72(5):1117–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen S, Parmigiani G (2007) Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 25(11):1329–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Breast Cancer Linkage C (1999) Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst 91(15):1310–1316 [DOI] [PubMed] [Google Scholar]
- 88.Campeau PM, Foulkes WD, Tischkowitz MD (2008) Hereditary breast cancer: new genetic developments, new therapeutic avenues. Hum Genet 124(1):31–42 [DOI] [PubMed] [Google Scholar]
- 89.Walsh T et al. (2006) Spectrum of mutations in BRCA1, BRCA2, CHEK2, and TP53 in families at high risk of breast cancer. JAMA 295(12):1379–1388 [DOI] [PubMed] [Google Scholar]
- 90.National Cancer Institute (2016) Genetics of breast and gynecologic cancers (PDQ®) – health professional version, http://www.cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq#section/_88 [PubMed]
- 91.Antoniou AC et al. (2014) Breast-cancer risk in families with mutations in PALB2. N Engl J Med 371(6):497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Genetics of Breast and Gynecologic Cancers (PDQ(R)): Health Professional Version, in PDQ Cancer Information Summaries; 2002: Bethesda: [PubMed] [Google Scholar]
- 93.Lee IO, Oguma Y (2006) Physical activity In: Schottenfeld D, Fraumeni JF (eds) Cancer Epidemiology and Prevention, 3rd edn Oxford University Press, New York [Google Scholar]
- 94.Yedjou CG et al. (2017) Assessing the racial and ethnic disparities in Breast Cancer mortality in the United States. Int J Environ Res Public Health 14(5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eliassen AH et al. (2010) Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med 170(19):1758–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fournier A et al. (2014) Recent recreational physical activity and breast cancer risk in postmenopausal women in the E3N cohort. Cancer Epidemiol Biomark Prev 23(9):1893–1902 [DOI] [PubMed] [Google Scholar]
- 97.Hildebrand JS et al. (2013) Recreational physical activity and leisure-time sitting in relation to postmenopausal breast cancer risk. Cancer Epidemiol Biomark Prev 22(10):1906–1912 [DOI] [PubMed] [Google Scholar]
- 98.Wu Y, Zhang D, Kang S (2013) Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat 137(3):869–882 [DOI] [PubMed] [Google Scholar]
- 99.Fintor L (1999) Exercise and Breast Cancer risk: lacking consensus. JNCI: J Natl Cancer Inst 91(10):825–827 [DOI] [PubMed] [Google Scholar]
- 100.Connolly BS et al. (2002) A meta-analysis of published literature on waist-to-hip ratio and risk of breast cancer. Nutr Cancer 44(2):127–138 [DOI] [PubMed] [Google Scholar]
- 101.Rose DP, Komninou D, Stephenson GD (2004) Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev 5(3):153–165 [DOI] [PubMed] [Google Scholar]
- 102.Lamon-Fava S et al. (2005) Differences in serum sex hormone and plasma lipid levels in Caucasian and African-American premenopausal women. J Clin Endocrinol Metab 90(8):4516–4520 [DOI] [PubMed] [Google Scholar]
- 103.Sephton SE et al. (2000) Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst 92(12):994–1000 [DOI] [PubMed] [Google Scholar]
- 104.Centers for Disease, C. and Prevention (2007) Prevalence of regular physical activity among adults – United States, 2001 and 2005. MMWR Morb Mortal Wkly Rep 56(46):1209–1212 [PubMed] [Google Scholar]
- 105.Hair BY et al. (2014) Racial differences in physical activity among breast cancer survivors: implications for breast cancer care. Cancer 120(14):2174–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thompson CL et al. (2014) Race, age, and obesity disparities in adult physical activity levels in breast cancer patients and controls. Front Public Health 2:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Winzer BM et al. (2011) Physical activity and cancer prevention: a systematic review of clinical trials. Cancer Causes Control 22(6):811–826 [DOI] [PubMed] [Google Scholar]
- 108.Stout NL et al. (2012) A prospective surveillance model for rehabilitation for women with breast cancer. Cancer 118(8 Suppl):2191–2200 [DOI] [PubMed] [Google Scholar]
- 109.Kotepui M (2016) Diet and risk of breast cancer. Contemp Oncol (Pozn) 20(1):13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chlebowski RT et al. (2006) Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst 98(24):1767–1776 [DOI] [PubMed] [Google Scholar]
- 111.Pierce JP et al. (2007) Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 298(3):289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.George SM et al. (2011) Postdiagnosis diet quality, the combination of diet quality and recreational physical activity, and prognosis after early-stage breast cancer. Cancer Causes Control 22(4):589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.George SM et al. (2010) Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer Epidemiol Biomark Prev 19(9):2220–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paxton RJ et al. (2011) Was race a factor in the outcomes of the Women’s Health Eating and Living Study? Cancer 117(16):3805–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Paxton RJ et al. (2012) Associations among physical activity, body mass index, and health-related quality of life by race/ethnicity in a diverse sample of breast cancer survivors. Cancer 118(16):4024–4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Davidson JA et al. (2007) Avoiding the looming Latino/Hispanic cardiovascular health crisis: a call to action. J Cardiometab Syndr 2(4):238–243 [DOI] [PubMed] [Google Scholar]
- 117.Egede LE, Dagogo-Jack S (2005) Epidemiology of type 2 diabetes: focus on ethnic minorities. Med Clin North Am 89(5):949–975, viii [DOI] [PubMed] [Google Scholar]
- 118.Hollar D, Agatston AS, Hennekens CH (2004) Hypertension: trends, risks, drug therapies and clinical challenges in African Americans. Ethn Dis 14(4):S2–23–5 [PubMed] [Google Scholar]
- 119.Colditz GA, Bohlke K (2014) Priorities for the primary prevention of breast cancer. CA Cancer J Clin 64(3):186–194 [DOI] [PubMed] [Google Scholar]
- 120.Optenberg SA et al. (1995) Race, treatment, and long-term survival from prostate cancer in an equal-access medical care delivery system. JAMA 274(20):1599–1605 [PubMed] [Google Scholar]
- 121.Eley JW et al. (1994) Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA 272(12):947–954 [DOI] [PubMed] [Google Scholar]
- 122.Carey LA et al. (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer study. JAMA 295(21):2492–2502 [DOI] [PubMed] [Google Scholar]
- 123.Elledge RM et al. (1994) Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst 86(9):705–712 [DOI] [PubMed] [Google Scholar]
- 124.Harlan LC et al. (1993) Estrogen receptor status and dietary intakes in breast cancer patients. Epidemiology 4(1):25–31 [DOI] [PubMed] [Google Scholar]
- 125.ACS, American Cancer Society (2011) Cancer facts and figures for African Americans 2011–2012. American Cancer Society, Atlanta, 2011 [Google Scholar]
- 126.van Ryn M, Burke J (2000) The effect of patient race and socio-economic status on physicians’ perceptions of patients. Soc Sci Med 50(6):813–828 [DOI] [PubMed] [Google Scholar]
- 127.Yao S, Ambrosone CB (2013) Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J Steroid Biochem Mol Biol 136:337–341 [DOI] [PubMed] [Google Scholar]
- 128.Printz C (2013) Racial and ethnic disparities in breast cancer: experts gain new clues about differences in mortality rates among racial groups. Cancer 119(21):3739–3741 [DOI] [PubMed] [Google Scholar]
- 129.Powe BD et al. (2005) Perceptions about breast cancer among African American women: do selected educational materials challenge them? Patient Educ Couns 56(2):197–204 [DOI] [PubMed] [Google Scholar]
- 130.Friedman LC et al. (1995) Breast cancer screening: racial/ethnic differences in behaviors and beliefs. J Cancer Educ 10(4):213–216 [DOI] [PubMed] [Google Scholar]
- 131.Phillips JM, Wilbur J (1995) Adherence to breast cancer screening guidelines among African-American women of differing employment status. Cancer Nurs 18(4):258–269 [PubMed] [Google Scholar]
- 132.Miller AM, Champion VL (1997) Attitudes about breast cancer and mammography: racial, income, and educational differences. Women Health 26(1):41–63 [DOI] [PubMed] [Google Scholar]
- 133.Miller LY, Hailey BJ (1994) Cancer anxiety and breast cancer screening in African-American women: a preliminary study. Womens Health Issues 4(3):170–174 [DOI] [PubMed] [Google Scholar]
- 134.Lannin DR et al. (1998) Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA 279(22):1801–1807 [DOI] [PubMed] [Google Scholar]
- 135.LaVeist TA, Carroll T (2002) Race of physician and satisfaction with care among African-American patients. J Natl Med Assoc 94(11):937–943 [PMC free article] [PubMed] [Google Scholar]
- 136.LaVeist TA et al. (2002) Physician referral patterns and race differences in receipt of coronary angiography. Health Serv Res 37(4):949–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vernon SW et al. (1993) Factors associated with perceived risk of breast cancer among women attending a screening program. Breast Cancer Res Treat 28(2):137–144 [DOI] [PubMed] [Google Scholar]
- 138.Dawson DA, Thompson GB (1990) Breast cancer risk factors and screening: United States, 1987. Vital Health Stat 10(172):iii–iiv, 1–60 [PubMed] [Google Scholar]
- 139.Wray CJ et al. (2013) The effect of age on race-related breast cancer survival disparities. Ann Surg Oncol 20(8):2541–2547 [DOI] [PubMed] [Google Scholar]
- 140.DeSantis C et al. (2014) Breast cancer statistics, 2013. CA Cancer J Clin 64(1):52–62 [DOI] [PubMed] [Google Scholar]
- 141.ACS, American Cancer Society (2017) Cancer facts and figures 2017. 2017. American Cancer Society, Atlanta [Google Scholar]
- 142.Iqbal J et al. (2015) Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 313(2):165–173 [DOI] [PubMed] [Google Scholar]