Abstract
Extracellular vesicles (EVs) are lipid-bilayer-enclosed vesicles of submicron size that are secreted by various cells. As mediators of intercellular communication, EVs can alter the physiological state of recipient cells by delivering encapsulated proteins and nucleic acids. Incontestably, growing evidence has shown important biological roles and the clinical relevance of EVs. The use of stem cell-derived EVs as a cell-free therapeutic modality for skin treatment has emerged as a promising application in dermatology. However, the moderate isolation efficiency of prevalent ultracentrifugation and low secretion rate make the massive low-cost production of EVs difficult. Here, we report development of engineered EVs (eEV) derived from human umbilical cord mesenchymal stem cells (hucMSCs) for skin treatment. Ultrasonication was used to shear intact hucMSCs for only 1 min, followed by regular centrifugation and filtration for producing nanoscale eEVs. This approach has ∼20-fold higher yield and ∼100-fold faster production than that of naturally secreted EVs (nsEV), while the production cost decreased to less than 10%. The eEVs have similar morphology, size distribution, and typical protein markers compared to nsEVs. Moreover, in vitro, both nsEVs and eEVs promote the proliferation and migration of dermal fibroblasts and increase in the expression of collagen, elastin, and fibronectin, whereas the matrix metalloproteinases-1 (MMP-1) and MMP-3 production can be significantly reduced. The wound-healing study in mice showed that both nsEVs and eEVs promote wound recovery in comparison with the controls. In sum, our results indicate that hucMSC-derived eEVs prepared by ultrasonication potentially can be used to increase skin extracellular matrix and enhance skin rejuvenation.
1. Introduction
Due to self-renewal property, multilineage differentiation potential, paracrine effects, and immunosuppressive properties, mesenchymal stem cells (MSCs) are attractive and promising cells for regenerative medicine.1,2 MSCs can be harvested from different adult (adipose tissue, peripheral blood, bone marrow) and neonatal tissues (particular parts of the placenta and umbilical cord).3 Among the source-dependent MSCs, human umbilical cord mesenchymal stem cells (hucMSCs) are the youngest and most primitive MSCs that function in various physiological activities.4 While the isolation of MSCs from adult tissues requires an invasive procedure, hucMSCs can be easily obtained from an extra-embryonic tissue after birth and they are available in potentially large quantities. Moreover, hucMSCs have a relatively rapid proliferation rate and harbor strong immunomodulatory properties compared to their adult counterparts and have intermediate properties between embryonic and adult stem cells.5,6 Recent studies demonstrated that the use of hucMSCs can promote skin regeneration and rejuvenation, have antiaging/wrinkles effects, inhibit skin pigmentation, and have other biological functions.7,8 In brief, hucMSCs have demonstrated advantages in skin and facial treatments. However, the direct application of hucMSCs on the skin has raised safety concerns related to unwanted inflammatory response, potential tumorigenicity, vascular occlusion, etc.7,9 To overcome the disadvantages, strategies related to the use of hucMSC byproducts have been developed over the years. One such strategy involves the use of extracellular vesicles (EVs) derived from hucMSCs.
EVs are lipid-bilayer-enclosed vesicles secreted by cells. Based on their origin and size, EVs can further be classified as exosomes, microvesicles, and apoptotic bodies.10 Currently, exosomes (30–120 nm) and microvesicles (50–1000 nm) are under intense investigation. These nanoscale EVs are secreted from live cells. During their release procedure cytosolic proteins, membrane proteins, and nucleic acids can be selectively wrapped.10 These EVs are capable of mediating intercellular communication while transferring contents, and thus are known as signal modulators.11,12 The suitable nanosize enables them to cross physiological barriers and have relatively longer blood circulation times, allowing efficient access to other tissues or organs.13 Also, direct implantation of derived EVs would have minimum side effects compared to intact live allogenic hucMSCs. These unique advantages make them a novel and promising cell-free therapeutic modality for skin treatment. Nevertheless, the relatively slow growth rate of hucMSCs, the low yield of EVs, and the moderate isolation efficiency of prevalent ultracentrifugation (UC) make the massive low-cost production of EVs very challenging. Hence, it is difficult to translate hucMSC-derived EVs into practical applications.
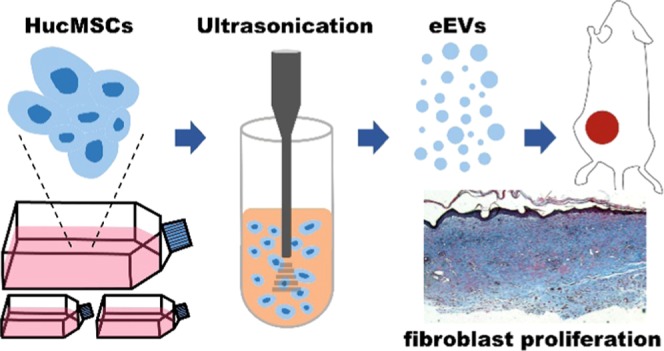
In this study, we used ultrasonication to shear intact live hucMSC to generate engineered EVs (eEV) in only 1 min, followed by regular centrifugation and filtration. The eEVs were formed as a result of the disruption of the cell membrane using shear or frictional forces, release of biological molecules from inside a cell, and reorganization of lipid bilayer-forming proteins/nucleic acid-encapsulated EVs in seconds. Through investigation of the biological functions of eEVs and comparison with naturally secreted EVs (nsEV), no significant difference was found in their morphology and size. Both can promote the proliferation and migration of human dermal fibroblasts (HDFs), aid in healing the wounds, and increase the expression of extracellular matrix (ECM) proteins. Our results indicate that eEVs can potentially be used for skin regeneration and rejuvenation and they can be efficiently and rapidly prepared with ultrasonication.
2. Results
2.1. Optimization of Ultrasonication
Approximately, 1 × 106 hucMSCs were used to optimize the amplitude and time of ultrasonication. To avoid potential break of the tapered microtip, the maximum amplitude was set to 40%. After ultrasonication at 20, 30, and 40% for 1 min, followed by centrifugation, filtration, and radioimmunoprecipitation assay (RIPA) lysis, the average protein concentrations in the respective groups were determined as 88.7 ± 2.6, 86.8 ± 10.6, and 91.9 ± 5.4 μg/mL. No significant difference was found among the three groups [analysis of variance (ANOVA), p > 0.05]. Meanwhile, the generated eEV average amount was (9.4 × 109) ± (1.2 × 108), (1.1 × 1010) ± (4.7 × 108), and (1.6 × 1010) ± (5.9 × 108) (ANOVA, p < 0.05). In each group, the peak concentration at 133, 128, and 106 nm was observed. The higher amplitude generated more eEVs with smaller average size. Given that the total protein mass in the three groups was close, we speculated that cytosolic and membrane proteins may not be efficiently encapsulated into eEVs when 40% amplitude was used to generate eEVs. We further speculated that these free proteins might not be protected from lipid envelope from degradation by proteinase. Thereby, 20% amplitude was selected for further trials. Next, 1, 3, and 5 min ultrasonication at 20% amplitude was optimized with ∼1 × 106 hucMSCs. The average protein concentrations in the 3 and 5 min groups were determined as 102.7 ± 16.8 and 226.6 ± 26.9 μg/mL (ANOVA, p < 0.05), respectively, indicating that cells can be significantly homogenized into nanosize that cannot be pelleted with regular centrifugation. The generated eEV average amounts in the 3 and 5 min groups were (1.7 × 1010) ± (4.1 × 108) and (3.3 × 1010) ± (6.5 × 108) (ANOVA, p < 0.05), respectively. Moreover, in the 3 and 5 min groups, the peak concentration at 124 and 94 nm was observed. Similarly, the extended treatment time increased the eEV yield but decreased the average size. Through the analysis of eEV amount, protein mass, and size, we determined that 1 min ultrasonication can efficiently package proteins into generated eEVs. Altogether, 20% amplitude and 1 min ultrasonication were used for eEV preparation.
2.2. EV Characterization
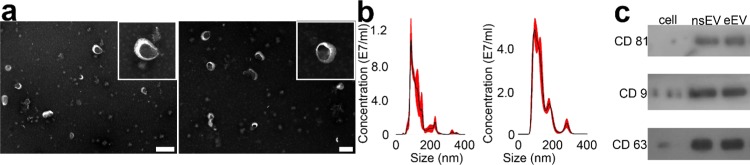
Under electron microscopy, there was no significant difference in the morphology between nsEVs and eEVs (Figure 1a). Both displayed a typical saucer-shaped morphology. After filtration, the size of nsEVs and eEVs ranged from 50 to 500 nm, and the average sizes of nsEVs and eEVs were 122.9 ± 2.3 and 133.3 ± 1.8 nm, respectively (Figure 1b), indicating that eEVs are larger than nsEVs (t-test, p < 0.001). Based on the measured concentration of nsEVs and eEVs, we determined that ∼1.5 × 1011 nsEVs were collected from ∼3 × 108 hucMSCs after 48 h fetal bovine serum (FBS)-free culture and 4 h UC processing. By contrast, ∼3.5 × 1011 eEVs were prepared with ∼3.3 × 107 hucMSCs after 1 min ultrasonication and 30 min centrifugation. The productivity was improved ∼18.5-fold, while the processing time decreased ∼104-fold. In addition, with ultrasonication, we only need to culture ∼3.3 × 107 hucMSCs with six T-225 flasks. Based on the respective diameter and counts of eEV and hucMSC, we calculated that the eEV generation efficiency was ∼30.2%, indicating that a small proportion of cell membrane was utilized to generate eEVs during ultrasonication. Of note, in our previous study,14 the eEV generation efficiency of ultrafiltration was ∼20%. In comparison, six 10-layer T-225 cell factories were needed to culture ∼3 × 108 hucMSCs. The cell expansion from six flasks to six cell factories would also take approximately 2 weeks. The overall cost for cell expansion and culture was also decreased to less than 10%. Next, cargo proteins extracted from ∼1 × 1011 nsEVs and eEVs were analyzed by Western blot. Three EV membrane proteins, including CD9, CD63, and CD81, were identified in both samples (Figure 1c), which further confirmed their identity as EVs.
Figure 1.
Characterization of nsEV and eEV. (a) Transmission electron microscopy (TEM) images of nsEVs and eEVs, respectively. The scale bar represents 500 nm. (b) Size distribution of nsEVs and eEVs measured by Nanosight NS300. (c) Western blot analysis of EV surface marker proteins on nsEVs and eEVs.
2.3. Proliferation and Migration of HDFs
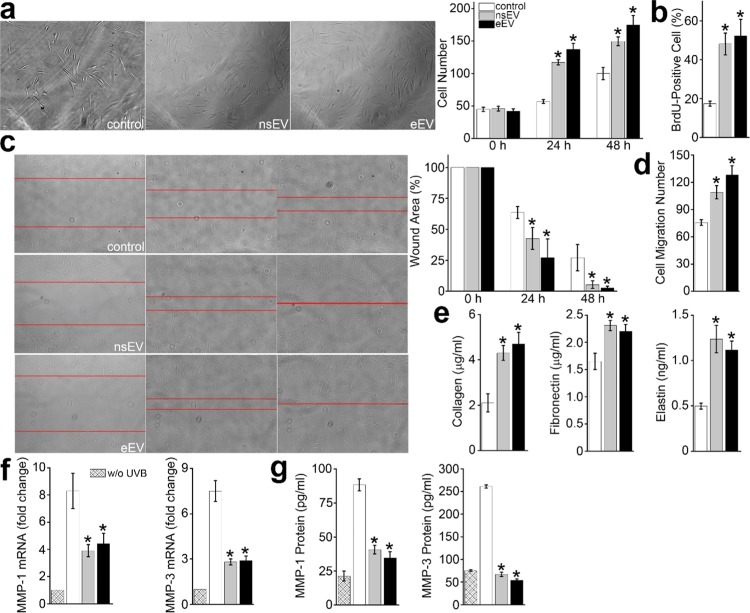
The nsEVs or eEVs educated HDFs and counterpart were seeded into a 96-well plate, respectively, to determine whether EVs can promote the proliferation of HDFs. To better visualize and quantify the cell expansion, 2% FBS was used to maintain HDFs. In the control group, the average number of HDFs increased from 44.8 ± 3.2 at the 0 h timepoint, to 56.7 ± 2.9 at the 24 h timepoint, and further to 100 ± 9.4 at the 48 h timepoint. In the nsEV group, the average number of HDFs increased from 45.8 ± 3.6 to 117.2 ± 4.1, and to 149.6 ± 6.8. In the eEV group, the numbers increased from 42 ± 3.6, to 137.2 ± 9.4, and to 174.7 ± 14.5 (Figure 2a). Both nsEVs and eEVs educated HDFs showed a higher proliferation rate compared to the control (t-test, p < 0.01). Meanwhile, BrdU staining at the 48 h timepoint indicates 17.4 ± 1.3, 48.1 ± 5.6, and 52.3 ± 8.5% HDFs cells undergoing division in the control, nsEV, and eEV groups (t-test, p < 0.01) (Figure 2b). Next, a wound-healing assay was performed to investigate the migration of nsEVs or eEVs educated HDFs and counterpart. After 48 h culture, the wound width in the control group was 27.1 ± 10.6%. In contrast, the values in the nsEV and eEV groups were 5.3 ± 3.1 and 2.7 ± 1.3%, respectively (t-test, p < 0.05) (Figure 2c). In addition, transwell migration assay was also performed. After 48- h cell culture, the number of cells at the bottom of the membrane in the control, nsEV, and eEV groups were 75.7 ± 3.1, 109 ± 7.3, and 128.3 ± 10.1, respectively (Figure 2d). The number of HDFs that crossed the micropore increased ∼1.4- and ∼1.7-fold by nsEVs and eEVs, respectively (t-test, p < 0.01). Together, the above findings indicate that both nsEVs and eEVs can promote proliferation and migration in HDFs in comparison with the control group, while it is inconclusive whether eEVs are superior to nsEVs and vice versa.
Figure 2.
Biofunctions of nsEVs and eEVs in vitro. (a) Proliferation of HDFs treated with nsEVs and eEVs, respectively. Cells were continuously photographed at 0, 24, and 48 h timepoints. The cell number was counted by ImageJ. The nsEVs- and eEVs-treated HDFs showed increased proliferation at 24 and 48 h timepoints (p < 0.01). (b) BrdU-positive percentage confirmed increased proliferation of HDFs treated with nsEVs or eEVs (p < 0.01). (c) Wound-healing assay of nsEVs- and eEVs-treated HDFs (p < 0.05). Red lines indicate wound boundary. (d) In transwell migration assay, cells at the bottom of the membrane were recorded and counted with ImageJ. Compared to the control group, more nsEVs- and eEVs-treated HDFs can translocate through the pores (p < 0.01). (e) Measurement of matrix proteins, including collagen, fibronectin, and elastin in HDFs treated with nsEVs or eEVs (p < 0.05). (f) Matrix metalloproteinases (MMP)-1 and MMP-3 activities at the mRNA level in HDFs treated with nsEVs and eEVs, respectively (p < 0.05). (g) MMP-1 and MMP-3 activities at the protein level in HDFs treated with nsEVs and eEVs, respectively (p < 0.05).
2.4. Production of ECM Proteins
The ECM, constituting over 70% of the skin, is the central hub for repair and regeneration of the skin.15 Collagen and elastin are key in the prevention of skin dehydration as well as in firmness and elasticity preservation. Fibronectin is known to cause cellular interactions with the ECM, and thus fibronectin plays an important role in epidermal–dermal adherence in human skin, proliferation, differentiation, and wound healing.16 Fragmentation of collagen, abnormal cross-linkages between the collagen fragments, amorphous elastin agglutinations, and degradation of the fibronectin can impede the ECM from its normal repair and regenerative capacity.15 Hence, we investigated the level of three key ECM proteins, including collagen, fibronectin, and elastin, with enzyme-linked immunosorbent assay (ELISA) kits to determine whether hucMSC-derived EVs can stimulate their expression. The collagen expression level in nsEV or eEV educated HDFs was 4.3 ± 0.33 and 4.7 ± 0.51 μg/mL, respectively, which was significantly higher than 2.1 ± 0.4 μg/mL in the control (Figure 2e) (t-test, p < 0.05). A significant increase in the production of fibronectin and elastin was also observed in nsEV or eEV educated HDFs. Compared to that of the control group, the fibronectin concentration in the neEV and eEV groups increased ∼1.4- and ∼1.3-fold, respectively, and the elastin concentration in neEV and eEV increased ∼2.5- and ∼2.2-fold, respectively. Moreover, we also investigated the MMP mRNA level in nsEV and eEV educated HDFs. MMPs are matrix-degrading enzymes that are activated by UV exposure or inflammation. These MMPs contribute to the breakdown of collagen while inhibiting new collagen formation, resulting in damage of the HDFs.17 It has been found that these aging fibroblasts can synthesize and secrete large amounts of MMP-1 and MMP-3 to degrade skin collagen matrix.18 Moreover, MMPs are particularly important to skin photoaging because of their collagenolytic activity. Seven of the 18 MMPs expressed in human skin can significantly elevate the levels of MMP due to UV radiation exposure, including MMP-1 and MMP-3.19,20 Therefore, we selected MMP-1 and MMP-3 to determine whether nsEV or eEV can inhibit their expression. Routinely, UVB was used to induce MMP-1 and MMP-3 expression in the short term.21 UVB irradiation increased the mRNA expression of MMP-1 and MMP-3 by 8.3 ± 1.3- and 7.5 ± 0.7-fold, respectively, in the control group. On the contrary, the expression levels of MMP-1 and MMP-3 were only increased 4.4 ± 0.8 and 2.9 ± 0.3 times in nsEV educated HDFs, and 3.9 ± 0.4 and 2.8 ± 0.2 times in eEV educated HDFs (Figure 2f). The findings indicated that nsEVs decreased the MMP-1 and MMP-3 expression by ∼47 and ∼61%, respectively (t-test, p < 0.05). Meanwhile, eEVs decreased the expression by ∼53 and ∼63%, respectively (t-test, p < 0.05). The ELISA test further confirmed that both nsEVs and eEVs educated HDFs showed reduced expression of MMP-1 and MMP-3. Of note, 54 and 69% reductions of MMP-1 and MMP-3 protein expression in the nsEV group were observed, and 61 and 75% reductions of MMP-1 and MMP-3 protein expression in the eEV group (Figure 2g). It is possible that the reduction of MMP-1 and MMP-3 mRNA expression peaked at an earlier timepoint compared with the reduction in protein expression. Together, the observed decrease in the mRNA level and protein downregulation confirms that using nsEVs and eEVs in vitro can inhibit the expression of MMP-1 and MMP-3 in HDFs irradiated with UVB. Controlling MMP activity with hucMSC-derived EVs could be one of the therapeutic strategies for treatment of photoaging.
2.5. Wound Healing in Mice
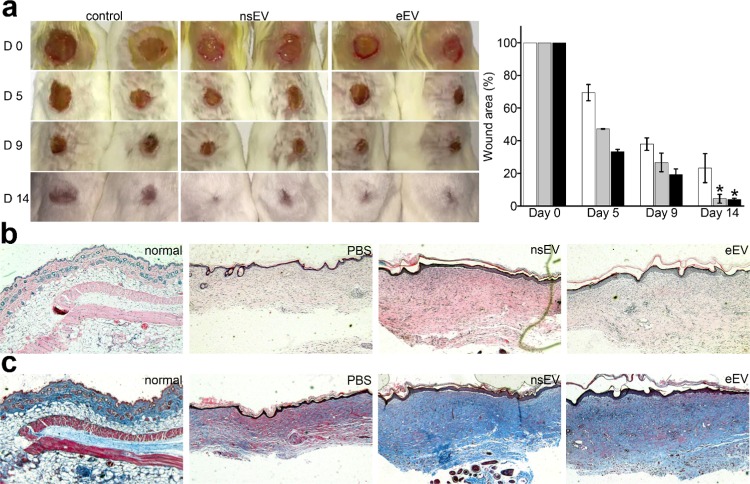
To investigate the effect of nsEVs and eEVs on wound healing, full-thickness excisional wounds of the same size were created on mice (Figure 3a). On day 9, wounds treated with nsEVs and eEVs decreased to 26.7 ± 5.7 and 19.3 ± 3.4%, respectively. In comparison, untreated wounds only decreased to 37.9 ± 13.8%, indicating that nsEVs and eEVs can promote wound healing (t-test, p < 0.05). On day 14, wounds treated with nsEVs and eEVs further decreased to 4.5 ± 2.7 and 4.1 ± 0.7%, respectively. Meanwhile, the untreated wound remained 23.2 ± 8.9%, confirming that both nsEVs and eEVs can significant promote wound healing (t-test, p < 0.005). Furthermore, the histologic structures of wounds from the respective group were analyzed (Figure 3a). Hematoxylin and eosin (H&E) staining revealed that the nsEVs- and eEVs-treated wounds showed relatively thick neo-epidermis and dermis than that of untreated wounds at day 14 post-wounding (Figure 3b). Masson’s staining showed larger amounts of fibroblast in the wounds treated with nsEVs or eEVs compared with the controls. The findings reconfirm that nsEVs and eEVs promote the wound-healing process in mice (Figure 3c).
Figure 3.
nsEVs and eEVs promote wound healing in an animal model. (a) Representative images of the healing process in wounds treated with phosphate-buffered saline (PBS), nsEVs, and eEVs. The wound-closure rates of each group at day 0, day 5, day 9, and day 14 were measured, respectively. (b) Representative H&E staining images of wounds treated with PBS, nsEVs, and eEVs at a magnification of 200×, respectively, on day 14. Normal dermal tissue without wound was used as an additional control. (c) Representative Masson’s trichrome staining of corresponding wound sections at a magnification of 200× in each group at day 14. Normal dermal tissue without wound was used as an additional control. Fibrocytes were stained in deep blue. The epidermis, dermis, subcutaneous tissue, and muscle layer were clearly displayed.
3. Discussion
Over the years, EVs have emerged as novel therapeutics capable of modulating a variety of cellular processes because selective packaging and transport of contents enable changes in recipient cells. Recent studies involving stem cell-derived EVs demonstrate that they are promising for cell-free therapy in skin treatments. For example, stem cell-derived EVs are capable of steering all three phases of wound healing by initially modulating inflammation and further promoting the migration and proliferation of fibroblasts.22 Moreover, the effects of natural aging can be reversed by treatment with stem cell-derived EVs. Skin aging is caused by the breakdown of connective tissue that is mediated by reduction in the proliferation of dermal cells and low levels of ECM proteins,23 while EVs can promote the proliferation of dermal cells and increase ECM protein levels responsible for stimulating skin regeneration and rejuvenation.24 In addition, photoaging effects caused by UV light damage to skin can be reversed by stem cell-derived EVs suppressing the expression of matrix-degrading proteins like MMPs and improving ECM protein production in photoaging.25 In brief, use of stem cell-derived EVs could serve as an excellent alternative therapeutic strategy for skin treatments. Although stem cell-derived EVs are very promising in skin regeneration and rejuvenation, the large-scale, rapid, low-cost production of EVs is very challenging. To address the low-yield issue, mechanical extrusion of stem cells for generating nanoscale EVs has been developed.26,27 Generally, in extrusion approaches, donor cells are harvested and resuspended in solution, followed by pushing cells through microconstrictions, such as micropores in a track-etched membrane, microchannels with fixed width in a microfluidic device, etc.27 There are two major shortcomings of the physical extrusion that need to be addressed. First, occlusion of the microconstrictions oftentimes happens, resulting in the failure of extrusion. To avoid frequent clogging, the concentration of cell suspension must be diluted to a proper range. The filtration flow rate or pressure also needs to be well controlled. Second, the throughput of cell processing is still limited. For instance, normally, ∼106 cells need to be continuously filtered with 10 and 5 μm track-etched membranes at least three times to improve the yield of generated EVs, and the whole procedure would take 30 min or longer. To extrude ∼107 cells with the track-etched membrane, multiple devices would be required for processing in parallel. Similarly, a microfluidic device inherently is designed for processing or analyzing microliter or even nanoliter samples. It is not suitable for large-scale production of EVs. In addition to extrusion, cryogenic grinding also has been used to generate EVs.28 It can well preserve the RNA and native protein at −80 °C or lower temperature. But it requires dry ice or liquid nitrogen to maintain the low temperature, and the sample loading/unloading is not convenient either. Moreover, the plastic or metal microbeads for intense grinding also raise the concern of plastic or metallic debris. Ultrasonication has been used for cell homogenization, liposome/micelle preparation, drug loading into vesicles, and other applications.29 The generated ultrasound waves cause periodical compression and rarefaction when propagating through the medium. The microbubbles formed during this process violently collapse within a few microseconds after reaching a critical size, inducing the occurrence of cavitation.30 The sudden collapse initiates powerful hydromechanical shear forces that can instantaneously shear and disassemble the cell membrane. The cytosolic materials, including various RNA and proteins, can be simultaneously released into the extracellular environment. Meanwhile, the hydrophobic/hydrophilic membrane lipid can automatically reassemble, forming vesicles, and during the reassembling, the surrounding RNAs and proteins can be randomly encapsulated. In the optimization step, higher amplitude and longer treatment time would better homogenize cells and generate relatively smaller vesicles. But based on the protein mass, EV amount, and size, we inferred that cargo loading was impaired by intense amplitude and long timescale. Therefore, we chose a 20% amplitude and a 1 min treatment with the 2 s on/off mode for producing eEVs, and the generated eEVs show similar morphology, size distribution, and typical EV protein markers compared to nsEVs. We directly resuspended hucMSCs in 2 mL of PBS for ultrasonication, followed by centrifugation and filtration, and the flow-through is ready to use. Of note, 20 000g centrifugation is a compulsory procedure. It removes big cellular debris, which facilitates the following filtration with a 0.22 μm filter. The more important consideration is to clear the generated apoptotic bodies and/or necrotic cell debris. In our previous study, we noticed that without high-speed centrifugation, the eEVs prepared by extrusion reversely can induce recipient cell apoptosis and animal death (data not shown). We speculated that this might be caused by the relatively large apoptotic bodies and/or necrotic cell debris in the supernatant. Once high-speed centrifugation is applied to remove debris and large microvesicles, the supernatant can promote recipient cell growth again. Moreover, the >30 min extrusion procedure would leave cells sufficient time to develop these apoptotic bodies and/or necrotic debris. In comparison, we used 1 min ultrasonication to generate eEVs and then immediatelycarried out centrifugation.
On the other hand, investigation of EV contents that can modulate cellular processes is also important as it would shed light on the underlying mechanisms responsible for skin treatments.31 Stem cell-derived EVs influence the recipient cells by delivering biologically active molecules like growth factors, transcription factors, and nucleic acids, of which RNA is most abundantly found in EVs in the form of mRNAs, miRNAs, long noncoding RNAs, and circular RNAs.20,32−34 Recent studies further demonstrated that EVs can effectively wrap dsDNA, ssDNA, mtDNA, and retrotransposon elements.33 The composition of these DNA materials has sparked interest in the potential for horizontal gene transfer by EVs. Nevertheless, while few reports have examined the DNA content of EVs, the RNA and protein contents of EVs have been studied extensively. Numerous studies have been done to understand the underlying mechanism of EVs in skin treatments, which has led to the identification of various EV contents like proteins and RNAs as well as signaling pathways. The delivery of these bioactive molecules then triggers a signaling pathway that can potentially cause EV-mediated skin treatment. For example, Ti et al. reported that miR-21, miR-146a, and miR-181 from hucMSC-derived EVs regulate inflammation and promote tissue regeneration with the help of TLR and IL-6 signaling pathways.35 Similarly, Zhang et al. reported that EVs derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through erk1/2 signaling. Fang et al. confirmed that EV miRNAs from umbilical cord-derived mesenchymal stem cells suppressed myofibroblast differentiation by inhibiting the TGF-β/Smad2 pathway during wound healing.36 Moreover, many other stem cell-derived molecules, including miR-21-3p,37 miR-125a,38 α-2-macroglobulin,39 Wnt4,40 MALAT1,41 and many others, have been identified that can promote skin wound healing.42−50 Based on these findings, EV RNAs and proteins seem to greatly contribute to EV-mediated skin treatment. The EV RNAs in recipient cells can regulate the expression of the target gene. Meanwhile, the presence of EV proteins can directly promote the proliferation and migration of recipient cells that stimulate skin regeneration. Of note, the mechanism in skin regeneration and rejuvenation is very complex and difficult to explain with a single molecule or signaling pathway alone. Thus, in future studies, it is necessary to investigate the potential mechanisms by exploring the full spectrum of signaling networks in detail along with developing a better understanding of biofunctions of various EV contents. Moreover, this approach and hucMSC-derived eEVs potentially can be translated into skin treatment, such as radiation-induced acute skin damage and diabetic foot ulcer.
4. Conclusions
Our results demonstrated that eEVs derived from hucMSC using ultrasonication can be efficiently, rapidly, and massively produced. This simple approach overcomes the low-yield concern. The generated eEVs have similar biological functions as nsEVs. In the following serial comparative tests, we demonstrated that eEVs can promote HDFs’ proliferation and migration, increase key ECM proteins’ expression, and inhibit the MMP-1/MMP-3 expression when HDFs receive high-dose UVB in vitro. We further demonstrated that these nsEVs can promote wound healing in animal models. To sum up, we conclude that hucMSC-derived eEVs with ultrasonication can preserve and deliver the useful components of hucMSCs to dermal tissue and thereby can promote skin regeneration and rejuvenation.
5. Experimental Section
5.1. Cell Culture
The hucMSCs and HDFs were purchased from ATCC. The hucMSCs were cultured with a low-glucose Dulbecco’s modified Eagle’s medium (DMEM) containing 2.4 nM l-glutamine, 2% fetal bovine serum (FBS), 10 ng/mL rhFGF, and 5 ng/mL rhEGF. The HDFs were cultured in DMEM with 5% FBS and penicillin/streptomycin. Both were cultured at 37 °C in a humidified atmosphere containing 5% CO2.
5.2. Isolation and Characterization of EVs
The hucMSCs with 80% confluence were cultured without FBS for an additional 48 h. In total, 400 mL of medium was centrifuged at 2500g for 15 min. The supernatant was subsequently filtered using a 0.22 μm filter, followed by UC at 350 000g and 4 °C for 4 h. The nsEV pellet was resuspended in 2 mL of PBS. To produce eEVs, ∼1 × 108 husMSCs were resuspended in 2 mL of PBS, followed by ultrasonication with an ultrasonic homogenizer (950E, Scientz) using a 0.125 in. tip at 4 °C. Power of 500 W was used for the generation of eEVs, and the frequency was 20–25 kHz. Ultrasonication was on for 2 s to shear cells and off for an additional 2 s to cool down the local temperature. The amplitude ranging from 20 to 40% was optimized first, and then treatment time ranging from 1 to 5 min was optimized. After ultrasonication, the sample was centrifuged at 20 000g for 30 min, followed by filtration with a 0.22 μm filter. All of the collected EV samples were then stored at −80 °C. Next, EVs were routinely characterized with TEM (Hitachi H-7650). Protein markers, including CD9, CD63, and CD81, were identified with Western blot. Nanosight NS300 was used to measure the size distribution and concentration of EVs.
5.3. Cell Assays and Measurement of ECM Proteins
HDFs that were educated with 200 μg/mL nsEVs or eEVs for 3 consecutive days served as the experimental group, whereas HDFs routinely cultured were used as the control. Approximately 2 × 103 HDFs were seeded per well in a 96-well plate and cultured with 2% FBS. At 0, 24, and 48 h timepoints, cells in each group were photographed, respectively, and counted by ImageJ. Transwell migration assay was also used to measure the migration ability of HDFs. Briefly, HDFs were plated at 2 × 104 cells/200 μL in FBS-free DMEM onto upper chambers in a transwell insert (Corning) and 500 μL of DMEM with 5% FBS were added to the lower chamber. After incubation for 48 h, nonmigration cells were removed with cotton swabs. Moreover, in the wound-healing assay, HDFs were seeded at 2 × 104 cells per well in a 96-well plate and cultured overnight. The cells were then scratched with a 1 mL pipette tip when merged completely, followed by rinsing with PBS to remove the detached cells. To evaluate wound closure, the width of the wound was recorded and analyzed with ImageJ. Cells at the bottom of the membrane were stained with neutral red dye for 10 min, followed by imaging. In each group, 3 × 105 HDFs were harvested and the soluble collagen and fibronectin in the supernatant and elastin were measured with the collagen assay kit (ab242291, Abcam), fibronectin immunoassay (DFBN10, R&D Systems), and the elastin ELISA kit (ab239433, Abcam), respectively.
5.4. UVB Irradiation and Measurement of MMP RNA
In each group, HDFs were plated in 24-well plates at 2 × 104 cells per well and cultured overnight. Cells were treated with UVB using an EL Series UV lamp 8 W (UVP) for 10 s at a dose of 0.05 J/cm2, with a spectral peak of 302 nm, once a day for 3 consecutive days. Thereafter, cells were thoroughly rinsed with PBS and further cultured with or without EVs in DMEM for 48 h. Then, cells were collected for RNA extraction. Sixty nanograms of mRNA of each sample was used for first-strand cDNA synthesis using the SuperScript First-Strand Synthesis System (Thermo Fisher). Primers for MMP-1 (F: CAT CGT GTT GCA GCT CAT GA; R: ATG GGC TGG ACA GGA TTT TG), MMP-3 (F: TGC TGC TCA TGA AAT TGG CC; R: TCA TCT TGA GAC AGG CGG AA), and β-actin (F: GTG GGG CGC CCC AGG CAC CAC; R: CTC CTT AAT GTC ACG CAC GAT TT) were purchased from IDT. Polymerase chain reaction (PCR) amplification was performed at 94 °C for 3 min, and then 45 cycles at 94 °C for 1 min, 53 °C for 1 min, and 72 °C for 1 min. Following amplification, the reactions were subjected to a thermal melt from 55 to 95 °C in 0.5 °C increments, and FAM fluorescence was monitored at each increment. The expression levels of candidate RNAs were normalized using actin as the endogenous control. The relative quantitative expression levels of RNAs were determined by the 2–ΔΔCt method. Concentrations of MMP-1 and MMP-3 were detected by the ELISA kit according to the manufacturer’s instructions (Cusabio).
5.5. Wound-Healing Model In Vivo
Six BALB/c mice divided into each group were used for the study. Mice were anesthetized and the dorsal hair was shaved. A full-thickness excisional wound of ∼4 mm diameter was created. Thereafter, mice were individually caged without dressing. Mice were then intradermally injected with 100 μL of PBS, 200 μg of eEVs in 200 μL of PBS, and 200 μg of nsEVs in 200 μL of PBS, respectively, at multiple sites around the wound. The wound-healing process was monitored by taking digital photographs of wounds on days 0, 5, 9, and 14. ImageJ was used to measure the area of the wound. The percentage of wound closure was calculated by
5.6. Statistical Analysis
Results are presented as mean ± standard deviation (SD). Statistical comparisons were performed by Student’s t-test or ANOVA. A p-value <0.05 was considered as statistically significant.
Acknowledgments
This work was partially supported by Binghamton University Faculty Startup Fund 910252-35, Binghamton University S3IP award ADLG195, Jiangsu Provincial Medical Youth Talent Award QNRC2016054, Nanjing Medical Science and Technology Development Foundation Youth Program ZDX16008, and Youth Programme of Natural Science Foundation of Jiangsu Province BK20170134.
Author Contributions
L.W. and K.K.A. contributed equally to this work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Law S.; Chaudhuri S. Mesenchymal stem cell and regenerative medicine: regeneration versus immunomodulatory challenges. Am. J. Stem Cells 2013, 2, 22–38. [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons R. E. B.; Mazurek M. S.; Soos A.; Simmons C. A. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018, 2018, 8031718 10.1155/2018/8031718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass R.; Kasper C.; Bohm S.; Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signaling 2011, 9, 12 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. W.; Staples M.; Shinozuka K.; Pantcheva P.; Kang S. D.; Borlongan C. V. Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int. J. Mol. Sci. 2013, 14, 11692–11712. 10.3390/ijms140611692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiał-Wysocka A.; Kot M.; Sulkowski M.; Badyra B.; Majka M. Molecular and Functional Verification of Wharton’s Jelly Mesenchymal Stem Cells (WJ-MSCs) Pluripotency. Int. J. Mol. Sci. 2019, 20, 1807 10.3390/ijms20081807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong C. Y.; Chak L. L.; Biswas A.; Tan J. H.; Gauthaman K.; Chan W. K.; Bongso A. Human Wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev. Rep. 2011, 7, 1–16. 10.1007/s12015-010-9166-x. [DOI] [PubMed] [Google Scholar]
- Kim Y. J.; Seo D. H.; Lee S. H.; Lee S. H.; An G. H.; Ahn H. J.; Kwon D.; Seo K. W.; Kang K. S. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells stimulate rejuvenation function in human skin. Biochem. Biophys. Rep. 2018, 16, 96–102. 10.1016/j.bbrep.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. S.; Jeon H. B.; Lim H.; Shin J. H.; Park S. J.; Jo Y. K.; Oh W.; Yang Y. S.; Cho D. H.; Kim J. Y. Conditioned Media from Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Inhibits Melanogenesis by Promoting Proteasomal Degradation of MITF. PLoS One 2015, 10, e0128078 10.1371/journal.pone.0128078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J.; Ludlow J. W. Exosomes for repair, regeneration and rejuvenation. Expert Opin. Biol. Ther. 2016, 16, 489–506. 10.1517/14712598.2016.1131976. [DOI] [PubMed] [Google Scholar]
- van der Pol E.; Boing A. N.; Harrison P.; Sturk A.; Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- Paolicelli R. C.; Bergamini G.; Rajendran L. Cell-to-cell Communication by Extracellular Vesicles: Focus on Microglia. Neuroscience 2019, 405, 148–157. 10.1016/j.neuroscience.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Colombo M.; Raposo G.; Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Maas S. L. N.; Breakefield X. O.; Weaver A. M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y.; Wang L.; Zhu C.; Zheng Q.; Wang G.; Tong J.; Fang Y.; Xia Y.; Cheng G.; He X.; Zheng S. Y. Aptamer-Conjugated Extracellular Nanovesicles for Targeted Drug Delivery. Cancer Res. 2018, 78, 798–808. 10.1158/0008-5472.CAN-17-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widgerow A. D.; Fabi S. G.; Palestine R. F.; Rivkin A.; Ortiz A.; Bucay V. W.; Chiu A.; Naga L.; Emer J.; Chasan P. E. Extracellular Matrix Modulation: Optimizing Skin Care and Rejuvenation Procedures. J. Drugs Dermatol. 2016, 15, s63–s71. [PubMed] [Google Scholar]
- Pankov R.; Yamada K. M. Fibronectin at a glance. J. Cell Sci. 2002, 115, 3861–3863. 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- Panwar P.; Butler G. S.; Jamroz A.; Azizi P.; Overall C. M.; Bromme D. Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol. 2018, 65, 30–44. 10.1016/j.matbio.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Rabe J. H.; Mamelak A. J.; McElgunn P. J.; Morison W. L.; Sauder D. N. Photoaging: mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. 10.1016/j.jaad.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Quan T.; Little E.; Quan H.; Qin Z.; Voorhees J. J.; Fisher G. J. Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: impact of altered extracellular matrix microenvironment on dermal fibroblast function. J. Invest. Dermatol. 2013, 133, 1362–1366. 10.1038/jid.2012.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittayapruek P.; Meephansan J.; Prapapan O.; Komine M.; Ohtsuki M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868 10.3390/ijms17060868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J. H.; Kim A.; Park J. M.; Kim S. H.; Chung A. S. Ultraviolet B-induced matrix metalloproteinase-1 and -3 secretions are mediated via PTEN/Akt pathway in human dermal fibroblasts. J. Cell. Physiol. 2006, 209, 775–785. 10.1002/jcp.20754. [DOI] [PubMed] [Google Scholar]
- Hu L.; Wang J.; Zhou X.; Xiong Z.; Zhao J.; Yu R.; Huang F.; Zhang H.; Chen L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993 10.1038/srep32993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W. S.; Park B. S.; Park S. H.; Kim H. K.; Sung J. H. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J. Dermatol. Sci. 2009, 53, 96–102. 10.1016/j.jdermsci.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Kim Y. J.; Yoo S. M.; Park H. H.; Lim H. J.; Kim Y. L.; Lee S.; Seo K. W.; Kang K. S. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem. Biophys. Res. Commun. 2017, 493, 1102–1108. 10.1016/j.bbrc.2017.09.056. [DOI] [PubMed] [Google Scholar]
- Oh M.; Lee J.; Kim Y. J.; Rhee W. J.; Park J. H. Exosomes Derived from Human Induced Pluripotent Stem Cells Ameliorate the Aging of Skin Fibroblasts. Int. J. Mol. Sci. 2018, 19, 1715 10.3390/ijms19061715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. S.; Svennerholm K.; Shelke G. V.; Bandeira E.; Lasser C.; Jang S. C.; Chandode R.; Gribonika I.; Lotvall J. Mesenchymal stromal cell-derived nanovesicles ameliorate bacterial outer membrane vesicle-induced sepsis via IL-10. Stem Cell Res. Ther. 2019, 10, 231 10.1186/s13287-019-1352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C.; Jeong D.; Kim B.; Jo W.; Kang H.; Cho S.; Kim K. H.; Park J. Mesenchymal Stem Cell Engineered Nanovesicles for Accelerated Skin Wound Closure. ACS Biomater. Sci. Eng. 2019, 5, 1534–1543. 10.1021/acsbiomaterials.8b01646. [DOI] [PubMed] [Google Scholar]
- Rutter B. D.; Innes R. W. Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiol. 2017, 173, 728–741. 10.1104/pp.16.01253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A.; Singla R.; Guliani A.; Yadav S. K. Nanoencapsulation for drug delivery. EXCLI J. 2014, 13, 265–286. 10.17877/DE290R-15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z.; Kumon R. E.; Deng C. X. Mechanisms of microbubble-facilitated sonoporation for drug and gene delivery. Ther. Delivery 2014, 5, 467–486. 10.4155/tde.14.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL Andaloussi S.; Mäger I.; Breakefield X. O.; Wood M. J. A. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discovery 2013, 12, 347–357. 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- Motavaf M.; Pakravan K.; Babashah S.; Malekvandfard F.; Masoumi M.; Sadeghizadeh M. Therapeutic application of mesenchymal stem cell-derived exosomes: A promising cell-free therapeutic strategy in regenerative medicine. Cell. Mol. Biol. 2016, 62, 74–79. 10.14715/cmb/2016.62.7.13. [DOI] [PubMed] [Google Scholar]
- Kawamura Y.; Yamamoto Y.; Sato T. A.; Ochiya T. Extracellular vesicles as trans-genomic agents: Emerging roles in disease and evolution. Cancer Sci. 2017, 108, 824–830. 10.1111/cas.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abels E. R.; Breakefield X. O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ti D.; Hao H.; Fu X.; Han W. Mesenchymal stem cells-derived exosomal microRNAs contribute to wound inflammation. Sci. China: Life Sci. 2016, 59, 1305–1312. 10.1007/s11427-016-0240-4. [DOI] [PubMed] [Google Scholar]
- Fang S.; Xu C.; Zhang Y.; Xue C.; Yang C.; Bi H.; Qian X.; Wu M.; Ji K.; Zhao Y.; Wang Y.; Liu H.; Xing X. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-beta/SMAD2 Pathway During Wound Healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. 10.5966/sctm.2015-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.; Rao S. S.; Wang Z. X.; Cao J.; Tan Y. J.; Luo J.; Li H. M.; Zhang W. S.; Chen C. Y.; Xie H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. 10.7150/thno.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X.; Zhang L.; Wang S.; Han Q.; Zhao R. C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 2016, 129, 2182–2189. 10.1242/jcs.170373. [DOI] [PubMed] [Google Scholar]
- Bakhtyar N.; Jeschke M. G.; Herer E.; Sheikholeslam M.; Amini-Nik S. Exosomes from acellular Wharton’s jelly of the human umbilical cord promotes skin wound healing. Stem Cell Res. Ther. 2018, 9, 193 10.1186/s13287-018-0921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.; Wang M.; Gong A. H.; Zhang X.; Wu X. D.; Zhu Y. H.; Shi H.; Wu L. J.; Zhu W.; Qian H.; Xu W. R. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168. 10.1002/stem.1771. [DOI] [PubMed] [Google Scholar]
- Cooper D. R.; Wang C.; Patel R.; Trujillo A.; Patel N. A.; Prather J.; Gould L. J.; Wu M. H. Human Adipose-Derived Stem Cell Conditioned Media and Exosomes Containing MALAT1 Promote Human Dermal Fibroblast Migration and Ischemic Wound Healing. Adv. Wound Care 2018, 7, 299–308. 10.1089/wound.2017.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiyalagan P.; Liang Y.; Kim D.; Misener S.; Thorne T.; Kamide C. E.; Klyachko E.; Losordo D. W.; Hajjar R. J.; Sahoo S. Angiogenic Mechanisms of Human CD34(+) Stem Cell Exosomes in the Repair of Ischemic Hindlimb. Circ. Res. 2017, 120, 1466–1476. 10.1161/CIRCRESAHA.116.310557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatina T.; Bruno S.; Tetta C.; Kalinina N.; Porta M.; Camussi G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun. Signal. 2014, 12, 26 10.1186/1478-811X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. D.; Johansson H. J.; Graham C. S.; Vesterlund M.; Pham M. T.; Bramlett C. S.; Montgomery E. N.; Mellema M. S.; Bardini R. L.; Contreras Z.; Hoon M.; Bauer G.; Fink K. D.; Fury B.; Hendrix K. J.; Chedin F.; El-Andaloussi S.; Hwang B.; Mulligan M. S.; Lehtio J.; Nolta J. A. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells 2016, 34, 601–613. 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montemurro T.; Vigano M.; Ragni E.; Barilani M.; Parazzi V.; Boldrin V.; Lavazza C.; Montelatici E.; Banfi F.; Lauri E.; Giovanelli S.; Baccarin M.; Guerneri S.; Giordano R.; Lazzari L. Angiogenic and anti-inflammatory properties of mesenchymal stem cells from cord blood: soluble factors and extracellular vesicles for cell regeneration. Eur. J. Cell Biol. 2016, 95, 228–238. 10.1016/j.ejcb.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Eirin A.; Zhu X. Y.; Puranik A. S.; Woollard J. R.; Tang H.; Dasari S.; Lerman A.; van Wijnen A. J.; Lerman L. O. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci. Rep. 2016, 6, 36120 10.1038/srep36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulski A. B. B.; Capriglione L. G.; Batista M.; Marcon B. H.; Senegaglia A. C.; Stimamiglio M. A.; Correa A. The Protein Content of Extracellular Vesicles Derived from Expanded Human Umbilical Cord Blood-Derived CD133(+) and Human Bone Marrow-Derived Mesenchymal Stem Cells Partially Explains Why both Sources are Advantageous for Regenerative Medicine. Stem Cell Rev. Rep. 2017, 13, 244–257. 10.1007/s12015-016-9715-z. [DOI] [PubMed] [Google Scholar]
- Ti D. D.; Hao H. J.; Fu X. B.; Han W. D. Mesenchymal stem cells-derived exosomal microRNAs contribute to wound inflammation. Sci. China: Life Sci. 2016, 59, 1305–1312. 10.1007/s11427-016-0240-4. [DOI] [PubMed] [Google Scholar]
- Kim Y. J.; Yoo S. M.; Park H. H.; Lim H. J.; Kim Y. L.; Lee S.; Seo K. W.; Kang K. S. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem. Biophys. Res. Commun. 2017, 493, 1102–1108. 10.1016/j.bbrc.2017.09.056. [DOI] [PubMed] [Google Scholar]
- Kou X. X.; Xu X. T.; Chen C.; Sanmillan M. L.; Cai T.; Zhou Y. H.; Giraudo C.; Le A.; Shi S. T. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci. Transl. Med. 2018, 10, eaai8524 10.1126/scitranslmed.aai8524. [DOI] [PMC free article] [PubMed] [Google Scholar]