Summary
Background
Influenza causes significant morbidity and mortality despite currently available treatments. Although prior studies suggest that anti-influenza immune plasma may provide benefit, those earlier studies were not designed as definitive trials. We aimed to prospectively evaluate the clinical efficacy of high-titre immune plasma in severe influenza A.
Methods
We conducted a randomised, blinded, controlled, phase 3 trial at 31 U.S. medical centres to assess the efficacy of high-titre anti-influenza plasma with hemagglutination inhibition (HAI) antibody titres of ≥ 1:80 compared to low titre plasma (HAI ≤ 1:10). Hospitalized children and adults with severe influenza A were randomly assigned (2:1) to receive either 2 units high-titre plasma or low-titre control plasma (or paediatric equivalent) and followed for 28 days. High-titre and low titre plasma had the same appearance. Randomization was stratified by severity (in the intensive care unit (ICU), non-ICU hospitalization requiring supplemental oxygen, or non-ICU hospitalization not requiring supplemental oxygen) and age (< 18 years of age, ≥ 18 years of age). The primary endpoint was clinical status as assessed by a 6-point ordinal scale (death, in the ICU, non-ICU hospitalization requiring supplemental oxygen, non-ICU hospitalization not requiring supplemental oxygen, not hospitalized but unable to resume normal activity, not hospitalized with full resumption of normal activity) on Day 7 assessed in a proportional odds model. The primary analysis used a modified intention-to-treat approach, excluding two participants who did not receive plasma. This study is registered with Clinicaltrials.gov number:
Findings
The study was conducted between January 2016, and May 2018. Of 200 participants enrolled, 140 met criteria for randomization. This was a relatively ill cohort, with 43% of participants enrolled in the ICU and 70% of the non-ICU patients requiring oxygen. 93% of planned plasma infusions were completed. The study was terminated in July 2018 when independent efficacy analysis revealed low conditional power to show an effect of high-titre plasma even if full accrual (target 150 participants) was achieved. The proportional odds ratio for improved clinical status on Day 7 was 1.22 (95% CI [0.65, 2.29], p=0.54). Forty-seven of 138 (38%) participants experienced a total of 88 SAEs – 32 participants (35%) with 60 SAEs in the high-titre arm, and 15 participants (32%) with 28 SAEs in the low-titre arm. The most common SAEs were for ARDS (affecting 4 participants (4%) vs 2 (4%)), allergic transfusion reactions (2 (2%) vs 2 (4%)), and respiratory distress (3 (3%) vs 0 (0%)). Sixty-five of 138 (47%) participants experienced a total of 183 adverse events - 42 participants (46%) with 126 adverse events in the high-titre arm, and 23 participants (49%) with 57 adverse events in the low-titre arm. The most common AEs were anaemia (affecting 4 participants (3%) vs (2 (4%)) and ARDS (4 (3%) vs 3 (5%)).
Interpretation
Despite encouraging results from prior studies, high-titre anti-influenza plasma conferred no statistically significant benefit over non-immune plasma. While this study did not have the precision to rule out a small effect that might be clinically relevant, the benefit is insufficient to justify the use of immune plasma for treating patients with severe influenza A.
Introduction
Seasonal and pandemic influenza remains a global health threat. One potential therapeutic approach that is frequently utilized, especially during pandemics or following the emergence of novel influenza subtypes, is the use of high-titre anti-influenza immune plasma derived either from convalescent or recently immunized individuals. Preclinical animal models have demonstrated the therapeutic efficacy of both polyclonal F(ab) fragments or polyclonal convalescent plasma. 1,2 A meta-analysis of reports from the 1918 influenza A/H1N1 pandemic concluded that early administration of convalescent blood products reduced the absolute risk of death from pneumonia by 21% from 37% to 16% (95% CI 15–27%).3 Following the re-emergence of H1N1 influenza in 2009, a cohort study was conducted evaluating the use of convalescent plasma for severe influenza A/H1N1/pdm09 infection. All participants were offered immune plasma, with a neutralizing antibody titre of >1:160. Twenty participants accepted the intervention, leaving 73 participants who did not accept the plasma as a contemporaneous control group. Mortality was 20.0% for those receiving high-titre immune plasma compared to 54.8% in those that received standard care alone (p=.01).4 However, the control arm mortality was significantly higher than anticipated for a similar severity of illness.5,6
We previously conducted a randomised, phase 2 study in which participants with influenza A or B that had severe disease (defined as having hypoxia or tachypnoea) were assigned to receive either two units of immune plasma plus standard care versus standard care alone.7 While the study did not demonstrate clear benefit in the primary endpoint of normalization of respiratory status by Day 28 (67% vs 53%, p = 0.069), multiple secondary endpoints were suggestive of efficacy, including fewer days in the hospital (median 6 vs. 11, p= 0.13), fewer participants admitted to the ICU (57% vs. 69%, p = 0.097), fewer days on mechanical ventilation (median 0 vs. 3, p=0.14), and better clinical status at Day 7 (clinical status assessed as death, in ICU, hospitalized on oxygen, hospitalized not on oxygen, not hospitalized but not returned to normal activities, or not hospitalized and returned to normal activities) (p=0.020). There were differences in baseline characteristics of the study groups that potentially contributed to these perceived benefits, and there was asymmetrical participant loss to follow up possibly due to the unblinded study design. However, the totality of these suggestive results supported development of the current phase 3 study of high-titre plasma for severe hospitalized influenza in order to address this question more definitively. The hypothesis was that treatment with high-titre immune plasma in addition to standard antiviral therapy would improve clinical outcomes in those with severe influenza.
Methods
Study Design
This was a randomised, double-blinded, multi-centre phase 3 trial initially designed to be conducted at 41 large medical centres in the United States. All study participants provided written informed consent. The study protocol (protocol document submitted to https://clinicaltrials.gov/ct2/show/NCT02572817) was approved by the institutional review board at each study site. The study planned to randomize a total of 150 eligible participants hospitalized with influenza A in a 2:1 ratio to receive either two units of high-titre anti-influenza plasma or a similar volume of control (low-titre) plasma. Paediatric participants < 56 kg received 8 mL/kg of assigned study plasma, not to exceed two units of plasma. This study is registered with Clinicaltrials.gov number: .
Participants
Participants of all ages, including children two weeks or older and pregnant women, hospitalized with influenza A (diagnosed locally by rapid antigen or polymerase chain reaction (PCR) tests) who had a National Early Warning (NEW)8 score ≥ 3 (or Paediatric Early Warning (PEW)9 ≥ 3 for children) with the onset of illness ≤ 6 days before randomization, were eligible for randomization. Exclusion criteria include treatment with other investigational anti-influenza agents, history of allergic reaction to blood or plasma products, medical conditions that increased risk for thrombosis, and medical conditions that could not tolerate a 450–700 mL infusion of plasma.
Participants with influenza B were excluded. In our previous plasma study (IRC002)7, the plasma-treated arm had minimal increase in HAI titres. As an increase in HAI was central to proposed treatment effect, it was thought inappropriate to enrol participants with influenza B into this study.
Randomization and masking
Randomization occurred by an online computer-generated randomization system. Randomization was stratified by severity (in the ICU, non-ICU hospitalization requiring supplemental oxygen, or non-ICU hospitalization not requiring supplemental oxygen), and age category (child (< 18 years of age) or adult (≥ 18 years of age)). High-titre and low titre plasma had the same appearance, having a standard International Society of Blood Transfusion (ISBT-128) plasma label with a modified product quadrant reflecting the investigational new drug (IND) status of the plasma. The randomization system noted the specific plasma unit number (donation identification number) to administer to a participant. No identifiers on the label would discern high-titre and low titre units. All participants, site staff, and the study team were masked to treatment allocation, and remained blinded until after final database lock.
Study Plasma
All high titre units were required to have a HAI titre of at least 1:80 towards the seasonal vaccine strain of H1N1 and H3N2 (specific strains varied by year). These control plasma units were required to have a HAI of ≤ 1:10. The study plasma was obtained from three large regional blood collection centres in Iowa, Minnesota, and Texas, and all units of plasma met standard release criteria and had prespecified hemagglutination inhibition (HAI) titre(s) measured by one central laboratory. See supplement for methods on HAI assay. Units were collected prior to and during a given influenza season, and sent to a central repository from which sites were supplied with plasma units. Units not used within one year of collection were destroyed.
Procedures
Participants were assessed on Day 0 (baseline) and on Days 1, 2, 3, 7, 14, and 28. For participants who were not hospitalized on Days 2, 14, and 28, contact could be performed by telephone. Blood samples were collected on Days 0, 1, 3, and 7. Oropharyngeal (OP) swabs for influenza PCR were obtained on Days 0 and 3. Given the limited utility of virology in the prior plasma study7, and the FDA position that the relation of viral shedding in relation to clinical outcomes is not well standardized10, additional virologic assessment was not included.
For RT-PCR, RNA isolated from oral swabs were used to determine positive for influenza A and further subtype to H1 and H3 as previously described. 11,12 The amount of influenza A viral RNA copies was determined by quantitative real-time PCR using in vitro generated influenza A viral RNA as the reference standard.
For hemagglutination inhibition (HAI), sera samples were treated with RDE (Denka Seiken) and hem-adsorbed before use in the HAI testing. Serial 2-fold dilution of the RDE and hem-adsorbed treated samples were prepared in V-bottom 96 well plates to which a fixed amount of influenza virus (4 HA units) was added and mixed. An equal volume of 0.5% turkey RBCs (Lampire Biological) suspension was added and plates were incubated for about one hour until HA activity was observed in virus control. The plates were tilted to read and the HAI titer was reported as the last dilution of sera with no hemagglutination activity. 13
Outcomes
In the prior anti-influenza plasma study7, the endpoint of resolution of hypoxia and tachypnoea had moderate variability, with 15% of the plasma arm meeting the endpoint after randomization and before plasma administration. Therefore, in this study, the primary outcome was chosen as the participants clinical status at Day 7, as measured by a 6-point ordinal scale: death, in the intensive care unit, not in the intensive care unit but requiring supplemental oxygen, not in the intensive care unit and not requiring supplemental oxygen, not hospitalized but unable to resume normal activities, and not hospitalized with full resumption of normal activities.
Secondary outcomes that were measured include ordinal outcome assessed at Days 1, 2, 3, 7, 14, and 28; duration of hospitalization; mortality; NEW score at Day 0, 3, and 7; incidence and duration of supplemental oxygen; incidence and duration of intensive care (if applicable); incidence and duration of mechanical ventilation (if applicable); incidence and duration of acute respiratory distress syndrome (if applicable); incidence and duration of ECMO (if applicable); SOFA score for age ≥18 years and PELOD score for age <18 years on Days 0, 3, and 7; disposition (home, rehabilitation, chronic nursing facility) following the initial hospitalization; quantitative PCR for influenza in oral swab on Day 0 and 3; grade 3 and 4 adverse events, and serious adverse events.
Statistical Analysis
The sample size was calculated based on a proportional odds ratio comparing high titre to low titre of 2.5. Details of the sample size justification are provided in the supplemental appendix. All participants were required to receive standard care antiviral treatment.
All results are presented using a modified intention-to-treat (mITT) approach, excluding two participants who did not receive plasma. A proportional odds model was used to compare the primary endpoint between treatments; this gives a proportional odds ratio (POR) as the measure of effect comparing high titre to low titre plasma administration. In a secondary analysis, this model was extended to include the repeated assessments at days 1, 2, 3, 7, 14 and 28, and fitted using the generalized estimating equations method (GEE) with robust standard errors. We assessed the treatment effect in this model based on the interaction of study day and treatment. Adverse event data were coded using MedDRA. Analysis was performed in SAS 9.4 (SAS Institute, Cary, NS, USA).
Data and Safety Monitoring Board (DSMB)
DSMB reviews of the IRC005 data occurred after each influenza season, and included information on study conduct, safety, and the primary efficacy endpoint. At the first two reviews, the Board determined that no safety concerns were identified and recommended that the study continue as designed. By the time of the third review, 140 participants had been randomised. As the accrual goal of 150 participants randomised had not quite been achieved, the DSMB reviewed the possibility of continuing the study into the next influenza season with a modest increase in sample size versus discontinuing the study at that time. The DSMB recommended stopping the study due to low conditional power to show a positive treatment effect of high titre plasma even with a modest sample size increase to the original accrual goal.
Role of Funding Source
Employees of the sponsor of the study were involved with study design, analysis, data interpretation, and the writing of the report. The sponsor had no role in data collection and did not have access to the raw data prior to database lock. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
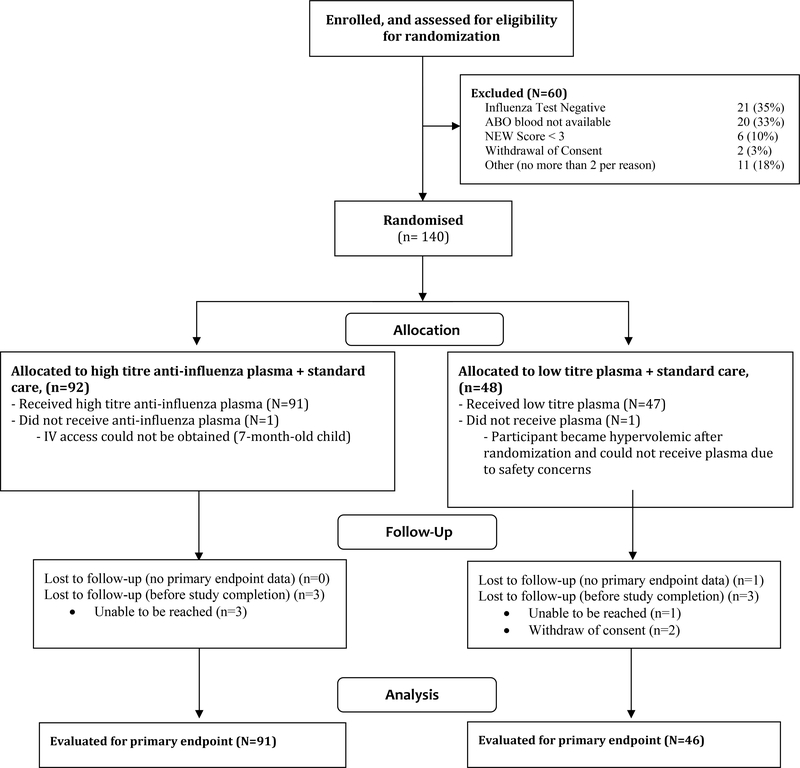
Between January 26, 2016 and April 19, 2018, a total of 200 participants (177 adults and 23 children) were enrolled in the study. Sixty participants were excluded prior to randomization: 21 (35%) did not have a positive test for influenza at the site, 20 (33%) had blood types for which ABO matched plasma was not available, 6 had a NEW score of < 3, 2 withdrew consent, and 11 did not qualify for other reasons (no more than 2 participants per reason). 31 of the 41 participating sites enrolled at least one participant into the trial.
One hundred forty participants were randomised (92 to receive high-titre plasma, 48 to receive low-titre plasma). Two participants were excluded from all analyses because they did not receive any plasma, and therefore the mITT population for analysis was 138 participants. The median age of the randomised participants was 60.5 years (range 5 months to 93 years). Thirteen children and one pregnant woman were randomised (Table 1). Participants had a median of 3 days of illness prior to enrolment, and 108 (78%) had received antivirals prior to randomization.
Table 1:
Demographics, and Baseline Characteristics
| Randomised treatment | ||||
|---|---|---|---|---|
| Total (N=138) | High titre (N=91) | Low titre (N=47) | ||
| Age (years) | N | 138 | 91 | 47 |
| Median (Q1, Q3) | 60.5 (45.0, 69.0) | 58 (47, 69) | 63 (44, 69) | |
| Min, Max | 0, 93 | 0, 93 | 1, 92 | |
| Age Category | <18 years | 13 (9%) | 8 (9%) | 5 (11%) |
| Sex | Female | 67 (49%) | 41 (45%) | 26 (55%) |
| Race | American Indian or Alaskan Native | 2 (1%) | 2 (2%) | 0 (0%) |
| Asian | 4 (3%) | 2 (2%) | 2 (4%) | |
| Black or African American | 24 (17%) | 16 (18%) | 8 (17%) | |
| White | 105 (76%) | 69 (76%) | 36 (77%) | |
| Other | 3 (2%) | 2 (2%) | 1 (2%) | |
| Ethnicity | Hispanic or Latino | 10 (7%) | 7 (8%) | 3 (6%) |
| Pregnant? | Yes | 1 (1%) | 1 (2%) | 0 (0%) |
| Influenza vaccination in the current season? | No | 71 (51%) | 48(53%) | 23 (49%) |
| Days of symptoms prior to randomization | Median (Q1, Q3) | 3 (2, 4) | 3 (2, 5) | 3 (2, 4) |
| Took any antivirals prior to randomization? | Yes | 108 (78%) | 69 (76%) | 39 (83%) |
| If yes, antiviral used | Oseltamivir | 105 (97%) | 67 (97%) | 38 (97%) |
| Influenza subtype (central lab testing) | Influenza A / H1N1 | 35 (25%) | 25 (27%) | 10 (21%) |
| Influenza A / H3N2 | 89 (64%) | 56 (62%) | 33 (70%) | |
| Indeterminate | 4 (3%) | 3 (3%) | 1 (2%) | |
| Not detected | 1 (1%) | 0 (0%) | 1 (2%) | |
| Not done | 9 (7%) | 7 (8%) | 2 (4%) | |
| Viral shedding (log10 copies/mL) | Median (Q1, Q3) | 4.3 (3.4,5.6) | 4.2 (3.3,5.4) | 4.6 (3.5,5.7) |
| Days from hospitalization to randomization | Median (Q1, Q3) | 2 (2, 3) | 2 (2, 3) | 2 (2, 3) |
| Clinical status | In the ICU | 60 (43%) | 40 (44%) | 20 (43%) |
| Hospitalized, requiring oxygen | 55 (40%) | 37 (41%) | 18 (38%) | |
| Hospitalized, not requiring oxygen | 23 (17%) | 14 (15%) | 9 (19%) | |
| Mechanical ventilation | Yes | 39 (28%) | 25 (27%) | 14 (30%) |
| ECMO | Yes | 6 (4%) | 5 (5%) | 1 (2%) |
| ARDS | Yes | 18 (13%) | 15 (16%) | 3 (6%) |
| NEW score (N=123 adults) | Median (Q1, Q3) | 5 (4, 7) | 5 (4, 8) | 5 (3, 7) |
| SOFA score (N=121 adults) | Median (Q1, Q3) | 3 (2, 6) | 3.0 (1.5, 6.0) | 3 (2, 7) |
| PEW score (N=13 children) | Median (Q1, Q3) | 8 (5, 12) | 9.0 (5.0, 11.5) | 8 (4, 13) |
| PELOD score (N=12 children) | Median (Q1, Q3) | 0.5 (0.0, 3.0) | 0 (0, 1) | 3.0 (1.5, 12.0) |
At baseline, 60 (43%) of participants were in the ICU, and 55 of 78 (71%) of those not in the ICU required oxygen. For adults, the median NEW score was 5, and the median Sequential Organ Failure Assessment (SOFA)14 score was 3. For children, the median PEW score was 8, and median Paediatric Logistic Organ Dysfunction (PELOD)15 score was 0.5. These baseline characteristics were not notably different between the two treatment arms.
One hundred twenty-nine of 138 (93%) of participants randomised to the plasma received the full planned treatment (87 or 91 (96%) for high-titre and 42 of 47 (89%) for low-titre). The plasma infusion was started in a median of 2.2 hours following randomization. The infused plasma for the high-titre arm had a median HAI to H1N1 of 1:160–1:320 (depending on tested strain), and 1:160–1:640 for H3N2. All of the low titre plasma units had a HAI to both strains of 1:10 or lower. During the study, 129 of 138 (93%) of participants had documented concomitant antiviral use, primarily oseltamivir (Supplemental Table 3).
The clinical status on Day 7 as measured on a 6-step ordinal scale is presented in Table 2. A total of 50 participants (55%) receiving high-titre plasma were no longer hospitalized, compared to 22 (47%) of those receiving low-titre plasma. From the proportional odds model applied to the ordinal scale, the proportional odds ratio (POR) was 1.22 (95% CI [0.65, 2.29], p=0.54). Sensitivity analysis using the last observation carried forward for the one participant with a missing status at Day 7, and comprising all randomised participants which included the two who did not receive study plasma, show similar results to the primary analysis (POR 1.25 and 1.22 respectively).
Table 2:
Clinical status on Day 7
| Randomized treatment | ||||
|---|---|---|---|---|
| Total (N=138) | High titre | Low titre | ||
| Clinical Status at Day 7 | N | 137 | 91 | 46 |
| Death | 4 (3%) | 2 (2%) | 2 (4%) | |
| In the ICU | 25 (18%) | 15 (16%) | 10 (22%) | |
| Non-ICU hospitalization, requiring supplemental oxygen | 23 (17%) | 16 (18%) | 7 (15%) | |
| Non-ICU hospitalization, not requiring supplemental oxygen | 13 (9%) | 8 (9%) | 5 (11%) | |
| Not hospitalized, but unable to resume normal activity | 41 (30%) | 30 (33%) | 11 (24%) | |
| Not hospitalized with full resumption of normal activity | 31 (23%) | 20 (22%) | 11 (24%) | |
Odds ratio 1.22. 95% CI [0.65, 2.29], p-value 0.54
An odds ratio of greater than one is indicative of improved clinical status for the high titer group versus the low titer group.
The POR for the primary ordinal outcome was determined for patients divided into subgroups according to characteristics at study entry (Table 3). Findings for most subgroups, including duration of symptoms prior to treatment, were consistent with the overall findings. The more severe population (as demonstrated by either ICU care, or higher NEW/PEW score) have slightly higher POR, but the p-value for interactions suggest these subgroup differences are not statistically significant. The POR was larger for H1N1 than H3N2, though neither of these subgroups are statistically significant.
Table 3:
Primary Endpoint by Demographic, Severity of Disease, and Virologic Subgroups
| Subgroup | N | Percentage in Group | Proportional Odds Ratio [95% CI] | p-value | P-value for treatment by subgroup interaction* |
|---|---|---|---|---|---|
| Age (category) | |||||
| Adult | 125 | 90.6 | 1.09 [0.56, 2.12] | 0.8 | 0.41 |
| Children | 13 | 9.4 | 2.64 [0.36, 19.38] | 0.34 | |
| Gender | |||||
| Male | 71 | 51.4 | 1.48 [0.60, 3.66] | 0.4 | 0.59 |
| Female | 67 | 48.6 | 1.04 [0.43, 2.52] | 0.93 | |
| Race | |||||
| White | 105 | 76.1 | 1.15 [0.56, 2.35] | 0.7 | 0.75 |
| Non-white | 33 | 23.9 | 1.47 [0.39, 5.53] | 0.57 | |
| Clinical Status at Day 0 | |||||
| Non-ICU no supplemental oxygen | 23 | 16.7 | 1.22 [0.23, 6.35] | 0.82 | 0.9 |
| Non-ICU with supplemental oxygen | 55 | 39.8 | 1.30 [0.46, 3.70] | 0.62 | |
| In the ICU | 60 | 43.5 | 1.74 [0.66, 4.62] | 0.27 | |
| Baseline NEW/PEW Score | |||||
| Below Median | 76 | 55.9 | 1.27 [0.54, 2.96] | 0.59 | 0.21 |
| Above Median | 60 | 44.1 | 3.01 [1.03, 8.81] | 0.045 | |
| Duration of Symptoms | |||||
| ≤ 4 days | 109 | 79.0 | 1.46 [0.72, 2.93] | 0.29 | 0.88 |
| > 4 days | 29 | 21.0 | 1.27 [0.25, 6.35] | 0.77 | |
| Subgroup | |||||
| A/H1N1 | 35 | 28.2 | 3.63 [0.96, 13.71] | 0.058 | 0.12 |
| A/H3N2 | 89 | 71.8 | 1.07 [0.49, 2.33] | 0.86 |
the interaction p-values shown are not adjusted for multiple testing
Evaluating the clinical status by day demonstrated a POR slightly less than 1 on Day 1 & 2 (POR 0.86 and 0.95 respectively), and then above 1 with the POR at Day 28 similar to the Day 7 findings (Table 4). There was no significant benefit of high titre plasma on any of the study days evaluated, or in the repeated assessments analysis over time.
Table 4:
The Clinical Status at Each Study Day it was Assessed
| Study Day | Randomized treatment | N | Death | In the ICU | Non-ICU hospitalization, requiring supplemental oxygen | Non-ICU hospitalization, not requiring supplemental oxygen | Not hospitalized, but unable to resume normal activity | Not hospitalized, with full resumption of normal activity | Missing | Odds ratio ** | 95% CI | p-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | High titre | 91 | 40 (44%) | 37 (41%) | 14 (15%) | |||||||
| Low titre | 47 | 20 (43%) | 18 (38%) | 9 (19%) | ||||||||
| 1 | High titre | 91 | 38 (42%) | 30 (33%) | 21 (23%) | 2 (2%) | 0 | 0.86 | [0.45, 1.66] | 0.66 | ||
| Low titre | 46 | 18 (39%) | 14 (30%) | 14 (30%) | 0 (0%) | 1 | ||||||
| 2 | High titre | 91 | 1 (1%) | 35 (38%) | 24 (26%) | 19 (21%) | 6 (7%) | 6 (7%) | 0 | 0.95 | [0.50, 1.80] | 0.87 |
| Low titre | 46 | 0 (0%) | 17 (37%) | 12 (26%) | 13 (28%) | 3 (7%) | 1 (2%) | 1 | ||||
| 3 | High titre | 90 | 1 (1%) | 29 (32%) | 20 (22%) | 17 (19%) | 11 (12%) | 12 (13%) | 1 | 1.26 | [0.65, 2.41] | 0.49 |
| Low titre | 43 | 0 (0%) | 15 (35%) | 10 (23%) | 12 (28%) | 4 (9%) | 2 (5%) | 4 | ||||
| 7 | High titre | 91 | 2 (2%) | 15 (16%) | 16 (18%) | 8 (9%) | 30 (33%) | 20 (22%) | 0 | 1.22 | [0.65, 2.29] | 0.54 |
| Low titre | 46 | 2 (4%) | 10 (22%) | 7 (15%) | 5 (11%) | 11 (24%) | 11 (24%) | 1 | ||||
| 14 | High titre | 86 | 4 (5%) | 10 (12%) | 3 (3%) | 6 (7%) | 26 (30%) | 37 (43%) | 5 | 1.07 | [0.55, 2.08] | 0.83 |
| Low titre | 45 | 4 (9%) | 6 (13%) | 2 (4%) | 1 (2%) | 12 (27%) | 20 (44%) | 2 | ||||
| 28 | High titre | 88 | 6 (7%) | 5 (6%) | 0 (0%) | 2 (2%) | 24 (27%) | 51 (58%) | 3 | 1.29 | [0.65, 2.56] | 0.47 |
| Low titre | 45 | 4 (9%) | 3 (7%) | 2 (4%) | 1 (2%) | 11 (24%) | 24 (53%) | 2 |
Proportional odds model
NOTE: an odds ratio of greater than one is indicative of improved clinical status for the high-titre group versus the low-titre group
Other measures of clinical support were also evaluated, including duration of initial hospitalization, duration of initial ICU admission (among those in the ICU at randomization), and duration of mechanical ventilation (among those on mechanical ventilation at randomization). While there were smaller durations for many of these parameters among those receiving high-titre plasma, none of these were statistically significant. (Table 5)
Table 5:
Duration of Hospitalisation, ICU, Mechanical Ventilation, ECMO, and ARDS Among Those Requiring the Support at Randomisation, and Escalation of Clinical Support Measures During the Study.
| High-titre (n=91) | Low-titre (n=47) | p value | |
|---|---|---|---|
| Duration of initial in-hospital treatment (days) | |||
| Patients receiving intervention at randomisation (n) | 91 | 47 | ·· |
| Median (IQR) | 5 (3–12) | 6 (4–12) | 0·30 |
| Duration of initial intensive care treatment (days) | |||
| Patients receiving intervention at randomisation (n) | 40 | 20 | ·· |
| Median (IQR) | 5·0 (3·0–12·5) | 8 (4–25) | 0·32 |
| Duration of initial mechanical ventilation (days) | |||
| Patients receiving intervention at randomisation (n) | 25 | 14 | ·· |
| Median (IQR) | 9 (4–16) | 15·5 (7·0–29·0) | 0·22 |
| Duration of extracorporeal membrane oxygenation (days)* | |||
| Patients receiving intervention at randomisation (n) | 5 | 1 | ·· |
| Median (IQR) | 16 (14–18) | 20 (20–20) | ·· |
| Duration of acute respiratory distress syndrome (days)* | |||
| Patients with condition at randomisation (n) | 15 | 3 | ·· |
| Median (IQR) | 9 (4–15) | 8 (4–29) | ·· |
p values were calculated with Wilcoxon’s test.
Estimates from Wilcoxon’s test are not provided because of the small population size of the low-titre group.
Day 3 OP swabs were obtained from 125 of 138 participants. Twenty (24%) of those in the high-titre arm had undetectable virus on Day 3 compared to 5 of 47 (13%) who received low-titre plasma (OR 0.47 [0.13, 1.43], p=0.23; Supplemental Table 2).
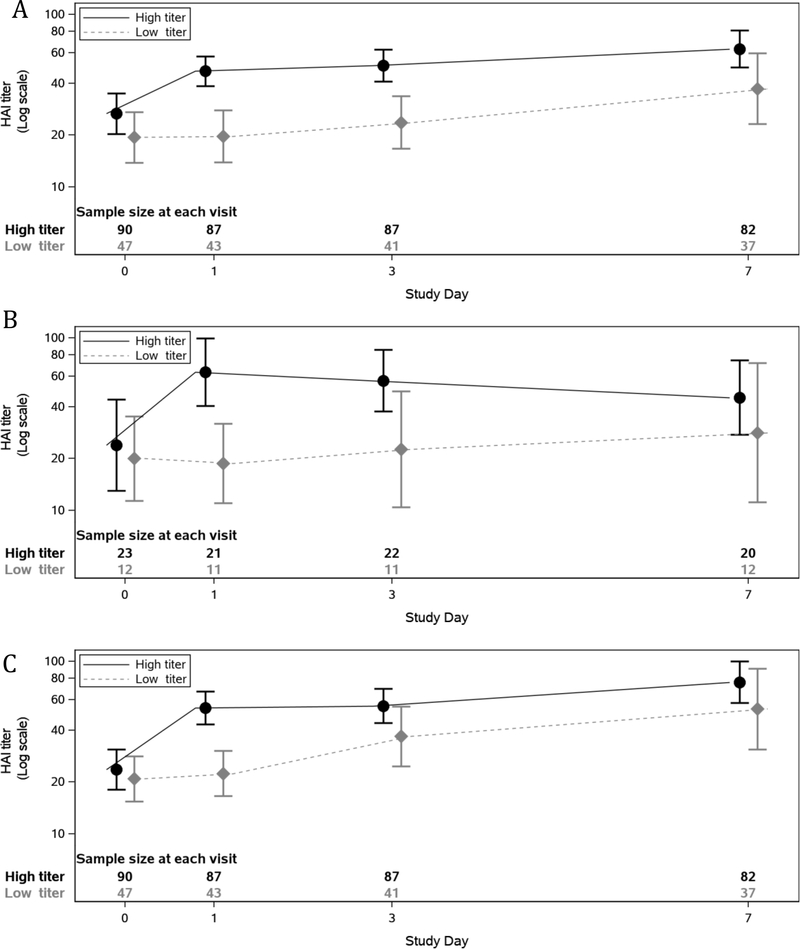
The HAI titre achieved by infused plasma cannot be determined separately from the participant’s pre-existing immunity and immune response. For H1N1 2009, which was a strain in circulation throughout the study, the geometric mean HAI titre in participants at baseline was 1:26.7 vs. 1:19.4 (high-titre versus low-titre), 1:46.9 vs. 1:19.7 at Day 1, and by Day 7 it was 1:63.1 vs. 1:37.1 (Figure 2A). Similar results were seen for H3N2 titres (Figures 2B and 2C).
Figure 2.
HAI titres over time by randomised treatment. (In dilutions (1:x); boxes span from the lower limit of the 95% CI to upper limit of the 95% CI)
A) influenza A/California/7/2009 H1N1
B) influenza A/Switzerland/9715293/2013 H3N2
C) influenza A/Hong Kong/4801/2014 H3N2
Ten participants died during the study: 6 of 91 (7%) in the high-titre arm, compared to 4 of 47 (9%) among participants randomised to low-titre plasma (p = 0.73). The most common cause of death was worsening of the ARDS (4 participants, 2 in each arm). Three additional participants were known to have died shortly after they had completed study participation (Day 28 visit), two in the high-titre arm and one in the low-titre arm. Although these deaths occurred after the final study visit, their information was recorded through the serious adverse event (SAE) reporting system.
Forty-seven of 138 (38%) participants experienced a total of 88 SAEs – 32 participants (35%) with 60 SAEs in the high-titre arm, and 15 participants (32%) with 28 SAEs in the low-titre arm. The most common SAEs were for ARDS (affecting 4 participants (4%) vs 2 (4%)), allergic transfusion reactions (2 (2%) vs 2 (4%)), and respiratory distress (3 (3%) vs 0 (0%)). All other SAEs occurred in 2 or fewer participants (Supplemental Table 4). Given the severity of the underlying illness, the protocol required reporting of all grade 3 or grade 4 AEs, and all SAEs, as well as any AE leading to plasma discontinuation (regardless of grade). Sixty-five of 138 (47%) participants experienced a total of 183 adverse events (capturing only grade 3 or grade 4 AEs, and any AE leading to plasma discontinuation regardless of grade) - 42 participants (46%) with 126 adverse events in the high-titre arm, and 23 participants (49%) with 57 adverse events in the low-titre arm. The most common AEs were anaemia (affecting 4 participants (3%) vs (2 (4%)) and ARDS (4 (3%) vs 3 (5%)). All other AEs occurred in less than 2% of the population. (Supplemental Table 5).
Discussion
Over the past 100 years, polyclonal antibody-based therapeutics have often been considered for use in treating severe infectious diseases for which few other therapeutic options were available. In the setting of a rapidly spreading epidemic, convalescent plasma therapy can be generated rapidly and used to provide specific passive immunity against a particular pathogen, while a more conventional response is implemented, if available. This approach has been used with influenza when clinicians have recognized severe disease, from the emergence of H1N1 in 19183, to avian H5N1 in 2006 16, pandemic H1N1 in 2009,4 and H7N9 in 2015.17 Despite the frequent invocation of this therapeutic strategy, no studies have been adequately designed to assess the efficacy of plasma treatment in severe influenza. Even our previous randomised trial with high-titre immune plasma in severe influenza7, while suggestive of benefit, was limited by the unblinded study design. This trial failed to meet its primary endpoint, demonstrating that high-titre immune plasma provided a proportional odds ratio of 1.22 (95% CI [0.65, 2.29], p=0.54) compared to low-titre plasma when measured by an ordinal scale of clinical outcomes on Day 7. To our knowledge, this is the first randomised blinded controlled trial of immune plasma for the treatment of severe influenza.
Despite encouraging results from prior studies, this study was unable to demonstrate a favourable treatment benefit from high-titre immune plasma. The expected benefits of a therapeutic must outweigh its potential risks to patients18, and its costs. A larger benefit would need to be established to justify the risks and costs of using immune plasma as a therapeutic, and the modest POR of 1.22 observed in this study is likely not sufficient to justify the continued development of immune plasma for treating severe influenza A. Nevertheless, it is important to recognize that the upper limit of the 95% confidence interval for the POR was 2.29 and so our study cannot rule out effects of a magnitude that could change these considerations.
The reason this intervention did not demonstrate significant efficacy is not known. It is possible this is a consequence of patients having more advanced illness, but as all participants had detectable influenza virus it is difficult to discern the contributions of viral replication and injury, and host immune response. Early antiviral therapy with a neuraminidase inhibitor is associated with improved outcome in patients hospitalized with seasonal influenza19,20, but a significant number of deaths still occur despite administration of antivirals.21 Therefore, antivirals alone may not be sufficient and strategies targeting host inflammation may be needed.22
Several secondary endpoints demonstrated a trend towards benefit with high-titre plasma treatment (e.g. duration of ICU, duration of mechanical ventilation). Given the large variability and sample size, the results were statistically insignificant. These endpoints were chosen following U.S. Food & Drug Administration guidance that notes “For seriously ill influenza patients requiring hospitalization, a primary endpoint should include clinical signs and symptoms, duration of hospitalization, time to normalization of vital signs and oxygenation, requirements for supplemental oxygen or assisted ventilation, and mortality”.10 The significant variability in these clinical parameters, however, require a large sample size to demonstrate a definitive treatment effect.
In the development of this trial, several considerations were made regarding the study design, treatment, and population. Inasmuch as the prior unblinded plasma study suffered from asymmetrical loss to follow-up7, the decision was made that this study must be blinded. Pharmacy-derived control infusion (e.g. albumin in saline) were considered. Even if the saline-albumin solution could be supplied in plasma bags with ISBT plasma labels, it would likely still not be blinding as albumin is typically a clear bright yellow and plasma is more opaque. Ultimately it was determined the only treatment that would allow full study blinding would be low-tire human plasma.
There was concern if the low-titre plasma was truly an inactive comparator. Plasma units were screened using HAI titres of 1:80 or above for high-titre units and 1:10 or below for low-titre units. The evidence for using HAI is derived from a live influenza virus challenge trial conducted in the 1970s.23 It has been argued that other markers of immunity such as neutralizing titres and anti-NA titres may better correlate with disease severity metrics.24 If both high-titre and low-titre units had similar levels of other protective factors, then no difference in the two arms would be expected. However, in a study evaluating high-titre anti-influenza immune globulin compared to saline placebo in a similar population with the same primary endpoint of clinical status by ordinal scale on Day 7, the POR was almost the same as that demonstrated in the current study (POR 1.25 (95% CI [0.79, 1.97], p=0.33) (Davey et al, also in peer review at Lancet Resp Med). Thus, using low-titre plasma as the control infusion is likely not obscuring a treatment effect with plasma that would have been revealed if we had used a saline control. Similarly, it is unlikely that non-antiviral, immunomodulation is contributing to the efficacy, as this too would have shown benefit in the IVIG study and not the current plasma study.
Lastly, there was considerable discussion defining the population for which the risk of a plasma therapeutic would be appropriate. This had to be balanced by the practical considerations and challenges of enrolling a population hospitalized with influenza, which are very difficult studies to enrol with many sites not enrolling any participants.25 We believe the NEW score of 3 or greater defined a population with physiologic aberrations and risk for poor outcomes, while still being a study than can be fully enrolled. It is possible that focusing on the more severe population may have led to different study outcomes, but we likely would not know this answer yet.
Of interest is the ordinal status by day. Ordinarily, the POR just below 1 (0.86 on Day 1, 0.95 on Day 2, 1.26 on Day 3, 1.22 on Day 7) with large p-values would not be given further consideration. However, the anti-influenza IVIG study demonstrated strikingly similar data (0.55 on Day 1, 0.78 on Day 2, 0.87 on Day 3, 1.22 on Day 4, and 1.23 on Day 7.). (Davey et al, in peer review). This raises at least the possibility that there might be mild worsening in clinical status shortly after receipt of polyclonal antibodies, converting to small benefit by Days 4–7.
The results of these two definitive polyclonal antibody trials (plasma and IVIG) are similar to many monoclonal studies. At least eight monoclonal antibodies have been tested in clinical studies.6 Some had encouraging early results, mostly from controlled human infection studies. However, many of these have stopped further clinical development due to mixed efficacy results in naturally occurring influenza.6
In conclusion, despite encouraging results from prior studies, the addition of high-titre anti-influenza immune plasma to standard care conferred a Day 7 clinical status POR of 1.22. This small statistically insignificant benefit is insufficient to justify the use of anti-influenza immune plasma for treating patients with severe influenza A.
Supplementary Material
Figure 1 –
Enrolment, Randomization and Treatment
Research in context.
Evidence before this study
We searched PubMed January 26, 2019, for studies using the search terms “plasma” and “influenza” in the title or abstract, restricting to the article type of “clinical trials”, and excluded trials that did not evaluate human plasma as the intervention. In 2009, a cohort study with convalescent plasma in the treatment of pandemic H1N1 influenza resulted in a mortality of 20% in the treatment group versus 54% the control group. However, mortality in the control group in this study was higher than expected raising the concern for bias in patient selection. In 2017, a randomised, phase 2 study of participants with severe influenza A or B (with hypoxia or tachypnoea) were assigned to either immune plasma plus standard care versus standard care alone. This small study was unable to demonstrate clear benefit in the primary endpoint of normalization of respiratory status by Day 28, but multiple secondary endpoints were suggestive of efficacy. Additionally, there were baseline imbalances that may be contributing to these perceived benefits. No previous randomised blinded studies were identified for the treatment of influenza with convalescent plasma.
Added value of this study
To our knowledge, our study is the first blinded treatment study to investigate the use of immune plasma in the treatment of severe seasonal influenza. Our findings showed that patients with influenza A treated with convalescent plasma had no statistically significant clinical benefit from treatment with the immune plasma.
Implications of all the available evidence
The evidence from this study suggest there is insufficient benefit to justify the use of immune plasma for treating patients with severe influenza A.
Acknowledgements
The study was funded and sponsored by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, Bethesda, MD. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contracts HHSN261200800001E and HHSN261201500003I. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The identification of specific products or scientific instrumentation are considered an integral part of the scientific endeavour and does not constitute endorsement or implied endorsement on the part of the author, DoD, or any component agency. The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
Funding The study was funded and sponsored by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, Bethesda, MD.
Declaration of interests
MDH report other funding from NIAID during the conduct of this study; KR reports other funding from the Department of Defense Infectious Diseases Clinical Research Program during the conduct of the study; all authors declare no competing interests.
Footnotes
Data sharing: The data collected for the study, including individual deidentified participant data, will be made available to other researchers upon reasonable request, beginning at the time of publication. Related documents (study protocol, statistical analysis plan, informed consent form) are also available. Requests for access to data should be addressed to the corresponding author, and will require a signed data sharing agreement.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John H Beigel, National Institute of Allergy and Infectious Diseases, Bethesda, MD.
Evgenia Aga, Harvard T.H. Chan School of Public Health, Boston, MA.
Marie-Carmelle Elie-Turenne, University of Florida, Gainesville, FL.
Josalyn Cho, Massachusetts General Hospital, Boston, MA.
Pablo Tebas, University of Pennsylvania, Philadelphia, PA.
Carol L Clark, Beaumont Hospital - Royal Oak, Royal Oak, MI.
Jordan P. Metcalf, University of Oklahoma Health Sciences Center, Oklahoma City, OK
Caroline Ozment, Duke University Medical Center, Durham, NC.
Kanakatte Raviprakash, Naval Medical Research Center, Silver Spring, MD.
Joy Beeler, Leidos Biomedical Research, Frederick, MD.
H. Preston Holley, Jr., Leidos Biomedical Research, Frederick, MD
Stephanie Warner, Social & Scientific Systems, Inc., Silver Spring, MD.
Carla Chorley, Leidos Biomedical Research, Frederick, MD.
H. Clifford Lane, National Institute of Allergy and Infectious Diseases, Bethesda, MD.
Michael D. Hughes, Harvard T.H. Chan School of Public Health, Boston, MA
Richard T Davey, National Institute of Allergy and Infectious Diseases, Bethesda, MD.
References
- 1.Lu J, Guo Z, Pan X, et al. Passive immunotherapy for influenza A H5N1 virus infection with equine hyperimmune globulin F(ab’ )2 in mice. Respir Res 2006; 7(1): 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanson BJ, Boon ACM, Lim APC, Webb A, Ooi EE, Webby RJ. Passive immunoprophylaxis and therapy with humanized monoclonal antibody specific for influenza A H5 hemagglutinin in mice. Respir Res 2006; 7: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med 2006; 145(8): 599–609. [DOI] [PubMed] [Google Scholar]
- 4.Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis 2011; 52(4): 447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duggal A, Pinto R, Rubenfeld G, Fowler RA. Global Variability in Reported Mortality for Critical Illness during the 2009–10 Influenza A(H1N1) Pandemic: A Systematic Review and Meta-Regression to Guide Reporting of Outcomes during Disease Outbreaks. PLoS One 2016; 11(5): e0155044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beigel JH. Polyclonal and monoclonal antibodies for the treatment of influenza. Curr Opin Infect Dis 2018; 31(6): 527–34. [DOI] [PubMed] [Google Scholar]
- 7.Beigel JH, Tebas P, Elie-Turenne MC, et al. Immune plasma for the treatment of severe influenza: an open-label, multicentre, phase 2 randomised study. Lancet Respir Med 2017; 5(6): 500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Royal College of Physicians of London. National early warning score (NEWS): standardising the assessment of acute-illness severity in the NHS—report of a working party. 2012. 2018. (accessed December 3 2018).
- 9.Duncan H, Hutchison J, Parshuram CS. The Pediatric Early Warning System score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care 2006; 21(3): 271–8. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food & Drug Administration. Guidance for Industry - Influenza: Developing Drugs for Treatment and/or Prophylaxis 2011.
- 11.Shu B, Wu KH, Emery S, et al. Design and performance of the CDC real-time reverse transcriptase PCR swine flu panel for detection of 2009 A (H1N1) pandemic influenza virus. J Clin Microbiol 2011; 49(7): 2614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control - 510(k) Summary for Centers for Disease Control and Prevention human influenza virus real-time RT-PCR detection and characterization panel.. 2008. http://www.accessdata.fda.gov/cdrh_docs/pdf8/k080570.pdf (accessed December 26, 2018 2018).
- 13.World Health Organization - Manual for the laboratory diagnosis and virological surveillance of influenza.. Geneva: World Health Organization; 2011. [Google Scholar]
- 14.Jones AE, Trzeciak S, Kline JA. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med 2009; 37(5): 1649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet 2003; 362(9379): 192–7. [DOI] [PubMed] [Google Scholar]
- 16.Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med 2007; 357(14): 1450–1. [DOI] [PubMed] [Google Scholar]
- 17.Wu XX, Gao HN, Wu HB, Peng XM, Ou HL, Li LJ. Successful treatment of avian-origin influenza A (H7N9) infection using convalescent plasma. Int J Infect Dis 2015; 41: 3–5. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Food & Drug Administration. Benefit-Risk Assessment in Drug Regulatory Decision-Making. 2018. https://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM602885.pdf (accessed April 16 2019).
- 19.Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis 2009; 200(4): 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee N, Choi KW, Chan PK, et al. Outcomes of adults hospitalised with severe influenza. Thorax 2010; 65(6): 510–5. [DOI] [PubMed] [Google Scholar]
- 21.Louie JK, Yang S, Acosta M, et al. Treatment with neuraminidase inhibitors for critically ill patients with influenza A (H1N1)pdm09. Clin Infect Dis 2012; 55(9): 1198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hui DS, Lee N, Chan PK, Beigel JH. The role of adjuvant immunomodulatory agents for treatment of severe influenza. Antiviral Res 2018; 150: 202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972; 70(4): 767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Memoli MJ, Shaw PA, Han A, et al. Evaluation of Antihemagglutinin and Antineuraminidase Antibodies as Correlates of Protection in an Influenza A/H1N1 Virus Healthy Human Challenge Model. MBio 2016; 7(2): e00417–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beigel JH, Nam HH, Adams PL, et al. Advances in respiratory virus therapeutics - A meeting report from the 6th isirv Antiviral Group conference. Antiviral Res 2019; 167: 45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.