Abstract
A novel clay-reinforced polycaprolactone/chitosan/curcumin (PCl/CS/Clay/Cur) composite film was fabricated in this study. The prepared Cur-loading composite films were characterized with attenuated total reflection Fourier transformed infrared spectroscopy, scanning electron microscopy, atomic force microscopy, water contact angle, differential scanning calorimetry, thermogravimetric analysis, and X-ray diffraction, and the results showed good dispersion of clay in the composite films. The addition of nanoclay was found to significantly increase the tensile strength. Also, the clay-enhanced drug-loading films exhibited better controlled-release profiles of Cur than those membranes without clay. Skin disinfection test demonstrated that the curcumin-loaded film could protect wound from bacterial infection. Cytotoxicity analysis proved the good biocompatibility of the composite films. The clay-enhanced Cur-loading films might be promising candidates for wound care.
1. Introduction
Skin injuries are one of the most common injuries in daily life. Both physical and chemical damages cause destruction of the skin. In the process of wound healing, inflammation is a very fatal obstacle, especially for chronic wounds, which often leads to skin destruction and serious complications.1 Hence, to accelerate wound healing, it is crucial to protect the wound from inflammation. When suffering from inflammation, the human body will respond and release reactive oxygen species (ROS). ROS belongs to one of the well-investigated inflammatory mediators that are chemical species containing chemically reactive oxygen.2 It is believed that involvement of antioxidants against ROS will be beneficial to wound healing.3 Cur, the extract product of the root of Curcuma longa L., is a well-known anti-inflammatory, antimicrobial, and anticancer drug agent. The drug has been applied to treat diabetes, Alzheimer’s disease, arthritis, and other inflammatory diseases.4,5 Cur is a hydrophobic polyphenol component and has been widely used as traditional and culinary medicine in Asia. The pharmacological and biological efficacy of Cur has been clearly established by extensive research studies.5 Previous studies have evidenced that Cur can be capable of inhibiting macrophages and monocytes from releasing tumor necrosis factor-α (TNF-α) (an inflammatory cytokine)6,7 and interleukin-8 (IL-8), as well as regenerating and functional reconstructing skin tissue by facilitating the formation of transforming growth factor (TGF-β).8 However, concerning the potential cytotoxicity exhibited by Cur at a high concentration, controlled release of Cur by incorporating it with polymeric scaffolds might be a suitable method for application in wound dressing.9
Among polymeric scaffolds, polycaprolactone (PCl)/chitosan (CS) films have their significant advantages. PCl is a U.S. Food and Drug Administration-approved synthesis polymer. With the merits of being stabilized, elastomeric, and highly flexible, PCl is usually used as the matrix of wound dressing.10−12 CS, a naturally derived biopolymer, is the only positively charged polysaccharide. There are many reports regarding the improvement of the biocompatible, biodegradable, and antimicrobial properties for wound dressing and tissue scaffold.13−17 In addition, CS has good performance on the controlled release of drugs.18 The PCl/CS membranes exhibit important values on wound therapeutics. Salgado et al. had fabricated PCl/CS films for wound repair and found the membranes to be biocompatible and degradable.19 Poornima and Korrapati designed a PCl/CS nanofiber wound dressing, which could sustainably release ferulic acid until 120 h.20 However, the poor mechanical property of PCl/CS hinders its further development in the clinic.
In the last two decades, polymer nanocomposites have been widely investigated. Montmorillonite (MMT) clay is a kind of layered silicate that is abundant, cheap, and stiffly.21 In the human body, there exist many minerals, which are also the main components of clay. Thus, a clay nanosheet with noncytotoxicity and biodegradability is a promising candidate as nanoreinforcement.22 It is noteworthy that the charge found along the edges of clay is positive, while the charge on each face of clay is negative, making the clay nanosheet attractive.23 Owing to its unique structure, clay is equipped with enhanced interactions with cells or biopolymers and high drug loading capacity.
Therefore, in the present study, clay-enhanced PCl/CS/Cur composite films are fabricated through spin coating. The physicochemical properties are investigated by scanning electron microscopy (SEM), attenuated total reflection Fourier transformed infrared spectroscopy (ATR-FTIR), X-ray diffraction (XRD), thermogravimetric analysis (TGA), and differential scanning calorimetry (DSC) analysis. Skin disinfection test is applied here to assess the antibacterial activity. Finally, the in vitro cytotoxicity is evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) method.
2. Results and Discussion
All the drug loading solutions displayed an orange-yellow color. As shown in Figure 1, the color of PCl/CS/Cur was almost the same as PCl/Cur, and the solutions were transparent, which demonstrated that PCl, CS, and Cur were dissolved well in the HAc/H2O solvent. However, since clay did not dissolve in the solvent but dispersed in the solution, the addition of clay sheets changed the solutions. The more clay was added, the less transparent was the displayed appearance. In the end, well-dispersed solutions were obtained and deaerated before further use.
Figure 1.

Color of the Cur-loading solution. Numbers 1–6 represent PCl/Cur, PCl/CS/Cur, PCl/CS/Cur/Clay-1, PCl/CS/Cur/Clay-2, PCl/CS/Cur/Clay-3, and PCl/CS/Cur/Clay-4, respectively.
2.1. Characterization of the Cur-Loading Films
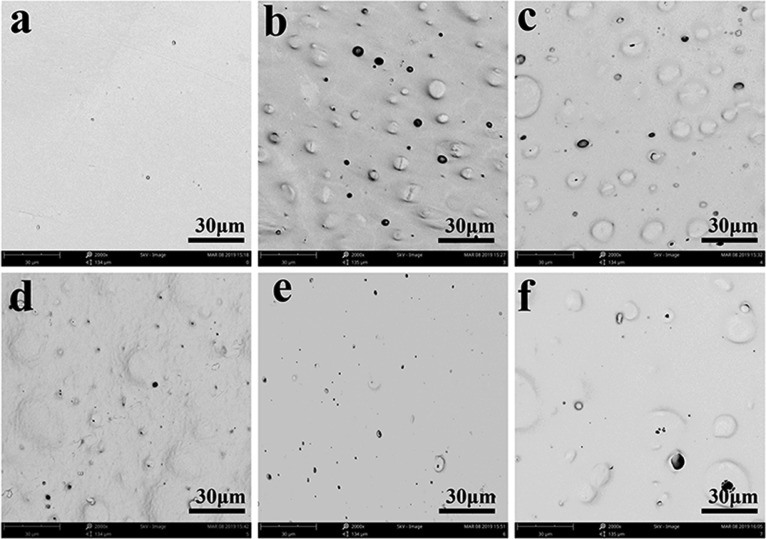
SEM was used to observe the morphology images of the surface and cross section. PCl/Cur was smoother than other cases with less little holes caused by different dissolution rates, as shown in Figure 2a. CS was dissolved in acid solution easily, leading to the formation of a rougher surface and bigger holes (Figure 2b) during the process of liquid–liquid phase separation. When clay was involved in the system, the situation began to change. According to Figure 2c–e, the surface became compact and smooth with the increase of clay, on account of the good blending of hydrophobic montmorillonite I.28E clay with PCl, which could help prevent the dissolution of CS. However, a raised surface can be found in Figure 2f, for there was excess clay in the system.
Figure 2.
Surface morphology images of (a) PCl/Cur, (b) PCl/CS/Cur, (c) PCl/CS/Cur/Clay-1, (d) PCl/CS/Cur/Clay-2, (e) PCl/CS/Cur/Clay-3, and (f) PCl/CS/Cur/Clay-4. Magnification: 2000×.
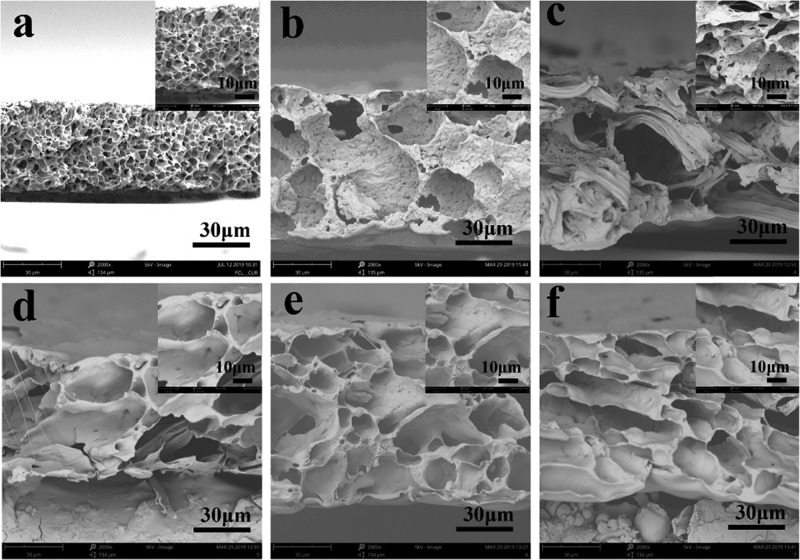
The cross-section morphology of the drug-loading films was investigated, and the results are shown in Figure 3. Significant differences were found between Cur-loading PCl, PCl/CS, and PCl/CS/Clay films. PCl/Cur exhibited a dense, spongy-like cross-section morphology with a disconnected structure (Figure 3a), while a well-connected spongy network was discovered in other films (Figure 3b–f). With the increase of clay, the aperture became smaller and homogeneous. That led to the formation of a tremendous surface area, which was beneficial to the controlled release of Cur.
Figure 3.
Cross-section images of (a) PCl/Cur, (b) PCl/CS/Cur, (c) PCl/CS/Cur/Clay-1, (d) PCl/CS/Cur/Clay-2, (e) PCl/CS/Cur/Clay-3, and (f) PCl/CS/Cur/Clay-4. Magnification: 2000× and 5000×.
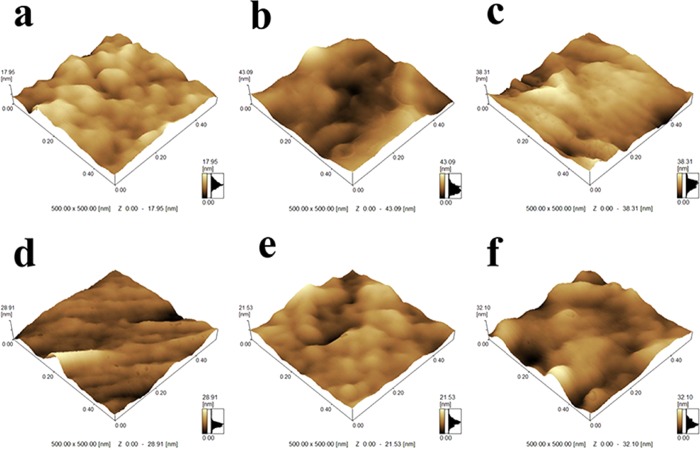
Atomic force microscopy (AFM) images (500 × 500 nm) were obtained to measure the surface roughness of prepared membranes, and different roughness parameters (Ra, Rq, and Rz) are summarized in Table 1. As shown in Figure 4 and Table 1, the film of PCl/Cur displayed a relatively smooth surface morphology. The roughness parameters of PCl/CS/Cur were higher than PCl/Cur. It can be seen that roughness decreased with the increase of clay, indicating the good dispersion of clay in PCl/CS/Cur. However, the surface becomes rougher for PCl/CS/Cur/Clay-4, which might be caused by the aggregates of clay on the surface.
Table 1. Summarized Roughness Parameters of Prepared Curcumin-Loaded Membranes.
| roughness
parameter |
|||
|---|---|---|---|
| sample name | Ra (nm) | Rq (nm) | Rz (nm) |
| PCl/Cur | 1.892 | 2.376 | 17.823 |
| PCl/CS/Cur | 5.400 | 6.644 | 43.126 |
| PCl/CS/Cur/Clay-1 | 5.015 | 6.143 | 38.330 |
| PCl/CS/Cur/Clay-2 | 2.602 | 3.675 | 28.878 |
| PCl/CS/Cur/Clay-3 | 2.233 | 2.849 | 21.496 |
| PCl/CS/Cur/Clay-4 | 3.661 | 4.672 | 32.076 |
Figure 4.
AFM images of (a) PCl/Cur, (b) PCl/CS/Cur, (c) PCl/CS/Cur/Clay-1, (d) PCl/CS/Cur/Clay-2, (e) PCl/CS/Cur/Clay-3, and (f) PCl/CS/Cur/Clay-4.
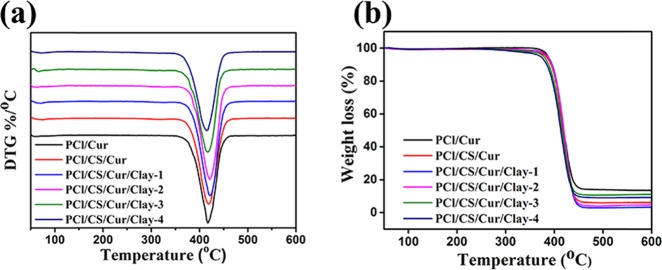
For commercial application, thermal stability should be evaluated.24 As shown in Figure 5, the results of TG and DTG were displayed. According to Figure 5a, the decomposition temperature of the films was around 410 °C, which was ascribed to the decomposition temperature of PCl. When mixing with CS or clay, the decomposition temperature had changed with a little blue shift but still met the requirements of sterilization in clinical practice. There existed a small peak around 70 °C, which belonged to the free water of films. As depicted in Figure 5b, all films underwent two weight loss processes. The first process began at the temperature below 100 °C, likely attributed to the decrease in humidity or the loss of water by evaporation. Obviously, the second weight loss process happened at about 410 °C, assigned to the decomposition of PCl/Cur, PCl/CS/Cur, or PCl/CS/Cur/Clay. In TG curves, overlapping was found between the investigated Cur-loading films, indicating that the polymers would co-decompose under the decomposition temperature.
Figure 5.
(a) DTG and (b) TG curves of the Cur-loading films.
The results of DSC analysis are presented in Figure 6. The typical melting point of PCl appeared at the temperature of 61 °C. It can be seen from Figure 6 that the incorporation of Cur and CS did not change the melting point. Note that the change was too small to influence the application in the clinical field.
Figure 6.
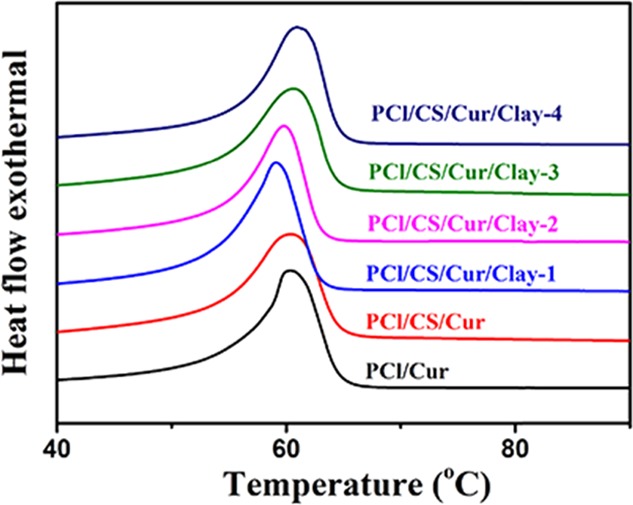
DSC curves of the prepared Cur-loading membranes.
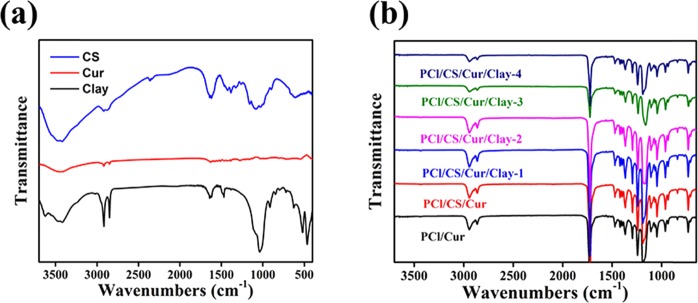
ATR-FTIR was used to assess the chemical compositions on the surface, and the results are shown in Figure 7. Typical FTIR peaks of PCl occurred around 2942–2865, 1732, 1471–1365, and 1239 cm–1. These peaks belonged to symmetric/asymmetric stretching vibrations of −CH2, stretching vibrations of —C=O, bending vibrations of −CH2, and vibrations of −COO, respectively. As shown in Figure 7a, the peaks of CS were observed at 3200–3400, 2872, 1650, and 1374 cm–1, related to the −OH/–NH2 stretch, aliphatic C–H stretch, NH group bending vibration, and −C–O stretching, respectively.25 Characteristic peaks at 3642 cm–1 (O–H stretching) and 1045 cm–1 (Si–O) corresponded to montmorillonite clay, in accordance with the study of Noori and colleagues.26 For Cur, the C=C benzene ring stretching vibrations showed a peak at 1521 cm–1, and C–H bond olefinic bending vibrations displayed a peak at 1430 cm–1, while the C–O vibration occurred at 858 cm–1. However, due to the low mass ratio and overlap exhibited by the high-intensity FTIR peaks of PCl, the characteristic peaks of CS, clay, and Cur were difficult to ascertain, as displayed in Figure 7b. When the mass ratio of CS increased to 10:2 and 10:4, the peaks at 3200–3400 and 1650 cm–1 were discovered (Figure S1), which demonstrated the above speculation.
Figure 7.
ATR-FTIR curves of (a) PCl, CS, and clay and (b) prepared Cur-loading films.
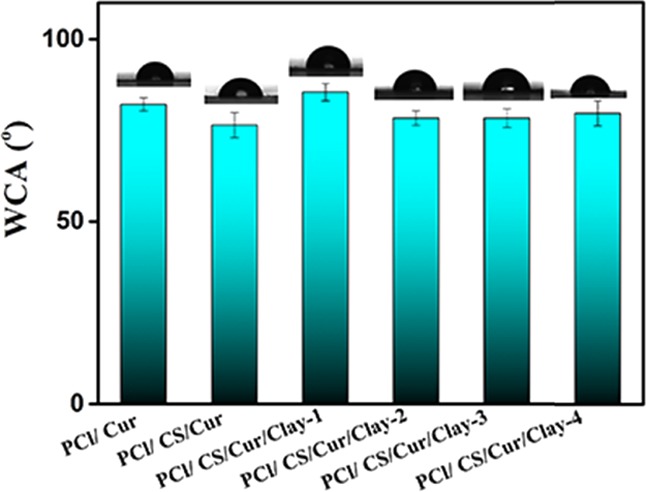
The hydrophobic–hydrophilic properties of the drug-loading films were investigated by water contact angle (WCA) analysis, and the results are exhibited in Figure 8. It is well known that PCl is a hydrophobic polymer with a WCA value of 125°. The addition of Cur decreased the WCA to 82°. For PCl/CS/Cur, the hydrophilic −OH and −NH2 groups of chitosan were transferred to the surface, which further slightly reduced the WCA of PCl/CS/Cur to 76°. For PCl/CS/Cur/Clay-1, the WCA value had a slight increase. The speculated reason was that, with small amounts of clay to CS, exfoliated structures were formed, resulting in the partial sealing of CS.27 With the increase of clay, the structures became intercalary, and the WCA decreased. No significant differences were found between groups of different PCl/CS/Cur/Clay (p > 0.05), indicating that the clay was mostly embedded in the interior of the drug-loading films.
Figure 8.
WCA analysis of curcumin-loaded films.
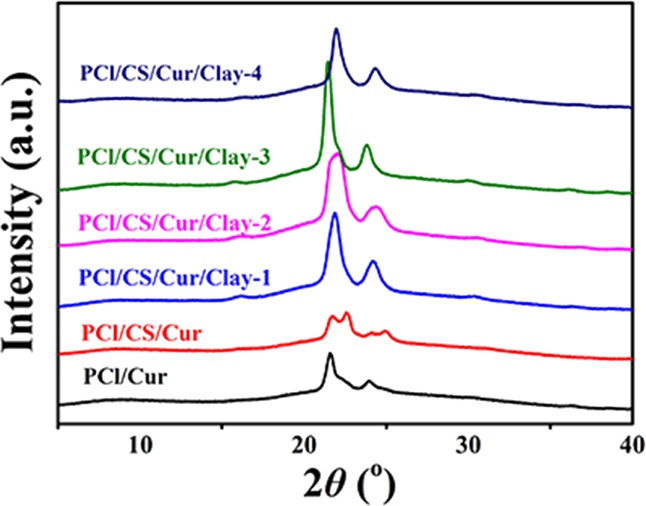
XRD analysis was conducted to measure the crystal structure of the Cur-loading films, and the XRD curves are shown in Figure 9. It was well known that angles at 21.4° and 23.8° were the typical XRD peaks of PCl, while Cur was highly crystalline,28 exhibiting many intense and sharp peaks mainly presented at 17.3°, 21.3°, 23.4°, and 24.7°. However, the typical peaks of Cur disappeared, suggesting the successful loading of Cur into PCl. In the report of Qi et al.,29 characteristic diffractograms of CS that occurred at around 21.8° were descripted. It can be seen from Figure 9, for PCl/CS/Cur, that the intension of PCl peaks decreased and the typical Cur peaks appeared. It was conjectured that the dissolution of CS led to the appearance of unsealed Cur. A previous study indicated that clay showed a diffraction profile at around 7.2°.30 In PCl/CS/Cur/Clay membranes, a clay pattern was not found, while the intensity of 2θ = 21.4° and 23.8° was strengthened with the increase of clay, except for PCl/CS/Cur/Clay-4, which revealed the good dispersibility of clay nanosheets in the fabricated Cur-loaded membranes. It was found that exfoliated clay played a role as a nucleating agent, resulting in increasing crystallinity. When intercalated into clay galleries, however, the crystallinity of PCl decreased.31
Figure 9.
XRD diagram of prepared Cur-loading membranes.
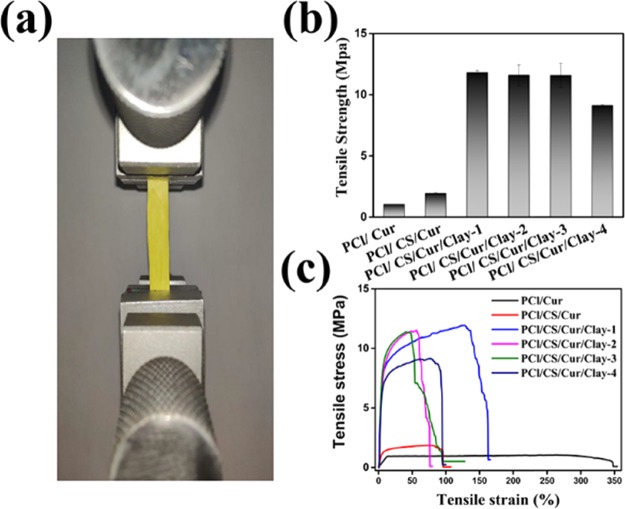
The mechanical properties of the Cur-loading films were assessed, and the results of tensile strength are displayed in Figure 10. A digital photo of PCl/CS/Cur/Clay-3 is displayed in Figure 10a. It can be discovered from Figure 10b,c that the poor tensile strength of PCl/CS (1.04 ± 0.01 MPa) and PCl/CS/Cur (1.94 ± 0.07 MPa) could not satisfy the requirements of native skin (5–30 MPa).25 The tensile strength of clay-enhanced Cur-loading films increased first and then decreased. Compared to films without clay, the tensile strength of clay-enhanced Cur-loading membranes were enhanced dramatically (p < 0.05), which was probably on account of the interaction between clay and PCl/CS and the increase in crystallinity. There were no significant differences between groups embedded with different amounts of clay (p = 0.699 and p = 0.967 for PCl/CS/Cur/Clay-1 vs PCl/CS/Cur/Clay-2 and PCl/CS/Cur/Clay-2 vs PCl/CS/Cur/Clay-3, respectively). Nevertheless, there was excess clay in PCl/CS/Cur/Clay-4, with the reduction of tensile strength, possibly due to the presence of clay agglomerates. These findings were consistent with XRD analysis.
Figure 10.
Mechanical properties of prepared Cur-loading membranes. (a) Digital photo of PCl/CS/Cur/Clay-3. (b) Effect of clay contents on the tensile strength of the Cur-loading membranes. (c) Typical tensile stress–strain curves of the Cur-loading membranes.
2.2. In Vitro Cur Release
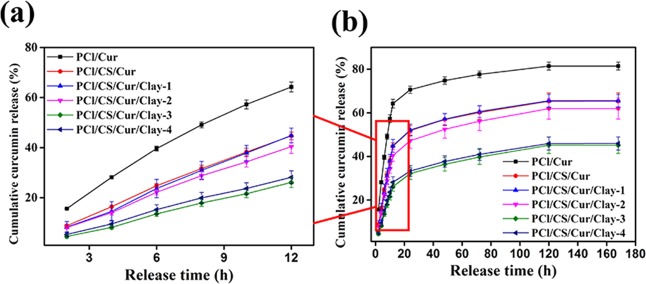
The release behavior of different Cur-loading membranes in PBS (pH 7.4) under the temperature of 37 °C is presented in Figure 11. In a controlled and sustained manner, Cur was released from the drug-loading films. It can be observed from Figure 11a that, within 12 h, over 64.21% of Cur was released from PCl/Cur, while 44.68% of Cur was released from PCl/CS/Cur owing to the property of controlled release exhibited by CS. Montmorillonite clay was found to be beneficial for controlled release of Cur while improving the textural properties (Figure 11b). The more clay was added, the less cumulative Cur was released. The release profile of PCl/CS/Cur/Clay-4 was similar to that of PCl/CS/Cur/Clay-3, suggesting the saturated capacity of clay.
Figure 11.
Release profiles ((a) within 12 h and (b) within 168 h) of Cur from the prepared membranes.
CS was a natural polysaccharide, which could wrap up drugs through a physical or chemical reaction and prevent them from quick release.32 Drugs would be released in a controlled manner with the degradation of CS.33 Owing to good absorbability and ion exchange capacity, the clay was able to inhibit or delay release of drugs.34,35 Via an ion exchange process, CS was capable of exchanging the metal interlayer cations of clay, generating a strong cross-linked structure in the hybrid film.36 Herein, the capability of controlled release was enhanced. This was proved by previous studies. Kevadiya et al. fabricated CS/clay/alginate composite hydrogel beads for oral drug delivery.37 Diclofenac sodium was found to be released in a controlled manner from the hydrogel. In the research of Hua et al., compared to pure chitosan beads, the incorporation of clay enhanced the drug entrapment and reduced the drug release.38
To study the release mechanism of curcumin from the prepared films, n, ln k, and R2 of the plot of ln(Mt/M∞) versus ln t were calculated. The obtained results are shown in Table 2. It was found that, within 12 h, the values of n of the drug-loading films were 0.5 < n < 1, indicating that curcumin was released via non-Fickian diffusion for all the tested films, which occurred when the diffusion and relaxation rates were comparable. However, after 12 h of release, the n values were lower than 0.5, which suggested that the release of curcumin was depending on Fickian diffusion.
Table 2. Analysis of in Vitro Curcumin Release Kinetics from the Prepared Films.
| sample name | time period (h) | n | ln k | R2 |
|---|---|---|---|---|
| PCl/Cur | 0–12 | 0.795 | 2.222 | 0.997 |
| 12–120 | 0.100 | 3.922 | 0.986 | |
| PCl/CS/Cur | 0–12 | 0.915 | 1.542 | 0.999 |
| 12–120 | 0.160 | 3.418 | 0.987 | |
| PCl/CS/Cur/Clay-1 | 0–12 | 0.963 | 1.411 | 0.996 |
| 12–120 | 0.160 | 3.422 | 0.987 | |
| PCl/CS/Cur/Clay-2 | 0–12 | 0.915 | 1.432 | 0.996 |
| 12–120 | 0.180 | 3.262 | 0.993 | |
| PCl/CS/Cur/Clay-3 | 0–12 | 0.996 | 0.788 | 0.995 |
| 12–120 | 0.230 | 2.709 | 0.989 | |
| PCl/CS/Cur/Clay-4 | 0–12 | 0.945 | 0.997 | 0.996 |
| 12–120 | 0.208 | 2.827 | 0.996 |
2.3. Skin Disinfection Test
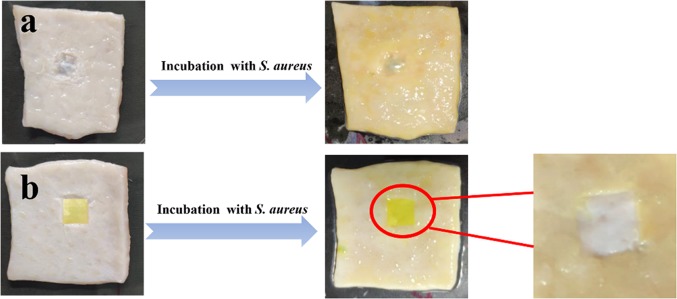
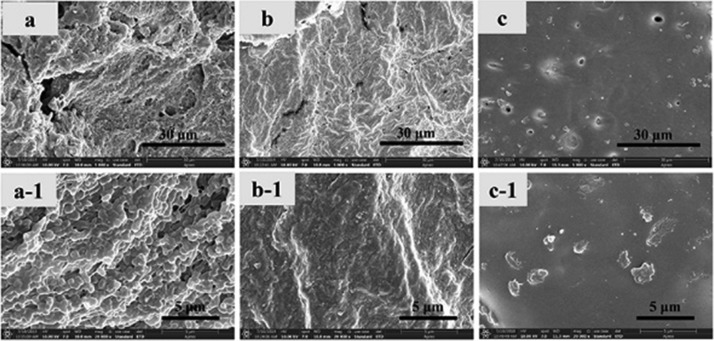
Pig skin is known to be similar with the composition and structure of human skin tissue. Therefore, pig skin disinfection tests are instructive and meaningful to clinical use on the human body in the future. The digital photographs and SEM images of wounds and curcumin-loaded film (PCl/CS/Clay-4) are illustrated in Figures 12 and 13. After 2 days of incubation, it is shown in Figure 12 that many yellow spots and mucus are seen on the control wound without protection. However, the wound protected by PCl/CS/Cur/Clay-3 had no visible yellow spot, suggesting the effective restraint on bacterial infection showed by PCl/CS/Cur/Clay-3. Further, the surface morphologies of the wound sites were observed by SEM. As displayed in Figure 13a,a-1, for the wound without protection, Staphylococcus aureus bacteria aggregated on the surface. The bacteria grew well on the wound and formed a thick biofilm. Nevertheless, there were barely any bacteria on the wound covered by a PCl/CS/Cur/Clay-3 film, which indicated that the film successfully protected the wound from bacterial infection (Figure 13b,b-1). It was discovered from Figure 13c,c-1 that a few bacteria were found to attach on the film surface and the cytomembranes of bacteria were broken, demonstrating the inhibiting effect of PCl/CS/Cur/Clay-3.
Figure 12.
Pictures of S. aureus infection for (a) pig skin with a wound without protection and (b) pig skin with a wound protected by a PCl/CS/Clay-3 film.
Figure 13.
SEM pictures of (a) the wound sites on the control, (b) a wound under the protection of a PCl/CS/Clay-3 film, and (c) bacteria attached on the surface of PCl/CS/Clay-3. Magnification: 5000× and 20,000×.
2.4. Cytocompatibility Studies
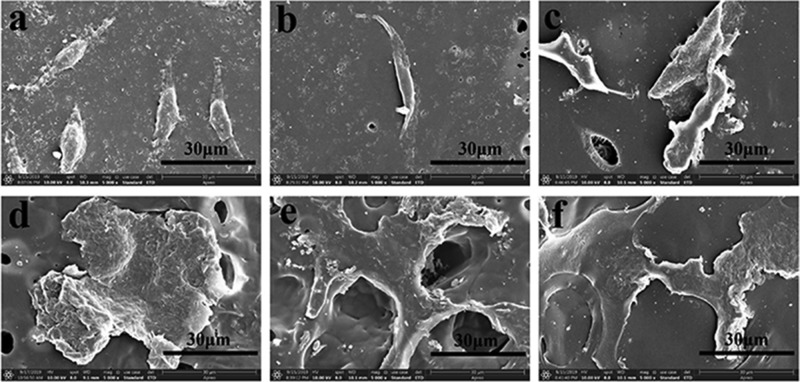
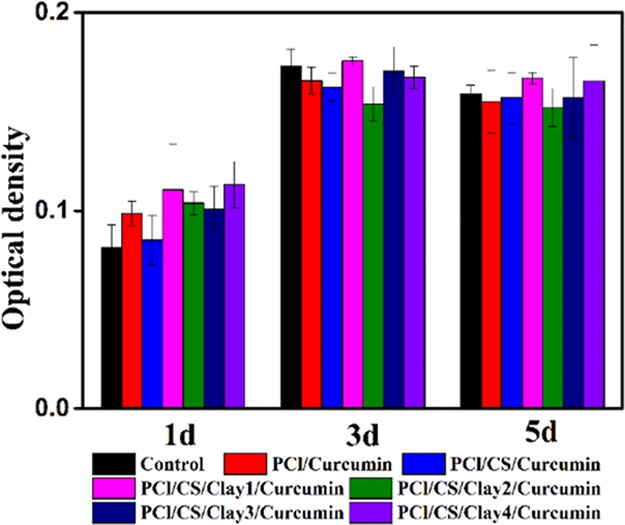
In vitro cytocompatibility, playing a crucial factor for wound dressing, was assessed by MTT assay and SEM observation in the present study. L929 fibroblasts were employed in this investigation and incubated in the 96 cell wells. The extracts of Cur-loading membranes were cultivated with the L929 cells for 1 day, 3 days, and 5 days. At each time point, the 96 cell wells were taken out to measure the optical density (OD). As illustrated in Figure 14, it could be seen that OD values increased with incubation time. After 3 days of cocultivation, the tested cells were less than the control cells but were still suitable for wound dressing application. It might due to the reason that it took time for cells to adapt to the growing environment. However, after 5 days of cultivation, the cell proliferation of investigated samples caught up with the cell proliferation of the control, indicating the good cytocompatibility of the Cur-loading films. The same finding was observed in the drug-loading groups equipped with different amounts of clay, demonstrating that the extract of clay was nontoxic. It was reported by Hsu et al. that clay/chitosan showed good in vivo compatibility.39 The histopathological study of Maisanba et al. showed that the PLA/Clay-1 nanocomposite displayed no toxic effects.40 Clay was a nanomaterial that showed no clear evidences of systemic toxicity even at doses of 5000 mg/kg.41 In this study, all the clay-involved drug-loading membranes showed no side effect on the cell proliferation. It could be speculated that the increase of clay will not exhibit cytotoxicity on L929 cells. Cells attached on the films were viewed by SEM, and the images are shown in Figure 15. L929 fibroblasts grew well on the curcumin-loading films, and the cells kept their fusiform or spread shapes. Cells on PCl/CS/curcumin were less but healthy, which might be due to the positive charge of CS. However, cells spread well on the clay-involved films on account of the increase of hydrophilicity. In vitro cytocompatibility analysis confirmed the biocompatibility of the Cur-loading membranes with/without clay enhancement, implying their potential in the field of wound dressing.
Figure 14.
OD values of L929 cells after cultivating for 1 day, 3 days, and 5 days.
Figure 15.
SEM morphology of L929 cells on the surface of prepared films. (a) PCl/Cur, (b) PCl/CS/Cur, (c) PCl/CS/Cur/Clay-1, (d) PCl/CS/Cur/Clay-2, (e) PCl/CS/Cur/Clay-3, and (f) PCl/CS/Cur/Clay-4. Magnification: 5000×.
3. Conclusions
In conclusion, clay-enhanced Cur-loading films with appropriate mechanical properties were fabricated through the method of spin coating. To evaluate the performance of the prepared membranes, PCl/Cur and PCl/CS/Cur were also produced. Clay-enhanced Cur-loading films were found to have a porous sponge-like morphology with disconnected structures. The SEM observations indicated their superior controlled-release property, which was demonstrated by the release profile in vitro. It was found that, within 12 h, curcumin was released via non-Fickian diffusion for all the tested films, which occurred when the diffusion and relaxation rates were comparable. However, after 12 h of release, the release of curcumin was depending on Fickian diffusion. Skin disinfection test demonstrated that the curcumin-loaded film could protect the wound from S. aureus infection. The physical and chemical properties were investigated by FTIR, TG, DSC, XRD, and WCA, showing the good dispersion of clay in the drug-loading films. Meanwhile, the addition of clay significantly increased the tensile strength of PCl/CS/Cur from 1.94 to 11.81 MPa (PCl/CS/Cur/Clay-1). Cur-loading membranes with/without clay exhibited noncytotoxicity on L929 cells. The clay-enhanced Cur-loading membrane is a promising candidate for wound dressing.
4. Materials and Method
4.1. Materials
The molecular weight of PCl applied in this study is 80,000. PCl was ordered from Sigma-Aldrich. CS (viscosity, 100–200 mPa·s; deacetylation, ≥95%) was bought from Aladdin Chemical Company. Montmorillonite I.28E clay (specific gravity, 1.8 g/cm3) with a particle size of 15–20 μm was obtained from Nanocor, Inc. Cur (CAS: 458-37-7, lot: K1606280) and acetic acid (HAc, analytically pure) were purchased from Chengdu Best Reagent Co., Ltd. MTT came from Thermo Fisher Scientific, Inc. Other chemicals mentioned in this investigation were bought from Sigma-Aldrich, if not specifically mentioned. The L929 cell line was obtained from the Shanghai Cellular Institute of China Scientific Academy.
4.2. Preparation of Clay-Enhanced PCl/CS/Cur Composite Films
PCl was dissolved in HAc/H2O (9:1, v/v) solvent mixture, and a 14% (w/v) solution was obtained. Then, CS (m(PCl)/m(CS) = 10:1) was added to the prepared PCl/CS solution. Different weights of clay sheets were mixed with the above PCl/CS solution. As shown in Table 3, the names of prepared samples and the dosages of chemicals utilized in this study were summarized.
Table 3. Summary of the Names of Prepared Samples and the Dosages of Chemicals.
| sample | PCl (g) | CS (g) | clay (mg) | Cur (mg) | HAc/H2O (mL) |
|---|---|---|---|---|---|
| PCl/Cur | 4.20 | 15 | 30 | ||
| PCl/CS/Cur | 4.20 | 0.42 | 15 | 30 | |
| PCl/CS/Cur/Clay-1 | 4.20 | 0.42 | 42.00 | 15 | 30 |
| PCl/CS/Cur/Clay-2 | 4.20 | 0.42 | 84.00 | 15 | 30 |
| PCl/CS/Cur/Clay-3 | 4.20 | 0.42 | 168.00 | 15 | 30 |
| PCl/CS/Cur/Clay-4 | 4.20 | 0.42 | 336.00 | 15 | 30 |
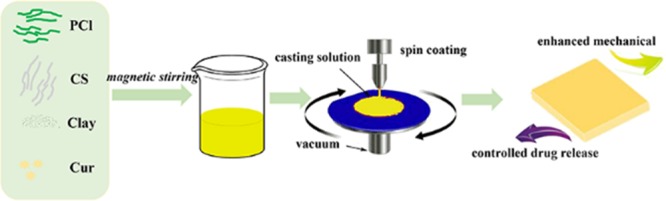
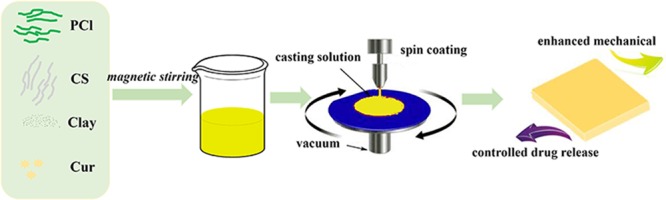
Before spin coating, the prepared solutions were vacuum-degassed, and the glass plates were washed with water and ethanol and underwent ultrasonication. The liquid drop was casted at the speed of 600 rpm for 10 s. After finishing spin coating, the glass sheets were immediately transferred into ultrapure water. Flat membranes were obtained through liquid–liquid phase separation. For comparison, PCl/Cur and PCl/CS/Cur were also fabricated. Schematic illustration in Scheme 1 depicts the fabrication process of membranes.
Scheme 1. Schematic Illustration of the Preparation of Cur-Loading with/without Clay-Enhanced Flat Membranes.
4.3. Characterizations of Cur-Loaded Membranes
Under the accelerating voltage with 5 kV, SEM (JSM-7500F, Japan) was applied to observe the morphology images of prepared Cur-loaded flat membranes.
Equipped with silicon TESP cantilevers (Shimadzu SPM-9600, Japan), a Dimension 3100 Nanoscope IV was used in this study to investigate AFM images and the surface roughness. At room temperature in air, the observations were conducted in noncontact (taping) mode.42
Scanning from 5° to 40°, XRD (Netherlands) analysis was performed under the current of 45 mA and the voltage of 40 kV.
Thermal stability was analyzed by DSC (DSC200-PC, Netzsch, Germany) and TG (Netzsch Co., Germany) tests. For DSC analysis, the test temperature ranged from 20 to 150 °C with a constant heating rate of 10 °C/min under a nitrogen flow. For TG measurement, the membranes were investigated at a heating rate of 15 °C/min from ambient temperature to 800 °C under a N2 atmosphere.
ATR-FTIR (Nicolet 560, America) was used to evaluate the chemical characteristics of prepared films. In a dry atmosphere at room temperature, the spectra were tested in the range of 400–4000 cm–1 with a resolution of 4.0 cm–1.
WCA with a video capture (Attension Theta, Biolin Scientific, Sweden) and TGA (Netzsch Co., Germany) were utilized to investigate the physicochemical properties. The samples were cut to a 1.5 cm2 square and attached on a glass slide. The static WCA results were measured after 10 μL of DI water dropping on the surface of the nanofiber mats.
4.4. Cur Release Profiles
Using UV spectroscopy, a serial dilution of Cur from 0.5 to 15 μg/mL was tested at 424 nm to obtain the calibration curve. A linear equation was fitted as A = 0.0503C – 0.0121 and R2 = 0.9991, where A and C represented absorbance and drug concentration, respectively.
Through the dialysis method, the Cur release profile was evaluated. Briefly, the receptor medium had 0.5% Tween-80 (v/v) in PBS (pH 7.4), and the tests were carried out at 37 °C with gentle vibration. The known amount of drug-loaded membranes was placed into 5 mL of receptor medium and then put in 15 mL centrifuge tubes for 7 days. The release medium was withdrawn to measure the absorbance at proper time intervals. The fresh medium was then added to keep the volume constant. All the tested samples were conducted in triplicate.
Mathematical modeling was applied to investigate the release behavior of curcumin release from the films. Calculated from eq 1, the release mechanism for drug release can be speculated.
| 1 |
In the equation, the fraction release of curcumin in time t was represented by Mt/M∞, the constant characteristic of the system was reflected by k, while the diffusion exponent characteristic of the release mechanism was represented by n. If n = 0.5, then the release mechanism was normal Fickian diffusion; if n = 1.0, then the release mechanism was case II diffusion; and if n = 0.5–1.0, then the release mechanism would be non-Fickian diffusion.43
4.5. Skin Disinfection Test
According to a previous study,44 the S. aureus infection model was conducted to evaluate the bacterial inhibition ability of the curcumin-loaded film with a little modification. Briefly, S. aureus bacteria were revived and cultivated at the temperature of 37 °C. The tested concentration of bacteria was set at 108 CFU/mL, which was confirmed by the absorbency at the wavelength of 600 nm. The bacterial dispersion was prepared before use. In vitro sterile pig skins were cut into 4 cm × 4 cm squares, and the groove-shaped square portion with the size of 1 cm × 1 cm was removed from the pig skin as the wound site. PCl/CS/Cur/Clay-3 was cut into a 1 cm × 1 cm square and placed on the wound site. Then, 10 μL of bacterial dispersion was added on the film. The pig skin with a wound site only was applied as control. All the pig skins were cultivated under the temperature of 37 °C. After 2 days of co-cultivation, the pig skins and films were taken out and gently washed three times with normal saline and photographed by a digital camera. Subsequently, the pig skins and films were fixed with glutaraldehyde (2.5%) and dehydrated with graded ethanol (0, 30, 50, 70, 80, 90, 95, and 100%). The bacteria morphology was visualized using SEM.
4.6. Cytotoxicity
By detecting the cell proliferation, the cytotoxicity of the prepared Cur-loading films was evaluated in vitro by the MTT method,45,46 and the cell morphology was studied by SEM observation. A total of 5 × 103 L929 fibroblast cells per well were seeded in 96-well tissue plates before use. Briefly, the tested cases were made to 2.5 × 2.5 cm2 squares before sterilization and co-cultivated with 2.1 mL of culture medium for 1 day. Then, 200 μL of the extractive medium was introduced into the 96-well plastic tissue plates. The incubation condition was kept constant with the temperature of 37 °C and 5% CO2. At different time intervals (1 day, 3 days, and 5 days), the cell-seeded plates were taken out from the incubator. MTT with the volume of 20 μL was introduced to each well. Blue formazan crystals were formed after 4 h of incubation. After dissolving the crystal with dimethyl sulfoxide (DMSO), the plates were shaken in the dark for 15 min. Utilizing a microplate (MK3, Labsystems, Finland), the absorbance values were measured at the wavelength of 492 nm. To measure the cell morphology on the drug-loading films, cells of 1 × 104 were seeded on the 1.5 cm2 square films for 4 h, and subsequently, 400 μL of medium was added. After incubating for 3 days, the films were fixed with paraformaldehyde (4%) and washed three times with PBS. Then, gradient ethyl alcohol solutions (30, 50, 75, 80, 90, 95, and 100%) were used to dehydrate. Finally, the films were sprayed with Au and viewed by SEM.
4.7. Statistical Analysis
In the present study, IBM SPSS Statistics 19.0 (SPSS, Inc., Chicago, Illinois, USA) was used to conduct the statistical analysis. Single-factor analysis of variance (ANOVA) was applied to determine the differences between groups. If the differences were significant, then the p value would be below 0.05.
Acknowledgments
We thank the financial support from the Fundamental Research Funds for the Central Universities and the generous help from our group member.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b02217.
(PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Lin Y. H.; Lin J. H.; Hong Y. S. Development of chitosan/poly-γ-glutamic acid/pluronic/curcumin nanoparticles in chitosan dressings for wound regeneration. J. Biomed. Mater. Res., Part B 2017, 105, 81–90. 10.1002/jbm.b.33394. [DOI] [PubMed] [Google Scholar]
- Aragona M.; Dekoninck S.; Rulands S.; Lenglez S.; Mascré G.; Simons B. D.; Blanpain C. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 2017, 8, 14684. 10.1038/ncomms14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M.; Zheng X.; Xu X.; Kong X.; Li X.; Guo G.; Luo F.; Zhao X.; Wei Y. Q.; Qian Z. Chitosan-alginate sponge: preparation and application in curcumin delivery for dermal wound healing in rat. J. Biomed. Biotechnol. 2009, 2009, 595126. 10.1155/2009/595126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell J. G.; McLaughlin S. W.; Tie L.; Laurencin C. T.; Chen A. F.; Nair L. S. Curcumin-loaded poly(ε-caprolactone) nanofibres: diabetic wound dressing with anti-oxidant and anti-inflammatory properties. Clin. Exp. Pharm. Physiol. 2009, 36, 1149–1156. 10.1111/j.1440-1681.2009.05216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G.; Li X.; Chen L.; Yang S.; Wu X.; Studer E.; Gurley E.; Hylemon P. B.; Ye F.; Li Y.; Zhou H. Synthesis and anti-inflammatory activities of mono-carbonyl analogues of curcumin. Bioorg. Med. Chem. Lett. 2008, 18, 1525–1529. 10.1016/j.bmcl.2007.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe Y.; Hashimoto S.; Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharm. Res. 1999, 39, 41. 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- Huang M. T.; Lysz T.; Ferraro T.; Abidi T. F.; Laskin J. D.; Conney A. H. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991, 51, 813–819. [PubMed] [Google Scholar]
- Vaughn A. R.; Haas K. N.; Burney W.; Andersen E.; Clark A. K.; Crawford R.; Sivamani R. K. Potential role of curcumin against biofilm-producing organisms on the skin: a review. Phytother. Res. 2017, 31, 1807–1816. 10.1002/ptr.5912. [DOI] [PubMed] [Google Scholar]
- Gopinath D.; Ahmed M. R.; Gomathi K.; Chitra K.; Sehgal P. K.; Jayakumar R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials 2004, 25, 1911–1917. 10.1016/S0142-9612(03)00625-2. [DOI] [PubMed] [Google Scholar]
- Ng K. W.; Achuth H. N.; Moochhala S.; Lim T. C.; Hutmacher D. W. In vivo evaluation of an ultra-thin polycaprolactone film as a wound dressing. J. Biomater. Sci., Polym. Ed. 2007, 18, 925–938. 10.1163/156856207781367693. [DOI] [PubMed] [Google Scholar]
- Al-Omair M. Synthesis of Antibacterial Silver–poly(ε-caprolactone)-methacrylic acid graft copolymer nanofibers and their evaluation as potential wound dressing. Polymer 2015, 7, 1464–1475. 10.3390/polym7081464. [DOI] [Google Scholar]
- Liu X.; Zheng S.; Dan W.; Dan N. Ultrasound-mediated preparation and evaluation of a collagen/PVP-PCL micro-and nanofiber scaffold electrospun from chloroform/ethanol mixture. Fibers Polym. 2016, 17, 1186–1197. 10.1007/s12221-016-6400-4. [DOI] [Google Scholar]
- Ramasamy P.; Shanmugam A. Characterization and wound healing property of collagen–chitosan film from sepia kobiensis (Hoyle, 1885). Int. J. Biol. Macromol. 2015, 74, 93–102. 10.1016/j.ijbiomac.2014.11.034. [DOI] [PubMed] [Google Scholar]
- Lin C. W.; Chen Y. K.; Lu M.; Lou K. L.; Yu J. Photo-crosslinked keratin/chitosan membranes as potential wound dressing materials. Polymer 2018, 10, 987. 10.3390/polym10090987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Dan N.; Wang L.; Liu X.; Dan W. Study on the cross-linking effect of a natural derived oxidized chitosan oligosaccharide on the porcine acellular dermal matrix. RSC Adv. 2016, 6, 38052–38063. 10.1039/C6RA03434A. [DOI] [Google Scholar]
- Liu T.; Dan W.; Dan N.; Liu X.; Liu X.; Peng X. A novel grapheme oxide-modified collagen-chitosan bio-film for controlled growth factor release in wound healing applications. Mater. Sci. Eng., C 2017, 77, 202–211. 10.1016/j.msec.2017.03.256. [DOI] [PubMed] [Google Scholar]
- Liu X.; Dan N.; Dan W.; Gong J. Feasibility study of the natural derived chitosan dialdehyde for chemical modification of collagen. Int. J. Biol. Macromol. 2016, 82, 989–997. 10.1016/j.ijbiomac.2015.11.015. [DOI] [PubMed] [Google Scholar]
- Gupta K. C.; Ravi Kumar M. N. V. Preparation, characterization and release profiles of pH-sensitive chitosan beads. Polym. Int. 2000, 49, 141–146. . [DOI] [Google Scholar]
- Salgado C. L.; Sanchez E. M. S.; Mano J. F.; Moraes A. M. Characterization of chitosan and polycaprolactone membranes designed for wound repair application. J. Mater. Sci. 2012, 47, 659–667. 10.1007/s10853-011-5836-6. [DOI] [Google Scholar]
- Poornima B.; Korrapati P. S. Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr. Polym. 2017, 157, 1741–1749. 10.1016/j.carbpol.2016.11.056. [DOI] [PubMed] [Google Scholar]
- Podsiadlo P.; Kaushik A. K.; Arruda E. M.; Waas A. M.; Shim B. S.; Xu J.; Nandivada H.; Pumplin B. G.; Lahann J.; Ramamoorthy A.; Kotov N. A. Ultrastrong and stiff layered polymer nanocomposites. Science 2007, 318, 80–83. 10.1126/science.1143176. [DOI] [PubMed] [Google Scholar]
- Dawson J. I.; Oreffo R. O. C. Clay: new opportunities for tissue regeneration and biomaterial design. Adv. Mater. 2013, 25, 4069–4086. 10.1002/adma.201301034. [DOI] [PubMed] [Google Scholar]
- Han L.; Lu X.; Liu K.; Wang K.; Fang L.; Weng L. T.; Zhang H.; Tang Y.; Ren F.; Zhao C.; Sun G.; Liang R.; Li Z. Mussel-inspired adhesive and tough hydrogel based on nanoclay confined dopamine polymerization. ACS Nano 2017, 11, 2561–2574. 10.1021/acsnano.6b05318. [DOI] [PubMed] [Google Scholar]
- Li Y.; Qing S.; Zhou J.; Yang G. Evaluation of bacterial cellulose/hyaluronan nanocomposite biomaterials. Carbohydr. Polym. 2014, 103, 496–501. 10.1016/j.carbpol.2013.12.059. [DOI] [PubMed] [Google Scholar]
- Figueira D. R.; Miguel S. P.; de Sá K. D.; Correia I. J. Production and characterization of polycaprolactone- hyaluronic acid/chitosan- zein electrospun bilayer nanofibrous membrane for tissue regeneration. Int. J. Biol. Macromol. 2016, 93, 1100–1110. 10.1016/j.ijbiomac.2016.09.080. [DOI] [PubMed] [Google Scholar]
- Noori S.; Kokabi M.; Hassan Z. M. Poly(vinyl alcohol)/chitosan/honey/clay responsive nanocomposite hydrogel wound dressing. J. Appl. Polym. Sci. 2018, 135, 46311. 10.1002/app.46311. [DOI] [Google Scholar]
- Xu Y.; Ren X.; Hanna M. A. Chitosan/clay nanocomposite film preparation and characterization. J. Appl. Polym. Sci. 2005, 99, 1684–1691. 10.1002/app.22664. [DOI] [Google Scholar]
- Yallapu M. M.; Gupta B. K.; Jaggi M.; Chauhan S. C. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J. Colloid Interface Sci. 2010, 351, 19–29. 10.1016/j.jcis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Qi L.; Xu Z.; Jiang X.; Hu C.; Zou X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. 10.1016/j.carres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Yeum J. H. Novel poly (vinyl alcohol)/clay nanocomposite microspheres via suspension polymerization and saponification. Polym.-Plast. Technol. Eng. 2011, 50, 1149–1154. 10.1080/03602559.2011.566302. [DOI] [Google Scholar]
- Chen B.; Evans J. R. G. Poly (ε-caprolactone)– clay nanocomposites: structure and mechanical properties. Macromolecules 2006, 39, 747–754. 10.1021/ma052154a. [DOI] [Google Scholar]
- Lai P.; Daear W.; Löbenberg R.; Prenner E. J. Overview of the preparation of organic polymeric nanoparticles for drug delivery based on gelatine, chitosan, poly( d , l -lactide-co-glycolic acid) and polyalkylcyanoacrylate. Colloid Surf., B 2014, 118, 154–163. 10.1016/j.colsurfb.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Soares P. I. P.; Sousa A. I.; Silva J. C.; Ferreira I. M. M.; Novo C. M. M.; Borges J. P. Chitosan-based nanoparticles as drug delivery systems for doxorubicin: Optimization and modelling. Carbohydr. Polym. 2016, 147, 304–312. 10.1016/j.carbpol.2016.03.028. [DOI] [PubMed] [Google Scholar]
- Ianchis R.; Ninciuleanu C. M.; Gifu I. C.; Alexandrescu E.; Nistor C. L.; Nitu S. G.; Petcu C. Hydrogel-clay nanocomposites as carriers for controlled release. Curr. Med. Chem. 2018, 10.2174/0929867325666180831151055. [DOI] [PubMed] [Google Scholar]
- Joshi G. V.; Kevadiya B. D.; Patel H. A.; Bajaj H. C.; Jasra R. V. Montmorillonite as a drug delivery system: intercalation and in vitro release of timolol maleate. Int. J. Pharm. 2009, 374, 53–57. 10.1016/j.ijpharm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Yuan Q.; Shah J.; Hein S.; Misra R. D. K. Controlled and extended drug release behavior of chitosan-based nanoparticle carrier. Acta Biomater. 2010, 6, 1140–1148. 10.1016/j.actbio.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Kevadiya B. D.; Rajkumar S.; Bajaj H. C. Application and evaluation of layered silicate–chitosan composites for site specific delivery of diclofenac. Biocybern. Biomed. Eng. 2015, 35, 120–127. 10.1016/j.bbe.2014.08.004. [DOI] [Google Scholar]
- Hua S.; Yang H.; Wang W.; Wang A. Controlled release of ofloxacin from chitosan–montmorillonite hydrogel. Appl. Clay Sci. 2010, 50, 112–117. 10.1016/j.clay.2010.07.012. [DOI] [Google Scholar]
- Hsu S. H.; Wang M. C.; Lin J. J. Biocompatibility and antimicrobial evaluation of montmorillonite/chitosan nanocomposites. Appl. Clay Sci. 2012, 56, 53–62. 10.1016/j.clay.2011.09.016. [DOI] [Google Scholar]
- Maisanaba S.; Gutiérrez-Praena D.; Puerto M.; Llana-Ruiz-Cabello M.; Pichardo S.; Moyano R.; Blanco A.; Jordá-Beneyto M.; Jos Á. In vivo toxicity evaluation of the migration extract of an organomodified clay-poly(lactic) acid nanocomposite. J. Toxicol. Environ. Health 2014, 77, 731–746. 10.1080/15287394.2014.890987. [DOI] [PubMed] [Google Scholar]
- Maisanaba S.; Pichardo S.; Puerto M.; Gutiérrez-Praena D.; Cameán A. M.; Jos A. Toxicological evaluation of clay minerals and derived nanocomposites: a review. Environ. Res. 2015, 138, 233–254. 10.1016/j.envres.2014.12.024. [DOI] [PubMed] [Google Scholar]
- Liu X.; Dan N.; Dan W. Preparation and characterization of an advanced collagen aggregate from porcine acellular dermal matrix. Int. J. Biol. Macromol. 2016, 88, 179–188. 10.1016/j.ijbiomac.2016.03.066. [DOI] [PubMed] [Google Scholar]
- Singh B.; Sharma D. K.; Gupta A. A study towards release dynamics of thiram fungicide from starch-alginate beads to control environmental and health hazards. J. Hazard. Mater. 2009, 161, 208–216. 10.1016/j.jhazmat.2008.03.074. [DOI] [PubMed] [Google Scholar]
- Fan X.; Yang F.; Nie C.; Yang Y.; Ji H.; He C.; Cheng C.; Zhao C. Mussel-inspired synthesis of NIR-responsive and biocompatible Ag-graphene 2D nano-agents for versatile bacterial disinfections. ACS Appl. Mater. Interfaces 2017, 10, 296–307. 10.1021/acsami.7b16283. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Dan N.; Dan W.; Zhao W.; Bai Z.; Chen Y.; Yang C. Facile fabrication of gelatin and polycaprolactone based bilayered membranes via spin coating method with antibacterial and cyto-compatible properties. Int. J. Biol. Macromol. 2019, 124, 699–707. 10.1016/j.ijbiomac.2018.11.262. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Dan N.; Dan W.; Zhao W.; Bai Z.; Chen Y.; Yang C. Bilayered antimicrobial nanofiber membranes for wound dressings via in situ cross-Linking polymerization and electrospinning. Ind. Eng. Chem. Res. 2018, 57, 17048–17057. 10.1021/acs.iecr.8b03122. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.