Abstract
Background/aim
mmune checkpoint inhibition (CPI) has an increasing impact in the multimodal treatment of locally advanced non-small cell lung cancer (LA-NSCLC). Increasing evidence suggests treatment outcome depending on tumor cell PD-L1 expression. The purpose of this retrospective study was to investigate the prognostic value of PD-L1 expression on tumor cells in combination with CD8+ tumor stroma-infiltrating lymphocyte (TIL) density in inoperable LA-NSCLC treated with concurrent chemoradiotherapy (CRT).
Patients and method
We retrospectively assessed clinical characteristics and initial tumor biopsy samples of 31 inoperable LA-NSCLC patients treated with concurrent CRT. Prognostic impact of tumor cell PD-L1 expression (0% versus ≥1%) and CD8+ TIL density (0–40% vs. 41–100%) for local control, progression-free (PFS) and overall survival (OS) as well as correlations with clinicopathological features were evaluated.
Results
Median OS was 14 months (range: 3–167 months). The OS rates at 1- and 2 years were 68 and 20%. Local control of the entire cohort at 1 and 2 years were 74 and 61%. Median PFS, 1-year and 2-year PFS were 13 ± 1.4 months, 58 and 19%. PD-L1 expression < 1% on tumor cells was associated with improved OS, PFS and local control in patients treated with concurrent CRT. Univariate analysis showed a trend towards improved OS and local control in patients with low CD8+ TIL density. Evaluation of Tumor Immunity in the MicroEnvironment (TIME) appears to be an independent prognostic factor for local control, PFS and OS. The longest and shortest OS were achieved in patients with type I (PD-L1neg/CD8low) and type IV (PD-L1pos/CD8low) tumors (median OS: 57 ± 37 vs. 10 ± 5 months, p = 0.05), respectively.
Conclusion
Assessment of PD-L1 expression on tumor cells in combination with CD8+ TIL density can be a predictive biomarker in patients with inoperable LA-NSCLC treated with concurrent CRT.
Keywords: TILs, PDL1, Chemoradiotherapy, Prognostic factors, Checkpoint inhibition
Introduction
Lung cancer remains the leading cause of cancer-related mortality worldwide [1–3]. Locally-advanced non-small cell lung cancer (LA-NSCLC) represents as a heterogeneous disease including large tumor volume, extensive lymph node involvement, tumor-related atelectasis and infiltration of the thoracic wall, mediastinum and spine [4–6]. The majority of LA-NSCLC patients are inoperable and multimodal approaches are considered a cornerstone of treatment [7–10]. Historically, administering platinum-based chemotherapy concurrently to thoracic irradiation resulted in modest improvements of local control, metastasis-free and overall survival (OS) compared to radiotherapy alone [11, 12]. In the last years, the role of immune checkpoint inhibition (CPI) in the multimodal treatment of LA-NSCLC has evolved [9, 12]. In 2015, the first programmed cell death protein 1 (PD-1) inhibitor (nivolumab) was approved by the Food and Drug Administration (FDA) for advanced or metastatic NSCLC in the second-line setting following progression during or after platinum-based chemotherapy [13, 14]. Subsequently in 2016, the FDA approved monotherapy with the PD-1 inhibitor pembrolizumab in the first-line setting for patients with metastatic NSCLC with programmed cell death 1 ligand 1 (PD-L1) Tumor Proportion Score (TPS) ≥ 50% and expanded the indication in April 2019 based on the results of the KEYNOTE-042 trial for the first-line treatment of patients with stage III patients who are not candidates for surgical resection or definitive CRT or metastatic NSCLC with TPS ≥ 1% determined by an FDA-approved test. Patients’ tumors had no Epidermal Growth Factor Receptor (EGFR) or Anaplastic lymphoma kinase (ALK) genomic aberrations [15, 16].
The addition of pembrolizumab to chemotherapy resulted in significantly higher rates of response and longer PFS than chemotherapy alone in a phase 2 cohort of the KEYNOTE-021 trial [17] and the FDA granted accelerated approval in May, 2017. CPI and chemotherapy combination therapy was also tested in the first-line setting in the KEYNOTE-189 and KEYNOTE-407 trials for metastatic nonsquamous NSCLC without sensitizing EGFR or ALK mutations and squamous NSCLC, respectively [18, 19]. Both studies reporting significantly improved OS and progression-free survival (PFS) than chemotherapy alone. Furthermore, the IMpower150 trial demonstrated superior PFS and OS for carboplatin/paclitaxel, bevacizumab and the PD-L1 blocking antibody atezolizumab vs. carboplatin/paclitaxel and bevacizumab in metastatic nonsquamous NSCLC, regardless of PD-L1 status and EGFR or ALK genetic alteration status [20]. Both combinations have been approved by the FDA.
Vis-à-vis stage III NSCLC, as a result of the PACIFIC trial, maintenance treatment with PD-L1 inhibitor durvalumab after successful completion of platinum-based concurrent chemoradiotherapy (CRT) has demonstrated significantly improved PFS and OS and became a new standard of care in inoperable stage III NSCLC [8, 9]. Currently, predictors for response to CPI are unclear and potential biomarkers are under investigation including PD-L1 expression of tumor cells, tumor-infiltrating lymphocytes (TIL), T-effector-interferon-γ-associated gene expression and tumor mutational burden (TMB) [21–23]. High mutation load has been shown to correlate with an immunogenic tumor microenvironment with increased expression of tumor-specific neo-antigens that can be targeted by activated immune cells e.g. cytotoxic CD8+ TILs [24, 25].
Considering the importance of PD-L1 expression on tumor cells and CD8 TIL density in defining the tumor immune microenvironment, we aimed to study PD-L1 expression alone and in combination with CD8 TIL density with relation to clinicopathologic characteristics and survival in patients treated with concurrent CRT.
Methods
Patients and samples
This study included 31 patients who received concurrent CRT for locally advanced or metastatic NSCLC. From their medical records, we retrieved patients’ clinical data, such as sex, age, histologic type and grading, pack years and TNM stage (using the 8th UICC TNM Staging System of lung cancer). Evaluation of EGFR/ALK genomic aberrations was performed in nonsquamous metastatic patients and was negative. All patients were closely followed-up according to an in-house protocol - every 3 months in the first 2 years, every 6 months up to 5 years and afterwards once per year. Expert pathologists (J.N. and S.R.) re-reviewed hematoxylin-eosin–stained slides from all cases, and corresponding formalin-fixed, paraffin-embedded specimens and performed the immunohistochemical staining.
Immunohistochemistry
All immunohistochemical stainings were done on 5 μm whole standard tissue sections of formaldehyde-fixed paraffin-embedded tissue (FFPE) tumor samples (see Fig. 1). For the detection of PD-L1 prediluted PD-L1 rabbit monoclonal antibody (SP263; Ventana Medical Systems, Oro Valley, Arizona) was used as the primary antibody. Immunohistochemical staining for CD8 was carried out with an anti- CD8α mouse monoclonal antibody (C8/144B, Cell Marque, Rocklin, California, dilution 1:50) as the primary antibody. Both stainings were performed on a Ventana Benchmark Ultra autostainer using the UltraView diaminobenzidine kit (Ventana Medical Systems, Oro Valley, AZ).
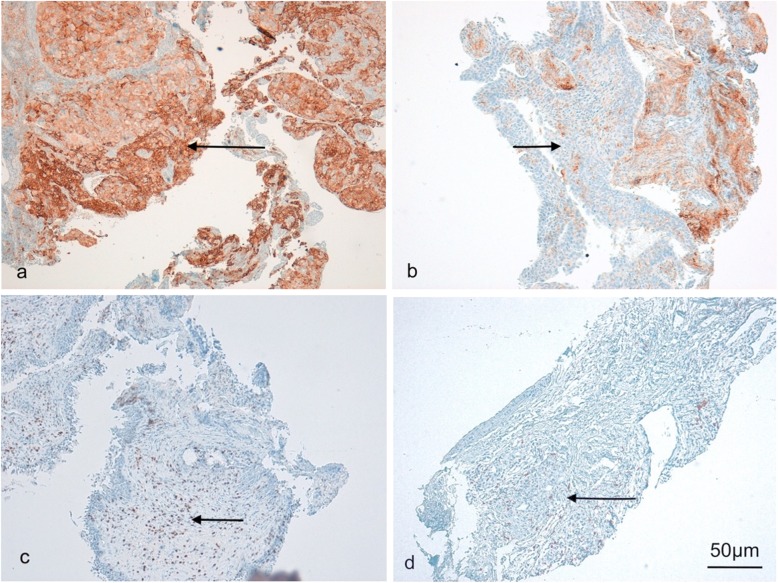
Fig. 1.
PD-L1 expression on tumor cells and CD8 expression on tumor infiltrating lymphocytes. Representative images under a microscope with a 10x enlargement of adenocarcinomas with positive (a, 80%) and negative (b, 0%) PD-L1 staining on tumor cells and adenocarcinomas with positive (c, 70%) and negative (d, 2%) CD8 staining on tumor infiltrating lymphocytes. The arrows indicate positive (a,c) or negative (b,d) staining
Assessment of PD-L1 expression
PD-L1 expression on tumor cells was measured quantitatively using an established immunohistochemistry assay (Ventana SP 263) which had been used recently published randomized phase III studies [8, 9, 26]. All of the stained sections were scored in five randomly selected areas containing tumor cells, which showed membranous and cytoplasmic staining. The percentage of positive tumor cells was graded on a scale of 0–2: 0 (< 1%), 1 (1–5%); 2 (> 5%). The intensity of staining was scored as follows: 0 (no staining), 1 (weak staining), 2 (moderate or strong staining). The H-score, ranging from 0 to 12, was calculated by multiplying the percentage of positive tumor cells by the intensity of staining on the tissue sections. The H-scores were categorized as follows: 0: negative (−), 1–4: weak positive (+), 5–8: moderately positive (+ +), 9–12: strong positive (+ + +).
Assessment of CD8+ TIL density
Assessment of CD8+ TIL density was performed according to established breast cancer protocols [23]. In literature, common cut-off points ranged between 2.5 and 40% in order to differentiate between high and low CD8+ TIL density. In our study we divided the patient cohort in two subgroups (low and high density of CD8+ TILs: 0–40% vs. 41–100%).
Assessment of tumor immunity in the MicroEnvironment (TIME)
Based on previous studies, four different types of tumour immune microenvironment have been identified according to PD-L1 expression of tumor cells and presence or absence of TILs in the tumor microenvironment [27, 28]. These included type I (PD-L1 − with no TILs indicating immune ignorance), type II (PD-L1 + with TILs implying adaptive immune resistance), type III (PD-L1 − with TILs suggesting the role of other suppressor(s) in promoting immune tolerance) and type IV (PD-L1 + with no TILs indicating intrinsic induction). All patients were stratified according to TIME classification and TIME subgroups were evaluated for prognostic outcome, local control, PFS and OS.
Statistical analysis
Each clinicopathologic characteristic was evaluated using Pearson’s chi-squared test or Fisher’s exact test (categorical variables). OS was measured from the date of the initial diagnosis until the date of death. The Kaplan-Meier method and log-rank test were applied to assess OS. In multivariate analysis, the Cox regression proportional hazard model was used to assess the clinicopathologic characteristics significantly related to OS with HRs and 95% CIs. A two-sided p value of ≤0.05 was considered statistically significant. All statistical analyses were performed using SPSS 25 software (IBM, Armonk, NY).
Results
The clinicopathologic characteristic of all patients are shown in Table 1. Median age was 65 years (range: 51–76 years). Histopathological biopsy was taken before treatment by all patients and reviewed by pathology specialists. Sixteen (52%) patients were diagnosed with squamous cell carcinoma, 9 (29%) patients with adenocarcinoma and 6 (19%) with a non-specified non-small cell lung cancer. Twenty-eight (90.3%) patients had stage III NSCLC according to the 8th UICC TNM Staging System of lung cancer and 3 (9.7%) patients were diagnosed with stage IV NSCLC due to pleural involvement or malignant pleural effusion. All 3 stage IV patients were without sensitizing EGFR or ALK mutations. At diagnosis, 23 (74.2%) patients were heavy smokers (median pack years (PY):40) and 8 (25.8%) patients never smokers.
Table 1.
patient characteristics
| Number of patients (%) |
|
|---|---|
| Age | |
| ≤ 65 years | 16 (52) |
| > 65 years | 15 (48) |
| Gender | |
| Female | 26 (84) |
| Male | 5 (16) |
| Karnofsky performance status | |
| > 80% | 11 (35) |
| ≤ 80% | 20 (65) |
| UICC stage | |
| III | 28 (90) |
| IV | 3 (10) |
| T category | |
| 1–2 | 6 (19) |
| 3–4 | 25 (81) |
| N category | |
| 0–1 | 3 (10) |
| 2–3 | 28 (90) |
| Histology | |
| Squamous cell carcinoma | 16 (52) |
| Non-squamous cell carcinoma | 15 (48) |
| Tobacco consumption (PY) | |
| 0 | 8 (26) |
| 20–40 | 8 (26) |
| > 40 | 15 (48) |
| Grading | |
| Moderately differentiated | 2 (6) |
| Poorly differentiated | 27 (87) |
| anaplastic | 2 (6) |
| TIME | |
| I | 10 (32) |
| II | 5 (16) |
| III | 5 (16) |
| IV | 7 (23) |
All patients were treated with definitive concurrent CRT. Twenty-five (81%) patients received platinum-based chemotherapy. A taxane-based combination was applied in 16 (52%) patients. Median biologically equivalent dose (EQD2) to the primary tumor and involved nodes was 65Gy (range: 50-70Gy). Follow-up was conducted as per in-house protocol every 3 months in the first 2 years, every 6 months up to 5 years and afterwards once per year.
The median overall survival in the entire patient collective was 14 months (range: 3–167 months). The 1-year and 2-year OS rates were 67.7 and 19.4%, respectively. The 1 and 2-year actuarial local control rates were 74 and 61%, respectively. Median PFS, 1-year and 2-year PFS were 13 ± 1.4 months, 58 and 19%, respectively.
Correlations of PD-L1 expression and clinicopathologic characteristics
Correlations of PD-L1 expression and clinicopathologic characteristics are shown in Table 2. PD-L1 inversely correlates with Karnofsky performance status (p = 0.023) and positively with CD8+ TIL density (p = 0.020).
Table 2.
Correlations of PD-L1 expression and clinicopathologic characteristics
| Positive, n (%) | Negative, n (%) | p-value | |
|---|---|---|---|
| Age | |||
| ≤ 65 years | 8 (50) | 8 (50) | |
| > 65 years | 8 (57) | 6 (43) | 0.834 |
| Gender | |||
| Female | 13 (52) | 12 (48) | |
| Male | 4 (80) | 1 (20) | 0.513 |
| Karnofsky performance status | |||
| 90–100% | 8 (80) | 2 (20) | |
| 70–80% | 8 (40) | 12 (60) | 0.023 |
| UICC stage | |||
| III | 15 (56) | 12 (44) | |
| IV | 1 (33) | 2 (67) | 0.447 |
| T category | |||
| 1–2 | 4 (67) | 2 (33) | |
| 3–4 | 12 (50) | 12 (50) | 0.073 |
| N category | |||
| 0–1 | 2 (67) | 1 (33) | |
| 2–3 | 14 (52) | 13 (48) | 0.402 |
| Histology | |||
| Squamous cell carcinoma | 9 (60) | 6 (40) | |
| Non- Squamous cell carcinoma | 7 (47) | 8 (53) | 0.864 |
| Tobacco consumption (PY) | |||
| 0 | 5 (63) | 3 (38) | |
| 20–40 | 3 (38) | 5 (63) | |
| > 40 | 8 (57) | 6 (43) | 0.105 |
| Grading | |||
| Moderately differentiated | 1 (50) | 1 (50) | |
| Poorly differentiated | 14 (54) | 12 (46) | |
| anaplastic | 1 (50) | 1 (50) | 0.223 |
| CD8+ TILs density | |||
| ≤ 40% | 5 (50) | 5 (50) | |
| > 40% | 10 (59) | 7 (41) | 0.020 |
Correlations of CD8+ TIL density and clinicopathologic characteristics
Correlations of CD8+ TIL density and clinicopathologic characteristics are shown in Table 3. CD8+ TIL density inversely correlates with Karnofsky performance status (p = 0.038) and positively with PD-L1 expression (p = 0.020).
Table 3.
Correlations of CD8+ TILs density and clinicopathologic characteristics
| high, n (%) | low, n (%) | p-value | |
|---|---|---|---|
| Age | |||
| ≤ 65 years | 12 (80) | 3 (20) | |
| > 65 years | 6 (46) | 7 (54) | 0.403 |
| Gender | |||
| Female | 14 (61) | 9 (39) | |
| Male | 4 (80) | 1 (20) | 0.384 |
| Karnofsky performance status | |||
| > 80% | 9 (90) | 1 (10) | |
| ≤ 80% | 9 (50) | 9 (50) | 0.038 |
| UICC stage | |||
| III | 17 (65) | 9 (35) | |
| IV | 1 (50) | 1 (50) | 0.409 |
| T category | |||
| 1–2 | 3 (60) | 2 (40) | |
| 3–4 | 15 (65) | 8 (35) | 0.751 |
| N category | |||
| 0–1 | 2 (67) | 1 (33) | |
| 2–3 | 16 (64) | 9 (36) | 0.899 |
| Histology | |||
| Squamous cell carcinoma | 8 (62) | 5 (39) | |
| Non- Squamous cell carcinoma | 10 (67) | 5 (33) | 0.681 |
| Tobacco consumption (PY) | |||
| 0 | 6 (75) | 2 (25) | |
| 20–40 | 5 (71) | 2 (29) | |
| > 40 | 7 (54) | 6 (46) | 0.11 |
| Grading | |||
| Moderately differentiated | 0 (0) | 2 (100) | |
| Poorly differentiated | 16 (67) | 8 (33) | |
| anaplastic | 2 (100) | 0 (0) | 0.067 |
| PD-L1 expression | |||
| 0% | 7 (58) | 5 (42) | |
| ≥ 1% | 10 (67) | 5 (33) | 0.02 |
Prognostic impact of PD-L1 expression for local control, PFS and OS
Univariate and multivariate analysis for OS, PFS and local control concerning PD-L1 expression are shown in Tables 4, 5 and 6. Univariate analysis for OS showed significance (p = 0.048). However, multivariate analysis with cox regression failed (p = 0.648). In univariate analysis for PFS and local control, PD-L1 expression was associated with improved PFS (p = 0.006) and improved local control rate (p = 0.017).
Table 4.
univariate and multivariate survival analysis
| Survival | p-value | |||
|---|---|---|---|---|
| at 12 months (%) | at 24 months (%) | univariate Analysis | multivariate Analysis | |
| Age | ||||
| ≤ 65 years | 56 | 19 | ||
| > 65 years | 80 | 27 | 0.676 | |
| Gender | ||||
| Female | 80 | 20 | ||
| Male | 65 | 23 | 0.629 | |
| Karnofsky performance status | ||||
| > 80% | 75 | 30 | ||
| ≤ 80% | 55 | 10 | 0.041 | 0.077 |
| UICC stage | ||||
| III | 64 | 21 | ||
| IV | 100 | 33 | 0.537 | |
| T category | ||||
| 1–2 | 67 | 0 | ||
| 3–4 | 68 | 28 | 0.395 | |
| N category | ||||
| 0–1 | 33 | 0 | ||
| 2–3 | 71 | 25 | 0.299 | |
| Histology | ||||
| Squamous cell carcinoma | 69 | 25 | ||
| Non- Squamous cell carcinoma | 67 | 20 | 0.935 | |
| Tobacco consumption (PY) | ||||
| 0 | 75 | 25 | ||
| 20–40 | 50 | 12,50 | ||
| > 40 | 73 | 27 | 0.758 | |
| Grading | ||||
| Moderately differentiated | 50 | 50 | ||
| Poorly differentiated | 67 | 19 | ||
| anaplastic | 100 | 50 | 0.758 | |
| PD-L1 expression | ||||
| 0% | 86 | 29 | ||
| ≥ 1% | 50 | 19 | 0.048 | 0.648 |
| CD8+ TILs density | ||||
| ≤ 40% | 70 | 40 | ||
| > 40% | 61 | 17 | 0.055 | |
| TIME type | ||||
| I | 100 | 60 | ||
| II | 50 | 20 | ||
| III | 71 | 14 | ||
| IV | 40 | 20 | 0.05 | 0.048 |
Table 5.
univariate and multivariate analysis of local control
| Local control | p-value | |||
|---|---|---|---|---|
| at 12 months (%) |
at 24 months (%) |
univariate Analysis |
multivariate Analysis |
|
| Age | ||||
| 65 years | 58 | 58 | ||
| > 65 years | 83 | 63 | 0.380 | |
| Gender | ||||
| Female | 68 | 57 | ||
| Male | 80 | 80 | 0.941 | |
| Karnofsky performance status | ||||
| > 80% | 73 | 67 | ||
| ≤ 80% | 66 | 33 | 0.233 | |
| UICC stage | ||||
| III | 66 | 62 | ||
| IV | 100 | 67 | 0.862 | |
| T category | ||||
| 1–2 | 66 | 62 | ||
| 3–4 | 100 | 67 | 0.970 | |
| N category | ||||
| 0–1 | 33 | 33 | ||
| 2–3 | 75 | 64 | 0.154 | |
| Histology | ||||
| Squamous cell carcinoma | 70 | 60 | ||
| Non- Squamous cell carcinoma | 70 | 62 | 0.766 | |
| Tobacco consumption (PY) | ||||
| 0 | 83 | 83 | ||
| 20–40 | 51 | 34 | ||
| > 40 | 72 | 60 | 0.417 | |
| Grading | ||||
| Moderately differentiated | 100 | 100 | ||
| Poorly differentiated | 70 | 59 | ||
| anaplastic | 50 | 50 | 0.487 | |
| PD-L1 expression | ||||
| 0% | 92 | 79 | ||
| ≥ 1% | 44 | 44 | 0.017 | 0.045 |
| CD8+ TILs density | ||||
| ≤ 40% | 86 | 75 | ||
| > 40% | 62 | 62 | 0.092 | |
| TIME type | ||||
| I | 100 | 80 | ||
| II | 41 | 41 | ||
| III | 83 | 83 | ||
| IV | 67 | 67 | 0.05 | 0.694 |
Table 6.
univariate and multivariate analysis of progression free survival (PFS)
| PFS | P-value | |||
|---|---|---|---|---|
| at 12 months (%) |
at 24 months (%) |
univariate Analysis |
multivariate Analysis |
|
| Age | ||||
| ≤ 65 years | 50 | 19 | ||
| > 65 years | 67 | 20 | 0.925 | |
| Gender | ||||
| Female | 80 | 20 | ||
| Male | 54 | 19 | 0.868 | |
| Karnofsky performance status | ||||
| > 80% | 65 | 25 | ||
| ≤ 80% | 46 | 9 | 0.134 | |
| UICC stage | ||||
| III | 54 | 18 | ||
| IV | 100 | 33 | 0.458 | |
| T category | ||||
| 1–2 | 67 | 0 | ||
| 3–4 | 56 | 20 | 0.292 | |
| N category | ||||
| 0–1 | 33 | 0 | ||
| 2–3 | 61 | 20 | 0.235 | |
| Histology | ||||
| Squamous cell carcinoma | 56 | 19 | ||
| Non- Squamous cell carcinoma | 60 | 20 | 0.855 | |
| Tobacco consumption (PY) | ||||
| 0 | 63 | 25 | ||
| 20–40 | 38 | 13 | ||
| > 40 | 67 | 20 | 0.633 | |
| Grading | ||||
| Moderately differentiated | 50 | 50 | ||
| Poorly differentiated | 59 | 15 | ||
| anaplastic | 50 | 50 | 0.831 | |
| PD-L1 expression | ||||
| 0% | 86 | 29 | ||
| ≥ 1% | 31 | 13 | 0.006 | 0.061 |
| CD8+ TILs density | ||||
| ≤ 40% | 70 | 30 | ||
| > 40% | 50 | 17 | 0.201 | |
| TIME type | ||||
| I | 100 | 60 | ||
| II | 30 | 20 | ||
| III | 71 | 14 | ||
| IV | 40 | 0 | 0.035 | 0.144 |
Prognostic impact of CD8+ TIL density for local control, PFS and OS
Univariate and multivariate analysis for OS, PFS and local control concerning CD8+ TIL density are shown in Tables 4, 5 and 6. Univariate analysis showed a trend for improved OS and better local control in patients with low CD8+ TIL density (p = 0.055; p = 0.092).
Prognostic impact of tumor immunity in the MicroEnvironment (TIME)
According to the Tumor Immunity in the MicroEnvironment (TIME) classification [27, 28], TIME subgroups were evaluated for prognostic outcome for OS, PFS and local control. The longest and shortest OS were achieved in patients with type I (PD-L1neg/CD8low) and type IV (PD-L1pos/CD8low) (median OS: 57 ± 37 vs. 10 ± 5 months, p = 0.05). In univariate and multivariate analysis for OS, TIME subgroups had significant differences (p = 0.05; p = 0.048) as well as in univariate analysis for PFS and local control (p = 0.05; p = 0.035).
Discussion
LA-NSCLC represents a heterogeneous disease which can include large tumor volumes, extensive lymph node involvement and infiltration of the thoracic wall, mediastinum and spine [4–6]. An interdisciplinary strategy is required to define optimal multimodal approaches based on disease stage, patients’ general condition and treatment options according to the latest evidence [29]. The majority of these patients are inoperable due to comorbidities and lymph node involvement. In this situation, multimodal treatment including concurrent application of chemo- and radiotherapy is associated with a moderate toxicity profile and improved patient outcome compared to sequential CRT or radiotherapy alone [7].
Based on the results of the PACIFIC trial, consolidation PD-L1 inhibition with durvalumab is currently considered as standard of care for stage III NSCLC patients without progressive disease following platinum-based concurrent CRT [8, 9]. In stage IV disease, patients with initial TPS ≥ 50% can be offered pembrolizumab monotherapy. Stage IV patient with tumor cell PD-L1 expression < 1% and good PS can receive a combination of platinum-based chemotherapy with PD-1 or PD-L1 inhibition [17–19].
Previous studies suggest that PD-L1 expression can be a potential biomarker for efficacy of NSCLC treatment including surgery, radiotherapy and checkpoint inhibition [19, 21, 30–32]. Retrospective post-hoc analysis of PACIFIC data suggests that outcome of patients appears to depend on initial PD-L1 expression [9, 33].
The principal finding of our study confirms the statement that initial tumor cell PD-L1 expression can be a prognostic factor for inoperable LA-NSCLC treated with concurrent CRT alone. In the study by Vrankar et al., the prognostic relevance of PD-L1 expression was evaluated in 102 patients with stage III NSCLC treated with concurrent chemoradiotherapy [30]. PD-L1 expression ≥5% on tumor cells resulted in significantly unfavorable PFS and OS. However, several limitations of this study need to be taken into account: only a very small patient number (n = 7) was considered PD-L1 positive. In addition, negative and unknown states of PD-L1 expression were evaluated together. In our study, 52% of all patients were considered PD-L1 positive according to the cut-off value in the PACIFIC trial.
Data of the predictive value of PD-L1 expression on tumor cells in combination with CD8+ tumor-infiltrating lymphocyte (TIL) density in patients with locally advanced NSCLC is limited [34, 35]. Tokito et al. found CD8+ TIL density is an independent prognostic factor for OS [34]. Interestingly, PD-L1 expression (≥5%) on tumor cells has shown no prognostic role in this study in contrast to previous reports [19, 32, 36]. Indeed, patients with low or no PD-L1 expression on tumor cells could respond to PD-1/PD-L1 inhibition as well and show a durable response [22, 37]. In addition, PD-L1 expression can vary between tumor cells, surrounding non-malignant tissue and peripheral immune cells [38–40]. Treatment modality appears to have an impact on PD-L1 expression [41, 42]. Fujimoto et al. evaluated PD-L1 expression on tumor cells before and after CRT and found that alteration of PD-L1 expression was associated with survival in patients with LA-NSCLC [42].
As a result, the interaction of tumor and immune cells in the treatment and immune response is still poorly understood. Based on preclinical and clinical data, the involvement of CD8+ TILs plays a crucial role in tumor-associated immune response [43]. The CD8+ TIL density in the tumor microenvironment has been suggested to predict the oncologic outcome in different cancer types such as colorectal cancer, malignant melanoma and anal cancer [28, 44, 45]. Based on previous studies, four different types of tumor immune microenvironment have been identified according to PD-L1 expression of tumor cells and presence or absence of TILs in the tumor microenvironment. These included type I (PD-L1neg with no TILs indicating immune ignorance), type II (PD-L1pos with TILs implying adaptive immune resistance, type III (PD-L1neg with TILs suggesting a role of other suppressor(s) in promoting immune tolerance) and type IV (PD-L1pos with no TILs indicating intrinsic induction). In our study, the longest OS was achieved in patients with type I (PD-L1neg/CD8low) in contrast to previous studies investigating the prognostic value of PD-L1 expression combined with CD8+ TIL density. In the studies of Tokito et al. and El-Guindy et al., patients with PD-L1neg/CD8high had the longest OS and according to Yang et al. the patient subgroup with PD-L1pos/CD8high showed the longest OS [34, 35, 46]].
The shortest OS in our study was seen in patients with type IV (PD-L1pos/CD8low) and well in accordance with the published literature [33, 34, 45]. This finding could be explained by the lack of immune-mediated tumor response. Tumor cells can decrease their immunogenicity through interaction of PD-L1 with PD-1 on T cells. As a result, the tumor can evade the immune surveillance. In addition, a lack of CD8+ TILs can account for most non-responders to PD-1/PD-L1 inhibition [28].
Several limitations of this study need to be considered when interpreting the results. Firstly, the retrospective nature of this study and the possibility of unknown biases. Secondly, the relatively small number of patients included in the analysis and lastly, all patients were treated at a single center. However, we are convinced that our findings supporting the assessment of CD8+ TIL density combined with PD-L1 expression, instead of PD-L1 expression alone is of important clinical relevance and requires special consideration in future trials.
Conclusion
Initial PD-L1 expression on tumor cells can be a prognostic factor for local control, PFS and OS and correlates with CD8+ TILs density in inoperable LA-NSCLC. Assessment of PD-L1 expression in combination with CD8+ TILs density, instead of PD-L1 expression alone, appears to be of strong prognostic relevance in patients treated with concurrent CRT. Future prospective studies are warranted to verify our findings.
Acknowledgements
The piece has not been previously published and is not under consideration elsewhere. The persons listed as authors have given their approval for the submission.
Authors’ contributions
LK, KG, JN, CE, OR, MO, MK, AT, SR, CB and FM analysed and interpreted the data, performed the statistical analysis and wrote the manuscript. LK, KG, JT and FM helped with the statistical analysis and editing the manuscript. All authors helped in drafting the manuscript. All authors read and gave their stamp of approval for the submission of the final version of the manuscript.
Funding
The study was funded by the German Center for Lung Research (DZL).
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All patients gave express written informed consent. This retrospective analysis is in compliance with the principles of the Declaration of Helsinki and its subsequent amendments. This work was approved by the Ethics Committee of the Ludwig Maximilian University of Munich.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kathrin Gennen and Lukas Käsmann contributed equally to this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson AG, Chansky K, Crowley J, Beyruti R, Kubota K, Turrisi A, Eberhardt WE, Van Meerbeeck J, Rami-Porta R, Goldstraw P. The International Association for the Study of Lung Cancer lung Cancer staging project: proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:300–311. doi: 10.1016/j.jtho.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Käsmann L, Niyazi M, Blanck O, Baues C, Baumann R, Dobiasch S, Eze C, Fleischmann D, Gauer T, Giordano FA. Predictive and prognostic value of tumor volume and its changes during radical radiotherapy of stage III non-small cell lung cancer. Strahlenther Onkol. 2018;194:79–90. doi: 10.1007/s00066-017-1221-y. [DOI] [PubMed] [Google Scholar]
- 4.Postmus P, Kerr K, Oudkerk M, Senan S, Waller D, Vansteenkiste J, Escriu C, Peters S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv1–iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 5.Choi HS, Jeong BK, Jeong H, Lee YH, Ha IB, Song JH, Kang KM. Application of the new 8th TNM staging system for non-small cell lung cancer: treated with curative concurrent chemoradiotherapy. Radiat Oncol. 2017;12:122. doi: 10.1186/s13014-017-0848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ettinger DS, Aisner DL, Wood DE, Akerley W, Bauman J, Chang JY, Chirieac LR, D’Amico TA, Dilling TJ, Dobelbower M. NCCN guidelines insights: non–small cell lung cancer, version 5.2018. J Natl Compr Cancer Netw. 2018;16:807–821. doi: 10.6004/jnccn.2018.0062. [DOI] [PubMed] [Google Scholar]
- 7.Walraven I, Damhuis R, Ten Berge M, Rosskamp M, van Eycken L, de Ruysscher D, Belderbos J. Treatment variation of sequential versus concurrent Chemoradiotherapy in stage III non-small cell lung Cancer patients in the Netherlands and Belgium. Clin Oncol. 2017;29:e177–e185. doi: 10.1016/j.clon.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 9.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 10.Käsmann L, Eze C, Dantes M, Roengvoraphoj O, Niyazi M, Belka C, Manapov F. State of clinical research of radiotherapy/chemoradiotherapy and immune checkpoint inhibitor therapy combinations in solid tumours—a German radiation oncology survey. Eur J Cancer. 2019;108:50–54. doi: 10.1016/j.ejca.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Roengvoraphoj O, Eze C, Wijaya C, Dantes M, Taugner J, Tufman A, Huber RM, Bartenstein P, Belka C, Manapov F. How much primary tumor metabolic volume reduction is required to improve outcome in stage III NSCLC after chemoradiotherapy? A single-Centre experience. Eur J Nucl Med Mol Imaging. 2018;45:2103–2109. doi: 10.1007/s00259-018-4063-7. [DOI] [PubMed] [Google Scholar]
- 12.Manapov F, Roengvoraphoj O, Dantes M, Marschner S, Li M, Eze C. Pneumonitis in irradiated lungs after nivolumab: a brief communication and review of the literature. J Immunother. 2018;41:96–99. doi: 10.1097/CJI.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 13.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mok TS, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Jr, Srimuninnimit V, Laktionov KK, Bondarenko I. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 16.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 17.Borghaei H, Langer CJ, Gadgeel S, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI. 24-month overall survival from KEYNOTE-021 cohort G: Pemetrexed and carboplatin with or without Pembrolizumab as first-line therapy for advanced nonsquamous non–small cell lung Cancer. J Thorac Oncol. 2019;14:124–129. doi: 10.1016/j.jtho.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 19.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 20.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 21.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 22.Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, Wu K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17:129. doi: 10.1186/s12943-018-0864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mino-Kenudson M. Programmed cell death ligand-1 (PD-L1) expression by immunohistochemistry: could it be predictive and/or prognostic in non-small cell lung cancer? Cancer Biol Med. 2016;13:157. doi: 10.20892/j.issn.2095-3941.2016.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Weimin, Green Michael, Rebecca Liu J., Lawrence Theodore S., Zou Weiping. Oncoimmunology. Cham: Springer International Publishing; 2017. CD8+ T Cells in Immunotherapy, Radiotherapy, and Chemotherapy; pp. 23–39. [Google Scholar]
- 25.Marciscano AE, Haimovitz-Friedman A, Lee P, Tran PT, Tomé WA, Guha C, Sahgal A, El Naqa I, Rimner A, Marks LB. Immunomodulatoryeffects of stereotactic body radiation therapy: preclinical insights and clinical opportunities. Int J Radiat Oncol Biol Phys. (2019). 10.1016/j.ijrobp.2019.02.046. [Epub ahead of print]. [DOI] [PubMed]
- 26.Rebelatto MC, Midha A, Mistry A, Sabalos C, Schechter N, Li X, Jin X, Steele KE, Robbins PB, Blake-Haskins JA. Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma. Diagn Pathol. 2016;11:95. doi: 10.1186/s13000-016-0545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Chen L. Classification of advanced human cancers based on tumor immunity in the MicroEnvironment (TIME) for cancer immunotherapy. JAMA Oncol. 2016;2:1403–1404. doi: 10.1001/jamaoncol.2016.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdel-Rahman O. Outcomes of surgery as part of the Management of Metastatic non–Small-Cell Lung Cancer: a surveillance, epidemiology and end results database analysis. Cancer Investig. 2018;36:238–245. doi: 10.1080/07357907.2018.1466895. [DOI] [PubMed] [Google Scholar]
- 30.Vrankar M, Stanic K. Long-term survival of locally advanced stage III non-small cell lung cancer patients treated with chemoradiotherapy and perspectives for the treatment with immunotherapy. Radiol Oncol. 2018;52:281–288. doi: 10.2478/raon-2018-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaira K, Shimizu K, Kitahara S, Yajima T, Atsumi J, Kosaka T, Ohtaki Y, Higuchi T, Oyama T, Asao T. 2-Deoxy-2-[fluorine-18] fluoro-d-glucose uptake on positron emission tomography is associated with programmed death ligand-1 expression in patients with pulmonary adenocarcinoma. Eur J Cancer. 2018;101:181–190. doi: 10.1016/j.ejca.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 32.Sui Hongshu, Ma Ningxia, Wang Ying, Li Hui, Liu Xiaoming, Su Yanping, Yang Jiali. Anti-PD-1/PD-L1 Therapy for Non-Small-Cell Lung Cancer: Toward Personalized Medicine and Combination Strategies. Journal of Immunology Research. 2018;2018:1–17. doi: 10.1155/2018/6984948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang A, Wang H, Liu Y, Zhao M, Zhang H, Lu Z, Fang Y, Chen X, Liu G. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol EJSO. 2015;41:450–456. doi: 10.1016/j.ejso.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Tokito T, Azuma K, Kawahara A, Ishii H, Yamada K, Matsuo N, Kinoshita T, Mizukami N, Ono H, Kage M. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer. 2016;55:7–14. doi: 10.1016/j.ejca.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 35.El-Guindy DM, Helal DS, Sabry NM, El-Nasr MA. Programmed cell death ligand-1 (PD-L1) expression combined with CD8 tumor infiltrating lymphocytes density in non-small cell lung cancer patients. J Egypt Natl Cancer Inst. 2018;30:125–131. doi: 10.1016/j.jnci.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Zhou C, Tang J, Sun H, Zheng X, Li Z, Sun T, Li J, Wang S, Zhou X, Sun H. PD-L1 expression as poor prognostic factor in patients with non-squamous non-small cell lung cancer. Oncotarget. 2017;8:58457. doi: 10.18632/oncotarget.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerr KM, Tsao M-S, Nicholson AG, Yatabe Y, Wistuba II, Hirsch FR, Pathology Committee IASLC. Programmed death-ligand 1 immunohistochemistry in lung cancer: in what state is this art? J Thorac Oncol. 2015;10:985–989. doi: 10.1097/JTO.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Kim TH, Fouladdel S, Zhang Z, Soni P, Qin A, Zhao L, Azizi E, Lawrence TS, Ramnath N. Pd-l1 expression in circulating tumor cells increases during radio (chemo) therapy and indicates poor prognosis in non-small cell lung cancer. Sci Rep. 2019;9:566. doi: 10.1038/s41598-018-36096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takamori S, Takada K, Toyokawa G, Azuma K, Shimokawa M, Jogo T, Yamada Y, Hirai F, Tagawa T, Kawahara A. PD-L2 expression as a potential predictive biomarker for the response to anti-PD-1 drugs in patients with non-small cell lung Cancer. Anticancer Res. 2018;38:5897–5901. doi: 10.21873/anticanres.12933. [DOI] [PubMed] [Google Scholar]
- 40.Reynders K, Wauters E, Moisse M, Decaluwé H, De Leyn P, Peeters S, Lambrecht M, Nackaerts K, Dooms C, Janssens W. RNA-sequencing in non-small cell lung cancer shows gene downregulation of therapeutic targets in tumor tissue compared to non-malignant lung tissue. Radiat Oncol. 2018;13:131. doi: 10.1186/s13014-018-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin Junghoon, Chung Jin-Haeng, Kim Se Hyun, Lee Kyu Sang, Suh Koung Jin, Lee Ji Yun, Kim Ji-Won, Lee Jeong-Ok, Kim Jin-Won, Kim Yu-Jung, Lee Keun-Wook, Kim Jee Hyun, Bang Soo-Mee, Lee Jong-Seok. Effect of Platinum-Based Chemotherapy on PD-L1 Expression on Tumor Cells in Non-small Cell Lung Cancer. Cancer Research and Treatment. 2019;51(3):1086–1097. doi: 10.4143/crt.2018.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujimoto D, Uehara K, Sato Y, Sakanoue I, Ito M, Teraoka S, Nagata K, Nakagawa A, Kosaka Y, Otsuka K. Alteration of PD-L1 expression and its prognostic impact after concurrent chemoradiation therapy in non-small cell lung cancer patients. Sci Rep. 2017;7:11373. doi: 10.1038/s41598-017-11949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamada T, Soong TR, Masugi Y, Kosumi K, Nowak JA, da Silva A, Mu XJ, Twombly TS, Koh H, Yang J. TIME (tumor immunity in the MicroEnvironment) classification based on tumor CD274 (PD-L1) expression status and tumor-infiltrating lymphocytes in colorectal carcinomas. Oncoimmunology. 2018;7:e1442999. doi: 10.1080/2162402X.2018.1442999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balermpas P, Martin D, Wieland U, Rave-Fränk M, Strebhardt K, Rödel C, Fokas E, Rödel F. Human papilloma virus load and PD-1/PD-L1, CD8+ and FOXP3 in anal cancer patients treated with chemoradiotherapy: rationale for immunotherapy. Oncoimmunology. 2017;6:e1288331. doi: 10.1080/2162402X.2017.1288331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang H, Shi J, Lin D, Li X, Zhao C, Wang Q, Zhang L, Jiang T, Zhao S, Liu X. Prognostic value of PD-L1 expression in combination with CD 8+ TIL s density in patients with surgically resected non-small cell lung cancer. Cancer Med. 2018;7:32–45. doi: 10.1002/cam4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.