Abstract
Aim:
This study aimed to determine the prevalence of gestational diabetes mellitus (GDM) among women attending public health centers in Rwanda using the World Health Organization (WHO) 2013 diagnostic criteria.
Methods:
A cross-sectional analysis was performed on 281 pregnant women attending antenatal care at urban and rural public health centers. Diagnostic testing was performed between 24 and 32 weeks gestation using a 75 g oral glucose tolerance test. Venous plasma glucose was centrifuged within one hour and measured at one of two laboratories. Descriptive statistics were used.
Results:
GDM prevalence was 3.2%, (4.28% urban and 2.13% rural). Women diagnosed with GDM were older, had higher BMI, hypertension, and glycosuria of ≥2+. None with HIV (14/281) had GDM. All women reported birth outcomes. All women with GDM (9/281) had normal glucose values postpartum and therefore it is unlikely that any women had overt diabetes.
Conclusion:
This study adds important information about the GDM prevalence in Rwanda, which is a resource-limited country. Although the prevalence of 3.2% was low, significant risk factors for GDM were identified. We anticipate that the risk factors for developing GDM will increase in the near future, similar to the global trend of obesity and diabetes, necessitating continued research and education in this important condition that carries a double burden of disease to both mothers and infants.
1. Introduction
The prevalence of diabetes worldwide doubled from 1980 to 2014 [1], and the rate in the general population parallels the rate in pregnancy [2]. Gestational diabetes mellitus (GDM) is a condition of carbohydrate intolerance first diagnosed in the second or third trimester of pregnancy, which is not pre-existing type 1 or type 2 diabetes (T2DM) [3,4]. In 2017, hyperglycemia in pregnancy was estimated to affect one in seven births globally [5]. Both GDM and T2DM confer risk to pregnant women and their offspring with both short and long-term complications related to hyperglycemia and hyperinsulinemia [6].
A systematic review of six countries in Africa estimated the overall prevalence of GDM to be 5% [7]; however, there is variability due to the availability of screening procedures and diagnostic criteria [7,8]. Two recent studies indicated a 2.9% prevalence rate in Kenya [9], and a 9.1% rate in South Africa [10]. Both studies used the WHO (2013) criteria based on the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) evidenced-based practice recommendations [3,6,11].
A clear relationship exists between GDM and the risk of adverse maternal, fetal, and neonatal outcomes, as demonstrated by the international Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) study [6]. The adverse maternal outcomes of GDM are well documented [6,12], and about half of the women diagnosed develop T2DM within five to ten years [5]. For the affected fetus, in addition to the genetic predisposition and immediate consequences, intrauterine exposure to hyperglycemia and the resultant hyperinsulinemia increases the risk of childhood obesity and T2DM later in life [13,14]. All pregnant women should be tested for GDM at 24–28 weeks gestation, if not previously diagnosed [4].
With increasing prevalence associated with the global epidemic of obesity and diabetes, more women in the prime of their life will be at risk for GDM and the attendant burden to their offspring [15]. This factor will likely affect not only women and children, but also families, communities and the country’s healthcare system. Women aware of their GDM positive status could be directed to attend prevention programs and reduce the risk of developing T2DM and the effect on their offspring [3–5]. Rwanda supports the International Diabetes Federation’s (IDF) Global monitoring framework (GMF) that tracks the progress of prevention and diabetes care programs [16]. The purpose of this study was to assess the prevalence of GDM in urban and rural health centers in Rwanda using the gold standard 75 g OGTT venous testing. Currently, pregnant women are not systematically screened for GDM in Rwanda. The Ministry of Health (MOH) 2012 Gynecology and Obstetric clinical protocol suggested that investigations for diabetes in pregnancy include a fasting blood sugar (capillary) and 50 g OGTT at 24–28 weeks gestation. No resources have been allocated to test systematically, however. Some clinicians, not all, will test for overt diabetes when confronted with obvious signs or symptoms or multiple risk factors.
2. Materials and methods
2.1. Participants
A cross-sectional design was used to guide data collection and analysis. Pregnant women were recruited from the general population attending antenatal clinics at specified public health centers in Rwanda between 10 April and 16 September 2017. Four health centers were located in or near the capital city of Kigali, under Muhima District Hospital catchment area and six were located in the rural catchment area of Rwamagana District Hospital, in the Eastern Province of Rwanda.
All women attending the study sites, 15 years or older with singleton pregnancies, between 24 and 28 weeks gestation, with a maximal range to 32 weeks [6] were eligible for inclusion. Gestational age and expected date of birth were based on Naegele’s rule and palpation of fundal height. If the last menstrual period date was unknown or there was a discrepancy between these two criteria, then an obstetric ultrasound was ordered, and the gestational age was based on the ultrasound result. Pregnant women with the human immunodeficiency virus (HIV) and on antiretroviral medications (ARVs) were included. Excluded were women with pre-existing diabetes mellitus, multiple gestations, or incomplete plasma glucose values. A ticket of 2000 Rwandan francs (about $2.50) was given to urban participants and 3000 Rwandan francs (about $3.50) for those in rural areas to cover transportation costs.
After the initial contact at the study site, women were followed up via phone by the research coordinator, a Clinical Officer, to alert them of the date and time of their OGTT. For the many women without phones, particularly in the rural area, the research coordinator communicated with them via their local Community Health Worker (CHW). In Rwanda, each small village has at least one CHW with a phone. The research coordinator called the CHW and requested a convenient time to gather the pregnant women in a small group to share information and address their questions about their upcoming OGTT.
We conducted a pilot study on 40 women using the urban health center laboratory in February and March 2017. The glucose results revealed very low values, and we discovered that the serum samples had not been immediately centrifuged and quickly decreased due to glycolysis. The adjoining hospital laboratory also was not suitable due to logistical issues and staff shortage. We, therefore, moved the study site to the Rwanda Diabetes Association (RDA) in Kigali where we obtained timely and accurate results using RDA’s laboratory.
An additional 281 pregnant women were recruited at the ten health centers, (140 from the urban area and 141 from the rural area), allowing for a 10% attrition rate. The sample size to detect prevalence of GDM was calculated to be 261 (95% CI, 5.2%; 0.052 ± 0.0261), based on the recommended IDF [17] regional interval rates of 5.62–12.83% (urban) and 0.7–5.9% (rural), and a systematic review estimation of 5% in Africa [7].
Additional women were included to cover for attrition and therefore the final sample size was 281.
2.2. Data collection
Information about the study was presented to the pregnant women and accompanying husbands attending the antenatal clinics, in addition to, flyers posted on the walls of the main rooms at the centers. Eligible women who agreed to participate returned early in the morning after an overnight fast at a convenient date, typically within a few days after initial contact.
The study began in the urban area, followed by recruitment in the rural area. In the urban area, women were given the option to be picked up at the health centers by taxi or come directly to the Rwanda Diabetes Association; however, most were picked up and driven the short distance to the association’s headquarters. Most women in the rural areas came directly to the hospital, though some were picked up at prearranged locations on the main road to the hospital.
After an overnight fast and arrival at the data collection site, all women were given further information and signed consent, before gathering demographic, anthropometric and metabolic data. Data on socio-demographics; obstetric, medical and family history; medications, including ARVs; height, weight, blood pressure, fetal heart tone, and fundal height measurements were obtained using a standard questionnaire. A 10-point urinalysis was done, with particular attention to glucose, protein, ketones, nitrites, leukocytes, and blood.
Serum glucose was drawn from the antecubital vein using a 21-gauge needle and vacutainer tubes. A 75-gram anhydrous oral glucose load was given, and serum glucose levels were collected after 1 h and 2 h post-load. The glucose was Excel brand, produced in Kenya, measured to 82.5 g for allowance of water in the granules, using the pharmacy scale, and dissolved in 250 ml of cooled boiled water, as advised by Dr Pastakia [9]. The venous blood samples were centrifuged on site within one hour of the blood draw and analyzed by the Humalyzer 3000 (Human Diagnostics Worldwide: Germany) at RDA; and the Cobas C311 (Roach: Switzerland) at Rwamagana District Hospital. Women who tested positive for diabetes were given a nutritional handout and referred to the obstetrician for an ultrasound and follow-up care. All women were given breakfast immediately after the third and final blood draw before the journey home.
2.3. Study outcomes
The primary outcome was the prevalence of gestational diabetes, based on the gold standard 75 g OGTT venous testing. A GDM diagnosis was given using the IADPSG/WHO 2013 criteria of a single threshold value: fasting plasma glucose (FPG) 5.1–6.9 mmol/l (92–125 mg/dl); or a 1-hour of ≥10.0 mmol/l (180 mg/dl) or a 2-h 8.5–11.0 mmol/l (153–199 mg/dl), post a 75 g oral glucose load [3].
The secondary outcomes were based on the women’s self-report, or observer report by the five trained and experienced clinician data collectors. A sixth team member (an OB/GYN) performed the dating ultrasounds to confirm gestational age if needed. Demographic data, client obstetrical, medical and family history were collected using a standard obstetric questionnaire. Anthropometric measurements and urinalysis were measured at the OGTT visit using standardized procedures and health center equipment. Birth outcomes included preterm/term; female/male; birth weight; delivery method; and NICU admission or not.
2.4. Statistical analyses
The prevalence of GDM was estimated by the proportion of women who were documented as having developed the condition during their pregnancy, and an exact 95% confidence interval was constructed around the prevalence estimate. Including n = 281 mothers in this study allowed us to make a relatively precise estimate of GDM prevalence, meaning that the 95% confidence interval would extend only about 3 or 4 percentage points from the estimate. Characteristics of study participants and birth outcomes were compared between women who did and did not develop GDM using Fisher’s exact tests (for categorical variables), 2-sample t-tests (for normally distributed continuous variables), and Wilcoxon rank sum tests (for non-normally distributed continuous variables). Glucose values were compared across BMI categories using Kruskal-Wallis analysis of variance tests or Fisher’s exact test, as appropriate. All hypothesis testing was 2-sided, and p-values < 0.05 were considered statistically significant. Analyses were conducted using SPSS version 25 (Armonk, NY: IBM Corp.) and SAS v9.4 (Cary, NC).
2.5. Ethical approval
The Institutional Review Boards of the University of Rwanda and the two district hospitals overseeing the ten antenatal care sites approved the study. Permission was also obtained from the RDA in Kigali for the urban population. All participants received detailed information about the study and provided written informed consent before data collection.
3. Results
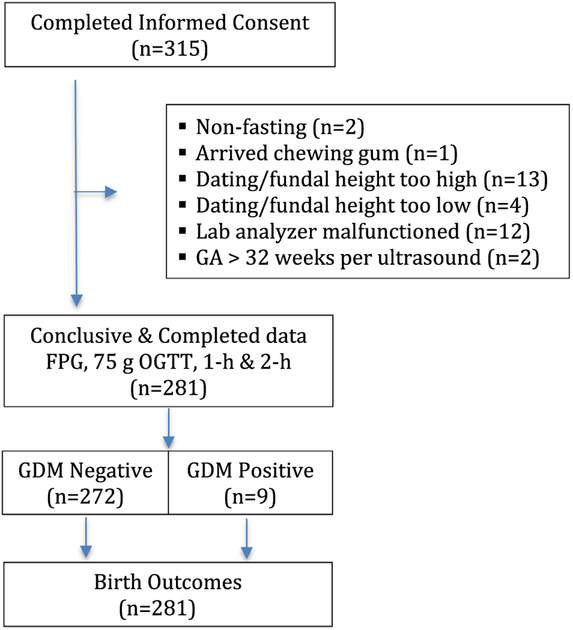
A flowchart showing the schema of the study was presented (Fig. 1). Pregnant women were recruited from the general public attending the ten health centers in Rwanda between April 10 and September 16, 2017. The characteristics of the 281 women who completed the FPG, 1-h and 2-h 75 g OGTT were presented (Table 1). Based on the WHO 2013 criteria [3], GDM was diagnosed in 9 (3.2%, 95% CI 1.5–6.0) women. Their follow-up glucose values after six weeks postpartum had returned to normal; eight had values of <5.5 mmol/l and one woman with 5.9 mmol/l, considered “prediabetes.” Therefore, it is unlikely that any of the women in the study had overt diabetes. All 281 women provided a self-report of birth outcomes, except for one mother who had a fetal demise and not given all information.
Fig. 1 –
Study schema.
Table 1 –
Characteristics of study participants.
| Characteristics | Total (n = 281) | Normal glucose (n = 272) | GDM Dx (n = 9) | P-Value |
|---|---|---|---|---|
| Study location | ||||
| Urban | 140 (49.8) | 134 (49.3) | 6 (66.7) | 0.335 |
| Rural | 141 (50.2) | 138 (50.7) | 3 (33.3) | |
| Age (years)a | 27.68 ± 5.86 | 27.49 ± 5.81 | 33.22 ± 4.84 | 0.004 |
| (27.00–28.37) | (26.75–28.31) | (29.50–36.94) | ||
| Gestational Age (Wks)a | 29.33 ± 1.9 | 29.32 ± 1.9 | 29.46 ±1.9 | 0.937 |
| (29.10–29.55) | (29.09–29.55) | (28.02–30.91) | ||
| Previous Births | ||||
| None | 89 (31.7) | 87 (32) | 2 (22.2) | 0.248 |
| 1–2 | 133 (47.3) | 130 (47.8) | 3 (33.3) | |
| ≥3 | 59 (21.0) | 55 (20.2) | 4 (44.4) | |
| LGA (≥4 kg) | 51 (18.1) | 49 (18.0) | 2 (22.2) | 0.669 |
| Previous AB or Stillbirth | 40 (14.3) | 37 (13.6) | 3 (33.3) | 0.122 |
| Medical Problems | ||||
| None | 256 (91.1) | 249 (91.5) | 7 (77.8) | 0.186 |
| HTN | 11 (3.9) | 9 (3.3) | 2 (22.2) | 0.043 |
| HIV | 14 (5.0) | 14 (5.2) | 0 (0) | 0.999 |
| Family History | ||||
| None | 254 (90.4) | 245 (90.1) | 9 (100) | |
| T2DM | 13 (4.6) | 13 (4.6) | 0 (0) | 0.804 |
| HTN | 18 (6.4) | 18 (6.6) | 0 (0) | |
| Height (cm)a | 160.22 ± 6.82 | 160.28 ± 6.82 | 158.56 ±6.77 | 0.378 |
| (159.4–161.0) | (159.5–161.1) | (153.4–163.8) | ||
| Weight (kg)ac | 62.44 ± 9.77 | 62.21 ± 9.65 | 69.33 ±11.73 | 0.057 |
| (61.29–63.59) | (61.06–63.36) | (60.32–78.35) | ||
| BMIc | 24.36 ± 3.47 | 24.25 ± 3.38 | 27.60 ± 4.86 | |
| (23.95–24.77) | (23.85–24.66) | (23.87–31.33) | 0.023 | |
| Underweightb | 2 (0.7) | 2 (0.7) | 0 (0) | 0.131 |
| Normal | 172 (61.2) | 169 (62.1) | 3 (33.3) | |
| Overweight | 89 (31.7) | 85 (31.3) | 4 (44.5) | |
| Obese | 18 (6.4) | 16 (5.9) | 2 (22.2) | |
| SBP > 140 or DBP > 90 | 21 (7.5) | 20 (7.4) | 1 (11.1) | 0.508 |
| Urine Glucose | ||||
| Negative | 261 (92.9) | 256 (94.1) | 5 (55.6) | <0.0001 |
| Trace | 10 (3.6) | 9 (3.3) | 1 (11.1) | |
| +1 | 5 (1.8) | 5 (1.8) | 0 (0) | |
| +2 or +3 | 5 (1.8) | 2 (0.8) | 3 (33.3) |
LGA Large for gestational age, GDM Gestational diabetes mellitus, HTN Hypertension, T2DM Type 2 Diabetes mellitus, SBP Systolic blood pressure, DBP Diastolic blood pressure, Wks Weeks.
Means and CI. (maternal age, gestational age).
Fischer’s Exact test.
Weight in Pregnancy at time of OGTT.
All women attending the 10 public antenatal clinics during the study period had the opportunity to be in the study if they met the inclusion criteria. The research coordinator initially recruited them at the clinic and then communicated by phone either directly or via a CHW. This communication system via the CHW was used both during the pregnancy to arrange an OGTT appointment, and during the postpartum period to obtain birth outcomes or arrange a FPG for those who tested positive for diabetes. Women who travelled very long distances were given additional compensation to cover higher transportation costs. Less than two percent of husbands accompanied their wife to the OGTT appointment; the study funding did not allow for transport compensation for family members, which likely limited the number of husbands in attendance.
The study population of 281 was relatively evenly divided between urban and rural. The mean age was 27 years with a range of 16–44 years. Women with a GDM diagnosis were older, had a history of HTN, higher BMI, and glycosuria, than women with normal glucose values. Fourteen (4.98%) were HIV positive and on ARVs; none of these women tested positive for diabetes.
Blood glucose values were analyzed and then stratified based on BMI categories, according to WHO BMI guidelines [18] (Table 2). Glucose values and prevalence varied significantly across the different categories, with the highest rates in the obese category (>30 kg/m2). All women were within the GDM WHO guidelines for the FPG, 1-h, and 2-h values. The women’s prepregnancy weights were not available, so the calculation was based on the height and weight of the measurements taken at the time of OGTT, and therefore, greater than the non-pregnant state.
Table 2 –
Glucose values across BMI categories.
| BMI (kg/m2) | |||||
|---|---|---|---|---|---|
| Under weight <18.5 (N =2) | Normal 18.5–24.99 (N = 172) | Overweight 25.00–29.99 (N = 89) | Obese ≥ 30.00 (N = 18) | p-value | |
| FPG (mmol/l) | 3.7 | 4.0 | 4.1 | 4.4 | <0.0001* |
| Median^ (mmol/l) | 3.6–3.7 | 3.7–4.3) | 3.9–4.5 | 4.2–4.8 | |
| 1-h GTT (mmol/l) | 6.1 | 5.7 | 6.2 | 7.1 | 0.001* |
| Median^ (mmol/l) | 5.9–6.1 | 5.0–6.5 | 5.3–7.1 | 6.7–7.5 | |
| 2-h GTT (mmol/l) | 5.4 | 5.3 | 5.4 | 6.0 | 0.084* |
| Median^ (mmol/l) | 4.7–5.4 | 4.7–6.0 | 5.1–6.0 | 5.2–6.2 | |
| GDM Diagnosis n(%) | 0/2 (0) | 3/172 (1.7) | 4/89 (4.5) | 2/18 (11.1) | 0.131** |
IQR.
p-value calculated using Kruskal-Wallis.
p-value calculated using Fisher’s exact test.
The majority of women with GDM (8/9; 88.9%) were diagnosed using only the FPG value according to the WHO (2013) criteria (Table 3). One other woman (11.1%) was diagnosed with an abnormal 1-h post 75 g OGTT. Based on these findings; the FPG test had an 88.9% sensitivity in this study population.
Table 3 –
GDM prevalence at specific thresholds using WHO (2013) criteria [3].
| Women diagnosed n(%) | |
|---|---|
| GDM diagnosis based on FPG | 8 (88.9) |
| FPG (5.1–6.9 mmol/l) alone | 4 (44.4) |
| FPG + 1-h (>10.0 mmol/l) | 1 (11.1) |
| FPG + 2-h (8.5–11.0 mmol/l) | 1 (11.1) |
| FPG + 1-h + 2 h post 75 g | 2 (22.2) |
| 1-h post 75 g OGTT (>10 mmol/l) | 1 (11.1) |
| Total GDM diagnosis | 9 (100) |
GDM Gestational diabetes mellitus, FPG Fasting plasma glucose, OGTT Oral glucose tolerance test.
Birth outcomes for all women are reported in Table 4. All birth outcomes were retrieved postpartum by January 6th, 2018, apart from one woman who had a fetal demise. Based on the gestational age, fundal height or ultrasound at the time of data collection, most neonates were at term (92.5%). The preterm births ranged from 31 weeks to 37.6 weeks gestation.
Table 4 –
Birth outcomes.
| Total (n = 281) n(%) | Normal glucose (n = 272) n(%) | GDM Diagnosis (n = 9) n(%) | P-Value | |
|---|---|---|---|---|
| Gestational age | ||||
| Term | 260(92.5) | 253(93.0) | 7(77.8) | 0.102 |
| Preterm | 21(7.5) | 19(7.0) | 2(22.2) | |
| Birth type | ||||
| Vaginal | 230(81.9) | 227(83.5) | 3(33.3) | 0.002 |
| Cesarean | 51(19.1) | 45(16.5) | 6(67.7) | |
| Birth weight (kg)* | ||||
| <2.5 | 5(1.8) | 4(1.5) | 1(11.1) | 0.009 |
| ≥2.5–3.9 | 265(94.6) | 259(95.6) | 6(66.7) | |
| ≥ 4.0 | 10(3.6) | 8(2.9) | 2(22.2) | |
| Gender* | ||||
| Female | 139(49.5) | 135(49.8) | 4(44.4) | 0.999 |
| Male | 141(50.2) | 136(50.2) | 5(55.6) | |
| NICU admission | 5(1.8) | 4(1.5) | 1(11.1) | 0.152 |
| Fetal demise* | 1(0.4) | 1(0.4) | 0(0) | 0.999 |
No data on the gender and weight of one newborn.
4. Discussion
The prevalence of GDM was 3.2% in the Rwandan population at the public health centers using the one-step IADPSG/WHO criteria. Of the nine women diagnosed with GDM, eight (89%) were diagnosed based on the FPG value of 5.1–6.9 mmol/l. All diagnosed with GDM were managed through nutrition modification alone, six from urban clinics and three from rural clinics. All women in the study gave a self-report of the birth outcomes, except for one who provided limited data due to a fetal demise believed to be unrelated to GDM. A FPG taken after six weeks postpartum revealed all serum glucose results had returned to normal values, indicating all nine had GDM, and therefore it is unlikely that any women had overt diabetes.
The prevalence rate of 3.2% (4.28% urban and 2.13% rural), was lower than anticipated, yet similar to the IDF prevalence estimate of 3.3% for adults in SSA [17], and a recent study in Kenya. Pastakia and colleagues [9] reported a slightly lower prevalence rate of 2.9% (95% Cl, 1.57–4.23%) in nearby Western Kenya. Our study population was quite similar, with women being highly active, even in pregnancy, and less likely to be obese. Macaulay and colleagues [10] reported a higher prevalence rate of 9.1% (95% CI 7.9–10.5) in a recent study in urban Soweto, South Africa. The authors cited a high obesity rate (47.5%) in the study population, which was parallel to the general female population. All three recent GDM studies conducted in SSA used the IADPSG/WHO diagnostic criteria [3].
Other studies conducted in Africa include a systematic review [7], four cross-sectional studies [19–22] and a prospective study [23]. Authors of the review examined 14 studies in six African countries, which indicated prevalence rates from 0% to 13.9% [7]. In contrast to our findings, a study conducted in urban Rwanda reported an 8.3% prevalence rate using the WHO 2006 criteria [19]. Several other studies in SSA reported rates of 8.1% [20], and 5.85% [21] in Nigeria, 8.4% in Tanzania [22], and 9% in Ghana [23], which varies according to diagnostic criteria.
The nine women diagnosed with GDM according to the WHO 2013 criteria [3], were significantly older, had higher BMI, history of hypertension, and glycosuria. The median age of participants was 33.22 (29.50–36.94) years for those with GDM and 27.49 (26.75–28.31) years without, a significant difference (p = 0.004). Similarly, other studies reported women over 30 years [24,25], or 35 years [10] were at higher risk.
Women with GDM were significantly more obese or overweight than those without diabetes (p = 0.023), which concurs with other studies reporting that diabetes is highly correlated with obesity [9,10,15,26,27] The women’s BMI was calculated based on their height and weight at the time of OGTT, and hence it was not an accurate measurement of their non-pregnant state.
Interestingly, women in the underweight category (<18.5 kg/m2) had 1-h, and 2-h glucose values nearer to the overweight category than the normal category (Table 2). Admittedly, there were only two women; however, in a study in Japan underweight young women had a 6.30-fold (2.26–17.59) higher risk of developing GDM than women with normal BMI [28]. The authors of that study postulated that nutritional deficiencies might have metabolic consequences that predispose to GDM.
Women who were HIV positive and on antiviral medications (ARVs) were included in the study, and none were diagnosed with GDM. Our results concur with other studies, indicating no relationship between GDM and HIV women on ARVs [29], though efavirenz is one of the three combination first-line ARV medications used for prevention of mother-to-child transmission (PMTCT) [30], which is known to cause dysglycemia [31].
All women gave a self-report of their birth outcomes. We had a low macrosomia rate (3.6%), with an equal ratio of female to male (5:5) newborns. The proportion of preterm newborns was 7.5%, but of interest was the number of large preterm newborns. One woman, who had two previous term newborns weighing 4.3 kg and 5.0 kg, gave birth to a 3.7 kg newborn at 36.3 weeks. A newborn that large may appear to be at term, and yet might be a late preterm neonate (34.0–36.6 weeks) with the attendant prematurity and potential difficult transition to extrauterine life.
Our results indicate that FPG was the most predictive, similar to other studies in SSA [9,10,20,23]. The Rwandan women came prepared to be tested at the designated time and place, in the fasting state and amenable to venipuncture. We, therefore, recommend a minimum of a FPG test be used as a GDM screening tool using the WHO 2013 criteria [3], particularly for those with risk factors, such as age 30 or older, BMI of 30 kg/m2 or higher, and a medical history of diabetes or hypertension, or family history of T2DM. Though including diabetic history could pose a problem; only one woman in those with a previous pregnancy (n = 192) could recall a prior glucose test, and family history would likely be even more remote. A recent study of 609 pregnant women in Tanzania [22] indicated a screening tool using three criteria: family history of T2DM, previous stillbirth and mid-upper arm circumference (MUAC) predicted 6 out of 10 pregnant women at risk for GDM.
The majority of Rwandan women are physically active outdoors throughout the year, though their lifestyle may acclimatize as they become more affluent. Rwanda is a hilly country in Central/Eastern Africa with summer-like temperatures year round, which not only aids regular exercise, but the climate may also contribute to a lower GDM prevalence in another way. A recent study in South Australia, [32] reported a seasonal variation of GDM, with lower incidence among pregnancies conceived in summer and higher during winter conceptions. Furthermore, a movement toward a more Westernized lifestyle, including motorized transportation, would likely lead to more obesity as seen in adults and children in developed countries [1]. A future study should include antenatal women attending private health clinics, and prepregnancy weight and MUAC measurements.
This study had several limitations. It was conducted at only 10 public health centers, and thus the prevalence is only an estimate of the true prevalence and not generalizable to pregnant women throughout Rwanda. Many women wanted to be included, but were outside the 24–32 week gestational range, though some who were early in their pregnancy waited until eligible. There was a potential for sampling bias even though all participants attended the public ANC centers and were considered on the same lower socio-demographic scale. Since the GDM testing was an additional activity, perhaps those who participated were more physically active, and therefore their glucose level might be lower than women who chose not to be tested.
5. Conclusion
This study adds important information about the prevalence of GDM in Rwanda, a resource-limited country. Although the prevalence of 3.2% was low, significant risk factors for GDM were identified. We anticipate that the risk factors for developing GDM will increase in the near future, similar to the global trend of obesity and diabetes, necessitating continued research and education in this important condition that carries a double burden of disease to both mothers and infants.
Supplementary Material
Acknowledgements
The authors would like to thank their collaborators at the 10 health centers, Muhima and Rwamagana Hospital, and Rwanda Diabetes Association; as well as Dr Sara Saravanan and Dr Sonak Pastakia for methodological advice, and all the study participants.
Source of funding
One anonymous donor funded the project. In addition, the following statistical support was provided: Dr Nietert’s time was covered by grants from the National Institutes of Health (National Center for Advancing Translational Sciences grant number UL1 TR001450 and the National Institute of General Medical Sciences grant number U54 GM104941).
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2019.03.035.
REFERENCES
- [1].Chan M. Global report on diabetes. World Heal Organ; [Internet]. 2016;1–88. Available from: http://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1. [Google Scholar]
- [2].Ben-Haroush A, Yogev Y, Hod M. Legitimate peripheral participation as a framework for conversation analytic work in second language learning. Diabetes Med 2004;21(2):103–13. [Google Scholar]
- [3].World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. World Heal Organ; [Internet]. 2013;1–6Available from: http://apps.who.int/iris/bitstream/10665/85975/1/WHO_NMH_MND_13.2_eng.pdf. [PubMed] [Google Scholar]
- [4].American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(January):S11–24.27979889 [Google Scholar]
- [5].International Diabetes Federation. Eighth edition 2017 [Internet]. 2017. 16–17 p. Available from: https://www.idf.org/e-library/welcome.html.
- [6].Metzger BE, Lowe LP, Dyer AR. N Engl J Med 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- [7].Macaulay S, Dunger DB, Norris SA. Gestational Diabetes Mellitus in Africa: A Systematic Review. Schillaci G, editor. PLoS One [Internet]. 2014. June 3 [cited 2017 Nov 7];9(6):e97871 Available from: http://dx.plos.org/10.1371/journal.pone.0097871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Farrar D, Duley L, Dowswell T, Lawlor DA. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev 2017;2017(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pastakia SD, Njuguna B, Onyango BA, Washington S, Christoffersen-Deb A, Kosgei WK, et al. Prevalence of gestational diabetes mellitus based on various screening strategies in western Kenya: a prospective comparison of point of care diagnostic methods. BMC Pregnancy Childbirth 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Macaulay S, Ngobeni M, Dunger DB, Norris SA. The prevalence of gestational diabetes mellitus amongst black South African women is a public health concern. Diabetes Res Clin Pract 2018;139:278–87. [DOI] [PubMed] [Google Scholar]
- [11].Metzger BE, Gabbe S, Persson B, Buchanan T, Pa C, Damm P. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33(3):676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marchetti D, Carrozzino D, Fraticelli F, Fulcheri M, Vitacolonna E. Quality of life in women with gestational diabetes mellitus: a systematic review. J Diabetes Res 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000. [DOI] [PubMed] [Google Scholar]
- [14].Deierlein AL, Siega-Riz AM, Chantala K, Herring AH. The association between maternal glucose concentration and child BMI at age 3 years. Diabetes Care 2011;34(2):480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Goldenberg RL, McClure EM, Harrison MS, Miodovnik M. Diabetes during pregnancy in low-and middle-income countries. Am J Perinatol 2016. [DOI] [PubMed] [Google Scholar]
- [16].Bouenizabila E. Global diabetes scorecard. Int Diabetes Federat 2015:146. [Google Scholar]
- [17].International Diabetes Federation. International Diabetes Federation. IDF Diabetes Atlas, 7th ed Brussels, Belgium: International Diabetes Federation, http://www.diabetesatlas.org [Internet]. International Diabetes Federation; 2015. 1–163 p. Available from: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/13-diabetes-atlas-seventh-edition.html. [Google Scholar]
- [18].World Health Organization. WHO|Mean Body Mass Index (BMI) [Internet]. WHO. World Health Organization; 2017. [cited 2018 Nov 24]. Available from: http://www.who.int/gho/ncd/risk_factors/bmi_text/en/#.W_jx7iReDpI.mendeley. [Google Scholar]
- [19].Mapira HT, Tumusiime DK, Yarasheski K, Rujeni N, Cade TW, Mutimura E. Strategy to improve the burden of gestational diabetes in African women: Rwandan perspective. Rwanda J 2017;4(1):36 Available from: https://www.ajol.info/index.php/rj/article/view/156410. [Google Scholar]
- [20].Olagbuji BN, Atiba AS, Olofinbiyi BA, Akintayo AA, Awoleke JO, Ade-Ojo IP, et al. Prevalence of and risk factors for gestational diabetes using 1999, 2013 WHO and IADPSG criteria upon implementation of a universal one-step screening and diagnostic strategy in a sub-Saharan African population. Eur J Obstet Gynecol Reprod Biol 2015;189:27–32. Available from: 10.1016/j.ejogrb.2015.02.030. [DOI] [PubMed] [Google Scholar]
- [21].Orru MI, Nwose EU, Bwititi PT, Igumbor EO. Screening for gestational diabetes : evaluation of prevalence in age-stratified subgroups at Central hospital Warri Nigeria. Int J Reprod Contraception, Obstet Gynecol 2018;7(1):63–8. [Google Scholar]
- [22].Nombo A, Wendelin A, Brouwer-brolsma EM, Ramaiya KL, Feskens EJM. Gestational diabetes mellitus risk score: a practical tool to predict gestational diabetes mellitus risk in Tanzania. Diabetes Res Clin Pract 2018;145:130–7. Available from: 10.1016/j.diabres.2018.05.001. [DOI] [PubMed] [Google Scholar]
- [23].Agbozo F, Abubakari A, Narh C, Jahn A. Accuracy of glycosuria, random blood glucose and risk factors as selective screening tools for gestational diabetes mellitus in comparison with universal diagnosing. BMJ Open Diabetes Res Care 2018;6(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kapur A, Divakar H, Seshiah V, Asia S. Perspectives on diagnostic strategies for hyperglycemia in pregnancy – dealing with the barriers and challenges in South Asia. Diabetes Res Clin Pract 2018;145:88–92. Available from: 10.1016/j.diabres.2018.01.025. [DOI] [PubMed] [Google Scholar]
- [25].Kuti MA, Abbiyesuku FM, Akinlade KS, Akinosun OM, Adedapo KS, Adeleye JO, et al. Oral glucose tolerance testing outcomes among women at high risk for gestational diabetes mellitus. J Clin Pathol 2011;64(8):718–21. [DOI] [PubMed] [Google Scholar]
- [26].Mwanri AW, Kinabo J, Ramaiya K, Feskens EJM. Gestational diabetes mellitus in sub-Saharan Africa: systematic review and metaregression on prevalence and risk factors. Trop Med Int Heal 2015;20(8):983–1002. Available from: http://doi.wiley.com/10.1111/tmi.12521. [DOI] [PubMed] [Google Scholar]
- [27].Short VL, Geller SE, Moore JL, McClure EM, Goudar SS, Dhaded SM, et al. The relationship between body mass index in pregnancy and adverse maternal, perinatal, and neonatal outcomes in Rural India and Pakistan. Am J Perinatol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yachi Y, Tanaka Y, Nishibata I, Sugawara A, Kodama S, Saito K, et al. Low BMI at age 20years predicts gestational diabetes independent of BMI in early pregnancy in Japan: Tanaka Women’s Clinic Study. Diabet Med 2013;30(1):70–3. [DOI] [PubMed] [Google Scholar]
- [29].Moore R, Adler H, Jackson V, Lawless M, Byrne M, Eogan M, et al. Impaired glucose metabolism in HIV-infected pregnant women: a retrospective analysis. Int J STD AIDS 2015;27(7):581–5. [DOI] [PubMed] [Google Scholar]
- [30].World Health Organization. WHO|Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. WHO. 2018. [cited 2018 Nov 23]; Available from: https://www.who.int/hiv/pub/arv/arv-2016/en/#.W_f1N-BcAio.mendeley.
- [31].Mouton JP, Cohen K, Maartens G. Key toxicity issues with the WHO-recommended first-line antiretroviral therapy regimen. Expert Rev Clin Pharmacol 2016;9(11):1493–503. Available from: 10.1080/17512433.2016.1221760. [DOI] [PubMed] [Google Scholar]
- [32].Verburg PE, Tucker G, Scheil W, Erwich JJHM, Dekker GA, Roberts CT. Seasonality of gestational diabetes mellitus: a South Australian population study. BMJ Open Diabetes Res Care 2016;4(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.