Abstract
Enyne metathesis (EM) has extensively been used to prepare diverse polycycles and heterocycles. EM in combination with Diels–Alder (DA) reaction has been used to prepare densely functionalized targets in a simple manner. In this mini-review, we discuss the various diversity-oriented approaches reported from our laboratory to prepare a variety of organic frameworks by a synergistic combination of EM and DA reactions. Some of the end products are useful intermediates for the synthesis of complex organic targets.
Introduction
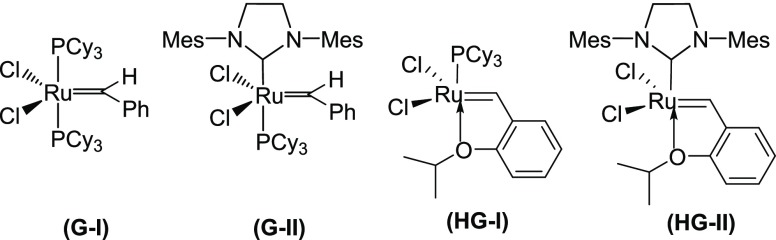
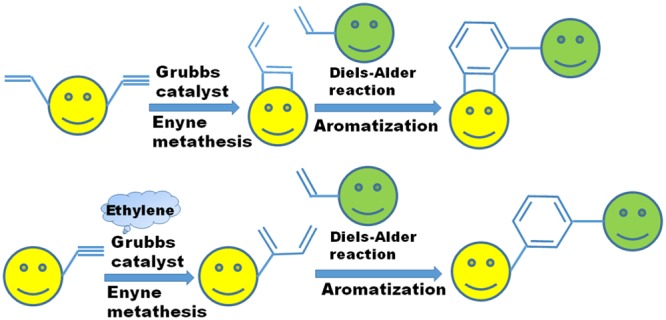
Enyne metathesis (EM)1 is a bond reorganization process between alkynes and alkenes to produce conjugated 1,3-dienes (Scheme 1). It involves the simultaneous bond cleavage and bond formation. This reaction is generally catalyzed by a ruthenium catalyst, Grubbs’ first-generation precatalyst (G-I), Grubbs’ second-generation precatalyst (G-II), and Hoveyda–Grubbs’ precatalysts (HG-I and HG-II) etc. (Figure 1). The intermolecular process is called a cross-enyne metathesis (CEM, Scheme 1), whereas the intramolecular reactions are referred to as ring-closing enyne metathesis (RCEM, Scheme 1). EM is a very expedient tool to generate a 1,3-diene moiety, which is a strategic component in the DA reaction to generate diverse polycyclic compounds.2 A large number of compounds containing a six-membered ring can be generated by utilizing the EM–DA approach. The designed approach is diversity-oriented, as a combinatorial library of target compounds could be assembled by varying the diene and the dienophile component in a DA reaction. The final six-membered ring compounds are the core structure of various biologically relevant drug-like molecules and structural analogues of bioactive natural products.3 More specifically, cross-metathesis (CM) and RCEM provide an easy access to generate various dienes containing polar functional groups. The generation of such intricate dienes is a difficult task by conventional methods.
Scheme 1. Intermolecular and Intramolecular Enyne Metathesis.
Figure 1.
Precatalysts used for the metathesis.
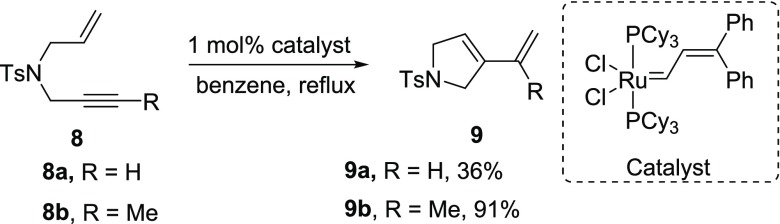
In 1985, Katz and Sivavec first reported the intramolecular RCEM with a tungsten carbene complex, where the diene 7 was generated from the enyne 6 by treating with a catalytic amount of tungsten carbene complex (Scheme 2).4a Later, in 1994, Mori and Kinoshita reported the first example of intramolecular enyne metathesis catalyzed by Grubbs’ catalyst, where the enyne building block 8 was treated with a ruthenium catalyst to generate the cyclic diene 9 (Scheme 3).4b
Scheme 2. Intramolecular Enyne Metathesis by a Tungsten Carbene Complex.
Scheme 3. Intramolecular Enyne Metathesis by a Ruthenium Carbene Complex.
Here, we summarize our efforts to prepare various diene building blocks via EM and their subsequent utilization in the synthesis of highly functionalized and intricate carbocyclic and heterocyclic frameworks. More specifically, the EM and DA sequence has been used to prepare various modified amino acid derivatives, polycycles, macroheterocycles, heterocycles, crownophanes, diphenylalkane derivatives, and spirocycles. Some of these building blocks were further incorporated into small peptides, thus generating a library of modified peptides. We have grouped the EM–DA reaction strategies based on the class of the final product, i.e., polycycles, heterocycles, amino acid derivatives, crownophanes, spirocycles, etc. As and when necessary, we have included others references also.
1. Indane-Based α-Amino Acid Derivatives and Natural Products
The Buchrer–Burg method is generally employed to generate indanyl glycine; however, this methodology is impractical for sensitive substrates due to the harsh conditions employed during the hydrolysis of hydantions. Therefore, a conceptually new approach by using EM and DA reaction in synergistic combination was utilized to assemble indanyl glycine derivatives (Scheme 4).5a,5b In this regard, O’Donnell Schiff base 10 was subjected to a propargylation, allylation, hydrolysis, and acetylation reaction sequence to generate the enyne building block 11.5c Later, enyne 11 was subjected to EM to generate the key inner–outer ring diene building block 12, which on DA reaction with various dienophiles and subsequent dehydrogenation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) delivered the indanyl glycine derivatives 13a,b and 14–15. The present strategy provides a “green route” to indanyl glycine derivatives as both EM and DA are atom-economic reactions. Later, this strategy has been used to generate various molecular frameworks.6
Scheme 4. Indanyl Glycine Derivatives.
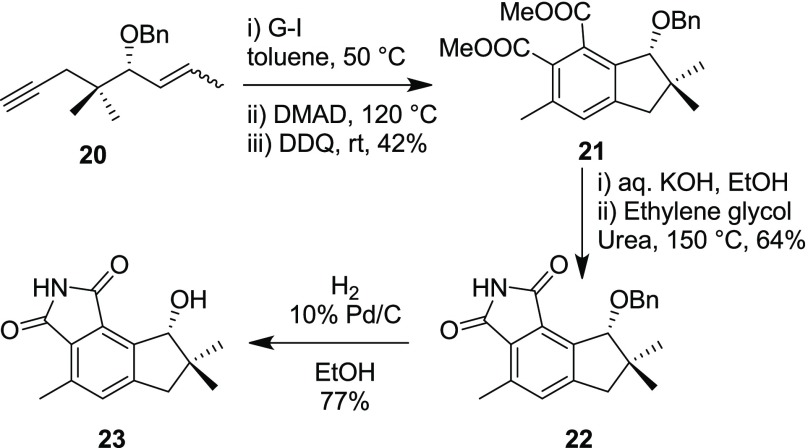
In 2011, Reddy’s group reported a first total synthesis of bicyclic diterpene, isofregenedadiol 19, by utilizing a one-pot reaction sequence involving RCEM, CM, DA reaction, and aromatization (Scheme 5).7 Later in 2014, Reddy and co-workers used a similar strategy to report the first total synthesis of a norsesquiterpene alkaloid, (R)-8-hydroxy-4,7,7-trimethyl-7,8-dihydrocyclopenta[e]isoindole-1,3(2H,6H)-dione 23. The synthetic strategy involves a RCEM, DA reaction, and aromatization sequence to generate the desired indane skeleton present in the natural product. The resulting indane-based diester 21 was transformed into a target anticancer agent by hydrolysis, conversion of diacid to imide 22, and subsequent removal of the benzyl group to deliver the compound 23 (Scheme 6).8
Scheme 5. Total Synthesis of Isofregenedadiol by EM–DA Strategy.
Scheme 6. Total Synthesis of a Norsesquiterpene Alkaloid by EM–DA Strategy.
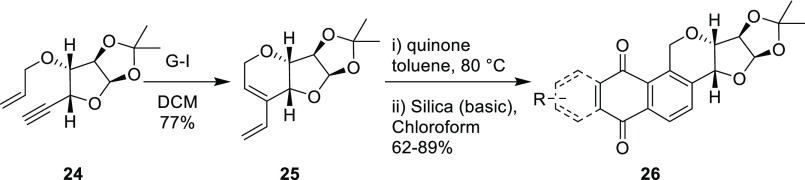
Recently, Kaliappan and Sayyad have reported the synthesis of a new class of sugar–oxasteroid–quinone hybrid molecules 26 via sequential EM–DA strategy (Scheme 7).9 By utilizing this approach with various enyne-containing sugar molecules, they have prepared a library of hybrid molecules with a steroid-like backbone.
Scheme 7. Sugar–Oxasteroid–Quinone Hybrid Molecules via EM–DA Strategy.
2. Tetrahydroisoquinoline-3-carboxylic Acid (Tic) Derivatives
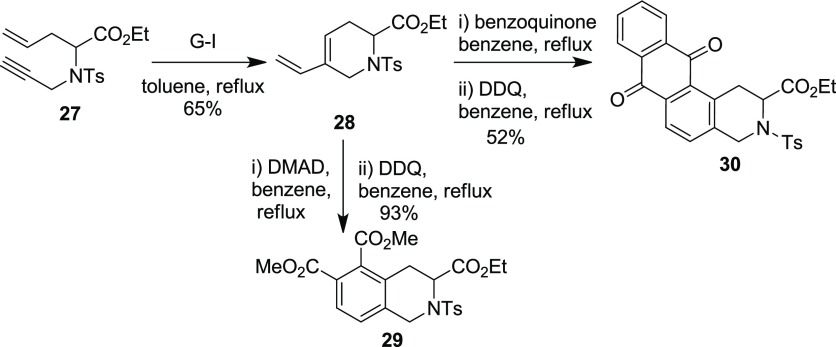
Later, the above strategy involving EM–DA reactions has been extended for the synthesis of topographically constrained Tic derivatives 29–30 (Tic, a constrained analogue of Phenylalanine (Phe), Scheme 8).10a
Scheme 8. Constrained Tetrahydroisoquinoline-3-carboxylic Acid (Tic) Derivatives.
Tic derivatives are conventionally prepared by Pictet–Spengler or Bischler–Napieralski reactions, which start with the preformed benzene derivatives. Moreover, these methods are not suitable for substrates containing electron-withdrawing groups due to the involvement of electrophilic aromatic substitution reaction. To realize the EM–DA strategy, the required enyne building block 27 was prepared from Schiff base ester 10 via C-allylation and hydrolysis reaction followed by the N-protection and N-propargylation. Later, EM of building block 27 using the G–I catalyst gave the required inner–outer ring diene building blocks 28. Finally, the DA reaction of the dienes 28 with dimethyl acetylenedicarboxylate (DMAD) and quinone, followed by subsequent oxidation of the DA adducts, generated the Tic derivatives 29 and 30, respectively, in good yields. The present methodology quickly improves the diversity and molecular complexity and provides access to intricate Tic derivatives.
Further, the above methodology has been extended to the synthesis of a highly substituted seven-membered analogue of Tic 34 (Scheme 9).10b In this context, initially the amide nitrogen in the compound 31 was N-alkylated with butenyl bromide, and then the compound 32 was subjected to EM to generate the diene 33, which on DA reaction with DMAD followed by oxidation using DDQ delivered the desired analogue of Tic 34. The present strategy is advantageous over the existing methods, as diverse substituents in the benzene ring can be incorporated by the careful selection of reacting partners.
Scheme 9. Seven-Membered Analogue of Tic Derivatives.
3. Modified Phenylalanine-Based α-Amino Acids and Peptides
The strategy involving CEM and DA reaction as key steps was further utilized for the synthesis of highly functionalized Phe derivatives (Scheme 10).10c,10d The beauty of this strategy is that Phe-based α-amino acid (AAA) derivatives are assembled from building blocks having no Phe moiety. To this end, the acetylenic building blocks 36 were prepared from the Schiff base 10 or 35, and later it was reacted with allyl acetate, a functionalized ethylene derivative to deliver the diene 37 as a mixture of isomers (1:1). No attempts were made to separate these isomers as the stereochemistry of the diene 37 is of no consequence for the synthesis of final Phe derivatives. The DA reaction of 37 with DMAD and subsequent oxidation of the DA adducts delivered the highly functionalized Phe derivatives 38. It is worthy to mention that the diene 37 could not be synthesized from CEM of alkynes and the allyl building blocks containing an AAA moiety.
Scheme 10. Synthesis of Functionalized Phenylalanine (Phe) Derivatives.
In another instance, the Schiff base 10 was subjected to a C-propargylation, hydrolysis, and protection sequence to generate the alkyne building block 31. Later, CEM of the alkyne building block 31 with ethylene as a cross-coupling partner gave the diene 39. Then, treatment of the diene 39 with various dienophiles and subsequent aromatization of the DA adduct delivered a highly functionalized Phe derivative 40 (Scheme 11).11 Further, the alkylation of ethyl isocyanoacetate (EICA) with 2-bromomethyl-1,3-butadiene 41 or sulfolene bromide 42 failed to deliver the diene 39 (Scheme 11).10c−10e In another report, the analogue of diene was prepared by using N-acyliminium ion chemistry.12a
Scheme 11. Highly Functionalized Phe Derivative.
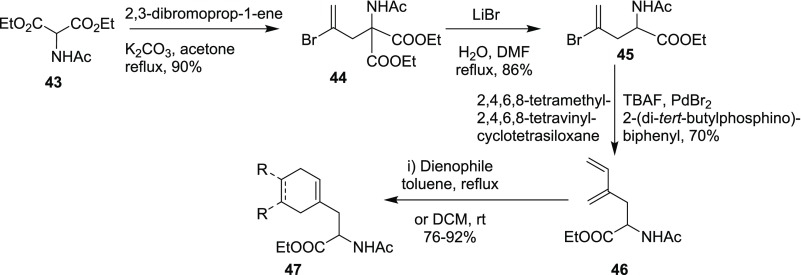
A similar diene containing the AAA derivative 46 was reported by Baldwin and co-workers using Denmark’s coupling reaction (Scheme 12).12b
Scheme 12. Generation of a Diene-Containing Amino Acid Moiety.
In another attempt, dicarba analogues of cystine derivatives such as 51 were prepared by using a CEM and DA approach (Scheme 13).11
Scheme 13. Dicarba Analogues of Cystine Derivatives.
The benzophenone imine glycine ester 10 was reacted with 1,4-dibromo-2-butyne (48), followed by hydrolysis, and acetylation gave the alkyne derivative 49 (two diastereomers in 1:1 ratio; RR/SS, and RS/SR). The bis(amino acid) derivative 49 was subjected to CEM in the presence of ethylene and HG-II catalyst to afford the desired diene 50. Finally, the DA reaction of diene 50 with various dienophiles gave the conformationally rigid dicarba analogues of cysteine 51.
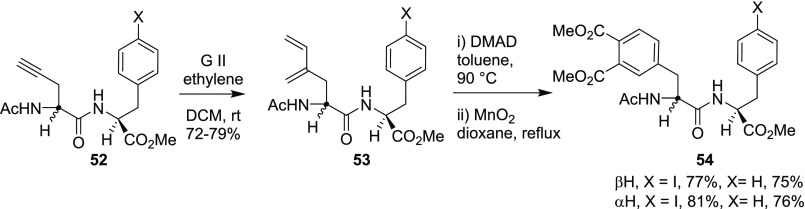
Further, the above strategy was extended toward the postassembly peptide modification.13a To realize the synthetic strategy, the alkyne-based dipeptide 52 was synthesized from diethyl acetamidomalonate (DEAM) (43). To the end, CEM of alkyne 52 with ethylene as a cross-coupling partner delivered the diene 53, which on treatment with DMAD followed by aromatization with MnO2 generated the desired modified Phe-based dipeptide 54 (Scheme 14). In addition, the same strategy was extended to tripeptide-based alkyne building block 55, and the DA reaction was also realized with 1,4-napthaquinone to establish the diversity of this approach (Scheme 15).
Scheme 14. Phe-Based Dipeptide.
Scheme 15. Phe-Based Tripeptide.
4. Crownophane
Recently, we reported a diversity-oriented approach to crownophanes by utilizing CEM and DA reactions as key steps (Scheme 16).13b Initially, the diene 59 was generated by CEM of alkyne precursor 58 in the presence of Grubb’s catalyst under an ethylene atmosphere. Later, the DA reaction of the diene 59 with DMAD followed by aromatization of the cycloadduct with DDQ gave the crownophane 60. It is worth noting that the present strategy involved the creation of eight new C–C bonds and thereby accomplishing step economy and atom economy. The above strategy was further employed to assemble ortho- and meta-crownophanes.
Scheme 16. Paracrownophanes via EM–DA Strategy.
Later, another derivative of crownophane 63 was assembled by utilization of the above strategy (Scheme 17).13b In this regard, the crownophane-based acetylenic derivative 61 was subjected to CEM under an ethylene atmosphere to generate the diene 62. Then, the DA reaction of diene 62 with DMAD followed by aromatization of the cycloadduct gave the desired crownophane 63. Similarly, this strategy was extended to ortho- and meta-crownophanes.
Scheme 17. Crownophane via EM–DA Strategy.
5. Diphenylalkane Derivatives
In 2009, a useful strategy has been realized to highly functionalized diphenylalkane derivatives via atom-economical processes such as [2 + 2 + 2] cyclotrimerization, CEM, and DA reaction as key steps (Scheme 18).13c
Scheme 18. Functionalized Diphenylalkane Derivatives.
To this end, the alkyne building blocks 66 were prepared by the [2 + 2 + 2] cyclotrimerization of dialkyne 64 and DMAD in the presence of Wilkinson’s catalyst [Rh(PPh3)3Cl]. Later, the CEM of the alkyne 66 in the presence of G-II catalyst under an ethylene atmosphere delivered the dienes 67, which on DA reaction with DMAD followed by aromatization gave the highly functionalized diphenylalkane derivatives 68. The beauty of the present strategy is that we accomplished desymmetrization in highly symmetrical starting materials, i.e., α,ω-diynes.
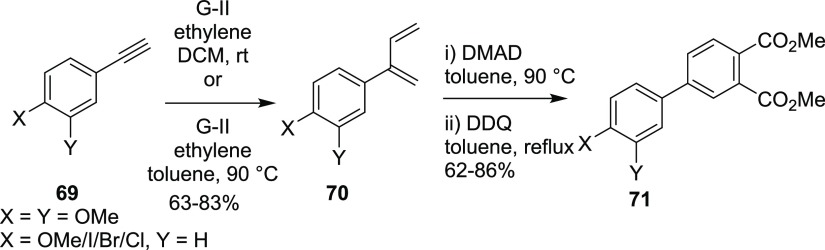
In another event, we have demonstrated that CEM of phenylacetylene derivatives with 1,5-hexadiene or ethylene followed by the DA reaction and aromatization sequence delivered biaryl derivatives (Scheme 19 and Scheme 20).14 To realize the synthetic design, phenyl acetylenes 69 were subjected to CEM in the presence of G-II catalyst under an ethylene atmosphere to generate the corresponding dienes 70. Later, the DA reaction of the dienes 70 with DMAD followed by aromatization with DDQ gave biphenyl derivatives 71. The halogen substituent present in the biaryl derivative is a useful handle to the Suzuki–Miyaura (SM) cross-coupling reaction15 to generate the terphenyl derivative. The present strategy can provide an easy access to a library of biaryl (or terphenyl) building blocks via Suzuki coupling by using a variety of commercially available boronic acids.
Scheme 19. Biaryl Derivatives via EM–DA Strategy.
Scheme 20. Homoallyl Derivatives via EM–DA Strategy.
Similarly, the phenylacetylene 69 was subjected to CEM with 1,5-hexadiene to generate the desired homoallyl diene derivatives 72 along with the benzoannulated product 73 (Scheme 20).14 The DA reaction of dienes 72 with DMAD gave the DA adduct, which on aromatization with DDQ generated the biphenyl derivatives 74. Later, this strategy has been extended toward the synthesis of amino acid derivatives 74a and 74b.16
6. Spirocycles
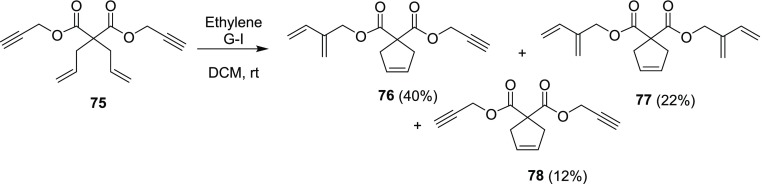
In 2007, we reported a unique example involving sequential RCM and CEM.17 Three products (76–78) were isolated when the enyne precursor 75 was treated with G-I in the presence of ethylene (Scheme 21). On the contrary, two products 79 and 80 were isolated when the enyne precursor 75 was treated with G-II in dichloromethane (DCM) at room temperature. The compound 79 has been derived by EM at one alkyne end and compound 80, due to EM at both alkyne ends. When the compound 75 was treated with the G-II catalyst under ethylene-free reaction conditions, no metathesis product was observed, but the G-I catalyst with in situ generated ethylene reacted with alkyne moieties. In this strategy, chemoselectivity was observed with G-I and G-II catalysts under an ethylene atmosphere. The reactions of enyne precursor 75 under G-I, G-II, and HG-II catalyst conditions failed to give the bis-spirocyclic diene 81 (Scheme 22).
Scheme 21. Sequential RCM and CEM.
Scheme 22. Attempt to Bis-spirocyclic Diene.
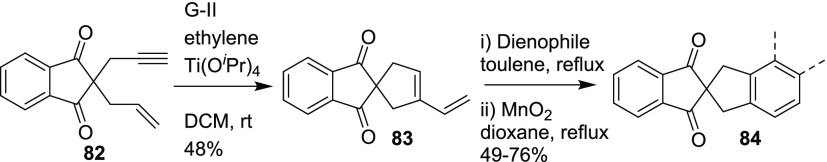
Recently, a simple diversity-oriented methodology for the synthesis of indane-based spirocycles has been developed via EM and DA reaction, as key steps (Scheme 23).18a The enyne building block 82 was assembled by monopropargylation followed by allylation of the indane-1,3-dione. Later, the enyne building block 82 was subjected to ring-closing enyne metathesis in the presence of G-II catalyst. A catalytic amount of titanium isopropoxide and ethylene atmosphere was found to increase the yield of 83. The diene 83 was treated with various dienophiles to produce the corresponding DA adducts. Then, the DA adducts were subjected to oxidation with MnO2 to deliver the corresponding aromatized spirocycles 84. This work has been highlighted in Synfacts.18b
Scheme 23. Indane-Based Spirocycles.
Dienophile: 1,4-benzoquinone (57%), 1,4-naphthoquinone (49%), 1,4-anthraquinone (55%), DMAD (76%), N-phenylmaleimide (65%).
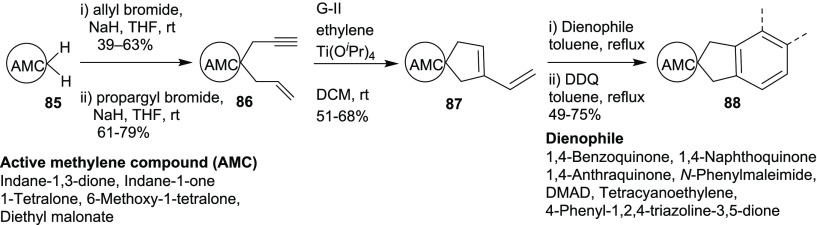
The above strategy was expanded to generate diverse spirocycles 88 by varying the active methylene compound (AMC), such as indane-1-one, 1-tetralone, 6-methoxy-1-tetralone, and diethyl malonate (Scheme 24).18c A variety of enyne building blocks 86 were prepared by the sequential allylation followed by propargylation of the corresponding active methylene compound 85. Later, the enyne building blocks were treated with G-II catalyst under an ethylene atmosphere in the presence of titanium tetraisopropoxide to generate dienes 87. These dienes were further utilized to generate a library of angularly fused spirocyclic compounds 88 by reacting with a variety of dienophiles such as DMAD, tetracyanoethylene, 1,4-benzoquinone, 1,4-naphthoquinone, 1,4-anthraquinone, 4-phenyl-1,2,4-triazoline-3,5-dione, and N-phenylmaleimide.
Scheme 24. Diverse Spirocycles.
7. Heterocycles
The strategy involving the EM–DA reaction was utilized by Dixneuf and co-workers for the synthesis of cyclic siloxanes 92 (Scheme 25).19 They have reported the intramolecular metathesis of enynes 89 containing the Si–O linkage with ruthenium-based three-component catalytic systems to generate the dienes 90. The resulting six-membered siloxane dienes 90 were utilized in DA reaction followed by aromatization of the resulting DA adduct 91, generating the aromatic compound 92 which is an important precursor for sol–gel materials and fine chemicals.
Scheme 25. Synthesis of Cyclic Siloxanes via EM–DA Reaction.
Similarly, Majumdar and co-workers have applied the EM–DA strategy to generate tricyclic oxepin-annulated pyrone derivatives 95 (Scheme 26).20 In this regard, the pyrone-based enyne derivatives 93 were subjected to RCEM to produce oxepin-annulated pyrone-based diene derivatives 94. These dienes 94 on DA reaction with dimethyl fumarate gave tricyclic oxepin-annulated pyrone derivatives 95 stereoselectively.
Scheme 26. Synthesis of Tricyclic Oxepin-Annulated Pyrone Derivatives.
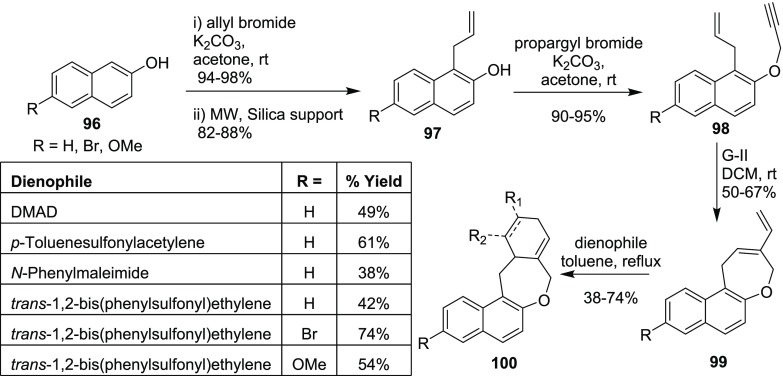
Kotha’s group has successfully utilized the EM and DA reaction strategy for the generation of heterocycles. In this regard, the required enyne building blocks 98 were prepared from the β-naphthols 96 by O-allylation followed by Claisen rearrangement (CR) and subsequent O-propargylation of 97. Later, these enynes 98 were successfully transformed into the required diene 99 by G-II catalyst. These dienes on DA reaction with various dienophiles generated the polycyclic frameworks such as 100 (Scheme 27).21
Scheme 27. Polycyclic Frameworks via EM–DA Strategy.
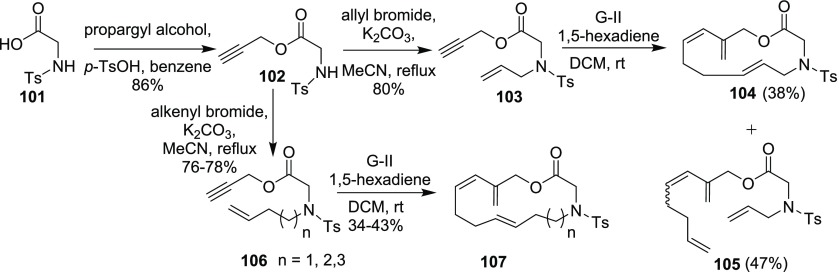
In 2007, tandem cross-enyne ring-closing metathesis (CE-RCM) was explored to generate densely functionalized macroheterocycles (Scheme 28).22a To realize the synthetic sequence, N-tosyl glycine 101 was subjected to esterification with propargyl bromide to yield the ester 102. The O-propargylated compound 102 was then allylated using allyl bromide to deliver the enyne precursor 103. Later, enyne 103 was subjected to CE-RCM with 1,5-hexadiene to deliver the desired 12-membered macrocyclic compound 104 along with an open-chain product 105. Further, the strategy was extended to generate macrocyclic systems of 13–16-membered ring size (107) by varying the size of alkenylation partner.
Scheme 28. Functionalized Macroheterocycles.
The above methodology was utilized to assemble C-α,α-functionalized macrocyclic AAA derivative 111 (Scheme 29).22b In this regard, a suitable enyne precursor 110 was synthesized from DEAM (43) in three steps. To begin with, DEAM was partially hydrolyzed and subjected to O-propargylation to deliver the ester 109, which was then subjected to C-allylation with allyl bromide to generate the required enyne precursor 110. Later, treatment of the enyne 110 with 1,5-hexadiene in the presence of a Grubb’s catalyst delivered the desired 12-membered macrocyclic AAA derivative 111 in 40% yield, along with the open-chain compound 112 in 54% yield. Systematic catalyst screening resulted in the selective formation of the desired macrocyclic AAA derivative, and with the HG-I catalyst, the cyclic AAA derivative 111 was obtained in 86% yield.
Scheme 29. Macrocyclic AAA Derivative.
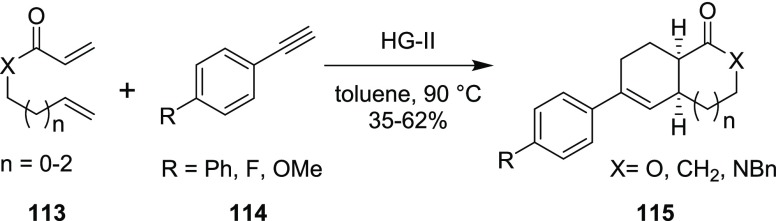
In 2015, Fustero’s group has reported a tandem CEM and intramolecular DA reaction methodology for the generation of linear bicyclic ketone, lactone, and lactam scaffolds in a simple manner with good diastereoselectivity (Scheme 30).23 In this regard, various conjugated ketones, esters, and amides bearing an alkene moiety 113 were reacted with different aromatic alkynes 114 in the presence of HG-II catalyst to obtain the bicyclic ketone, lactones, and lactams 115. The resulting bicyclic heterocycles 115 were formed via tandem CEM and an intramolecular DA reaction sequence.
Scheme 30. Bicyclic Scaffolds via EM–DA Strategy.
In 2014, Kotha’s group has developed a diversity-oriented approach to fused oxacycles 119 by using the enyne ring-rearrangement metathesis (ERRM) and DA reaction as key steps starting with a suitable propargylated derivative 117. The propargylated derivative 117 was derived from 1β-dicyclopentadienol 116. Then, ERRM of 117 in the presence of ethylene and G-I catalyst delivered the rearranged tricyclic diene 118, which on treatment with various dienophiles generated the cycloadducts such as 119 (Scheme 31).24a
Scheme 31. Oxacycles via EM–DA Strategy.
Similarly, our group has developed a simple synthetic strategy to construct the oxa-bowls containing dienes by utilizing ERRM as a key step (Scheme 32).24b In this regard, the required norbornene derivatives 121 were prepared by DA reaction of cyclopentadiene and quinone followed by the reduction of DA adduct 120. Then the diol 121 was subjected to O-propargylation using propargyl bromide to generate the dipropargylated norbornenes 122, which were subsequently converted to dienes 123 by ERRM. These dienes 123 can be further utilized for creating the polycycles by DA strategy, but these dienes are found to be unstable under normal DA reaction conditions.
Scheme 32. Enyne Ring-Rearrangement Metathesis (ERRM).
The ERRM strategy was extended toward the synthesis of oxa-bowl-based polycyclic compound 128. In this regard, tricyclic enone 124 was reduced to tricyclic diol 125, which was O-propargylated using propargyl bromide to generate an enyne building block 126. The ERRM of enyne building block 126 in the presence of a G-I catalyst gave the oxa-bowls containing diene 127. Later, the DA reaction of the diene 127 with N-phenylmaleimide generated the polycyclic compound 128 (Scheme 33). By varying the dienophile moiety, one can prepare a library of polycyclic compounds.
Scheme 33. Oxa-Bowl-Based Polycycle.
Along similar lines, a structurally intricate polycyclic compound 132 has been synthesized through ruthenium-catalyzed ring-rearrangement metathesis (RRM) of norbornene derivatives (Scheme 34 and Scheme 35).24c In this aspect, the diol 129 was prepared from dione 120 by treating it with allylmagnesium bromide. Then, the treatment of diol 129 with propargyl bromide generated the mono-O-propargyl derivative 130, which was subjected to the RCEM–RRM sequence in the presence of HG-II catalyst under an ethylene atmosphere to deliver the tetracyclic diene 131. When the compound 131a was reacted with tetracyanoethylene (TCE) in refluxing toluene, the dehydrated product 132 was isolated, which is derived from the DA adduct (Scheme 35).
Scheme 34. Polycyclic Diene through RCEM–RRM.
Scheme 35. Synthesis of Structurally Intricate Polycyclic Compound 132.
In 2017, a new synthetic strategy to fused nitrogen containing heterocycle 136 was demonstrated by using the ERRM–DA protocol (Scheme 36).24d In this context, when the N-propargyl derivative 133 was treated with a G-I catalyst in the presence of ethylene, a mixture of two products 134 and 135 was formed. The compound 134 was derived by ERRM and compound 135 by ring-opening reaction. The ring-opened product 135 underwent EM in the presence of ethylene and a G-I catalyst to deliver the desired diene 134 in good yield. Later, the DA reaction of the diene 134 with tetracyanoethylene (TCE) gave the cycloadduct 136. Interestingly, the pentacyclic compound 136 possesses the tricyclic core of the alkaloid, epimeloscine.
Scheme 36. ERRM–DA Protocol.
8. Miscellaneous
We have also reported the generation of dienes starting with protected, but-2-yne-1,4-diol derivatives 137 by CEM under ethylene atmosphere (Scheme 37).25 The acyl-protected diene 138a is a suitable starting material to assemble benzoannulated compound 139, which can be further utilized for the synthesis of polycyclic compounds through a regioselective DA reaction of the benzosultine-sulfone building block 140. The DA reaction of tosyl-protected diene 138b with various dienophiles under different conditions was unsuccessful. However, the DA reaction of acetyl derivative 138a with DMAD gave the expected DA adduct. Later, the DA adduct was subjected to aromatization using DDQ in benzene or MnO2 in dioxane under reflux conditions to generate the desired benzoannulated product 139.
Scheme 37. Benzoannulation via EM–DA Reaction.
Summary and Outlook
In this account, we have summarized various synthetic strategies to prepare diverse diene building blocks via EM and their use in assembling a variety of polycyclic compounds. The synergetic approach of the EM and DA sequence has been utilized to prepare various unnatural amino acid derivatives, modified peptides, polycycles, heterocycles, and spirocyclic compounds. This synergetic approach can be employed to prepare various biologically important molecules or chemical intermediates, which may inspire the synthesis of pharmaceutically important compounds.
Acknowledgments
We thank CSIR, UGC, and DST, New Delhi, for the financial support. S.K. thanks the Department of Science and Technology (DST), New Delhi, for the award of a J. C. Bose fellowship (SR/S2/JCB-33/2010), Praj Industries, Pune, for a Chair Professorship (Green Chemistry), and Council of Scientific and Industrial Research (CSIR), New Delhi [02(0272)/16/EMR-II], for the financial support. A.S.C. would like to thank Thakur College of Science and Commerce Kandivali, Mumbai, for providing an infrastructure. D.G. would like to thank Sri Guru Granth Sahib World University, Fatehgarh Sahib, Punjab, for providing an infrastructure.
Biographies

Sambasivarao Kotha graduated with an M.Sc. degree in Chemistry from University of Hyderabad and then obtained a Ph.D. in Organic Chemistry from University of Hyderabad in 1985. He continued his research at the University of Hyderabad as a postdoctoral fellow for one and half years. Later, he moved to UMIST Manchester, UK, and the University of Wisconsin, USA, as a research associate. Subsequently, he was appointed as a visiting scientist at Cornell University and as a research chemist at Hoechst Celanese Texas prior to joining IIT Bombay in 1994 as an Assistant Professor. In 2001, he was promoted to Professor. He has published 285 publications in peer-reviewed journals and was elected fellow of various academies (FNASc, FASc, FRSC, and FNA). He was also associated with the editorial advisory board of several journals. His research interests include: Organic synthesis, green chemistry, unusual amino acids, peptide modification, cross-coupling reactions, and metathesis. Currently, he occupies the Pramod Chaudhari Chair Professor in Green Chemistry.

Arjun S. Chavan graduated with an M.Sc. degree in Chemistry from Shivaji University, Kolhapur, and then obtained a Ph.D. in Organic Chemistry from IIT Bombay, India, in 2012 under the supervision of Prof. S. Kotha. Afterwards he joined National Chiao Tung University Hsinchu, Taiwan, and worked with Prof. S. C. Chuang as a Postdoctoral Fellow. Presently, he is Assistant Professor at Department of Chemistry, Thakur College of Science and Commerce Kandivali (E) Mumbai, India. His research interests include green chemistry, use of recyclable catalysts in organic synthesis, nanomaterials, and nanocatalysis.

Deepti Goyal obtained her M.Sc. (Honours School) degree from Panjab University, Chandigarh, in 2006 and a Ph.D. degree from Indian Institute of Technology (IIT), Bombay, Mumbai, India, in 2012 under the supervision of Prof. S. Kotha. She is presently working as an Assistant Professor in the Department of Chemistry, Sri Guru Granth Sahib World University, Fatehgarh Sahib, Punjab, India. The Science and Engineering Research Board (SERB), Government of India, awarded her a Young Scientists Research Grant in 2015. Her research interests focus on the design, synthesis, and evaluation of new molecular entities (small molecules, peptides, and peptidomimetics) as inhibitors of amyloid aggregation.
Author Contributions
# A.S.C. and D.G. equally contributed to this work.
The authors declare no competing financial interest.
References
- a Diver S. T.; Giessert A. J. Enyne Metathesis (Enyne Bond Reorganization). Chem. Rev. 2004, 104, 1317–1382. 10.1021/cr020009e. [DOI] [PubMed] [Google Scholar]; b Handbook of Metathesis (Vol. I-III), 2nd ed.; Grubbs R. H., Wenzel A. G., O’Leary D. J., Khosravi E., Eds.; Wiley-VCH Verlag Gmbh & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]; c Olefin Metathesis: Theory and Practice; Grela K., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, 2014. [Google Scholar]
- a Kotha S.; Meshram M.; Tiwari A. Advanced Approach to Polycyclics by A Synergistic Combination of Enyne Metathesis and Diels–Alder Reaction. Chem. Soc. Rev. 2009, 38, 2065–2092. 10.1039/b810094m. [DOI] [PubMed] [Google Scholar]; b Kotha S.; Chavan A. S.; Goyal D. Diversity–Oriented Approaches to Polycyclics and Bioinspired Molecules via the Diels–Alder Strategy: Green Chemistry, Synthetic Economy, and Beyond. ACS Comb. Sci. 2015, 17, 253–302. 10.1021/co500146u. [DOI] [PubMed] [Google Scholar]
- a Schreiber S. L. Target–Oriented and Diversity-Oriented Organic Synthesis in Drug Discovery. Science 2000, 287, 1964–1969. 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]; b O’Connor C. J.; Beckmann H. S. G.; Spring D. R. Diversity-Oriented Synthesis: Producing Chemical Tools for Dissecting Biology. Chem. Soc. Rev. 2012, 41, 4444–4456. 10.1039/c2cs35023h. [DOI] [PubMed] [Google Scholar]
- a Katz T. J.; Sivavec T. M. Metal-Catalyzed Rearrangement of Alkene-Alkynes and the Stereochemistry of Metallacyclobutene Ring Opening. J. Am. Chem. Soc. 1985, 107, 737–738. 10.1021/ja00289a054. [DOI] [Google Scholar]; b Kinoshita A.; Mori M. Ruthenium Catalyzed Enyne Metathesis. Synlett 1994, 1994, 1020–1022. 10.1055/s-1994-34973. [DOI] [Google Scholar]
- a Kotha S.; Sreenivasachary N.; Brahmachary E. Synthesis of Constrained α-Amino Acid Derivatives via Enyne Metathesis Reaction. Tetrahedron Lett. 1998, 39, 2805–2808. 10.1016/S0040-4039(98)00251-2. [DOI] [Google Scholar]; b Kotha S.; Sreenivasachary N.; Brahmachary E. Constrained Phenylalanine Derivatives by Enyne Metathesis and Diels–Alder Reaction. Eur. J. Org. Chem. 2001, 2001, 787–792. . [DOI] [Google Scholar]; c O'Donnell M. J.; Wojciechowski K.; Ghosez L.; Navarro M.; Sainte F.; Antoine J.-P. Alkylation of Protected R-Amino Acid Derivatives in the Presence of Potassium Carbonate. Synthesis 1984, 1984, 313–315. 10.1055/s-1984-30822. [DOI] [Google Scholar]
- a McReynolds M. D.; Dougherty J. M.; Hanson P. R. Synthesis of Phosphorus and Sulfur Heterocycles via Ring-Closing Olefin Metathesis. Chem. Rev. 2004, 104, 2239–2258. 10.1021/cr020109k. [DOI] [PubMed] [Google Scholar]; b Villar H.; Frings M.; Bolm C. Ring Closing Enyne Metathesis: A Powerful Tool for The Synthesis of Heterocycles. Chem. Soc. Rev. 2007, 36, 55–66. 10.1039/B508899M. [DOI] [PubMed] [Google Scholar]
- Kurhade S. E.; Sanchawala A. I.; Ravikumar V.; Bhuniya D.; Reddy D. S. Total Synthesis of Isofregenedadiol. Org. Lett. 2011, 13, 3690–3693. 10.1021/ol201336x. [DOI] [PubMed] [Google Scholar]
- Kashinath K.; Jadhav P. D.; Reddy D. S. Total Synthesis of An Anticancer Norsesquiterpene Alkaloid Isolated From The Fungus Flammulina Velutipes. Org. Biomol. Chem. 2014, 12, 4098–4103. 10.1039/C4OB00300D. [DOI] [PubMed] [Google Scholar]
- Sayyad A. A.; Kaliappan K. P. Sequential Enyne–Metathesis/Diels–Alder Strategy: Rapid Access to Sugar–Oxasteroid–Quinone Hybrids. Eur. J. Org. Chem. 2017, 34, 5055–5065. 10.1002/ejoc.201700599. [DOI] [Google Scholar]
- a Kotha S.; Sreenivasachary N. A New Synthetic Approach to 1,2,3,4-Tetrahydroisoquinoline-3-carboxylic Acid (Tic) Derivatives via Enyne Metathesis and the Diels-Alder Reaction. Chem. Commun. 2000, 503–504. 10.1039/a910217p. [DOI] [PubMed] [Google Scholar]; b Kotha S.; Khedkar P. Synthesis of a conformationally Constrained Phenylalanine Derivative by A Strategic Combination of Ring-Closing Enyne Metathesis and Diels–Alder Reaction. Synthesis 2008, 2008, 2925–2928. 10.1055/s-2008-1067237. [DOI] [Google Scholar]; c Kotha S.; Halder S.; Brahmachary E.; Ganesh T. Synthesis of Unusual α-Amino Acid Derivatives via Cross-Enyne Metathesis Reaction. Synlett 2000, 2000, 853–855. 10.1002/chin.200039082. [DOI] [Google Scholar]; d Kotha S.; Halder S.; Brahmachary E. Synthesis of Highly Functionalized Phenylalanine Derivatives via Cross-Enyne Metathesis Reactions. Tetrahedron 2002, 58, 9203–9208. 10.1016/S0040-4020(02)01178-X. [DOI] [Google Scholar]; e Kotha S.; Halder S. Ethyl Isocyanoacetate as Useful Glycine Equivalent. Synlett 2010, 2010, 337–354. 10.1055/s-0029-1219149. [DOI] [Google Scholar]
- Kotha S.; Mandal K.; Banerjee S.; Mobin S. M. Synthesis of Novel Quinone–Amino Acid Hybrids via Cross–Enyne Metathesis and Diels–Alder Reaction as Key Steps. Eur. J. Org. Chem. 2007, 2007, 1244–1255. 10.1002/ejoc.200600970. [DOI] [Google Scholar]
- a Berkheij M.; Dijkink J.; David O. R. P.; Sonke T.; Ijzendoorn D. R.; Blaauw R. H.; van Maarseveen J. H.; Schoemaker H. E. Synthesis of a Naturally Occurring Diene–Containing Amino Acid and Its Glutamyl Dipeptide via N–Acyliminium Ion Chemistry. Eur. J. Org. Chem. 2008, 2008, 914–924. 10.1002/ejoc.200701016. [DOI] [Google Scholar]; b Chen R.; Lee V.; Adlington R. M.; Baldwin J. E. A Facile Synthesis of Ethyl 2-Acetamido-4-methylenehex-5-enoate, a Versatile Diels–Alder Synthon for the Parallel Synthesis of Novel α-Amino Acid Derivatives. Synthesis 2007, 2007, 113–117. 10.1055/s-2006-958929. [DOI] [Google Scholar]
- a Kotha S.; Goyal D.; Thota N.; Srinivas V. Synthesis of Modified Phenylalanine Peptides by Cross Enyne Metathesis and a Diels–Alder Reaction as Key Steps. Eur. J. Org. Chem. 2012, 2012, 1843–1850. 10.1002/ejoc.201101744. [DOI] [Google Scholar]; b Kotha S.; Waghule G. T. Diversity Oriented Approach to Crownophanes by Enyne Metathesis and Diels–Alder Reaction as Key Steps. J. Org. Chem. 2012, 77, 6314–6318. 10.1021/jo300766f. [DOI] [PubMed] [Google Scholar]; c Kotha S.; Khedkar P. A Diversity-Oriented Approach to Diphenylalkanes by Strategic Utilization of [2 + 2+2] Cyclotrimerization, Cross-Enyne Metathesis and Diels–Alder Reaction. Eur. J. Org. Chem. 2008, 2008, 730–738. 10.1002/ejoc.200800924. [DOI] [Google Scholar]
- Kotha S.; Vittal S. Diversity-Oriented Synthesis of Biaryl Derivatives Using Cross-Enyne Metathesis, Diels–Alder Reaction, and Suzuki-Miyaura Cross-Coupling as Key Steps. Synlett 2011, 2011, 2329–2334. 10.1055/s-0030-1260315. [DOI] [Google Scholar]
- a Kotha S.; Lahiri K.; Kashinath D. Recent Applications of the Suzuki–Miyaura Cross-Coupling Reaction in Organic Synthesis. Tetrahedron 2002, 58, 9633–9695. 10.1016/S0040-4020(02)01188-2. [DOI] [Google Scholar]; b Kotha S.; Lahiri K. Expanding the Diversity of Polycyclic Aromatics Through a Suzuki–Miyaura Cross-Coupling Strategy. Eur. J. Org. Chem. 2007, 2007, 1221–1236. 10.1002/ejoc.200600519. [DOI] [Google Scholar]; c Kotha S.; Meshram M.; Chakkapalli C. Synergistic Approach to Polycycles Through Suzuki–Miyaura Cross Coupling and Metathesis as Key Steps. Beilstein J. Org. Chem. 2018, 14, 2468–2481. 10.3762/bjoc.14.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotha S.; Seema V.; Banerjee S.; Dipak M. K. Diversity Oriented Approach to Polycyclics via Cross-Enyne Metathesis and Diels-Alder Reaction as Key Steps. J. Chem. Sci. 2015, 127, 155–162. 10.1007/s12039-015-0765-6. [DOI] [Google Scholar]
- Kotha S.; Mandal K. Metathesis of a Novel Dienediyne System: A Unique Example Involving the Usage of In Situ Generated Ethylene as Cross-Enyne Metathesis Partner. J. Organomet. Chem. 2007, 692, 4921–4927. 10.1016/j.jorganchem.2007.07.009. [DOI] [Google Scholar]
- a Kotha S.; Ali R.; Tiwari A. Diversity-Oriented Approach to Novel Spirocyclics via Enyne Metathesis, Diels–Alder Reaction, and a [2 + 2+2] Cycloaddition as Key Steps. Synlett 2013, 24, 1921–1926. 10.1055/s-0033-1339489. [DOI] [Google Scholar]; b Swager T. M. A. Spirocycle Feast. Synfacts 2013, 9, 1172. 10.1055/s-0033-1339930. [DOI] [Google Scholar]; c Kotha S.; Ali R.; Tiwari A. Design and Synthesis of Angularly Annulated Spirocyclics via Enyne Metathesis and the Diels–Alder Reaction as Key Steps. Synthesis 2014, 46, 2471–2480. 10.1055/s-0034-1378280. [DOI] [Google Scholar]
- Sémeril D.; Cléran M.; Bruneau C.; Dixneuf P. H. New in situ Generated Ruthenium Catalyst for Enyne Metathesis: Access to Novel Cyclic Siloxanes. Adv. Synth. Catal. 2001, 343, 184–187. . [DOI] [Google Scholar]
- Majumdar K. C.; Rahaman H.; Muhuri S.; Roy B. Stereoselective Synthesis of Tricyclic Pyranoxepin Derivatives by Ruthenium-Catalyzed Enyne Metathesis/Diels-Alder Reaction. Synlett 2006, 2006, 0466–0468. 10.1055/s-2006-926238. [DOI] [Google Scholar]
- Kotha S.; Mandal K.; Tiwari A.; Mobin S. M. Diversity-Oriented Approach to Biologically Relevant Molecular Frameworks Starting with β-Naphthol and using the Claisen Rearrangement and Olefin Metathesis as Key Steps. Chem. - Eur. J. 2006, 12, 8024–8038. 10.1002/chem.200600540. [DOI] [PubMed] [Google Scholar]
- a Kotha S.; Singh K. Cross–Enyne and Ring–Closing Metathesis Cascade: A Building–Block Approach Suitable for Diversity–Oriented Synthesis of Densely Functionalized Macroheterocycles with Amino Acid Scaffolds. Eur. J. Org. Chem. 2007, 2007, 5909–5916. 10.1002/ejoc.200700744. [DOI] [Google Scholar]; b Kotha S.; Bansal D.; Singh K.; Banerjee S. Synthesis of A New Fluorescent Macrocyclic α-Amino Acid Derivative via Tandem Cross–Enyne/Ring–Closing Metathesis Cascade Catalyzed by Ruthenium Based Catalysts. J. Organomet. Chem. 2011, 696, 1856–1860. 10.1016/j.jorganchem.2011.02.019. [DOI] [Google Scholar]
- Miró J.; Sánchez-Roselló M.; Sanz Á.; Rabasa F.; del Pozo C.; Fustero S. Tandem Cross Enyne Metathesis (CEYM)–Intramolecular Diels–Alder Reaction (IMDAR). An Easy Entry to Linear Bicyclic Scaffolds. Beilstein J. Org. Chem. 2015, 11, 1486–1493. 10.3762/bjoc.11.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kotha S.; Ravikumar O. Diversity–Oriented Approach to Carbocycles and Heterocycles through Ring–Rearrangement Metathesis, Fischer Indole Cyclization, and Diels–Alder Reaction as Key Steps. Eur. J. Org. Chem. 2014, 2014, 5582–5590. 10.1002/ejoc.201402273. [DOI] [Google Scholar]; b Kotha S.; Ravikumar O. Design and Synthesis of Oxa-Bowls via Diels–Alder Reaction and Ring-Rearrangement Metathesis as Key Steps. Tetrahedron Lett. 2014, 55, 5781–5784. 10.1016/j.tetlet.2014.08.108. [DOI] [Google Scholar]; c Kotha S.; Ravikumar O. Ring–Rearrangement–Metathesis Approach to Polycycles: Substrate–Controlled Stereochemical Outcome during Grignard Addition. Eur. J. Org. Chem. 2016, 2016, 3900–3906. 10.1002/ejoc.201600596. [DOI] [Google Scholar]; d Kotha S.; Gunta R. Synthesis of Intricate Fused N-Heterocycles via Ring-Rearrangement Metathesis. J. Org. Chem. 2017, 82, 8527–8535. 10.1021/acs.joc.7b01299. [DOI] [PubMed] [Google Scholar]
- Kotha S.; Chavan A. S. Design and Synthesis of Benzosultine-sulfone as a o-Xylylene Precursor via Cross-enyne Metathesis and Rongalite: Further Expansion to Polycyclics via Regioselective Diels–Alder Reaction. J. Org. Chem. 2010, 75, 4319–4322. 10.1021/jo100655c. [DOI] [PubMed] [Google Scholar]