Abstract
Natural sugars combine energy supply and, except a few cases, a pleasant taste. On the other hand, exaggerated consumption may impact population health. This has busted the research for the synthesis of increasingly cheaper artificial sweeteners, with low energy content and intense taste. Here, we suggest that studies of the hydration properties of three disaccharides, namely, the natural sucrose and lactose and the artificial sucralose, may explain the difference by orders of magnitude among their sweetness. This is done by analyzing via Monte Carlo simulations the neutron diffraction differential cross sections of aqueous solutions of the three sugars and their isotopes. Our results show that the strength of the sugar–water hydrogen bond interaction is one of the factors influencing sweetness, another being the number of water molecules within the first neighboring shell of the sugar whether bonded or not.
Introduction
Although humans have evolved on a diet containing little to no refined carbohydrates,1 in recent years, the consumption of refined or artificial sugars has dramatically increased. Studies have explored the causes of such an increase, with fingers pointed at addiction-like behavior caused by dopamine and opioid released on sugar consumption,2 and ever-increasing concerns about its links to diabetes and other diseases.3
Recent discoveries have greatly advanced our understanding of the physiology of sweet taste perception,4 but the atomic-scale three-dimensional structure of the sweet taste receptor is currently unknown5 and alternative methodologies, such as homology models, are required to provide the details of the binding of sweet compounds.6
Investigating the hydration properties of sugars is motivated by the fact that (a) protein–ligand binding is often characterized in terms of a match between hydrophilic and hydrophobic surface regions both in the receptor pocket and on the target molecule7−10 and (b) the interaction between a molecule in a biological environment and its receptor is thought to be often mediated by the presence of an aqueous medium (see for example ref (11)). In short, we believe that knowing the experimentally determined hydration structure of common sugars may contribute to improving homology models for sugar–water interaction. Furthermore, understanding the sugar–water interaction also has implications on the ability of sugars to stabilize proteins in solution.12
We have recently investigated the correlation between the microscopic structure of monosaccharides in aqueous solutions and their ability to elicit sweet taste sensation.13−15 Specifically, in Bruni et al.,15 neutron diffraction has been employed to determine the structure of the hydration shell of fructose, glucose, and mannose. Small differences in stereochemistry between the different monosaccharides determine a significant change in polarity in a solution that is sufficient to influence the hydration shell of these molecules. We have observed a relationship between the sugar–water hydrogen bond length (and therefore strength) and the sugar’s perceived sweetness. In terms of their effect on the bulk water structure, the investigated monosaccharides all have a similar effect, at odd with the effect on the water of trehalose,16,17 with implications for bioprotective properties against environmental stresses.18
Among the sugars found in nature, sucrose (saccharose, beet sugar, or cane sugar) plays a dominant role in food processing and agriculture, as well as in international economics and politics.19 Sucrose is a disaccharide naturally present in many plants, in varying quantities, with the general formula C12H22O11, and is easily split by hydrolysis into the two monosaccharides glucose and fructose.
Lactose is primarily found in human and animal milk. It consists of a d-glucose and a d-galactose molecule joined by a β-1,4-glycoside linkage. Lactose has two isomeric forms, α- and β-lactose, which differ with respect to the steric configuration of the hydroxyl group of C-1 moiety of glucose. Lactose is a factor ∼10 less sweet than sucrose.20
Sucralose is an artificial sweetener of the intensive type, i.e., a small amount can substitute a large amount of sucrose. Sucralose has been chosen in this investigation as a comparison to the natural sugars for two main reasons: (a) it is much sweeter than sucrose (400–800 times21), while, at the same time, (b) not being hygroscopic,21 it is not expected to bind water very strongly. Based on our previous work in Bruni et al.,15 the sites forming hydrogen bonds (HBs) with water can also bind the hydrophilic ends of the receptor, and, as a consequence, the shorter (i.e., stronger) the HB, the sweeter the sugar. Sucralose, not being hygroscopic, is not expected to have a strong affinity for water. According to our previous work, sweetness correlates with strong sugar–water hydrogen bonds: therefore, a nonhygroscopic strongly sweet molecule challenges the theory we expressed in our previous publication.
To tackle this issue, we have employed a combination of neutron diffraction with isotopic substitution and a simulation method known as empirical potential structure refinement (EPSR)22 developed in the disordered materials group at the ISIS Neutron and Muon Source. In recent years, this combination has been successfully employed to investigate the ability of small molecules to form hydrophilic/phobic interactions in solution.23−27
Results
Hydrogen Bonding in Sucrose and Lactose
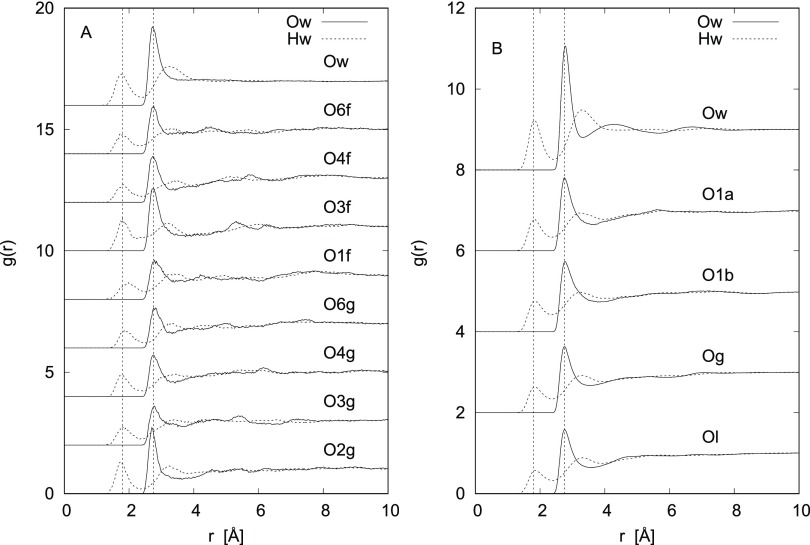
Looking at Figure 1, showing the Osugar–Hwater (dashed line) and Osugar–Owater (solid line) radial distribution functions, the first general observation is that sucrose and lactose have very similar HB patterns. All of the hydroxylic oxygens seem to have some degree of hydration for both sugars, with first peaks at ∼1.8 Å and in the 2.7–2.8 Å range, for the two functions, respectively. Similarly (Figures 2A and 3A), the hydroxyl hydrogens also show a clear sign of hydrogen bonding, with a peak at ∼1.80 Å. Conversely (Figures 2B and 3B), neither the ring oxygens (Org, Orf, Orl) nor the bridging oxygen (Ob) show significant signs of hydrogen bonding, as the radial distribution functions of these sites with water hydrogens (Hw) show peaks at distances larger than those typical of hydrogen bonding. Moreover, as expected, all methyl hydrogens (M) do not form hydrogen bonds (Figures 2A and 3A).
Figure 1.
Hydrogen-bonding interactions for the solutions of natural disaccharides: (A) for sucrose and (B) for lactose. The water–water radial distribution functions for the two samples are reported at the top of the graphic for comparison. The hydrogen–oxygen radial distribution functions are dotted and the oxygen–oxygen ones are solid. Vertical dashed lines at 1.80 and 2.75 Å evidence the position of the first peaks in the Ow–Hw and Ow–Ow correlations, respectively. Labeling is further described in Figures 8 and 9.
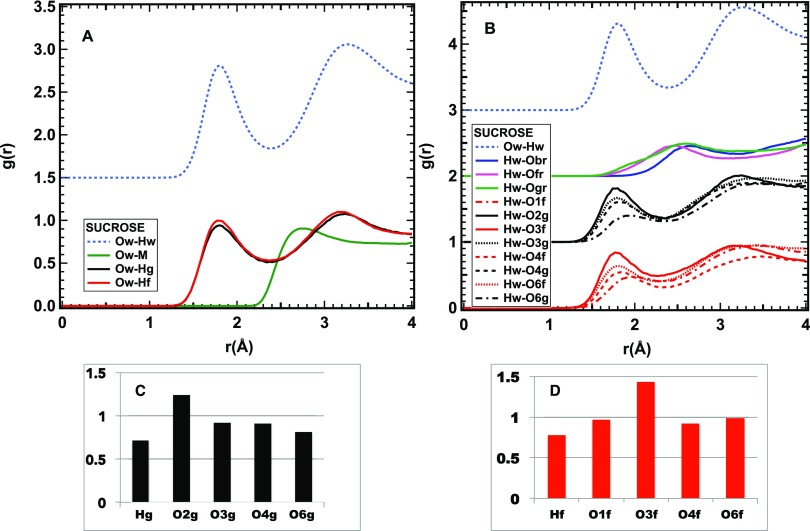
Figure 2.
Closer view of the HB interaction of sucrose and water. (A) Radial distribution functions of water oxygens and sucrose hydrogens. (B) Radial distribution functions of water hydrogens and sucrose oxygens. The radial distribution function of the Ow–Hw pair is reported as a light blue dashed line for comparison. The green line in (A) refers to methyl hydrogens, the red lines to O or H atoms on the fructose ring, and the black ones to those on the glucose ring. The blue, green, and magenta lines in (B) refer to the oxygens on and between the rings. (C) Number of HB contacts between water and hydrogen or oxygen sites on the glucose ring of sucrose. (D) Number of HB contacts between water and hydrogen or oxygen sites on the fructose ring of sucrose.
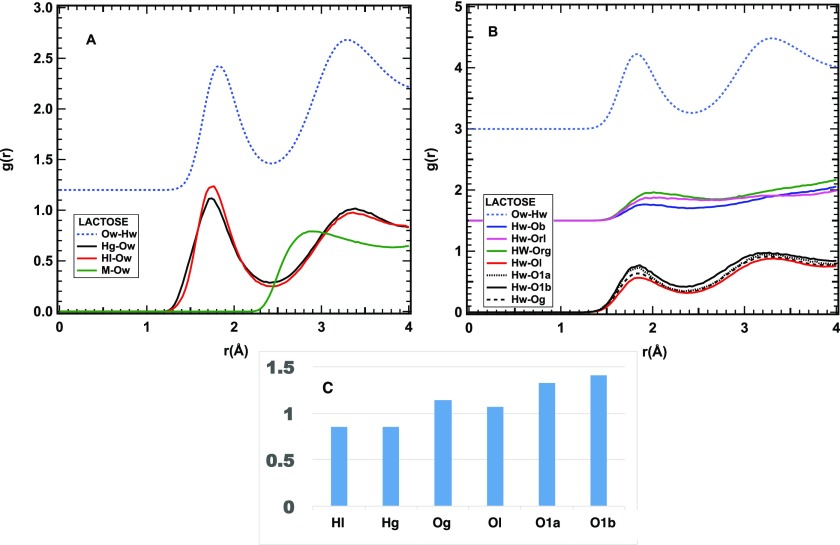
Figure 3.
Closer view of the HB interaction of lactose and water. (A) Radial distribution functions of water oxygens and lactose hydrogens. (B) Radial distributions of water hydrogens and sucrose oxygens. The radial distribution function of the Ow–Hw pair is reported as a light blue dashed line for comparison. The green line in (A) refers to the methyl hydrogens, the red lines to O or H atoms on the galactose ring, and the black ones to those on the glucose ring. The blue, green, and magenta lines in (B) refer to the oxygens on and between the rings. (C) Number of HB contacts between water and hydrogen or oxygen sites of lactose.
Consequently, similar to the monosaccharides, these molecules present both hydrophilic and hydrophobic interactions with water, which is one of the conditions required to elicit the sweet taste.7−10
A closer look (Figure 2) at sucrose hydration reveals the following features and subtle differences:
All of the hydroxyl hydrogens, contrarily to the methyl ones, strongly interact with water, as proved by a HB peak in the Ow–H radial distribution function at a short distance: ∼1.77 Å (Figure 2A).
Although all of the hydroxyl oxygens form HB with water, there are differences in the HB peak position and intensities of these peaks. In particular, the peak position varies from a minimum of 1.74 Å to a maximum of 1.92 Å (Figure 2B).
The number of HB contacts is similar for the two rings of sucrose and fluctuates between 0.7 and 1.2 at the individual sites (Figure 2C,D).
For lactose, an average over all OH hydrogens for each ring (Hg for the glucose ring and Hl for the galactose one) is presented in Figure 3A, along with the radial distribution function relative to the methyl hydrogens. The hydroxyl oxygens on the two rings have also been averaged as Og and Ol, respectively, with only the anomeric oxygen O1 on the glucosyl ring shown separately (the α and β anomers are distinguished as O1a and O1b). The bridging and ring oxygens have been labeled Ob, Org, and Orl, and also, in this case, their interaction with water is weaker than for the hydroxyl groups, although their hydrophobic character is less defined, as suggested by the low-intensity first peak at ∼2Å (see Figure 3B).
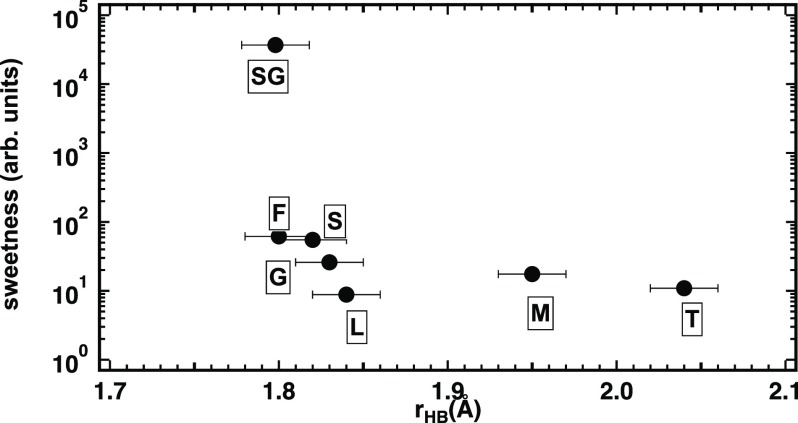
Overall, the HB peaks, relative to the hydroxyl groups, shown in Figure 3A,B are quite similar to what is observed in the case of sucrose, although with a larger average HB length. Interestingly, the average HB length of sucrose is intermediate between those of fructose and glucose, while that of lactose is longer than that of glucose and shorter than that of mannose (fructose < sucrose < glucose < lactose ≪ mannose). This observation suggests that, as in the case of monosaccharides, the strength of the HB is correlated with the sugar sweetness (see Figure 4).
Figure 4.
Relation between sweetness and average HB length for all of the sugars investigated so far. Individual data are labeled by the initial of the sugar name: F: fructose, S: sucrose, G: glucose, L: lactose, M: maltose,T: trehalose, and SG is the sucralose glucose-like ring.
Hydration of Sucralose
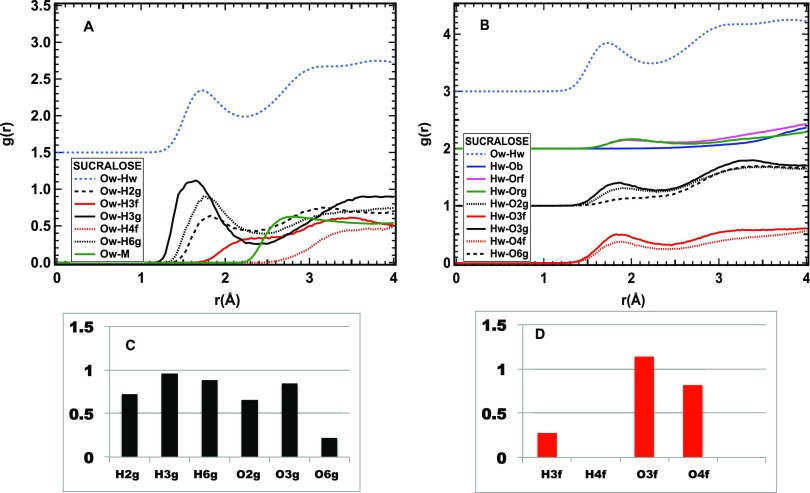
Hydrogen bonding between water and sucralose is shown in Figure 5. As expected, similar to sucrose and lactose, the ring and bridging oxygens and the methyl hydrogens are not hydrogen-bonded. Nevertheless, notable differences from those observed for the other disaccharides, and sucrose, in particular, are found:
Only the hydrogens of the glucose ring form hydrogen bonds, with lengths varying between 1.6 and 1.8 Å, while the two hydrogens of the fructose ring, H3f and H4f, show a first neighbor water oxygen at a distance longer than 2 or 3 Å, respectively (Figure 5A).
All hydroxyl oxygens form HB with lengths between ∼1.8 and ∼1.9 Å, although for O6g, the HB peak is very weak (Figure 5B).
The number of contacts made by water molecules with hydroxyl oxygens belonging to the glucose ring is quite similar to that observed in the case of sucrose, with the already mentioned exception of the O6g site (black dashed line in Figure 5B) (Figure 5C).
On the fructose ring, while O3f and O4f have almost the same number of contacts found at the corresponding sites for sucrose, the contacts at the H3f and H4f sites are strongly reduced or absent (Figure 5D).
Interestingly, the average HB length to hydrogens or oxygens on the glucose ring alone is shorter than 1.8 Å, which is shorter than that found for sucrose and lactose and for the monosaccharide glucose.15
Figure 5.
HB interaction of sucralose and water. (A) Radial distribution functions of water oxygens and sucralose hydrogens. (B) Radial distribution functions of water hydrogens and sucralose oxygens. The radial distribution function of the Ow–Hw pair is reported as a light blue dashed line for comparison. The green line in (A) refers to the methyl hydrogens, the red lines to O or H atoms on the fructose ring, and the black ones to those on the glucose ring. The blue, green, and magenta lines in (B) refer to the oxygens on and between the rings. (C) Number of HB contacts between water and hydrogen or oxygen sites on the glucose ring of sucralose. (D) Number of HB contacts between water and hydrogen or oxygen sites on the fructose ring of sucralose.
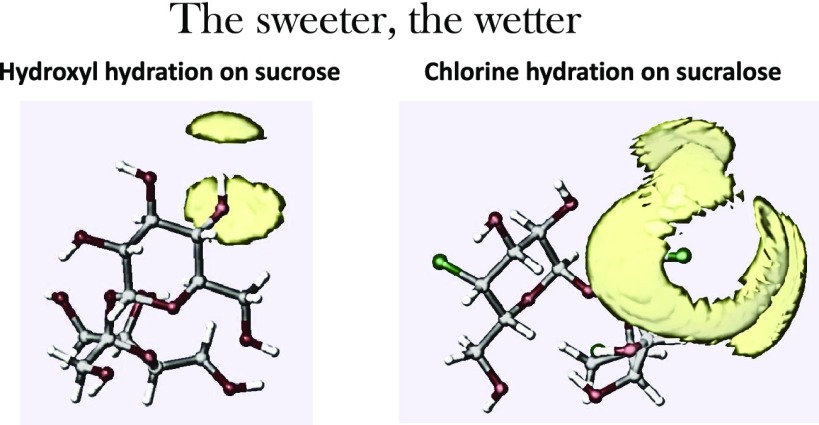
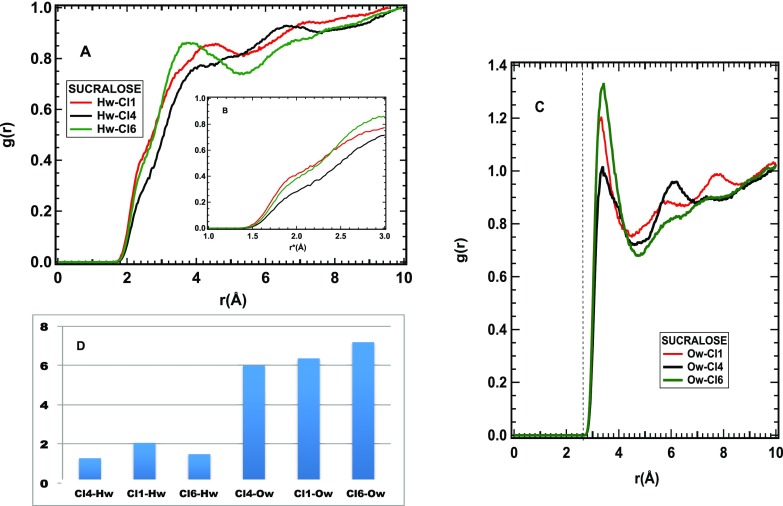
Figure 6 shows the hydration of the Cl sites in sucralose. All three gClHw(r) radial distribution functions increase toward a maximum at r ∼ 4 Å, with a shoulder between 2 and 3 Å, ascribable to the first neighbor water hydrogens (Figure 6A). The inset (Figure 6B) shows the same functions on a scale reduced according to the ratio σO/σCl: here, the shoulder is below 2 Å, a distance compatible with weak HBs. The Cl–OW first neighbor distance is quite well defined at 3.3 Å (Figure 6C), corresponding to 2.65 Å on the reduced scale (not shown). On the other hand, the broadness of the Hw–Cl peak and the almost flat distribution of the Cl–Hw–Ow angles (Figure 7) are indicative of the absence of directionality in the Cl–water interaction. The orientational disorder of water around the Cl sites is likely responsible for a large number of Cl–water oxygen contacts (see Figure 6D), making sucralose the wettest sugar among those investigated here.
Figure 6.
Interaction of sucralose Cl sites and water. (A) Radial distribution functions of water hydrogens and sucralose Cl sites. (B) Same functions as in (A), as a function of a reduced distance r* = r × (σOw/σCl). This reduced scale evidences the presence of water hydrogens at a distance from the Cl ones comparable with the OH distance typical of hydrogen bonding. (C) Radial distribution functions of water oxygens and sucralose Cl sites. The dashed line indicates the position of the first peak on the same reduced scale as in (B). (D) Number of contacts between water and Cl sites on sucralose, corresponding to the first neighbor peak.
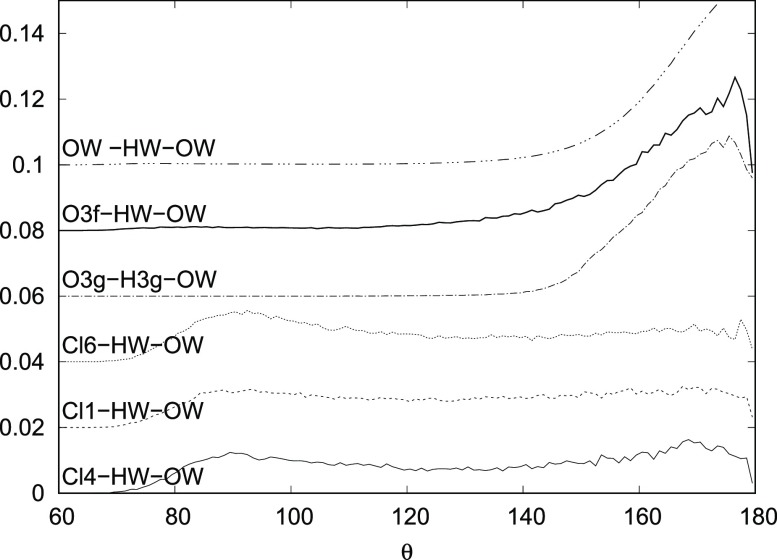
Figure 7.
Angular distributions for a subset of the sites on sucralose that display an interaction with water in the form of a peak at a distance compatible with a hydrogen bond. A directional hydrogen bond has a peak at very high angles, such as the case of HBs between water molecules. Chlorine atoms do not display any significant feature in their angular distribution.
Sucralose, although being the least hygroscopic among the investigated sugars, has the largest number of water molecules in its hydration shell. Hence, it does form fewer strong hydrogen bonds, and one of its rings does not form any at all. In fact, most of the molecules around sucralose are weakly bonded to its chlorine. For this reason, it can be soluble, and extremely sweet, but not necessarily hygroscopic.
Methods
The neutron diffraction experiments have been performed on the SANDALS diffractometer at the ISIS spallation neutron source, located at the Rutherford Appleton Laboratory (U.K.). SANDALS is a total scattering neutron diffractometer optimized for the study of liquids and amorphous samples containing light elements. To provide several independent determinations of the structure factor of the solutions, we have applied the H/D isotopic substitution method.28 This is based on the fact that the neutron scattering length bα changes sometimes quite significantly for different isotopes, while the atomic correlation does not vary within the sensitivity of the instruments; hence, isotopic substitution quite dramatically changes the shape of the measured function F(Q). In particular, by substituting 1H (bH = −3.740 fm) with 2H (bD = 6.671 fm), it is possible to highlight H–X correlations, where X is a nonsubstituted atom (e.g., oxygen).
For all three sugars, four isotopic compositions have been measured: (1) fully protiated, (2) fully deuteriated, (3) an equimolar mixture of (1) and (2), and (4) the so-called “null” mixture, i.e., 64% of (1) and 36% of (2). In mixture (4), the XH and HH correlations are canceled out, being the protiated molar fraction equal to cH = bD/(bH + bD). All sugars have been purchased from Sigma-Aldrich and used without further purification for the preparation of the fully protiated samples. The sugars used for the preparation of the fully deuteriated samples have been repeatedly solvated in D2O and freeze-dried, to exchange all of the hydroxyl hydrogens. Sucrose was measured at a concentration of 1 mole per 25 water moles (1:25), while both lactose and sucralose were measured at a lower concentration of 1 mole per 100 water moles (1:100). The different concentration is an experimental incident, and it is not expected to influence our comparison, as the structure of the first hydration shell, which is of interest for this study, is not affected by concentration when at least a complete first shell of water is available.17
All solutions have been exposed to the neutron beam inside standard flat Ti–Zr samples (1 mm thick). The standard corrections and normalizations have been applied to the data through the set of programs gathered under the graphical interface GudrunN. The theoretical background to the operations performed by the program is described in its manual.29
Models of the experimental data have been constructed using the empirical potential structure refinement (EPSR) program. The method has been described in detail elsewhere (see Soper22 and references therein) and therefore only a brief summary will be given here. The algorithm is based on a classical Monte Carlo simulation of the molecular system under study at a fixed concentration and density and employs an iterative algorithm that aims at building an atomistic three-dimensional model consistent with the scattering data. Details about the simulation (potential model employed, concentration of sugars and their anomers in the simulation box, and intramolecular structures) are available as the Supporting Information, along with the comparison between the measured and simulated F(Q) functions. Sucrose and lactose intramolecular configurations have been derived from the crystalline structure available in the literature; sucralose is a gas-phase optimization performed by the authors (full details in the Supporting Information). Both sucrose and sucralose have a folded “clam” structure, while lactose has a fully stretched link. Figures 8–10 show the Haworth projection for the three investigated disaccharides, as well as the atomic labels used throughout the paper.
Figure 8.
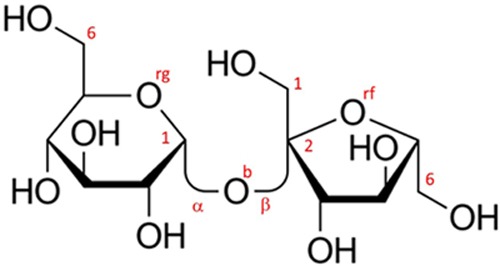
Haworth projection for sucrose alias α-d-glucopyranosyl-(1→2)-β-d-fructofuranoside. The figure also shows the labeling of some specific atomic sites; all labels (shown and not shown in the figure) are described as in the following paragraph. All carbon atoms (not shown) are labeled C and a clockwise (glucose) or anticlockwise (fructose) sequential number (C1 on glucose and C2 on fructose are marked as a reference); hydroxyl oxygen atoms are numbered like the nearest carbon (i.e., O2g is attached to C2 on the glucose side). The bridge oxygen is Ob, and the ring oxygens are Org and Orf for glucose and fructose rings, respectively. All other hydroxyl hydrogens are labeled H, while the carbonyl hydrogens (not shown) are labeled M.
Figure 10.
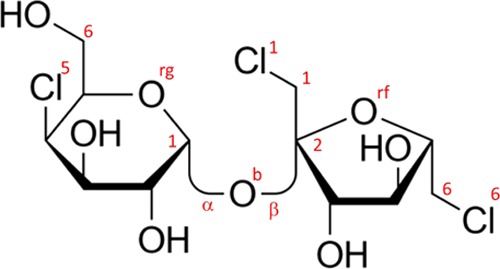
Haworth projection for sucralose. The figure also shows the labeling of some specific atomic sites; all labels (shown and not shown in the figure) are described as in the following paragraph. All labeling is similar to sucrose with the addition of chlorine atoms that are numbered according to the nearest carbon atoms Cl1 Cl5 and Cl6 as shown explicitly in the figure.
Figure 9.
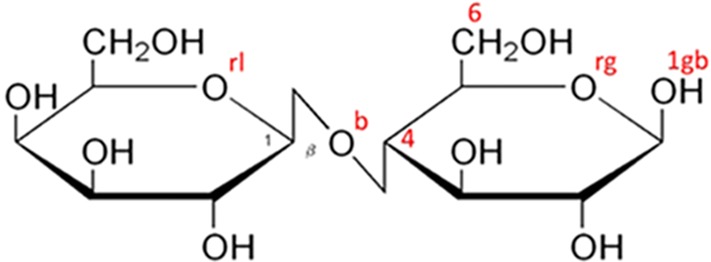
Haworth projection for β-lactose alias β-d-galactopyranosyl-(1→4)-d-glucopyranoside. The figure also shows the labeling of some specific atomic sites; all labels (shown and not shown in the figure) are described as in the following paragraph. All carbon atoms (not shown) are labeled C and a clockwise sequential number (C1 on the galactose ring and C4 on the glucose ring are marked as a reference); hydroxyl oxygen atoms are numbered like the nearest carbon (i.e., O2g is bonded to C2 on the glucose side). Note that the anomeric carbon has an additional label a or b for α (not pictured) and β (see O1gb in the figure) glucose anomers. The bridge oxygen is Ob, and the ring oxygens are Org and Orl for glucose and galactose rings, respectively. All other hydroxyl hydrogens are labeled H, while the carbonyl hydrogens (not shown) are labeled M.
Conclusions
For all natural sugars, we have demonstrated a correlation between the sweetness ranking and sugar–water hydrogen bond length, with the shorter bonds determining the sweeter perceived taste (see Figure 4). On the other hand, there is a minimum value of the HB length, under which hydrogen and oxygen cannot move closer to one another. Therefore, how is it possible to increase the sugar sweetness by one or two orders of magnitude, as in the case of artificial sweeteners? An additional mechanism is required, and the present measurement of sucralose solutions seems to suggest an answer. Sucralose, two orders of magnitude sweeter than sucrose, is obtained by substituting three of the hydroxylic groups of sucrose by chlorine atoms. One of the effects of this substitution is that only the glucose-like ring of sucralose forms hydrogen bonds with water. These are stronger bonds than the same ring of sucrose and the monosaccharide glucose.15 Additionally, we have found that all three chlorine sites of sucralose are surrounded by a large number of water molecules, at a relatively large distance and whose dipoles are not aligned to the radial field and that therefore cannot be considered hydrogen-bonded. (It is likely that the overcrowding of water molecules around the chlorine sites inhibits the formation of hydrogen bonds at the two hydroxylic sites on the fructose ring.) Thus, sucralose can be considered the wetter among the investigated sugars, although the number of actual hydrogen bonds formed with water is reduced by the substitution of three hydroxyl groups by chlorine atoms.
The findings from the current study, compared to those from the previous one on natural monosaccharides, are twofold: (a) on the one hand, they confirm the fact that the hydrogen bond length (with a caveat, see below) is correlated to sugar sweetness also for natural disaccharides and (b) the mechanism for artificial sugars could be more complicated and involve a number of weakly bonded water molecules, as well as strongly bonded water molecules (where short and strong are often used interchangeably when dealing with structural studies).
At this stage, we can only speculate about the microscopic mechanisms, which makes the wetter sugar be the sweeter sugar and how indeed this enhanced hydration contributes to how the molecules bind to the taste receptors in vivo. It could be speculated that the presence of orientationally disordered water molecules around the fructose ring favors the interaction with the sweet taste receptor in some way. Two among many things that could be happening include (a) that receptor–sugar interaction is facilitated toward the fructose ring, by the fact that weakly bound orientationally disordered water molecules are more easily removed, or impose weaker orientational constraints; and (b) that the lack of hydrogen bonds at the fructose side forces the receptor–sugar interaction toward the glucose ring, with strong hydrogen bonds. This implies a reduced number of possible sugar orientations within the receptor buds, suggesting the role of entropic forces in the binding mechanism.
Acknowledgments
Experiments at the ISIS Pulsed Neutron and Muon Source were supported by a beamtime allocation from the Science and Technology Facilities Council under the RB numbers 1520061 (sucrose and lactose) and 1800093 (sucralose). S.I. would like to thank all of the staff at the ISIS Neutron and Muon Source for support to this and many other experiments. This work has been performed within the Agreement No. 0018318 (02/06/2014) between STFC and CNR, concerning collaboration in scientific research at the spallation neutron source ISIS and with the partial financial support of CNR. F.B. and M.A.R. gratefully acknowledge the Grant of Excellence Departments, MIUR (ARTICOLO 1, COMMI 314–337 LEGGE 232/2016). S.E.M. and N.H.R. thank The Leverhulme Trust (U.K.) for the provision of research funding (RPG-2015-135).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b02794.
Details of the EPSR simulations; comparison of the EPSR-calculated vs experimental structure factors; sample composition for the three experiments; and Lennard Jones parameters and fractional charges of the individual sites (PDF)
Author Present Address
⊥ ACS International Ltd., Begbroke House, Wallbrook Court, North Hinksey Lane, Oxford OX2 0QS, United Kingdom.
The authors declare no competing financial interest.
Supplementary Material
References
- Yudkin J. Evolutionary and Historical Changes in Dietary Carbohydrates. Am. J. Clin. Nutr. 1967, 20, 108–115. 10.1093/ajcn/20.2.108. [DOI] [PubMed] [Google Scholar]
- Avena N. M.; Rada P.; Hoebel B. G. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci. Biobehav. Rev. 2008, 32, 20–39. 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S.; Yoffe P.; Hills N.; Lustig R. H. The Relationship of Sugar to Population-Level Diabetes Prevalence: An Econometric Analysis of Repeated Cross-Sectional Data. PLoS One 2013, 8, e57873 10.1371/journal.pone.0057873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N.; Roper S. D. The Cell Biology of Taste. J. Cell Biol. 2010, 190, 285–296. 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.; Owyang C. Sugars, Sweet Taste Receptors, and Brain Responses. Nutrients 2017, 9, 653 10.3390/nu9070653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi M.; Morini S.; Lucchesini M.; Mensuali-Sodi A. Effects of leaf soluble sugars content and net photosynthetic rate of quince donor shoots on subsequent morphogenesis in leaf explants. Biol. Plant. 2011, 55, 237–242. 10.1007/s10535-011-0034-6. [DOI] [Google Scholar]
- Shallenberger R. S.; Acree T. E. Molecular Theory of Sweet Taste. Nature 1967, 216, 480–482. 10.1038/216480a0. [DOI] [PubMed] [Google Scholar]
- Kier L. B. A Molecular Theory of Sweet Taste. J. Pharm. Sci. 1972, 61, 1394–1397. 10.1002/jps.2600610910. [DOI] [PubMed] [Google Scholar]
- Nofre C.; Tinti J.-M. Sweetness Reception in Man: the Multipoint Attachment Theory. Food Chem. 1996, 56, 263–274. 10.1016/0308-8146(96)00023-4. [DOI] [Google Scholar]
- Eggers S. C.; Acree T. E.; Shallenberger R. S. Sweetness Chemoreception Theory and Sweetness Transduction. Food Chem. 2000, 68, 45–49. 10.1016/S0308-8146(99)00154-5. [DOI] [Google Scholar]
- Sahai M. A.; Biggin P. C. Quantifying Water-Mediated ProteinLigand Interactions in a Glutamate Receptor: A DFT Study. J. Phys. Chem. B 2011, 115, 7085–7096. 10.1021/jp200776t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. J.; Debenedetti P. G. Structure and Dynamics in Concentrated, Amorphous Carbohydrate Water Systems by Molecular Dynamics Simulation. J. Phys. Chem. B 1999, 103, 7308–7318. 10.1021/jp9911548. [DOI] [Google Scholar]
- Maugeri L.; Busch S.; McLain S. E.; Pardo L. C.; Bruni F.; Ricci M. A. Structure-Activity Relationships in Carbohydrates Revealed by their Hydration. Biochim. Biophys. Acta, Gen. Subj. 2017, 1861, 1486–1493. 10.1016/j.bbagen.2016.12.017. [DOI] [PubMed] [Google Scholar]
- Rhys N. H.; Bruni F.; Imberti S.; McLain S. E.; Ricci M. A. Glucose and Mannose: A Link between Hydration and Sweetness. J. Phys. Chem. B 2017, 121, 7771–7776. 10.1021/acs.jpcb.7b03919. [DOI] [PubMed] [Google Scholar]
- Bruni F.; Di Mino C.; Imberti S.; McLain S.; Rhys N.; Ricci M. A. Hydrogen Bond Length as a Key to Understanding Sweetness. J. Phys. Chem. Lett. 2018, 9, 3667–3672. 10.1021/acs.jpclett.8b01280. [DOI] [PubMed] [Google Scholar]
- Pagnotta S. E.; McLain S. E.; Soper A. K.; Bruni F.; Ricci M. A. Water and Trehalose: How Much Do They Interact with Each Other. J. Phys. Chem. B 2010, 114, 4904–4908. 10.1021/jp911940h. [DOI] [PubMed] [Google Scholar]
- Soper A. K.; Ricci M. A.; Bruni F.; Rhys N. H.; McLain S. E. Trehalose in Water Revisited. J. Phys. Chem. B 2018, 122, 7365–7374. 10.1021/acs.jpcb.8b03450. [DOI] [PubMed] [Google Scholar]
- Sami F.; Yusuf M.; Faizan M.; Faraz A.; Hayat S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. 10.1016/j.plaphy.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Huberlant J.“Sucrose” in Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press, 2003; pp 5636–5641. [Google Scholar]
- Johnson J.; Conforti F.. “Lactose” in Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press, 2003; pp 3472–3476. [Google Scholar]
- Glória M.“Sweeteners – Others” in Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press, 2003; pp 5695–5702. [Google Scholar]
- Soper A. K. Computer simulation as a tool for the interpretation of total scattering data from glasses and liquids. Mol. Simul. 2012, 38, 1171–1185. 10.1080/08927022.2012.732222. [DOI] [Google Scholar]
- Steinke N.; Genina A.; Gillams R. J.; Lorenz C. D.; McLain S. E. Proline and Water Stabilization of a Universal Two-Step Folding Mechanism for -Turn Formation in Solution. J. Am. Chem. Soc. 2018, 140, 7301–7312. 10.1021/jacs.8b03643. [DOI] [PubMed] [Google Scholar]
- Tavagnacco L.; Brady J. W.; Bruni F.; Callear S.; Ricci M. A.; Saboungi M. L.; Cesaro A. Hydration of Caffeine at High Temperature by Neutron Scattering and Simulation Studies. J. Phys. Chem. B 2015, 119, 13294–13301. 10.1021/acs.jpcb.5b09204. [DOI] [PubMed] [Google Scholar]
- Shalaev E.; Soper A. K. Water in a Soft Confinement: Structure of Water in Amorphous Sorbitol. J. Phys. Chem. B 2016, 120, 7289–7296. 10.1021/acs.jpcb.6b06157. [DOI] [PubMed] [Google Scholar]
- Hayes R.; Imberti S.; Warr G. G.; Atkin R. How water dissolves in protic ionic liquids. Angew. Chem., Int. Ed. 2012, 51, 7468–7471. 10.1002/anie.201201973. [DOI] [PubMed] [Google Scholar]
- Headen T. F.; Howard C. A.; Skipper N. T.; Wilkinson M. A.; Bowron D. T.; Soper A. K. Structure of pi-pi interactions in aromatic liquids. J. Am. Chem. Soc. 2010, 132, 5735–5742. 10.1021/ja909084e. [DOI] [PubMed] [Google Scholar]
- Finney J. L.; Soper A. K. Solvent structure and perturbations in solutions of chemical and biological importance. Chem. Soc. Rev. 1994, 23, 1–10. 10.1039/cs9942300001. [DOI] [Google Scholar]
- Soper A. K.GudrunN and GudrunX: Programs for Correcting Raw Neutron and X-ray Diffraction Data to Differential Scattering Cross Section, Rutherford Appleton Laboratory report RAL-TR-2011-013 (July 2011).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.