Abstract
UV radiation (UVR) causing DNA damage is a well-documented risk factor for nonmelanoma skin cancer. Although poorly understood, UVR may also indirectly contribute to carcinogenesis by promoting immune evasion. To our knowledge, we report the first epidemiological study designed to investigate the association between quantitative measures of UVR, obtained using a spectrophotometer, and circulating T regulatory (Treg) cells. In addition to total Treg cells, the proportion of functionally distinct Treg cell subsets defined by CD45RA and CD27 phenotypic markers, graded expression of FOXP3 and CD25, and those expressing cutaneous lymphocyte–associated Ag and the chemokine receptor CCR4 were enumerated in 350 individuals undergoing routine skin cancer screening exams and determined not to have prevalent skin cancer. No associations were identified for UVR exposure or the overall proportion of circulating Treg cells; however, Treg cell subpopulations with an activation-associated phenotype, CD45RA−/CD27−, and those expressing cutaneous homing receptors were significantly positively associated with UVR. These subpopulations of Treg cells also differed by age, sex, and race. After stratification by natural skin tone, and adjusting for age and sex, we found that spectrophotometer-based measures of UVR exposure, but not self-reported measures of past sun exposure, were positively correlated with the highest levels of these Treg cell subpopulations, particularly among lighter-skinned individuals. Findings from this large epidemiologic study highlight the diversity of human Treg cell subpopulations associated with UVR, thus raising questions about the specific coordinated expression of CD45RA, CD27, CCR4, and cutaneous lymphocyte–associated Ag on Treg cells and the possibility that UVR contributes to nonmelanoma skin cancer carcinogenesis through Treg cell–mediated immune evasion.
Ultraviolet radiation (UVR) is an environmental factor that contributes to the development of nonmelanoma skin cancer (NMSC), one of the most frequently diagnosed cancers in the United States (1, 2). The two most common types of NMSC, squamous cell carcinoma and basal cell carcinoma, occur most often on areas of sun-exposed skin (2, 3). UVR is involved in several stages of carcinogenesis (1), including induction of DNA damage, and possibly through immune suppression, enabling malignant cells to grow unchecked by T cells or other immune population(s). Although the exact mechanism of the latter is not well understood, immune suppression associated with skin cancer is marked by both a reduction in conventional T cell functions (4, 5) independent of, and as a consequence of, T regulatory (Treg) cells (as reviewed in Ref. 6).
Treg cells, characterized by the expression of the transcription factor FOXP3, CD4, and the IL-2 receptor α-chain (CD25), are expanded systemically and within the tumor of various cancers, where they uniformly have negative prognostic significance (7-9). Differentiation markers on Treg cells have been studied in humans with autoimmune disease, viral infection (10-13), and cancer and include the protein8 tyrosine phosphatase (encoded by the PTPRC gene) CD45RA, CD62L (L-selectin), and CD27. Although the coordinated differentiation of conventional T cells in humans, and Treg cells in mice, have been well delineated, the differentiation path for Treg cells in humans is less well defined (as reviewed in Ref. 14). Both CD45RA and CD27, a costimulatory molecule involved in activation and memory development, have the potential to distinguish functionally distinct Treg cell subsets (15-17). All of these markers are expressed on naive, resting T cells and medullary thymocytes but are downregulated after TCR activation (18). Patterns of chemokine receptors are also useful in distinguishing functional Treg cell populations that exhibit directional localization within inflammatory environments, including the skin (19).
In mice, the frequency of neuropilin-1+, thymic-derived, natural Treg cells increased following exposure to low doses of UVB radiation in the absence of tumors (20). UVR-induced expansion of Treg cells is mediated by Ag activation (21), which, under specified conditions, enables their suppressive mechanisms and triggers tissue-homing to the skin (22, 23) (as reviewed in Ref. 20). Ag activation of Treg cells occurs through self-antigens and, in some tissues, the microbiome (24). The coordination of UVR exposure and Treg cell expansion suggests that both may contribute to tumor growth in keratinocyte carcinogenesis.
Functionally distinct Treg cell subpopulations characterized by specific phenotypic surface markers have been studied in various disease settings (11, 13, 25). Thymic-derived Treg cells expressing CD45RA decline with age in mice (26) during chronic viral infections (13) and following organ transplantation rejection (11). We found previously that CD45RA−/CD27− Treg cells were expanded prior to disease progression and were specifically associated with poor survival in myelodysplastic syndrome (25). Although the CD45RA−/CD27− Treg cell subset is more suppressive compared with CD45RA−/CD27+ Treg cell subtypes on an individual cell basis, Treg cell population dynamics in the context of UVR, age, sex, and race are poorly characterized (14, 25).
Epidemiological studies have reported associations between prevalence of chronic autoimmune diseases such as multiple sclerosis, lupus erythematosus, and rheumatoid arthritis and distance from the equator, thereby indicating a plausible role for UVR exposure in immune function (27-30). Other studies have used UVR as a treatment for multiple sclerosis and psoriasis and have reported decreased immune function as a result of UVR photochemical therapy, primarily by inducing Treg cells within the lymph nodes, followed by altering their skin migratory behavior (31-33). Among patients with psoriasis, dysfunction in circulating Treg cell populations was restored after treatment with photochemical therapy, suggesting an increase in immunosuppressive activity of Treg cells as a result of UVR (34).
To our knowledge, this is the first epidemiological study to investigate the association between UVR and immune response mediated by Treg cells among a cohort of individuals undergoing routine skin cancer screening exams using a quantitative, spectrophotometer-based measure of UVR. Circulating Treg cells were characterized using flow cytometry of cells in the peripheral blood, as defined previously on the basis of FOXP3, CD4, and CD25 (25, 35). The percent of total circulating conventional T cells and Treg cells was quantified (in addition to specific Treg cell subpopulations), defined by the expression of CD45RA and CD27 markers (11, 13, 25) and markers associated with bidirectional trafficking between lymph node and skin (36, 37) [including cutaneous lymphocyte–associated Ag (CLA) and the chemokine receptor CCR4 (11, 13, 22, 25, 33, 36, 38)], and graded expression levels of FOXP3 (35), as described previously. We hypothesized that UVR would be associated with higher levels of a circulating Treg cell population or a higher number of total Treg cells in this cohort.
Materials and Methods
Study design and population
Cross-sectional, baseline data from participants enrolled in the first year of the Viruses and Skin Cancer Study, an ongoing, 4-y prospective cohort study of UVR, cutaneous viral infections, and skin cancer being conducted at the Moffitt Cancer Center, were used for the current analysis. Individuals undergoing routine skin cancer screening exams at the University of South Florida Dermatology Clinic were eligible for the study if they were at least 60 y of age and had not had both squamous cell carcinoma and basal cell carcinoma previously. At the time of study enrollment, participants underwent a total-body skin examination, and suspicious lesions were biopsied as a part of routine clinical care. Study participants with a pathologically confirmed NMSC at baseline were excluded. Questionnaire data, peripheral blood samples, and skin pigmentation measurements were also required at study entry. Individuals that failed to complete these study-related activities were excluded from the analysis. The study was approved by the University of South Florida Institutional Review Board, and all patients provided written informed consent at the time of enrollment.
Spectrophotometer data collection
Skin pigmentation readings were obtained using a spectrophotometer (CM-600D; Konica Minolta Sensing Americas) with SpectraMagic NX Lite USB Ver. 2.5 software, using the specular component included mode of the instrument, which minimizes the influence of the gloss and texture of the skin-on-skin pigmentation readings, as described previously (39, 40). The instrument was calibrated against a white tile each morning, per manufacturer guidelines. The spectrophotometer readings were obtained by study personnel at the initial study visit when blood samples were collected. This instrument measures color on three different axes: lightness on a scale of 0 (black) to 100 (white), axis a, indicating color within the red through green range, and axis b, measuring color within the yellow through blue range, with increasing values on a- and b-axes, indicating saturation of color (41). Natural skin tone was assessed using spectrophotometer readings of the sun-unexposed underside of the upper arm (i.e., the axilla). The degree of recent tanning in response to recent UVR exposure was measured by calculating the difference between the color readings (ΔE × ab) on an area of sun-exposed skin (top of the forearm or forehead) and the axilla. Three readings were obtained at each anatomic site, and the average values were recorded. A strong, intraindividual correlation was observed between spectrophotometer readings obtained at the forearm and forehead (r = 0.6079; p < 2.2 × 10−16) (Supplemental Fig. 1). Therefore, the average of these two values was used as the single measurement of recent UVR exposure.
Blood collection and flow cytometry data acquisition and analysis
Blood samples were collected in three 10-ml heparin sodium–containing blood collection tubes. Samples were processed for isolation of PBMCs by Ficoll–Hypaque centrifugation using 10–20 ml of Lymphocyte Separation Media (Ficoll), according to manufacturer guidelines (Amersham Pharma Biotech, Piscataway, NJ). Freezing medium (10% DMSO and 90% FBS) was used to viably freeze PBMCs in preparation of the future cytometry analysis. Frozen PBMCs stored in liquid nitrogen were thawed on ice and placed into 1 ml PBS. Approximately 106 PBMCs were first labeled with the viability dye Ghost Dye Red 780 (1 μl; Tonbo Bioscience) for 20–30 min at room temperature to exclude dead cells from the analysis. PBMCs were then treated with 1 μl FcR Blocking Reagent (Miltenyi Biotec) and labeled with Abs to detect the following cell surface Ags: anti-CD3/allophycocyanin, anti-CD45RA/PerCPCy5.5 (Tonbo Bioscience), anti-CD4/BV785, anti-CCR4/BV421, anti-CLA/FITC, PD-1/PE (BioLegend), anti-CD25/BUV395 (BD Horizon), and anti-CD27/BUV737 (BD Horizon), using 1 μl each and for 30 min at 4°C. Cells were fixed with a Foxp3/Transcription Factor Staining Buffer Set (Thermo Fisher Scientific) according to the manufacturer’s protocol and labeled with 5 μl PE/Dazzle 594 anti-human FOXP3 Antibody (BioLegend) for 30 min.
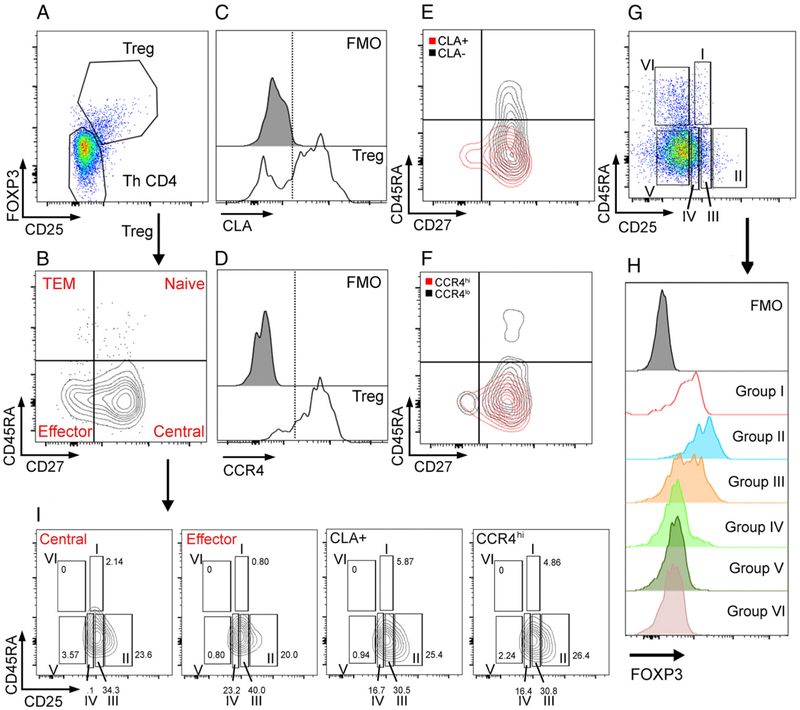
After staining and fixation, cell populations were analyzed within 24 h on a BD LSR II flow cytometry instrument (BD Biosciences) using the gating strategy defined in Fig. 1 and Supplemental Fig. 2 and quantified using FlowJo v9 (FlowJo). The PBMC samples from all patients were thawed and evaluated for viability. Those with <1.0 × 105 viable lymphocytes were excluded from the final data analysis (n = 31). In samples included in the analysis, 3.1 × 105 ± 2.2 × 105 viable cells were present. Samples were evaluated for CD8+ T cells and CD4+ T cells of CD3+ lymphocytes. CD4+ T cells were further defined by CD25−FOXP3− conventional Th cells (Th CD4) and total CD25+FOXP3+ Treg cell subpopulations: CD45RA+/CD27+ (naive) Treg cells, CD45RA−/CD27− activated, effector Treg cells (i.e., equivalent to conventional T effector cells), CD45RA+/CD27− (terminal effector memory) Treg cells (i.e., equivalent to conventional T cells with an exhausted phenotype), CD45RA−/CD27+ (central) Treg cells, CLA+ (skin migratory) Treg cells, and chemokine receptor CCR4hi (skin migratory) Treg cells, as previously described (11, 13, 22, 25, 33, 36, 38). All population data are described as a frequency of the parent population. In addition to the described gating strategy, CD4+ T cells samples were also divided into six populations, according to Miyara et al. (35) (Fig. 1D), to define CD45RA−CD25++FOXP3lo as resting Treg cells (Group I), CD45RA−CD25+++FOXP3hi (Group II) as activated Treg cells, and defined CD45RA−CD25++ FOXP3lo (Group III) as nonsuppressive Treg cells.
FIGURE 1.
Flow cytometry gating strategy for Treg cell analysis. PBMCs were gated first on viability and on CD3 and CD4 positivity. (A–D) (A) CD4+ Treg cells were defined by high expression of CD25, and intracellular FOXP3 and conventional CD4+ T cells (Th CD4) were defined by low expression of CD25 and FOXP3. (B) Treg cells were then analyzed for CD45RA and CD27 expression, and populations were defined by dual or single expression. (C–F) CLA and CCR4 expression was also analyzed among Treg cells. CLA- and CCR4-positive Treg cells were also confirmed to be CD45RA negative with mixed expression of CD27. (G–I) (E) Six groups of CD4+ T cells were defined by CD45RA positivity and various CD25 expression. (H) Each population was analyzed for FOXP3 expression. (I) Distribution of these populations was analyzed in CD45RA−/CD27+ (Central) Treg cells, CD45RA−/CD27− (Effector) Treg cells, CLA+ Treg cells, and CCR4hi Treg cells as defined in (B)–(D).
All gating strategies were confirmed by use of fluorescence-minus-one control samples in which cells are stained with all colors except the Ab noted (Supplemental Fig. 2A, 2B, 2E, 2F).
Statistical methods
Correlations between Treg cells and UVR were calculated using Spearman rank, and a trend line was created to visualize the associations. The correlations between T cell populations were described using Spearman rank correlation coefficient. Additionally, given that previous studies have reported effect modification of UVR-associated physiologic effects by one’s natural skin tone (42, 43), and analysis by lighter versus darker skin tone was performed, based on the median value of the spectrophotometer readings of the sun-unexposed axilla, to investigate whether the associations between UVR and Treg cell phenotypes differed by natural skin tone. Linear regression was used to examine the associations between baseline characteristics and Treg cell phenotypes, adjusted for age and sex. Differences in UVR by sex and age were examined using the Wilcoxon rank sum test and Spearman correlation, respectively. Logistic regression was used to calculate odds ratios and 95% confidence intervals to estimate the associations between recent UVR exposure and quartiles of the Treg cell phenotypes, adjusted for age and sex. Quartiles were used to account for the possible nonlinear relationship between Treg cells and recent UVR and to avoid use of data transformation within the multivariate models. Statistical analysis was conducted using R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Characterization of Treg cells associated with UV exposure
During the first year of recruitment (July 2014 through July 2015), 917 individuals were approached, of whom, 448 (49%) consented to participate. No significant differences were observed between study participants and individuals who declined participation, with respect to sex (p = 0.17) or age (p = 0.16). Participants were asked to complete a website-based questionnaire, including demographic and skin cancer risk factor information. In addition, blood samples and skin pigmentation measurements were obtained from each participant. Blood samples were collected from 409 patients, of which, 378 samples were found to have sufficient numbers of viable cells available for flow cytometry. Individuals with a pathologically confirmed NMSC at baseline (n = 23) or missing skin pigmentation readings (n = 5) were excluded. Therefore, the current analysis includes 350 individuals with complete information on both Treg cells and UVR exposure.
Skin pigmentation readings were used as a marker of recent UVR exposure and for natural skin tone, consistent with previous epidemiologic studies (30, 31). Males had significantly higher spectrophotometer readings (14.61 ± 3.12) than females (9.37 ± 2.62; p < 0.0001), whereas there was no association with age (p = 0.81).
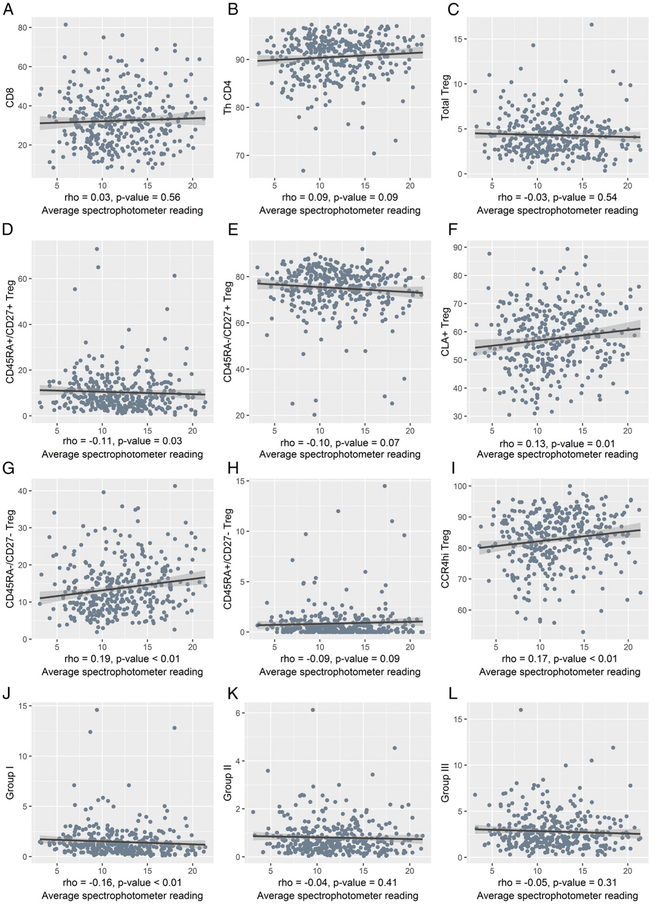
PBMC samples were analyzed by flow cytometry, as shown in Fig. 1 (25). First, CD8+ T cells and CD4+ T cells of CD3+ lymphocytes were identified, followed by CD4+ T cells that were further defined by CD25−FOXP3− conventional Th cells (Th CD4) and total CD25+FOXP3+ Treg cells. Low expression of CD127 on CD25+FOXP3+ Treg cells was confirmed, as described previously, but was not included in this analysis (data not shown) (25). Recent UVR exposure was plotted against each population of T cells (Fig. 2). The proportion of CD8 (33 ± 14.4), Th CD4 (66.1 ± 14.6), and total Treg cells (4.29 ± 2.15) were within normal range for peripheral blood of healthy individuals (25). CD8+ T cells (r = 0.03, p = 0.56), Th CD4+ T cells (r = 0.09, p = 0.09), and total percent of Treg cells among all CD4+ T cells (r = −0.03, p = 0.54) were not correlated with UV exposure (Fig. 2A-C). In addition to these populations, functionally distinct Treg cell subpopulations were examined, as defined in Fig.1B-F. Four phenotypically distinct Treg cell populations were evident by flow cytometry, with the vast majority (75.0 ± 10.5%) displaying a CD45RA−/CD27+ central memory phenotype, as described previously (25). CLA+ and CCR4hi Treg cells did not express CD45RA, consistent with prior Ag engagement, but consisted of both CD27+ and CD27− subpopulations (Fig. 1E, 1F), similar to conventional Th CD4+ T cells (Supplemental Fig. 2C, 2D). The CD45RA−/CD27− activated, effector Treg cells (Fig. 2G), CLA+ Treg cells (Fig. 2F), and CCR4hi Treg cells (Fig. 2I) were positively correlated, with recent UVR exposure among all individuals (r = 0.19, p < 0.01; r = 0.13, p = 0.01; r = 0.17, p < 0.01; respectively), whereas CD45RA+/CD27+ Treg cells (i.e., naive) showed a less-pronounced, negative correlation (r = −0.11, p = 0.03) (Fig. 2D), indicating that these specific Treg cell subsets, not total Treg cells or conventional T cells, correlated with recent UVR exposure as measured by spectrophotometer. Of note, no self-reported measures of past UVR exposures or sun-susceptibility factors were associated with these four Treg cell populations (Supplemental Table I).
FIGURE 2.
T cell populations in correlation with recent UV exposure among 350 individuals undergoing skin cancer screening. Correlations between Treg cells and UVR were calculated using Spearman rank, and a trend line was created to visualize the associations. (A–C) Scatterplot of spectrophotometer reading and CD8, Th CD4, and total Treg cells. (D–I) Scatterplot of spectrophotometer reading and phenotypes of Treg cells defined in Fig. 1A. (J–L) Scatterplot of spectrophotometer reading and Group I, II, and III Treg cell phenotypes defined in Fig. 1G.
To further explore the Treg cell populations and their association to UVR, CD4+ T cells were also segregated into six populations defined previously (Miyara et al.) using CD45RA, CD25, and levels of FOXP3 (Fig. 1G, 1H), with Groups I–III all expressing FoxP3, consistent with having a Treg cell phenotype (35). Group II (CD45RA−CD25+++FOXP3hi) and Group I (CD45RA+CD25++ FOXP3lo) are functionally suppressive, whereas Group III with a CD45RA−CD25++FOXP3lo phenotype was shown previously to be nonsuppressive, but capable of actively secreting cytokines. Although the CD45RA−/CD27−FOXP3 population showed an association to UVR exposure, no association was observed with the CD45RA− Treg cells defined as Group II or Group III (Fig. 2K, 2L, respectively). Interestingly, Group I Treg cells were negatively associated with recent UVR exposure (r = −0.16, p < 0.01) (Fig. 2J).
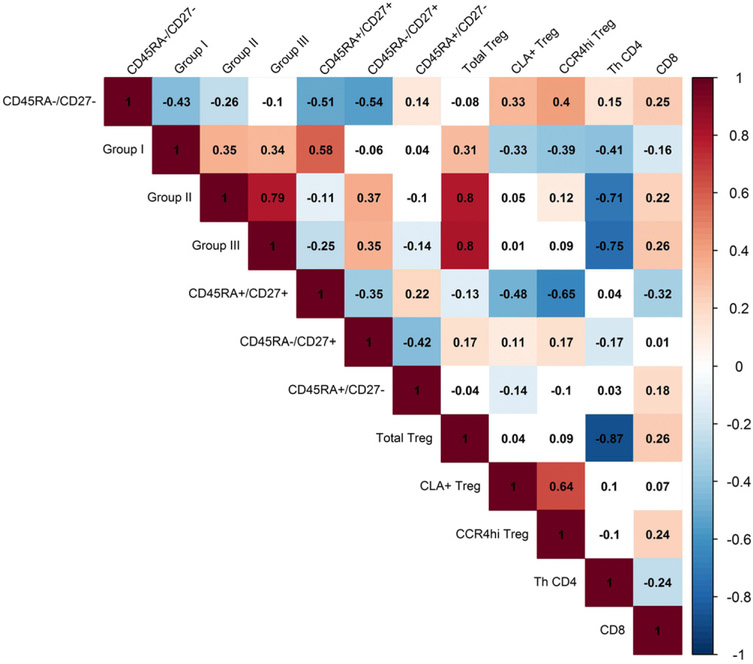
To further define the characteristics of the Treg cells and conventional T cells through association studies, a correlation matrix was prepared for all T cell populations identified. Group I and Group II Treg cell populations were highly correlated with total Treg cells, but were not associated with Treg cells defined by other surface markers, including CD27, CLA, and CCR4 (Fig. 3). Indeed, we found there to be a high positive correlation between CD45RA+/CD27+ Treg cells, naive Treg cells, and the Group I population, resting Treg cells (Fig. 3). These results are consistent with the negative association between both Group I and CD45RA+/CD27+ Treg cell populations. Moreover, overlays of the flow cytometry expression patterns reveal CD27, CLA, and CCR4 to be represented throughout Group II–IV populations (Fig. 1I), indicating that these surface markers further differentiate Treg cells. Gating on such populations within Group II–III may be necessary to observe correlations between Treg cells defined by this strategy and recent UVR exposure.
FIGURE 3.
Spearman rank correlation matrix for T cell populations among 350 skin cancer screening patients. Spearman correlation coefficients (rho) are presented for all T cell populations. The matrix cells are shaded only if they are significantly correlated at p < 0.05 level. Red shading represents a positive correlation, whereas blue shading represents a negative correlation.
Natural skin tone represents an effect modifier of the UVR and Treg cell association
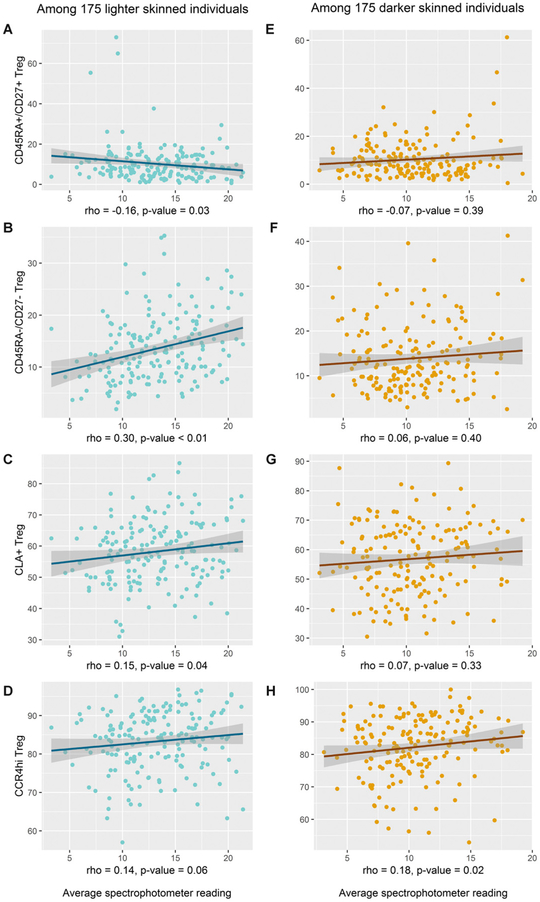
Previous studies of nevus development in children have reported effect modification of UVR-associated physiologic effects by one’s natural skin tone (42, 43). Therefore, we stratified the current analysis by lighter versus darker skin tone, based on the median value of the spectrophotometer readings of the sun-unexposed axilla, to investigate whether the associations between UVR and Treg cell phenotypes differed by natural skin tone. Interestingly, a significant positive correlation between recent UVR and the proportion of activated, effector CD45RA−/CD27− Treg cells (r = 0.30, p < 0.01) was observed only in individuals of lighter skin (Fig. 4). The CD45RA+/CD27+ naive Treg cell population was negatively correlated with recent UVR exposure in lighter-skinned individuals, although the trend was not as striking as the association with increased CD45RA−/CD27− Treg cells (Fig. 4). The positive correlation between UVR and CLA+ Treg cells was also restricted to the lighter-skinned individuals (r = 0.15, p = 0.04). However, UVR and CCR4hi Treg cells were positively correlated among both darker-skinned (r = 0.18, p = 0.02) and lighter-skinned individuals (r = 0.14, p = 0.06), with the former being statistically significant. These results suggest that individuals with a lighter natural skin tone may be at higher risk for UVR-induced changes in Treg cells.
FIGURE 4.
Effect modification by natural skin tone. (A–H) Scatterplot of spectrophotometer readings and Treg cell subpopulations that were found to have significant associations with UVR after stratification by natural skin tone as defined by median spectrophotometer readings of sun-unexposed underside of the upper arm (i.e., the axilla). Correlations between Treg cells and UVR were calculated using Spearman rank, and a trend line was created to visualize the associations.
Demographics are associated with Treg cells
Associations between demographic factors and Treg cell subpopulations, including naive CD45RA+/CD27+, activated effector CD45RA−/CD27−, CLA+, and CCR4hi Treg cells (based on results shown in Fig. 1), are presented in Table I. A statistically significant inverse trend was observed between age and naive CD45RA+/CD27+ Treg cells (p-trend < 0.01). Conversely, CD45RA−/CD27− Treg cells were positively associated with age (p-trend = 0.04). This is similar to the expected loss of conventional naive T cells because of thymic involution and shown previously for Treg cells in mice (26, 44-46). Significant positive trends were also observed between age and the two skin migratory Treg cells, CLA+ (p-trend = 0.02), and CCR4hi (p-trend = 0.01) Treg cell populations (Table I).
Table I.
Circulating Treg cell phenotypes and demographic characteristics
| Treg Cell Phenotypes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD45RA+/CD27+ Treg Cells |
CD45RA−/CD27− Treg Cells |
CLA+ Treg Cells |
CCR4hi Treg Cells |
||||||||||
| n (%) | Mean (SD) | Coef.a | p Valuea | Mean (SD) | Coef. | p Value | Mean (SD) | Coef. | p Value | Mean (SD) | Coef. | p Value | |
| Ageb | |||||||||||||
| 60–64 | 78 (22.3) | 12 (9.3) | −0.0031 | < 0.01 | 12.4 (6.9) | 0.0042 | 0.04 | 55.4 (10.8) | 0.2228 | 0.02 | 80.5 (9.1) | 383549 | 0.01 |
| 65–69 | 125 (35.7) | 10.8 (10.3) | 14.2 (7.5) | 57.4 (10.6) | 82.7 (8.4) | ||||||||
| 70–74 | 78 (22.3) | 9.8 (6.9) | 13.7 (6.4) | 58.1 (11.7) | 83.7 (8.1) | ||||||||
| 75–79 | 41 (11.7) | 9.5 (6.3) | 13.6 (6.6) | 58 (11.7) | 83.1 (8.5) | ||||||||
| 80–89 | 28 (8.0) | 6.4 (4.3) | 15.5 (6.3) | 62 (11.5) | 86 (8.2) | ||||||||
| Genderc | |||||||||||||
| Female | 187 (53.4) | 10.8 (9.1) | 13.0 (7.0) | 56.0 (11.3) | 81.2 (8.5) | ||||||||
| Male | 163 (46.6) | 9.8 (8.2) | −0.0187 | 0.15 | 14.5 (6.8) | 0.0588 | 0.02 | 59.3 (10.7) | 3.1290 | 0.01 | 84.5 (8.4) | 7335781 | <0.01 |
| Race | |||||||||||||
| White | 340 (97.1) | 10.3 (8.6) | 13.5 (6.8) | 57.8 (11.1) | 82.8 (8.4) | ||||||||
| Others | 10 (2.9) | 10.7 (10.8) | −0.0515 | 0.18 | 19.9 (9.6) | 0.2225 | <0.01 | 51.0 (11.9) | −6.1680 | 0.09 | 80.1 (13.4) | −1106525 | 0.85 |
| Ethnicity | |||||||||||||
| Non-Hispanic | 330 (94.8) | 10.4 (8.6) | 13.7 (6.9) | 57.5 (11.2) | 82.6 (8.6) | ||||||||
| Hispanic or Latino | 18 (5.2) | 10.2 (9.8) | −0.0166 | 0.57 | 14.5 (7.9) | 0.0293 | 0.61 | 59.0 (11.3) | 1.7580 | 0.51 | 84.2 (8.4) | 4063970 | 0.34 |
Bold indicates significance (p < 0.05) and italic represents statistical results.
Coefficients and p values were calculated using linear regression, adjusted for age, CD45RA− (categorical), and sex with transformed Treg cell data as follows: CD45RA+/CD27+ Treg cells (eighth root), CD45RA−/CD27− Treg cells (fourth root), CLA+ Treg cells, and CCR4hi Treg cells (fourth power).
Coefficient and p-trend were calculated using linear regression with continuous age, presented in years, and adjusted for sex, with transformed Treg cell data.
The p values were calculated using linear regression adjusted for age (categorical).
Coef, coefficient.
Interestingly, Treg cell populations differed significantly by sex after adjustment for age, including higher numbers of CD45RA−/CD27− Treg cells in males (14.5 ± 6.8 in males versus mean 13.0 ± 7.0 in females; p = 0.02), higher CLA+ Treg cells in males (59.3 ± 10.7 in males versus mean 56.0 ± 11.3 in females; p = 0.01), and higher CCR4hi Treg cells in males (84.5 ± 8.4 in males versus 82.8 ± 8.4 in females; p < 0.01). Race was also associated with CD45RA−/CD27− Treg cells (mean 13.5 ± 6.8 in white versus mean 19.9 ± 9.6 in others; p < 0.01). Ethnicity was not associated with Treg cell populations (Table I). Treg cells defined as Group I–III (Fig. 1D) were also analyzed by demographic information. No association was found among Group I–III populations with age, sex, or ethnicity. Group II populations were significantly associated with race, with white participants having higher numbers (Table II). This finding is consistent with the significant racial difference observed for the distinct Treg cell subpopulation defined by CD45RA−/CD27− expression, which was strongly negatively correlated with age, independent of Group I–III distribution (Fig. 3).
Table II.
Circulating Treg cell phenotypes and demographic characteristics
| Group I |
Group II |
Group III |
|||||
|---|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | p Value | Mean (SD) | p Value | Mean (SD) | p Value | |
| Agea,b | |||||||
| 60–64 | 78 (22.3) | 1.6 (2) | 0.10 | 0.7 (0.4) | 0.44 | 2.6 (1.5) | 0.62 |
| 65–69 | 125 (35.7) | 1.5 (1.6) | 0.9 (0.8) | 3.1 (2.4) | |||
| 70–74 | 78 (22.3) | 1.5 (1.4) | 0.7 (0.6) | 2.5 (1.4) | |||
| 75–79 | 41 (11.7) | 1.5 (1.1) | 0.9 (1) | 3 (1.9) | |||
| 80–89 | 28 (8.0) | 0.9 (0.6) | 0.8 (0.8) | 2.6 (1.7) | |||
| Genderb,c | |||||||
| Female | 187 (53.4) | 1.5 (1.7) | 0.7 (0.6) | 2.8 (2) | |||
| Male | 163 (46.6) | 1.4 (1.4) | 0.06 | 0.9 (0.8) | 0.15 | 2.9 (1.9) | 0.46 |
| Race | |||||||
| White | 340 (97.1) | 1.5 (1.6) | 0.8 (0.7) | 2.8 (2) | |||
| Others | 10 (2.9) | 1.3 (1.1) | 0.22 | 0.4 (0.2) | 0.04 | 2.4 (0.8) | 0.74 |
| Ethnicity | |||||||
| Non-Hispanic | 330 (94.8) | 1.5 (1.6) | 0.8 (0.7) | 2.8 (1.9) | |||
| Hispanic or Latino | 18 (5.2) | 1.6 (1.7) | 0.76 | 0.8 (0.7) | 0.93 | 3.2 (2.4) | 0.51 |
The p-trend was calculated using linear regression with continuous age, presented in years, and adjusted for sex.
The p values were calculated using linear regression, adjusted for age (categorical) and sex, with transformed Treg cell data: Group I (eighth root), Group II (fourth root), and Group III (fourth root).
The p values were calculated using linear regression adjusted for age (categorical).
Recent UVR and Treg cells are independently associated
Associations between recent UVR and quartiles of circulating Treg cells are presented in Table III. We observed a positive trend between recent UVR and quartiles of CD45RA−/CD27− Treg cells in all individuals (p-trend = 0.02) (Table III). After stratification by natural skin tone, the UVR association with CD45RA−/CD27− Treg cells was particularly pronounced among lighter-skinned individuals, with each unit increase in spectrophotometer readings associated with a 32% increase in odds of being in the uppermost versus lowermost quartile of CD45RA−/CD27− Treg cells (odds ratio = 1.32, 95% confidence interval = 1.12–1.61), after adjustment for age and sex. The strength of this association supports its biological relevance. Interestingly, we observed a significant inverse trend with recent UVR exposure and quartiles of CD45RA−/CD27+ Treg cells among all individuals (p-trend = 0.04) and lighter-skinned individuals (p-trend = 0.01), but not among darker-skinned individuals (p-trend = 0.22). Among darker-skinned individuals, UVR exposure was significantly lower among those in the second and third quartiles of CD45RA+/CD27− Treg cells compared with the first, although no clear trend was observed, taking into account the highest quartile (p-trend = 0.11). No associations were observed between UVR exposure and quartiles of total Treg cells, CLA+ Treg cells, and CCR4hi Treg cells, overall or after stratification, by natural skin tone (Supplemental Table II).
Table III.
Quartiles of circulating Treg cell subpopulations and UV spectrophotometer readings among all 350 patients undergoing skin cancer screening
| Average UV Spectrophotometer Reading | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n = 350) |
Lighter Skinc (n = 175) |
Darker Skin (n = 175) |
|||||||
| Treg Cell Phenotypesa | n | Mean (SD) | OR (95% CI)b | n | Mean (SD) | OR (95% CI) | n | Mean (SD) | OR (95% CI) |
| CD45RA+/CD27+ Treg cells | |||||||||
| Q1 | 88 | 12.63 (3.96) | 1.0 (ref.) | 46 | 14.19 (3.83) | 1.0 (ref.) | 42 | 10.92 (3.36) | 1.0 (ref.) |
| Q2 | 87 | 11.58 (3.74) | 0.91 (0.81–1.01) | 41 | 12.67 (3.93) | 0.84 (0.70–0.98) | 46 | 10.61 (3.30) | 0.99 (0.83–1.17) |
| Q3 | 87 | 11.50 (3.86) | 0.91 (0.81–1.01) | 43 | 12.91 (3.91) | 0.86 (0.73–1.01) | 44 | 10.12 (3.31) | 0.93 (0.77–1.12) |
| Q4 | 88 | 11.53 (3.89) | 0.92 (0.83–1.02) | 45 | 12.75 (3.89) | 0.88 (0.75–1.02) | 43 | 10.25 (3.49) | 0.95 (0.79–1.13) |
| p-trendd: 0.24 | p-trend: 0.18 | p-trend: 0.62 | |||||||
| CD45RA−/CD27− Treg cells | |||||||||
| Q1 | 88 | 10.88 (3.40) | 1.0 (ref.) | 44 | 11.48 (3.78) | 1.0 (ref.) | 44 | 10.27 (2.89) | 1.0 (ref.) |
| Q2 | 87 | 11.50 (3.64) | 1.00 (0.89–1.12) | 42 | 12.96 (3.79) | 1.08 (0.92–1.27) | 45 | 10.13 (2.94) | 0.92 (0.75–1.11) |
| Q3 | 89 | 12.09 (3.87) | 1.05 (0.94–1.17) | 47 | 13.41 (3.81) | 1.20 (1.03–1.42) | 42 | 10.61 (3.39) | 0.84 (0.67–1.04) |
| Q4 | 86 | 12.80 (4.34) | 1.13 (1.01–1.27) | 42 | 14.79 (3.65) | 1.32 (1.12–1.61) | 44 | 10.89 (4.11) | 0.99 (0.83–1.20) |
| p-trend: 0.02 | p-trend: < 0.001 | p-trend: 0.91 | |||||||
| CD45RA−/CD27+ Treg cells | |||||||||
| Q1 | 88 | 12.36 (4.15) | 1.0 (ref.) | 39 | 13.78 (3.70) | 1.0 (ref.) | 49 | 11.23 (4.18) | 1.0 (ref.) |
| Q2 | 88 | 11.72 (3.92) | 0.94 (0.84–1.04) | 43 | 13.30 (3.90) | 0.94 (0.79–1.10) | 45 | 10.21 (3.33) | 0.88 (0.75–1.03) |
| Q3 | 87 | 12.42 (3.96) | 0.92 (0.82–1.02) | 53 | 13.79 (4.04) | 0.85 (0.70–1.00) | 34 | 10.30 (2.74) | 0.83 (0.69–0.99) |
| Q4 | 87 | 10.73 (3.22) | 0.88 (0.78–0.98) | 40 | 11.53 (3.59) | 0.77 (0.63–0.93) | 47 | 10.05 (2.72) | 0.93 (0.78–1.10) |
| p-trend: 0.04 | p-trend: 0.01 | p-trend: 0.22 | |||||||
| CD45RA+/CD27− Treg cells | |||||||||
| Q1 | 88 | 12.84 (3.75) | 1.0 (ref.) | 45 | 13.60 (4.38) | 1.0 (ref.) | 43 | 12.05 (2.80) | 1.0 (ref.) |
| Q2 | 89 | 11.26 (3.56) | 0.91 (0.81–1.02) | 41 | 13.33 (3.25) | 1.01 (0.86–1.19) | 48 | 9.49 (2.80) | 0.72 (0.57–0.89) |
| Q3 | 85 | 11.18 (3.67) | 0.86 (0.77–0.96) | 43 | 12.32 (3.76) | 0.89 (0.76–1.04) | 42 | 10.01 (3.22) | 0.77 (0.63–0.93) |
| Q4 | 88 | 11.95 (4.30) | 0.99 (0.88–1.10) | 46 | 13.33 (4.09) | 1.05 (0.90–1.23) | 42 | 10.43 (4.04) | 0.87 (0.70–1.05) |
| p-trend: 0.41 | p-trend: 0.93 | p-trend: 0.11 | |||||||
Treg cells were categorized into quartiles using the following cutoffs: CD45RA+/CD27+ Treg cells: 4.89–8.34–13.5; CD45RA−/CD27− Treg cells: 8.44–12.25–17.4; CD45RA−/CD27+ Treg cells: 71.43–76.9–80.9; CD45RA+/CD27− Treg cells: 0.12–0.43–1.03.
ORs and 95% CIs for average spectrophotometer readings were calculated using logistic regression, comparing each risk group to the reference group Q1.
Natural skin tone based on median spectrophotometer readings in sun-unexposed underside of the upper arm, as described in Materials and Methods.
The p-trend was calculated using ordinal logistic regression.
CI, confidence interval; OR, odds ratio; Q, quartile; ref., reference.
Discussion
NMSC is the most common malignancy in the United States with older age (>60 y), representing an important risk factor (47). To our knowledge, this is the first epidemiologic study of recent UVR exposure and Treg cells in a population of individuals age 60 y and older at high risk for NMSC. In 350 individuals undergoing skin cancer screening, recent UVR exposure was positively associated with activated, effector CD45RA−/CD27− Treg cells, whereas an inverse association was observed between recent UVR and CD45RA−/CD27+ Treg cells. Furthermore, these associations were specific to lighter-skinned individuals. No associations were observed between recent UVR exposure and the total percentage of Treg cells relative to conventional CD4+ or CD8+ T cells.
To the best of our knowledge, previous findings of UVR-related changes in Treg cells have been exclusively reported in in vitro or in mouse models in vivo, in which subsets of T cells were not specifically studied (18, 36, 48). Treg cells are largely thought of as migratory populations both in circulation and in lymph nodes, but many are retained in cutaneous tissue, where they curb autoimmune diseases of the skin and contribute to tumor progression, allergic responses, and microbial immunity (as reviewed in Refs. 49 and 50). In cutaneous tissues, overrepresentation or increased suppressive functions of Treg cells may contribute to immune evasion and development of skin cancer.
We have reported previously that CD45RA−/CD27− Treg cells represent a more suppressive subset on a per-cell basis in T cell suppression assays (25). Because of the similarity between CD45RA−/CD27− Treg cells and conventional T effector cells, these cells were termed effector Treg cells. Downregulation of CD45RA and CD27 is aligned with acute Ag activation in the context of MHC class II, which, in the case of Treg cells, is largely induced by stimulatory autoantigens (51-53). This precise Treg cell subset was also expanded in a higher risk cohort of patients with a premalignant human disease myelodysplastic syndrome characterized by a high rate of leukemia transformation (25). In this disease, the total number of Treg cells increases during later stages of leukemia progression, suggesting that an activation-associated phenotypic change and expansion occur at different points of the disease, suggesting that Ag activation may precede the accumulation of Treg cells as a whole (54, 55). Collectively, our results show an increase in the CD45RA−/CD27− Treg cells in association with UVR exposure, which may occur through local tissue damage and Ag release. This increase is in tandem to low UV exposure in individuals with higher levels of naive CD45RA+/CD27+ Treg cells.
Alternative gating strategies on CD4 T cells by CD25 and CD45RA to define Treg cells, rather than by FOXP3 and CD25, resulted in no significant findings among this cohort (Figs. 1G, 1H, 2K, 2L, Table II). These populations have been previously shown to be phenotypically distinct, in which CD45RA−CD25+++ CD4+ cells, defined as Group II, exhibit more repressive activity on conventional T cells than other Treg cell populations (35). Indeed, these populations expressed the highest intracellular FOXP3 expression in the cohort presented in this study, but no association was found with recent UVR exposure. This population correlated with populations of total Treg cells defined by FOXP3 and CD25, which also showed no correlation with recent UVR exposure. Given that CD45RA−/CD27− Treg cells were found to be present within Groups II, III, and IV, loss of CD27 could contribute to a distinct functional attribute with clinical relevance to UVR exposure. Analysis of this process could yield a better understanding of Treg cell activity in response to UVR exposure.
Two skin-homing receptors were also measured on Treg cells in the context of UVR, chemokine receptor CCR4, and CLA (23, 56). CLA is a carbohydrate epitope induced by the α-(1,3)-fucosyltransferase VII gene and by cytokines and Ag stimulation (57-59), which is consistent with higher CD45RA−/CD27− Treg cells. In our data set, all CLA+ cells lacked CD45RA expression, confirming that these are activated Treg cells (Fig. 1C). Recently, CLA expression on the Treg cell surface was shown to occur after exposure to NO, a chemical that is released into the skin upon UVR exposure (56). CCR4 is another skin-associated T cell marker that has been shown to increase following UVB exposure in mice, suggesting that not only are Treg cells activated, but that they are stimulated to express both homing receptors significant for infiltration into cutaneous tissues (23, 60). Most studies suggest that Treg cells can migrate from lymph nodes to skin and then re-enter the circulation (60). Transcriptional signatures and phenotypes suggest that the migratory versus resident populations can be distinguished (49, 50). However, conjoined parabiotic surgery of two mice that share the same vasculature compartment showed that there are T cells in the skin epidermis that do not equilibrate through blood and are permanently resident in skin (61-64). Because many resident Treg cells remain in the dermis and do not recirculate after activation and migration to skin, Treg cells may accumulate through repeated sun exposure; a well-defined risk factor for skin cancer (65, 66). Although positive correlations were observed between UV exposure and CLA+ and CCR4hi Treg cells, after adjusting for age and sex, these associations were no longer statistically significant, indicating that the strongest independent marker is the accumulation of CD45RA−/CD27− Treg cells. Although it is difficult to delineate the origins of tissue resident cells, future studies are needed to define whether UVR contributes to the population of tissue resident memory Treg cells in human skin.
Although several mechanisms control the suppressive functions of Treg cells, UV promotes IL-10 secretion and reportedly exert suppressive activity largely through IL-10 production and express the glucocorticoid-induced TNF family–related receptor [(67, 68), reviewed in Ref. 69]. In cancer studies, engagement of glucocorticoid-induced TNF family–related receptor with agonistic Abs potentiates antitumor immunity by destabilizing FOXP3 expression and reducing Treg cells (70). Given that studies have shown that adoptively transferred UV-activated Treg cells into host mice suppress tumor-immune responses, CD4+ T cell proliferation, and contact hypersensitivity (68, 71-73), it is likely that UV exposure increases the suppressive activity of Treg cells through Ag activation. This is congruent with the data reported in this study in that distribution of total Treg cells is not associated with recent UV exposure (Fig. 2C, Supplemental Table I). The mechanism for both Treg cell expansion and increased suppressive activity has often pointed to the UV/vitamin D production axis (74). Vitamin D is both acquired through the diet and through activation of the first synthesis step by radiation in the UVB range (reviewed in Ref. 74). Topical application of vitamin D’s active form increases the suppressive activity of Treg cells (75) and causes comparable effects to UVB radiation in mice. This method for UV-induced immune suppression is controversial given that vitamin D receptor (VDR) knockout mice are still susceptible to UV-activated Treg cells (76). Furthermore, there are reports that VDR is not highly expressed in mouse FoxP3+CD4+ Treg cells, but there is an inverse relationship between VDR-dependent signaling and FoxP3 regulation (77-80) (as reviewed in Ref. 81). Vitamin D production by UVB exposure is particularly high in lighter-skinned individuals, potentially because of the protectiveness of melanin in individuals with natural dark skin (80, 82-84), which may explain the more pronounced associations between recent UVR exposure and Treg cells among lighter-skinned individuals in our cohort. Given the controversial evidence for vitamin D and its role in UV-induced immune suppression, investigation of vitamin D or VDRs in the context of UVR exposure and Treg cells is an important future step.
The cross-sectional nature of this analysis cannot establish the presence of UVR exposure prior to Treg cell measurement, although the UVR-associated changes in skin pigmentation had to have occurred as a result of exposure prior to the time of blood draw. The exact timing of UVR exposure is unknown, and therefore, the temporal relationship between UVR exposure and associated changes in circulating Treg cells requires further investigation. In mouse studies, UVB-induced Treg cell activation in skin lasted for 2 wk after exposure and then later contributed to expansion of these cells in the peripheral blood (23). Some previous studies of UVR exposure and immune-related conditions have relied on self-report of sun exposures and ecologic measures, such as latitude, to estimate UVR exposure (27, 29, 30, 85). Other studies have demonstrated that Treg cell populations are altered with UVR exposure in a small cohort of patients presenting with autoimmune disorders (31, 34), including one such study that demonstrated that activated CD45RA− Treg cells are increased in psoriasis patients with recent UV exposure (32). Our study builds upon these findings, suggesting that Treg cell activation occurs with recent UVR in this cohort.
The spectrophotometer readings used in the current study provide an objective and quantitative measure of recent UVR exposure. Future analysis of this prospective cohort will focus on the associations between baseline UVR exposure, Treg cells, and subsequent development of NMSC. In this review, to our knowledge, we report the first quantitative UVR exposure study associated with subpopulations of Treg cells in peripheral blood. Future studies are necessary to understand if UVR leads to the accumulation of tissue-resident Treg cell populations and whether that, in turn, contributes to carcinogenesis. Additionally, our study reports differences in Treg cell subpopulations by age, sex, and race, with potential importance in other immune-related diseases.
Supplementary Material
Acknowledgments
We sincerely thank the staff of the Flow Cytometry, Survey Methods, and Tissue Cores of the Moffitt Cancer Center.
This work was supported by the National Cancer Institute in conjunction with a Career Enhancement Award to support R.S.H. from Moffitt Skin Cancer Specialized Programs of Research Excellence Grant P50CA168536, the National Cancer Institute at the National Institutes of Health (Grant R01-CA177586), and a Moffitt Cancer Center Team Science grant. The Flow Cytometry, Survey Methods, and Tissue Cores of the Moffitt Cancer Center were supported by funds from the National Cancer Institute Cancer Center (Support Grant P30-CA076292) and by funds from the State of Florida to the Moffitt Cancer Center and Research Institute.
Abbreviations used in this article:
- CLA
cutaneous lymphocyte–associated Ag
- NMSC
nonmelanoma skin cancer
- Treg
T regulatory
- UVR
UV radiation
- VDR
vitamin D receptor
Footnotes
Disclosures
The authors have no financial conflicts of interest.
The online version of this article contains supplemental material.
References
- 1.Didona D, Paolino G, Bottoni U, and Cantisani C. 2018. Non melanoma skin cancer pathogenesis overview. Biomedicines 6: E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leiter U, Eigentler T, and Garbe C. 2014. Epidemiology of skin cancer. Adv. Exp. Med. Biol 810: 120–140. [DOI] [PubMed] [Google Scholar]
- 3.Raasch BA, Buettner PG, and Garbe C. 2006. Basal cell carcinoma: histological classification and body-site distribution. Br. J. Dermatol 155: 401–407. [DOI] [PubMed] [Google Scholar]
- 4.Rana S, Byrne SN, MacDonald LJ, Chan CY, and Halliday GM. 2008. Ultraviolet B suppresses immunity by inhibiting effector and memory T cells. Am. J. Pathol 172: 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li-Weber M, Treiber MK, Giaisi M, Palfi K, Stephan N, Parg S, and Krammer PH. 2005. Ultraviolet irradiation suppresses T cell activation via blocking TCR-mediated ERK and NF-kappa B signaling pathways. J. Immunol 175: 2132–2143. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz T 2008. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochem. Photobiol 84: 10–18. [DOI] [PubMed] [Google Scholar]
- 7.Dunn GP, Old LJ, and Schreiber RD. 2004. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21: 137–148. [DOI] [PubMed] [Google Scholar]
- 8.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, and June CH. 2001. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 61: 4766–4772. [PubMed] [Google Scholar]
- 9.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, and Linehan DC. 2002. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J. Immunol 169: 2756–2761. [DOI] [PubMed] [Google Scholar]
- 10.Maldonado A, Mueller YM, Thomas P, Bojczuk P, O’Connors C, and Katsikis PD. 2003. Decreased effector memory CD45RA+ CD62L− CD8+ T cells and increased central memory CD45RA− CD62L+ CD8+ T cells in peripheral blood of rheumatoid arthritis patients. Arthritis Res. Ther 5: R91–R96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaier M, Seissler N, Schmitt E, Meuer S, Hug F, Zeier M, and Steinborn A. 2012. DR(high+)CD45RA(−)-Tregs potentially affect the suppressive activity of the total Treg pool in renal transplant patients. PLoS One 7: e34208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponchel F, Morgan AW, Bingham SJ, Quinn M, Buch M, Verburg RJ, Henwood J, Douglas SH, Masurel A, Conaghan P, et al. 2002. Dysregulated lymphocyte proliferation and differentiation in patients with rheumatoid arthritis. Blood 100: 4550–4556. [DOI] [PubMed] [Google Scholar]
- 13.Hartling HJ, Gaardbo JC, Ronit A, Knudsen LS, Ullum H, Vainer B, Clausen MR, Skogstrand K, Gerstoft J, and Nielsen SD. 2012. CD4+ and CD8+ regulatory T cells (Tregs) are elevated and display an active phenotype in patients with chronic HCV mono-infection and HIV/HCV co-infection. Scand. J. Immunol 76: 294–305. [DOI] [PubMed] [Google Scholar]
- 14.Rosenblum MD, Way SS, and Abbas AK. 2016. Regulatory T cell memory. Nat. Rev. Immunol 16: 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, and Borst J. 2000. CD27 is required for generation and long-term maintenance of T cell immunity. Nat. Immunol 1: 433–440. [DOI] [PubMed] [Google Scholar]
- 16.Hendriks J, Xiao Y, and Borst J. 2003. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J. Exp. Med 198: 1369–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendriks J, Xiao Y, Rossen JW, van der Sluijs KF, Sugamura K, Ishii N, and Borst J. 2005. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J. Immunol 175: 1665–1676. [DOI] [PubMed] [Google Scholar]
- 18.Schenkel JM, and Masopust D. 2014. Tissue-resident memory T cells. Immunity 41: 886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duhen T, Duhen R, Lanzavecchia A, Sallusto F, and Campbell DJ. 2012. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood 119: 4430–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holick MF 2014. Sunlight, ultraviolet radiation, vitamin D and skin cancer: how much sunlight do we need? Adv. Exp. Med. Biol 810: 1–16. [PubMed] [Google Scholar]
- 21.Maeda A, Beissert S, Schwarz T, and Schwarz A. 2008. Phenotypic and functional characterization of ultraviolet radiation-induced regulatory T cells. J. Immunol 180: 3065–3071. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz A, Navid F, Sparwasser T, Clausen BE, and Schwarz T. 2011. In vivo reprogramming of UV radiation-induced regulatory T-cell migration to inhibit the elicitation of contact hypersensitivity. J. Allergy Clin. Immunol 128: 826–833. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki S, Nishioka A, Kasuya S, Ohkura N, Hemmi H, Kaisho T, Taguchi O, Sakaguchi S, and Morita A. 2014. Homeostasis of thymus-derived Foxp3+ regulatory T cells is controlled by ultraviolet B exposure in the skin. J. Immunol 193: 5488–5497. [DOI] [PubMed] [Google Scholar]
- 24.Ben Ya’acov A, Lichtenstein Y, Zolotarov L, and Ilan Y. 2015. The gut microbiome as a target for regulatory T cell-based immunotherapy: induction of regulatory lymphocytes by oral administration of anti-LPS enriched colostrum alleviates immune mediated colitis. BMC Gastroenterol. 15: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mailloux AW, Sugimori C, Komrokji RS, Yang L, Maciejewski JP, Sekeres MA, Paquette R, Loughran TP Jr., List AF, and Epling-Burnette PK. 2012. Expansion of effector memory regulatory T cells represents a novel prognostic factor in lower risk myelodysplastic syndrome. J. Immunol 189: 3198–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozlowska E, Biernacka M, Ciechomska M, and Drela N. 2007. Age-related changes in the occurrence and characteristics of thymic CD4(+) CD25(+) T cells in mice. Immunology 122: 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson S Jr., Blizzard L, Otahal P, Van der Mei I, and Taylor B. 2011. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J. Neurol. Neurosurg. Psychiatry 82: 1132–1141. [DOI] [PubMed] [Google Scholar]
- 28.Ravanat JL, Douki T, and Cadet J. 2001. Direct and indirect effects of UV radiation on DNA and its components. J. Photochem. Photobiol. B 63: 88–102. [DOI] [PubMed] [Google Scholar]
- 29.Vieira VM, Hart JE, Webster TF, Weinberg J, Puett R, Laden F, Costenbader KH, and Karlson EW. 2010. Association between residences in U.S. northern latitudes and rheumatoid arthritis: a spatial analysis of the Nurses’ health study. Environ. Health Perspect 118: 957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponsonby AL, Lucas RM, and van der Mei IA. 2005. UVR, vitamin D and three autoimmune diseases--multiple sclerosis, type 1 diabetes, rheumatoid arthritis. Photochem. Photobiol 81: 1267–1275. [DOI] [PubMed] [Google Scholar]
- 31.Breuer J, Schwab N, Schneider-Hohendorf T, Marziniak M, Mohan H, Bhatia U, Gross CC, Clausen BE, Weishaupt C, Luger TA, et al. 2014. Ultraviolet B light attenuates the systemic immune response in central nervous system autoimmunity. Ann. Neurol 75: 739–758. [DOI] [PubMed] [Google Scholar]
- 32.Saito C, Maeda A, and Morita A. 2009. Bath-PUVA therapy induces circulating regulatory T cells in patients with psoriasis. J. Dermatol. Sci 53: 231–233. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz A, Maeda A, and Schwarz T. 2007. Alteration of the migratory behavior of UV-induced regulatory T cells by tissue-specific dendritic cells. J. Immunol 178: 877–886. [DOI] [PubMed] [Google Scholar]
- 34.Furuhashi T, Saito C, Torii K, Nishida E, Yamazaki S, and Morita A. 2013. Photo(chemo)therapy reduces circulating Th17 cells and restores circulating regulatory T cells in psoriasis. PLoS One 8: e54895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, et al. 2009. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30: 899–911. [DOI] [PubMed] [Google Scholar]
- 36.Matsushima H, and Takashima A. 2010. Bidirectional homing of Tregs between the skin and lymph nodes. J. Clin. Invest 120: 653–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding Y, Xu J, and Bromberg JS. 2012. Regulatory T cell migration during an immune response. Trends Immunol. 33: 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rallón NI, López M, Soriano V, García-Samaniego J, Romero M, Labarga P, García-Gasco P, González-Lahoz J, and Benito JM. 2009. Level, phenotype and activation status of CD4+FoxP3+ regulatory T cells in patients chronically infected with human immunodeficiency virus and/or hepatitis C virus. Clin. Exp. Immunol 155: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.John EM, Schwartz GG, Koo J, Van Den Berg D, and Ingles SA. 2005. Sun exposure, vitamin D receptor gene polymorphisms, and risk of advanced prostate cancer. Cancer Res. 65: 5470–5479. [DOI] [PubMed] [Google Scholar]
- 40.John EM, Schwartz GG, Koo J, Wang W, and Ingles SA. 2007. Sun exposure, vitamin D receptor gene polymorphisms, and breast cancer risk in a multiethnic population. Am. J. Epidemiol 166: 1409–1419. [DOI] [PubMed] [Google Scholar]
- 41.Clarys P, Alewaeters K, Lambrecht R, and Barel AO. 2000. Skin color measurements: comparison between three instruments: the chromameter(R), the DermaSpectrometer(R) and the mexameter(R). Skin Res. Technol 6: 230–238. [DOI] [PubMed] [Google Scholar]
- 42.Gallagher RP, McLean DI, Yang CP, Coldman AJ, Silver HK, Spinelli JJ, and Beagrie M. 1990. Suntan, sunburn, and pigmentation factors and the frequency of acquired melanocytic nevi in children. Similarities to melanoma: the Vancouver Mole Study. Arch. Dermatol 126: 770–776. [PubMed] [Google Scholar]
- 43.Aalborg J, Morelli JG, Mokrohisky ST, Asdigian NL, Byers TE, Dellavalle RP, Box NF, and Crane LA. 2009. Tanning and increased nevus development in very-light-skinned children without red hair. Arch. Dermatol 145: 989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goronzy JJ, and Weyand CM. 2003. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity catalysts of autoimmunity and chronic inflammation. Arthritis Res. Ther 5: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goronzy JJ, and Weyand CM. 2005. T cell development and receptor diversity during aging. Curr. Opin. Immunol 17: 468–75. [DOI] [PubMed] [Google Scholar]
- 46.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, and Goronzy JJ. 2005. The influence of age on T cell generation and TCR diversity. J. Immunol 174: 7446–7452. [DOI] [PubMed] [Google Scholar]
- 47.Rogers HW, Weinstock MA, Feldman SR, and Coldiron BM. 2015. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 151: 1081–1086. [DOI] [PubMed] [Google Scholar]
- 48.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, et al. 2013. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 339: 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ali N, and Rosenblum MD. 2017. Regulatory T cells in skin. Immunology 152: 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW, Yang SH, Anthony BA, Sverdrup FM, Krow-Lucal E, et al. 2014. Memory regulatory T cells reside in human skin. J. Clin. Invest 124: 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, and Laufer TM. 2001. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. J. Exp. Med 194: 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, and Caton AJ. 2001. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol 2: 301–306. [DOI] [PubMed] [Google Scholar]
- 53.van Santen HM, Benoist C, and Mathis D. 2004. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J. Exp. Med 200: 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamdi W, Ogawara H, Handa H, Tsukamoto N, Nojima Y, and Murakami H. 2009. Clinical significance of regulatory T cells in patients with myelodysplastic syndrome. Eur. J. Haematol 82: 201–207. [DOI] [PubMed] [Google Scholar]
- 55.Kordasti SY, Ingram W, Hayden J, Darling D, Barber L, Afzali B, Lombardi G, Wlodarski MW, Maciejewski JP, Farzaneh F, and Mufti GJ. 2007. CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS). Blood 110: 847–850. [DOI] [PubMed] [Google Scholar]
- 56.Yu C, Fitzpatrick A, Cong D, Yao C, Yoo J, Turnbull A, Schwarze J, Norval M, Howie SEM, Weller RB, and Astier AL. 2017. Nitric oxide induces human CLA(+)CD25(+)Foxp3(+) regulatory T cells with skin-homing potential. J. Allergy Clin. Immunol 140: 1441–1444.e6. [DOI] [PubMed] [Google Scholar]
- 57.Malý P, Thall A, Petryniak B, Rogers CE, Smith PL, Marks RM, Kelly RJ, Gersten KM, Cheng G, Saunders TL, et al. 1996. The alpha(1,3) fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86: 643–653. [DOI] [PubMed] [Google Scholar]
- 58.Nakayama F, Teraki Y, Kudo T, Togayachi A, Iwasaki H, Tamatani T, Nishihara S, Mizukawa Y, Shiohara T, and Narimatsu H. 2000. Expression of cutaneous lymphocyte-associated antigen regulated by a set of glycosyl-transferases in human T cells: involvement of alpha1, 3-fucosyltransferase VII and beta1,4-galactosyltransferase I. J. Invest. Dermatol 115: 299–306. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi T, Ohshima K, Tsuchiya T, Suehuji H, Karube K, Nakayama J, Suzumiya J, Yoshino T, and Kikuchi M. 2003. The comparison of expression of cutaneous lymphocyte-associated antigen (CLA), and Th1- and Th2-associated antigens in mycosis fungoides and cutaneous lesions of adult T-cell leukemia/lymphoma. Eur. J. Dermatol 13: 553–559. [PubMed] [Google Scholar]
- 60.Ferran M, Romeu ER, Rincón C, Sagristà M, Giménez Arnau AM, Celada A, Pujol RM, Holló P, Jókai H, and Santamaria-Babí LF. 2013. Circulating CLA+ T lymphocytes as peripheral cell biomarkers in T-cell-mediated skin diseases. Exp. Dermatol 22: 439–442. [DOI] [PubMed] [Google Scholar]
- 61.Iijima N, and Iwasaki A. 2014. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, and Kupper TS. 2012. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 483: 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schenkel JM, Fraser KA, Vezys V, and Masopust D. 2013. Sensing and alarm function of resident memory CD8+ T cells. Nat. Immunol 14: 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park CO, and Kupper TS. 2015. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med 21: 688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu S, Han J, Laden F, and Qureshi AA. 2014. Long-term ultraviolet flux, other potential risk factors, and skin cancer risk: a cohort study. Cancer Epidemiol. Biomarkers Prev 23: 1080–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu S, Han J, Li WQ, Li T, and Qureshi AA. 2013. Basal-cell carcinoma incidence and associated risk factors in U.S. women and men. Am. J. Epidemiol 178: 890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghoreishi M, and Dutz JP. 2006. Tolerance induction by transcutaneous immunization through ultraviolet-irradiated skin is transferable through CD4 +CD25+ T regulatory cells and is dependent on host-derived IL-10. J. Immunol 176: 2635–2644. [DOI] [PubMed] [Google Scholar]
- 68.Wang L, Toda M, Saito K, Hori T, Horii T, Shiku H, Kuribayashi K, and Kato T. 2008. Post-immune UV irradiation induces Tr1-like regulatory T cells that suppress humoral immune responses. Int. Immunol 20: 57–70. [DOI] [PubMed] [Google Scholar]
- 69.Imabun S, Miyata M, Tanaka Y, Hashimoto T, Yamaguchi T, Sunada S, Kitagawa T, and Kawashima Y. 1991. Recurrent valvular pneumoperitoneum caused by a minute gastric ulcer--a case report. Jpn. J. Surg 21: 571–575. [DOI] [PubMed] [Google Scholar]
- 70.Schaer DA, Budhu S, Liu C, Bryson C, Malandro N, Cohen A, Zhong H, Yang X, Houghton AN, Merghoub T, and Wolchok JD. 2013. GITR pathway activation abrogates tumor immune suppression through loss of regulatory T cell lineage stability. Cancer Immunol. Res 1: 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwarz A, Beissert S, Grosse-Heitmeyer K, Gunzer M, Bluestone JA, Grabbe S, and Schwarz T. 2000. Evidence for functional relevance of CTLA-4 in ultraviolet-radiation-induced tolerance. J. Immunol 165: 1824–1831. [DOI] [PubMed] [Google Scholar]
- 72.Toda M, Wang L, Ogura S, Torii M, Kurachi M, Kakimi K, Nishikawa H, Matsushima K, Shiku H, Kuribayashi K, and Kato T. 2011. UV irradiation of immunized mice induces type 1 regulatory T cells that suppress tumor antigen specific cytotoxic T lymphocyte responses. Int. J. Cancer 129: 1126–1136. [DOI] [PubMed] [Google Scholar]
- 73.Schwarz A, Maeda A, Wild MK, Kernebeck K, Gross N, Aragane Y, Beissert S, Vestweber D, and Schwarz T. 2004. Ultraviolet radiation-induced regulatory T cells not only inhibit the induction but can suppress the effector phase of contact hypersensitivity. J. Immunol 172: 1036–1043. [DOI] [PubMed] [Google Scholar]
- 74.Mora JR, Iwata M, and von Andrian UH. 2008. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat. Rev. Immunol 8: 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gorman S, Kuritzky LA, Judge MA, Dixon KM, McGlade JP, Mason RS, Finlay-Jones JJ, and Hart PH. 2007. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J. Immunol 179: 6273–6283. [DOI] [PubMed] [Google Scholar]
- 76.Schwarz A, Navid F, Sparwasser T, Clausen BE, and Schwarz T. 2012. 1,25-Dihydroxyvitamin D exerts similar immunosuppressive effects as UVR but is dispensable for local UVR-induced immunosuppression. J. Invest. Dermatol 132: 2762–2769. [DOI] [PubMed] [Google Scholar]
- 77.Mayne CG, Spanier JA, Relland LM, Williams CB, and Hayes CE. 2011. 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur. J. Immunol 41: 822–832. [DOI] [PubMed] [Google Scholar]
- 78.Joshi S, Pantalena LC, Liu XK, Gaffen SL, Liu H, Rohowsky-Kochan C, Ichiyama K, Yoshimura A, Steinman L, Christakos S, and Youssef S. 2011. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol. Cell. Biol 31: 3653–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang JH, Cha HR, Lee DS, Seo KY, and Kweon MN. 2010. 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. [Published erratum appears in 2010 PLoS One 5.] PLoS One 5: e12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bonilla C, Ness AR, Wills AK, Lawlor DA, Lewis SJ, and Davey Smith G. 2014. Skin pigmentation, sun exposure and vitamin D levels in children of the avon longitudinal study of parents and children. BMC Public Health 14: 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hayes CE, Hubler SL, Moore JR, Barta LE, Praska CE, and Nashold FE. 2015. Vitamin D actions on CD4(+) T cells in autoimmune disease. Front. Immunol 6: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Armas LA, Dowell S, Akhter M, Duthuluru S, Huerter C, Hollis BW, Lund R, and Heaney RP. 2007. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J. Am. Acad. Dermatol 57: 588–593. [DOI] [PubMed] [Google Scholar]
- 83.Libon F, Cavalier E, and Nikkels AF. 2013. Skin color is relevant to vitamin D synthesis. Dermatology (Basel) 227: 250–254. [DOI] [PubMed] [Google Scholar]
- 84.D’Orazio J, Jarrett S, Amaro-Ortiz A, and Scott T. 2013. UV radiation and the skin. Int. J. Mol. Sci 14: 12222–12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hollingworth S, Walker K, Page A, and Eadie M. 2013. Pharmacoepidemiology and the Australian regional prevalence of multiple sclerosis. Mult. Scler 19: 1712–1716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.