Abstract
Context:
Mitoxantrone (MTX) is an antracyclin drug that is used for treatment of patients with chronic refractory multiple sclerosis (MS). Congestive heart failure (CHF) is a rare complication of this drug that may occur early, during therapy, or late, months or years after termination of therapy.
Aims:
The aim of this study is to evaluate the long-term adverse effect of MTX on cardiac function.
Methods:
The study involved 49 MS patients on MTX therapy because of their disease was refractory to other treatments (18 men and 31 women). They were treated in two canters related to Esfahan University of Medical Sciences. The mean age was 34.65 ± 9.56 years. Systolic and diastolic left ventricular (LV) functions were measured by echocardiography. The baseline echocardiographic data were collected from patients' file. Echocardiography was repeated by a single cardiologist in 2016.
Results:
After MTX therapy, one patient's ejection fraction (EF) reduced below 50% (2%). In spite of their normal diastolic function before therapy, two patients developed diastolic dysfunction (4%). Nonparametric binominal analysis reveals that MTX therapy increased the probability of developing systolic dysfunction, early or late P < 001.
Conclusions:
MS patients treated with MTX are at increased risk of developing early and late-LV dysfunction, so all patients on MTX therapy must be periodically evaluated for these late complications.
Keywords: Heart failure, mitoxantrone, multiple sclerosis
Introduction
Multiple sclerosis (MS) is a chronic, autoimmune disease of central nervous system in which axonal damage in brain and spinal cord is observed. It is one of the most common cause of disability in young adults in West Europe and North America.[1] There are 2.5 million people suffered from MS worldwide.[2] In a case-controlled study on patients with worsening relapsing–remitting MS mitoxantrone (MTX), 12 mg/m2 was generally well tolerated and reduced progression of disability and clinical exacerbations.[3]
MTX (Novantrone), a synthetic anthracenedione derivative, is an antineoplastic, immunomodulatory, and anti-inflammatory agent. Its presumed mechanism of action in patients with MS is via immunomodulatory mechanisms, although these remain to be fully elucidated. MTX interfered with the antigen-presenting capabilities of monocyte-derived dendritic cells and induced their apoptosis at low concentrations, whereas higher concentrations caused cell lysis.[4,5]
MTX also exerts both cytotoxic and immunomodulatory effects on microglia in the CNS, the latter being mediated by increased levels of IL-10 production and impaired IL-23p19 production.
Intravenous MTX treatment improved neurological disability and delayed progression of MS in patients with worsening relapsing–remitting or secondary-progressive disease.[4,5,6,7,8,9,10]
MTX is licensed in the United States and some European countries, as a disease-modifying therapy for MS.[2]
The drug was allowed in treatment of leukemia. It is also used in treatment of breast, prostate, ovarian, stomach, and liver cancer. MTX inhibits topoisomerase II activity, matches to DNA molecule, and damage her structure. The drug inhibits lymphocyte T, B, and macrophages activity and antibody synthesis.[11]
MTX monthly for 6 months as induction therapy followed by maintenance treatment showed sustained clinical benefit for up to 5 years with an acceptable adverse event profile in patients with aggressive relapsing–remitting MS.[12,13]
There is, however, concern that it may cause irreversible cardiomyopathy with reduced left ventricular (LV) ejection fraction (EF) and congestive heart failure (CHF).[8] CHF, potentially fatal, may occur during therapy with MTX or months to years after termination of therapy. Cardiotoxicity risk increases with cumulative MTX dose and may occur whether or not cardiac risk factors are present.[4,5,8,9,10] Presence or history of cardiovascular disease, radiotherapy to the mediastinal/pericardial area, previous therapy with other anthracyclines or anthracenediones, or use of other cardiotoxic drugs may increase this risk. The aim of this study is to evaluate the lung-term adverse effect of MTX on cardiac function of MS patients.
Methods
We examined long-term cardiotoxicity of MTX on MS patients treated with this drug. Cases selected from MS patients that were treated in two centers related to Isfahan University of Medical Sciences (al-Zahra Hospital and Isfahan MS Center). The time span between end of treatment and fallow-up evaluation was between 1 and 5 years.
Inclusion criteria
All MS patients that had at least two sessions of MTX therapy and had no exclusion criteria.
Exclusion criteria
Patients with congenital heart disease, valvular heart disease, heart failure, coronary hear disease, pregnant patients, and patients who were treated with other cardiotoxic drugs excluded from the study.
Demographic and baseline data were collected by a questionnaire. The baseline echocardiographic data were collected from patients file and echocardiography was repeated by a single cardiologist in 2016.
Echocardiography
Systolic function evaluated by measurement of ejection fraction (EF) diastolic function evaluated by measurement of E/A, deceleration time E'/A', and E/E'. Pulmonary arterial pressure was estimated with measurement of trans-tricuspid gradient.[14] All new cardiac adverse events during treatment including reduction of resting LVEF measured by transthoracic echocardiogram (<50% or an absolute reduction of ≥10% from the baseline), diastolic and systolic contraction abnormalities, and newly emerging arrhythmias that could not be attributed to any other origin were regarded as therapy-related cardiac dysfunction. Mean values and standard deviations are given for the descriptive analyses, because of small sample size, the data were analyzed by nonparametrical binominal test of significance. P value <0.05 was considered significant.
All statistical analyses were performed using SPSS version 15.0.
This article is the output of research project NO. 395109, registered by the Isfahan University of Medical Sciences, and it was also approved by local ethic committee of university NO 1395.3.109.
Results
Forty-nine patients (18 men and 31 women) enrolled in this study. The mean age was 34.65 ± 9.56 [Table 1].
Table 1.
Cardiovascular complication of mitoxantrone therapy
| Characteristics | n (%) or mean±SD |
|---|---|
| Age | 34.65±9.56 |
| Sex | |
| Male | 18 (36.7) |
| Female | 31 (63.3) |
| Cardiovascular complication | |
| No complication | 45 (92) |
| Systolic dysfunction | 1 (2) |
| Diastolic dysfunction | 2 (4) |
| Arrhythmia | 1 (2) |
| Pericardial effusion | 0 |
| Total | 4 (8) |
SD=Standard deviation
Out of 31 female patients, only one person developed systolic heart failure (reduced EF below 50%); this means that 3.2% of female or 2% of all patients developed this complication. The EF reduction was over 10% and patient had no clinical symptom. Two other patients developed diastolic dysfunction in spite of normal diastolic function before therapy [Table 2 and Figure 1].
Table 2.
The frequency off cardiovascular complication according to number of sessions of mitoxantrone therapy
| Number of injection | Complication | |||
|---|---|---|---|---|
| Diastolic dysfunction, n (%) | Systolic dysfunction, n (%) | Arrhythmia, n (%) | Total number | |
| 2 | 0 | 0 | 0 | 3 |
| 3 | 0 | 0 | 0 | 5 |
| 4 | 0 | 0 | 0 | 3 |
| 5 | 1 (2.9) | 1 (2.9) | 1 (2.9) | 34 |
| 6 | 1 (25.0) | 0 | 0 | 4 |
Figure 1.
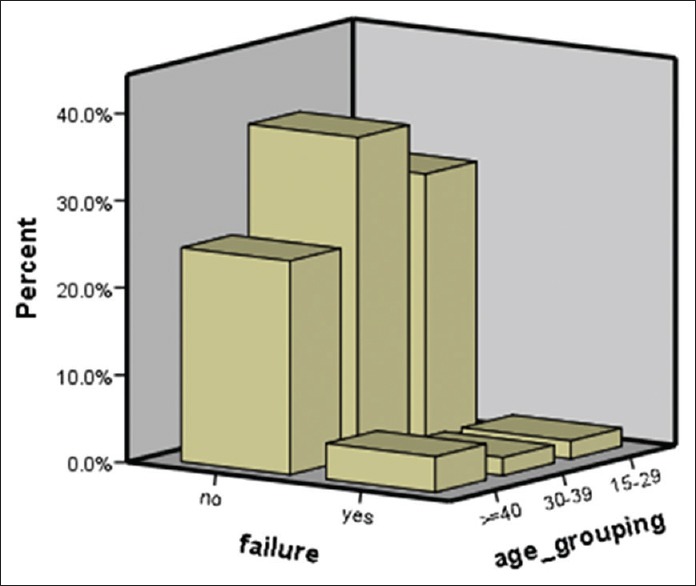
Probability of heart failure after mitoxantrone therapy in age groups
None of our patients had pericardial effusion or arrhythmia as a complication of drug therapy [Table 3].
Table 3.
Age distribution of complication of mitoxantrone therapy
| Sex | Age | Frequency | Diastolic dysfunction | Systolic dysfunction | Arrhythmia |
|---|---|---|---|---|---|
| Male | 15-29 | 3 | 0 | 0 | 0 |
| 30-39 | 9 | 0 | 0 | 0 | |
| ≥40 | 6 | 0 | 0 | 0 | |
| Female | 15-29 | 13 | 0 | 1 | 0 |
| 30-39 | 0 | 0 | 0 | 0 | |
| ≥40 | 8 | 2 | 0 | 0 |
Nonparametric binominal analysis reveals that after MTX therapy the probability of developing systolic dysfunction early or late is 2% [Table 4].
Table 4.
Binominal analysis for probability of cardiac complication
| Category | n | Observed_prop | Test_prop | P | |
|---|---|---|---|---|---|
| Complication | Yes | 5 | 0.1 | 0.05 | <0.001 |
| Systolic dysfunction_ early | Yes | 1 | 0.02 | 0.01 | <0.001 |
| Systolic dysfunction_ late | Yes | 1 | 0.02 | 0.01 | <0.001 |
Discussion
Subclinical cardiotoxicity is commonly defined on cardiac imaging as clinically asymptomatic left ventricular systolic dysfunction with a fall in LVEF by >10% to a value of EF <50%.[14,15] One patient out of our 49 patients (2%) developed late subclinical cardiotoxicity and another one developed early cardiotoxicity that healed after discontinuation of drug. None of our patients had clinically symptomatic heart failure. Two patients also developed grade II diastolic dysfunction. Diastolic dysfunction is a physiological process that is caused by age-related reduction in cardiac compliance. These patients were 37- and 40-year old, so this mild reduction on diastolic function may not be a complication of MTX therapy.
Paul reported that from a total number of 18 patients with secondary progressive MS who were treated with MTX at their institution in 2-year follow-up, 4 patients developed the temporary and considerable decrease in LVEF (22%).[16]
In a multicenter retrospective cohort study performed at two MS centers in Germany between 1995 and 2010, Fleischer reported that from 639 MS patients, who were treated with MTX, none of them developed symptomatic CHF. However, the frequency of patients experiencing cardiac dysfunction of milder forms was 4.1% (26 patients) among all patients.[17]
Registry to Evaluate Novantrone Effects in worsening multiple sclerosis overall was a 5-year, phase IV study in which the safety of MTX was monitored in patients from the United States. About 509 patients were enrolled and treated at 46 MS centers. The objective of the study was to evaluate the long-term safety profile of MTX in patients with secondary progressive MS.[18]
In this study, left ventricular ejection fraction reduction under 50% was reported in 27 (5.3%) patients during the treatment phase (n =509) and 14 (5.6%) patients during the annual follow-up phase (n =250). Signs and symptoms of CHF were observed in 10 (2.0%) patients. Post-hoc analyses of the risk for cardiotoxicity outcomes revealed that cumulative dose exposure is the primary risk factor associated with the risk of cardiac toxicity with MTX.
In our study, the cumulative dose for patients who had developed systolic dysfunction was 60 mg/m2 or higher.
MTX is used in the chemotherapy of several cancer types, including solid and non-solid malignancies, such as breast cancer, leukemia, lymphomas, and sarcomas. These drugs interact with iron to generate reactive oxygen species (ROS), target topoisomerase 2 (Top2), and impair mitochondria. This is one of the mechanisms through which these drugs induce late cardiomyopathy.[19]
The cardiotoxic mechanisms of anthracyclines may involve the dual pathways of reactive oxidation species and Top2-beta and a final common pathway of calcium overload, lipid peroxidation, and mitochondrial dysfunction.[20]
In patients treated with this drug for cancer chemotherapy, clinically symptomatic heart failure occurs in 2%–4%, asymptomatic fall in LVEF in 9%–11%, arrhythmia in ≥12%, and cardiac biomarker rise in 30%–35% of treated patients. Predictors of cardiotoxicity include cumulative dose, cardiovascular risk factors, and age of treatment.[20,21]
Comparing the cardiotoxicity of MTX in MS patients with cancer patients, MTX is slightly less cardiotoxic in MS patients. This may be due to lower therapeutic dose of drug in MS patients; another description of this difference may be different in mean age of patient. MS patients are usually younger than cancer patients and have less comorbid disease.
So, MTX is an appropriate drug for MS patients who have worsening relapsing–remitting MS despite prior therapy. Clinical trials show that MTX would reduce relapse, the number of new lesions visualized on magnetic resonance imaging, and stop or reduce the progression of the disease in many patients.[22,23]
The important limitations of our study were the small number of patients and variability on follow-up duration and inequality in cumulative dose of drug.
Conclusions
MTX is a disease-modifying drug that could be used in patients with progressive MS that are not controlled by other drugs late cardio-toxicity is a rare but significant complication that must be detected early by appropriate screening of cardiac function at least during first 5 years after therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank the participating personnel of neurologic Department of Isfahan alzahra hospital, and Isfahan MS center especially Mrs. Afsaneh Hadi and Mrs. Raheleh Janghorban, and thanks to Dr. Nilfrooshzadeh for his cooperation, and Ms. Taheri from statistical department of Isfahan Cardiovascular Research Center for data analysis. This article is the output of research project NO. 39471.
References
- 1.Edan G, Miller D, Clanet M, Confavreux C, Lyon-Caen O, Lubetzki C, et al. Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: A randomised multicentre study of active disease using MRI and clinical criteria. J Neurol Neurosurg Psychiatry. 1997;62:112–8. doi: 10.1136/jnnp.62.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pastuszak Ż, Stępień A, Tomczykiewicz K, Piusińska-Macoch R, Durka-Kęsy M. Mitoxantrone role in treatment of primary progressive multiple sclerosis. Pol Merkur Lekarski. 2016;40:66–9. [PubMed] [Google Scholar]
- 3.Hartung HP, Gonsette R, König N, Kwiecinski H, Guseo A, Morrissey SP, et al. Mitoxantrone in progressive multiple sclerosis: A placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360:2018–25. doi: 10.1016/S0140-6736(02)12023-X. [DOI] [PubMed] [Google Scholar]
- 4.Hanly A, Petito CK. HLA-DR-positive dendritic cells of the normal human choroid plexus: A potential reservoir of HIV in the central nervous system. Hum Pathol. 1998;29:88–93. doi: 10.1016/s0046-8177(98)90395-1. [DOI] [PubMed] [Google Scholar]
- 5.Luessi F, Zipp F, Witsch E. Dendritic cells as therapeutic targets in neuroinflammation. Cell Mol Life Sci. 2016;73:2425–50. doi: 10.1007/s00018-016-2170-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millefiorini E, Gasperini C, Pozzilli C, D'Andrea F, Bastianello S, Trojano M, et al. Randomized placebo-controlled trial of mitoxantrone in relapsing-remitting multiple sclerosis: 24-month clinical and MRI outcome. J Neurol. 1997;244:153–9. doi: 10.1007/s004150050066. [DOI] [PubMed] [Google Scholar]
- 7.van de Wyngaert FA, Beguin C, D'Hooghe MB, Dooms G, Lissoir F, Carton H, et al. A double-blind clinical trial of mitoxantrone versus methylprednisolone in relapsing, secondary progressive multiple sclerosis. Acta Neurol Belg. 2001;101:210–6. [PubMed] [Google Scholar]
- 8.Scott LJ, Figgitt DP. Mitoxantrone: A review of its use in multiple sclerosis. CNS Drugs. 2004;18:379–96. doi: 10.2165/00023210-200418060-00010. [DOI] [PubMed] [Google Scholar]
- 9.Cohen BA, Mikol DD. Mitoxantrone treatment of multiple sclerosis: Safety considerations. Neurology. 2004;63:S28–32. doi: 10.1212/wnl.63.12_suppl_6.s28. [DOI] [PubMed] [Google Scholar]
- 10.Mather FJ, Simon RM, Clark GM, Von Hoff DD. Cardiotoxicity in patients treated with mitoxantrone: Southwest Oncology Group phase II studies. Cancer Treat Rep. 1987;71:609–13. [PubMed] [Google Scholar]
- 11.Hamzehloo A, Etemadifar M. Mitoxantrone-induced cardiotoxicity in patients with multiple sclerosis. Arch Iran Med. 2006;9:111–4. [PubMed] [Google Scholar]
- 12.Avasarala JR, Cross AH, Clifford DB, Singer BA, Siegel BA, Abbey EE, et al. Rapid onset mitoxantrone-induced cardiotoxicity in secondary progressive multiple sclerosis. Mult Scler. 2003;9:59–62. doi: 10.1191/1352458503ms896oa. [DOI] [PubMed] [Google Scholar]
- 13.Ghalie RG, Edan G, Laurent M, Mauch E, Eisenman S, Hartung HP, et al. Cardiac adverse effects associated with mitoxantrone (Novantrone) therapy in patients with MS. Neurology. 2002;59:909–13. doi: 10.1212/wnl.59.6.909. [DOI] [PubMed] [Google Scholar]
- 14.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH, et al. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: Application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 15.Mauch E, Kornhuber HH, Krapf H, Fetzer U, Laufen H. Treatment of multiple sclerosis with mitoxantrone. Eur Arch Psychiatry Clin Neurosci. 1992;242:96–102. doi: 10.1007/BF02191555. [DOI] [PubMed] [Google Scholar]
- 16.Paul F, Dörr J, Würfel J, Vogel HP, Zipp F. Early mitoxantrone-induced cardiotoxicity in secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2007;78:198–200. doi: 10.1136/jnnp.2006.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleischer V, Salmen A, Kollar S, Weyer V, Siffrin V, Chan A, et al. Cardiotoxicity of mitoxantrone treatment in a German cohort of 639 multiple sclerosis patients. J Clin Neurol. 2014;10:289–95. doi: 10.3988/jcn.2014.10.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivera VM, Jeffery DR, Weinstock-Guttman B, Bock D, Dangond F. Results from the 5-year, phase IV RENEW (Registry to evaluate novantrone effects in worsening multiple sclerosis) study. BMC Neurol. 2013;13:80. doi: 10.1186/1471-2377-13-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damiani RM, Moura DJ, Viau CM, Caceres RA, Henriques JA, Saffi J, et al. Pathways of cardiac toxicity: Comparison between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch Toxicol. 2016;90:2063–76. doi: 10.1007/s00204-016-1759-y. [DOI] [PubMed] [Google Scholar]
- 20.McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM, et al. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31:63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hequet O, Le QH, Moullet I, Pauli E, Salles G, Espinouse D, et al. Subclinical late cardiomyopathy after doxorubicin therapy for lymphoma in adults. J Clin Oncol. 2004;22:1864–71. doi: 10.1200/JCO.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 22.Jeffery DR. The argument against the use of cyclophosphamide and mitoxantrone in the treatment of multiple sclerosis. J Neurol Sci. 2004;223:41–6. doi: 10.1016/j.jns.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Murray TJ. The cardiac effects of mitoxantrone: Do the benefits in multiple sclerosis outweigh the risks? Expert Opin Drug Saf. 2006;5:265–74. doi: 10.1517/14740338.5.2.265. [DOI] [PubMed] [Google Scholar]


