Abstract
In the present manuscript, we report the kinetic and spectroscopic analysis of six new pinch-porphyrins: protoporphyrin-picpenta 1, mesoporphyrin-picpenta 2, deuteroporphyrin-picpenta 3, protoporphyrin-picocta 4, mesoporphyrin-picocta 5, and deuteroporphyrin-picocta 6. The Michaelis–Menten enzymatic pathway and the guaiacol test confirmed the ability of the compounds to function like new peroxidase models. UV–vis, 1H NMR, and electron spin resonance studies are in accordance with porphyrin-Fe(III) molecules with the quantum phenomena called quantum mixed spin (qms, s = 3/2, s = 5/2). Importantly, the influence of the presence of the s = 3/2 spin state in the compounds and its critical role for the catalytic capacity is proven here, which was the original hypothesis in our research group. The compounds with higher populations of the s = 3/2 spin state have increased peroxidase activity.
Introduction
Most physiological reactions occur within the cell, and diverse enzymes catalyze them.1 Particularly, the peroxidase enzymes from plants and animals are of interest to many researchers because of their promising biocatalytic capability for oxidizing a wide range of aromatic substrates including various industrial dyes.2 The peroxidases belong to the group of hemeproteins. This group carries out several relevant biological functions, and among them, we find O2 transport, storage and reduction, electron transfer, redox catalysis, and O2 and CO sensing.3 The peroxidases belong to the biggest superfamily of biocatalyzers and are evolutionarily related monomeric proteins, usually with a molecular weight ranging from 35 000 to 45 000 Da.4−6 Moreover, peroxidase enzymes at the resting state exhibit a single heme b cofactor with a high-spin s = 5/2 penta-coordinated Fe(III), with axial coordination via the Nε of conserved proximal histidine. The imidazole ring of this histidine is approximately perpendicular to the porphyrin plane, giving the molecule mobility within the plane and allowing the coordination to a possible sixth substituent.7−9 This feature has a key role for O2 release on Fe(III), and, hence, for life itself.10
The magnetic state of Fe(III) in hemoproteins is a consequence of the porphyrin coordination. In this sense, when porphyrin is axially coordinated to strong, weak, or middle field ligands, it presents all three different possibilities of magnetic structures. The electronic arrangement of Fe(III) corresponds to the five unpaired 3d electrons, giving various possible spin states such as low spin s = 1/2, the not observed intermediate spin s = 3/2, high spin s = 5/2, and the quantum mixed spin (qms) s = 3/2, s = 5/2. In a low spin octahedral environment, the unpaired electron is initially assigned to dx2–y2 and partially delocalized in the dxy orbital.11 It is well known that if the native protein horseradish peroxidase (HRP) lacks a substituent in the sixth coordination position, the energy gap of the nondegenerated dx2–y2 and dz2 orbitals is narrower. However, the coordination of a sixth ligand can result in the displacement of the Fe ion located nearest to the porphyrin plane. This displacement results in a complex with a distorted octahedral geometry. This “in–out” movement between the highest and the lowest spin of the iron ion will direct the qms contribution for the different spin state mixtures of Fe(III) according to the crystal field theory.12 A quantic mixture of pure spin states in a hemoprotein means that the wave function representing the electronic configuration of the Fe-hemo contains contributions of two or more unequal spin states,13−15 which is the case for this work as the different spectroscopic tools prove.
Additionally, Reyes-Ortega et al. studied the proportion of the qms in many peroxidase models.16 Those models presented the qms phenomenon for the Fe(III) ion, which is also present in some native peroxidases such as the horseradish peroxidases A2 and C2, Japanese peroxidase, and myeloperoxidases.13 Interestingly, the length of the aliphatic chain in the pinch ligand seemed to play an important role in the s = 3/2 proportion in the qms of Fe(III) and in the enzymatic kinetics of the models, as shown in Table 1.
Table 1. Kinetic and qms Percentages of Previously Studied Pinch-Porphyrin Compounds16,20 (with Permission of Reyes-Ortega, Y. Dalton Trans. 1998,4, 667–674 and Sánchez-Sandoval, A. et al. Biophys. Chem. 2003,106, 253–265).
| # of atoms in the chain of the pinch-ligand | percentage of s = 3/2 in the intermediate spin portion | kcat (M–1 s–1) | references | |
|---|---|---|---|---|
| protoporphyrin-Fe(III), PP | 89 | 13 000 | ||
| deuteroporphyrin-Fe(III), DP | 11 | 3930 | ||
| mesoporphyrin-Fe(III), MP | 8 | 1670 | ||
| PP picdien | 9 | 92 | 7 620 000 | (16) |
| DP picdien | 93 | 90 500 | ||
| MP picdien | 8 | 437 000 | ||
| PP picpropilen | 7 | 85 | 910 | (20) |
| DP picpropilen | 87 | 491 | ||
| MP picpropilen | 16 | 364 | ||
| PP picdipropilen | 11 | 90 | 1000 | (20) |
| DP picdipropilen | 14 | 315 | ||
| MP picdipropilen | 14 | 270 |
As discussed later in this article, the new compounds included here support the hypothesis of the length of the aliphatic chain of the pinch ligand affecting the qms ratio, and even the lack of nitrogen inside this chain is crucial for their efficiency as catalysts. In this work, we use compounds 1–6 which are obtained from the chemical reaction of proto, meso, and deuteroporphyrin with the novel ligand picpenta (seven atoms of length chain) and picocta (11 atoms of length chain), which do not contain the mid-chain nitrogen atom, as presented in Scheme 1.
Scheme 1. Synthesis Reaction of 1–6.
Results and Discussion
Ultraviolet–Visible Spectra
The absorption spectra of free porphyrins have a unique shape containing four Q bands and the Soret band, having the D2h symmetry and increasing it to D4h in the metallic coordination sphere when an ionic metal is added to the porphyrin ring center.17 When a pinch ligand coordinates to the iron cation in the porphyrins, we can expect a system of penta or hexa coordination; thus, these changes should be evident in the UV–vis plots [Figure S2]. A summary of the more representative changes is reported in Table 2.
Table 2. Selected Absorption Bands for 1–6(16) (with Permission of Reyes-Ortega, Y. et al. Dalton Trans. 1998,4, 667–674).
| porphyrin Fe(III) | shoulder (nm) | Soret (nm) | Q1 (nm) | Q2 (nm) |
|---|---|---|---|---|
| protoporphyrin Fe(III)16 | 356.0 | 397.5 | 487.5 | 594.5 |
| 1 | 358.0 | 398.0 | 481.5 | 594.0 |
| 4 | 357.0 | 398.0 | 484.5 | 594.0 |
| mesoporphyrin Fe(III)16 | 352.0 | 391.5 | 481.5 | 590.0 |
| 2 | 352.0 | 392.0 | 482.0 | 591.5 |
| 5 | 350.5 | 392.5 | 478.5 | 589.0 |
| deuteroporphyrin Fe(III)16 | 346.5 | 388.5 | 484.5 | 587.0 |
| 3 | 343.0 | 391.0 | 476.0 | 583.0 |
| 6 | 344.0 | 389.0 | 487.0 | 586.0 |
The typical UV–vis bands for Fe(III) containing qms are identifiable in Figure S2, in the range of 580–550 nm for all the pinch-porphyrin compounds studied herein.18 Furthermore, the Soret band for 1–6 decreases because the intensity of the transition in the system is lower due to the change in the geometry of the molecule, which becomes axially coordinated to the pinch ligand.19,20
NMR 1H Spectroscopy
To further analyze the formation of the complexes and their electronic spin configuration, NMR proton measurements were performed in CD3OD at different temperatures. The chemical shifts in the paramagnetic region presented in Table 3 for 1–6 are in accordance with previous assignments of similar pinch-porphyrin complexes.16 However, the dynamics of the porphyrin Fe(III) compounds at 300 K do not allow us to convey in a simple conclusion of a six-coordinated Fe(III) complex. Thus, experiments at low temperature were needed, Figure S1. The series of spectra show the lineshape of six-coordinated-like porphyrin complexes.16−20 Furthermore, the heme-ethyl groups in these kinds of compounds have been well studied, as well as their influence in the porphyrin’s pyrrole rings protons. The admixture of the spin could be established on the basis of the electronic and magnetic interaction over the longitudinal axis (dx2–y2 orbital) with the electronic σ-spin delocalization, resulting in different chemical shifts. The dependence of the methyl-heme and meso-protons chemical shift has been associated with the presence of the Fe(III) qms phenomenon. It has been shown that the picdien-free porphyrin complexes present a S = 5/2 Fe configuration that is the result of the π-electron system of the pyrrole conjugation. In this context, when the pinch ligand is coordinated to the Fe(III), it is expected that the amine groups, being a weak ligand field, open up the energy gap of the d orbitals, provoking an admixture of Fe(III) states that could vary depending of the type of porphyrin used. Then, the porphyrinic ring is magnetically perturbed as well, yielding a more anisotropic environment, for example, when the axial orbitals are unoccupied in the case of a five-coordinated compound. We could study the contribution of this magnetic effect in the ligand field of the pinch ligand to the qms using the Qassym parameter which is calculated from the chemical shift in the 1H NMR at different temperatures. It has been studied that a six-coordinated value of Qassym is around 0.14.16,17
Table 3. Chemical Shifts of 1–6 at 300 K of Selected H and Their Calculated Parameter Qassym.
| picpenta |
picocta |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| δ (ppm) | spread | average | Qassym | δ (ppm) | spread | average | Qassym | |||
| heme CH3 | δ (ppm) H meso | heme CH3 | δ (ppm)H meso | |||||||
| proto | 42.25 | 40.68 | 0.07 | –32.33 | 47.7 | 46.0 | 0.1 | –35.52 | ||
| 41.23 | 3 | 46.0 | 2.6 | |||||||
| 40 | 45.1 | |||||||||
| 39.25 | 45.1 | |||||||||
| meso | 44.23 | 5.21 | 40.97 | 0.13 | –30.59 | 38.4 | 5.3 | 35.7 | 0.1 | –37.98 |
| 41.39 | 36.5 | |||||||||
| 39.25 | 34.9 | |||||||||
| 39.02 | 33.1 | |||||||||
| deutero | 50.36 | 6.13 | 47.65 | 0.13 | –29.28 | 49.6 | 2.7 | 48.2 | 0.1 | –28.37 |
| 48.65 | 48.3 | |||||||||
| 47.36 | 48.0 | |||||||||
| 44.23 | 46.9 | |||||||||
Figure 1 shows the chemical shift versus the reciprocal temperature describing the linear behavior of 1–6 in the CH3, vinyl-Hα, α-CH2, and 2,4-H signals, which according to the literature, demonstrates that six-coordinated compounds are formed because they deviate from the Curie behavior seen for five-coordinated compounds.
Figure 1.

Curie law plots of heme-CH3, H-meso, and α-CH2 of complexes 1–6 vs T–1.
Additionally, the Walker–Nikolai equation (eq 1)
| 1 |
is used to fit the data obtained from the NMR experiments, which allows us to calculate the H-meso parameters F1 and F2 (Tables 4–6), which are the indicators of the dipolar and fermi contacts in the ground and excited states of the pyrrolic protons, directly affecting their chemical shifts,20−22 where δ(n) is the chemical shift in ppm of the lines in the NMR 1H experiment, W1 is the statistical weight (multiplicity) of the ground state, W2 is the statistical weight (multiplicity) of the excited state, F1 is the Curie factor of the ground state, F2 is the Curie factor of the excited state, and ΔE/KBT is the energy gap.
Table 4. Curie Factors and Energy Separations, ΔE, of the Meso-Protons Isotropic Shifts from 1–6 Complexes for W2/W1 = 1.
| compound | δ (ppm) H-meso | 10–3F1 (ppm K) | 10–3F2 (ppm K) | |ΔE| (cm–1) | 10–3 σ, (ppm K) |
|---|---|---|---|---|---|
| 1 | –32.33 | –29.45 | –1.36 | 55.68 | 0.6 |
| 2 | –30.59 | –15.39 | 0 | 75.36 | 0.5 |
| 3 | –29.28 | –17.35 | 2.39 | 88.09 | 0.6 |
| 4 | –35.52 | –29.11 | 1.06 | 75.73 | 0.6 |
| 5 | –37.98 | –40.72 | –15.32 | 77.06 | 0.6 |
| 6 | –28.37 | –9.26 | –10.57 | 100.98 | 0.3 |
Table 6. Curie Factors and Energy Separations, ΔE, of the Meso-Protons Isotropic Shifts from 1–6 Complexes for W2/W1 = 4/6.
| compound | δ (ppm) H-meso | 10–3F1 (ppm K) | 10–3F2 (ppm K) | |ΔE| (cm–1) | 10–3 σ, (ppm K) |
|---|---|---|---|---|---|
| 1 | –32.33 | –30.25 | 1.02 | 142.36 | 0.8 |
| 2 | –30.59 | –25.23 | –3.99 | 266.78 | 0.5 |
| 3 | –29.28 | –10.32 | 1.02 | 39.45 | 0.2 |
| 4 | –35.52 | –11.54 | 0 | 67.6 | 0.2 |
| 5 | –37.98 | –36.98 | 0 | 77.12 | 0.9 |
| 6 | –28.37 | –40 | –4.48 | 79.36 | 1 |
Table 5. Curie Factors and Energy Separations, ΔE, of the Meso-Protons Isotropic Shifts from 1–6 Complexes for W2/W1 = 6/4.
| compound | δ (ppm) H-meso | 10–3F1 (ppm K) | 10–3F2 (ppm K) | |ΔE| (cm–1) | 10–3 σ, (ppm K) |
|---|---|---|---|---|---|
| 1 | –32.33 | –12.85 | 5.6 | 25.72 | 0.2 |
| 2 | –30.59 | –21.35 | –2.36 | 32 | 0.2 |
| 3 | –29.28 | –87.37 | –0.06 | 45.15 | 0.4 |
| 4 | –35.52 | –55.02 | 1.74 | 63.39 | 0.6 |
| 5 | –37.98 | –48.87 | 4.06 | 52.38 | 0.6 |
| 6 | –28.37 | –60.39 | 1.38 | 41.88 | 0.2 |
Factor F1 for 1–6 for the H-heme signals are always negative, being this typically displayed for unoccupied dx2–y2 orbitals and a vast electron density delocalization over the π orbitals. In this case, the spin state s = 3/2 is energetically the lowest level. The values obtained for F2 are negative or near to zero for 1–6 because of no changes in σ localization through the variance of temperature. A different analysis of Figure 2 was made through the linearization of temperature dependence, in other words, through the elimination of the spin state s = 5/2.
Figure 2.

Chemical shifts of 1–6 as a function of inverse temperature and fits to the equation of Walker–Nikolai.18
The Curie factors obtained for this adjustment
are as follows (ppm
K): F(1) = −6.98 × 10–3F(2) = −19.06
× 10–3F(3) =
−3.58 × 10–3F(4) = −11.74 × 10–3F(5) = −6.52 × 10–3F(6) = −10.29 × 10–3. The resulting Curie factors are clear evidence that the spin state s = 3/2 in the Fe(III) species has enormous contribution
when the magnetic levels are independent of the thermic energy or
when the temperature tends towards 0 K. The discussed parameters were
obtained by setting the statistical weigh state 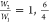 and 4/6. This group of values is representing
the spin states: S1 = S2 = 3/2, S1 = 3/2, S2 = 5/2 and S1 =
5/2 S2 = 3/2.
and 4/6. This group of values is representing
the spin states: S1 = S2 = 3/2, S1 = 3/2, S2 = 5/2 and S1 =
5/2 S2 = 3/2.
Electron Spin Resonance Spectroscopy
The electron spin resonance (ESR) spectra of 1–6 are displayed in Figure 3, where three species of Fe(III) were identified: species A, B, and C. For precursor porphyrins, the ESR spectra showed a rhombic species, Figure 3, with g’s of ∼12, 5, and 2. Two axial spectra have been reported for porphyrinic systems with the qms s = 3/2, s = 5/2 ground state.13,16,20 ESR spectra of pinch-porphyrin Fe(III) complexes showed a new lineshape and several Fe(III) species, corresponding to the different spin states as discussed earlier. The Fe(III) pinch-porphyrins showed a rhombic line shape after the addition of the pinch ligand for 1–6, which is different from those showed for free porphyrins, indicating that the axial coordination of the pinch ligand modified the ligand field around Fe(III).17 The signals corresponding to species B presented g⊥ values between 6.5 and 5.3 and for species C, the value was ≈ 4.2. The cc values for species A were between 4.3 and 4.05.11,20 Values of g⊥ = 6 and g∥ = 2 are reported to belong to the pure high spin, s = 5/2, while the signals with g⊥ = 4 and g∥ < 2 value would correspond to the pure intermediate spin, s = 3/2. Signals from the species with s = 1/2 are not analyzed in the present work because high spin s = 5/2 and low spin s = 1/2 compounds have been largely studied.7,21
Figure 3.
ESR spectra of the porphyrin-Fe(III) complexes used in this work and of their corresponding pinch complexes 1–6 at 77 K in the X-band.
Moreover, we performed the Maltempo–Moss and Cammack,13 correlation curves treatment of the crystal splitting λ/Δ versus g⊥ giving values of λ/Δ among −1.2 and −2.3 for 1–6. The g⊥ values of species A, B, and C are taken from Figure 3 to obtain the admixtures present in the novel compounds. The proportion of the s = 5/2 state in the qms s = 5/2, s = 3/2 of Fe(III) resulted, for the species A and for 1–6, larger than 70%, species A being in higher proportion with respect to species B and C.22 According to Nesset et al.23 for 1–6, it is concluded that the Fe(III) ion is moved in the range of 0.10–0.30 Å out of the porphyrin plane for s = 5/2. Thus, species B of 1–6, showing qms, with more than 70% s = 3/2 may contain the Fe(III) ion moved out of the plane ≈0.30 Å. For species C in 1–6, the treatments resulted in an admixture larger than 72.5% of s = 3/2, which coincides with previous studies for this species and suggests that the iron ion might be displaced out of the plane a distance of 0.10 Å. All these observations confirmed the fact that the Fe(III) compounds have, indeed, a qms, but the B and C species, in all compounds, have a major contribution of s = 3/2 in the quantic mixture. As we will see further in the discussion, when similar porphyrinic systems are analyzed throughout kinetic parameters, a major contribution of the spin state s = 3/2 will determine the reaction rate.16,20
The ESR analysis helped us correlate the Michaelis constant values and the kcat with the amount of quantic mixture in the system (Table 8). This correlation shows (a) when the predominant spin state is s = 3/2 over 5/2 in the major species of Fe(III) with qms, the catalytic peroxidase activity is higher and thus, (b) the lowest kcat values for these type of models are directly related to the percentage of species C. In the present work, the low catalytic activity of 1–6 results from the fact that species with more s = 3/2 are the less abundant part of the mixture.
Table 8. Comparison of Catalytic Activity of the Porphyrin-Fe(III) Complexes Used in This Work, Their Corresponding Pinch Complexes 1–6, and Its Correlation with the Proportion of qms16 (with Permission of Reyes-Ortega, Y. et al. Dalton Trans. 1998,4, 667–674).
| comp. | KH2O2 (M) | Kguaiacol (M) | kcat (M–1 s–1) | qms A s = 5/2, 3/2 | qms B s = 5/2, 3/2 | qms C s = 5/2, 3/2 |
|---|---|---|---|---|---|---|
| protoporphyrin Fe(III) | 1.2 × 10–2 | 2.45 × 10–4 | 1.30 × 104 | 74% [70%, 30%] | 26% [11%, 89%] | |
| 1 | 6.67 × 10–2 | 4.00 × 10–4 | 2.00 × 104 | 90% [84%, 16%] | <7% [<15%, >85%] | <3% [<20%, >80%] |
| 4 | 2.30 × 10–2 | 1.27 × 10–4 | 4.58 × 102 | 85% [73%, 26%] | 10% [<8%, >92%] | <5% [<9%, >91%] |
| mesoporphyrin Fe(III) | 2.75 × 10–2 | 3.48 × 10–4 | 4.34 × 102 | 85% [85%, 15%] | 15% [92%, 8%] | |
| 2 | 4.2 × 10–1 | 5.3 × 10–4 | 1.5 × 103 | 90% [86%, 14%] | <9% [<15%, >85%] | <1% [<12%, >88%] |
| 5 | 3.03 × 10–2 | 3.05 × 10–5 | 1.90 × 102 | 67% [<8%, >92%] | 18% [67%, 33%] | 15% [<52%, >48%] |
| deuteroporphyrin Fe(III) | 4.44 × 10–2 | 6.67 × 10–4 | 3.33 × 103 | 92% [87%, 13%] | 9% [89%, 11%] | |
| 3 | 1.3 × 10–1 | 4.3 × 10–4 | 1.9 × 102 | 90% [76%, 24%] | <10% [<15%, >85%] | |
| 6 | 1.65 × 10–2 | 3.36 × 10–4 | 3.58 × 102 | 79% [>92%, <8%] | 21% [11%,88%] | |
| (HRP) | 1.3 × 10–1 | 1.8 × 107 |
The ESR and UV–vis analysis ensure that the coordination of the pinch-ligand happened, however, according to a preliminary density functional theory (DFT) relaxed structure and previous studies,16,20 one of the axial coordination is made by the methanol solvent on the account of stress that will be imposed on the structure in case two axial coordination of pyridines are forced, Figure 4.
Figure 4.

A model of the relaxed structure by DFT of the novel compounds. Note the Hmeso···Npicdien interaction which stabilizes the pinch-porphyrin structure.
Magnetism
The interpretation of the Fe(III) spin states was examined by magnetic measurements for complex 1–6 using SQUID magnetometry because of the exact predictions of the spin states of the compounds over the temperature range 2–300 K. Sample solutions at ca. 0.001 mM of 2–6 were found in fact to exhibit χMT ≈ 1.57 cm3 K/mol at 300 K (Table 7), which are in accordance with the Fe(III) χMT spin-only values for S = 3/224 except for 1 which is χMT ≈ 2.5 cm3 K/mol and stays steady during the temperature variation.
Table 7. Magnetic Susceptibilities, χMT cm3 K/mol, of 1–6 at Selected Temperaturesa.
| pinch-porphyrin complexes | ||||||
|---|---|---|---|---|---|---|
| T (K) | 1 | 2 | 3 | 4 | 5 | 6 |
| 77 | 0.77 | 1.10 | 0.84 | 0.76 | 0.82 | 0.77 |
| 100 | 0.82 | 1.30 | 0.93 | 0.86 | 0.94 | 0.88 |
| 150 | 0.91 | 1.72 | 1.09 | 1.06 | 1.18 | 1.08 |
| 200 | 0.97 | 2.13 | 1.23 | 1.25 | 1.41 | 1.28 |
| 250 | 1.02 | 2.52 | 1.35 | 1.42 | 1.62 | 1.46 |
| 300 | 1.04 | 2.89 | 1.45 | 1.57 | 1.81 | 1.62 |
Spin only expected values for S = 3/2, χMT = 1.875 cm3 K/mol and for S = 1/2, χMT = 0.375 cm3 K/mol.
Moreover, a variation of χMT values is also shown while the temperature decreases as seen in Figure 5. This decay could be attributed to the porphyrin-Fe(III) plane that at low temperatures lacks mobility, and it is stabilized toward the low spin configuration Fe(III), S = 1/2 with χMT ≈ 0.37 cm3 K/mol.25 This behavior of the Fe(III) species is shown, as well as plots of effective magnetic moment (μeff) versus the temperature (Figure S6) for 1–6.
Figure 5.

Temperature dependence of the magnetic susceptibility of 1–6.
Kinetic Behavior
To confirm the peroxidase-like behavior, guaiacol tests18 were performed for 1–6, followed by UV–vis, resulting in λmax (nm) for the guaiacol oxidation products (Scheme S1), proving their formation. Additionally, the data from UV–vis spectra allowed us to perform a Michaelis–Menten study for each compound, proving that the compounds behave as enzymes ruled by this model.26,27 Complementarily, the guaiacol test proves even further that 1–6 are indeed the peroxidase model. By adjusting the guaiacol test graphs with the Michaelis–Menten model V0 = Vmax[S]/(Km + [S]), we are able to emphasize two different regions: (a) the linear area which is the initial part and exhibits a first-order kinetic and (b) the asymptotic part which is the end area and has a zero-order kinetic, from which the three concentrations of guaiacol were used to quantify the kinetic constants (Table S1).
The complete kinetic parameters of 1–6 are calculated from the Michaelis–Menten plots (Figures S3–S6). The 10 optimum concentrations of 1–6 were obtained by fixing one H2O2 concentration and one guaiacol concentration (Table S1). Equivalent experiments were made to determine the 10 optimum concentrations for guaiacol and H2O2.
Therefore, according to the classic ping-pong peroxidase kinetic model, it is possible to obtain a primary plot (Figure 6) which is adjusted with eq 2.28
| 2 |
where [E]0 is the compound’s concentration and KH2O2 and Kguaiacol are the Michaelis constants, and kcat is the theoretical maximum rate constant. A straight line results from the previous equation, being KH2O2/kcat the slope, and by using three different concentrations of guaiacol, we obtained two more parallel lines. The primary intercepts (PIs) to [E]/V0 were obtained from the classic ping-pong model as well for each of the guaiacol concentration producing the secondary plot.
Figure 6.
Primary plots for 1–6.
From the PI plots versus 1/[guaiacol] (secondary plots), kcat for 1–6 is calculated. The low affinity that compounds 1–6 have for H2O2 is clear, Table 8. Furthermore, Kguaiacol for 1–6 is smaller than that for KH2O2; hence, their affinity for guaiacol is higher, being in accordance with the native peroxidase mechanism, where once the enzyme is oxidized, it will immediately oxidize the substrate. Then, from the PI plots versus 1/[guaiacol] (secondary plots), kcat for 1–6 are calculated. Finally, the complexes showed kcat in the same order of magnitude (102 M–1 s–1) and fall into the same class of complexes already reported, and the complete set of kinetic parameters can be found in Table 8.
Table 8 helped us complete an extensive study in our research group. It becomes clearer that there is an optimal length of the aliphatic chain in the pinch ligand to achieve better catalytic effect of the compounds (Figure 7 and Table 8). Another fundamental characteristic in the pinch ligand is the influence of the nitrogen atom present in the middle of the chain because this can stabilize the mobility of the structure. The trend of the plot in Figure 7 shows that the larger the chain, the least catalytically efficient the model is; however, as we can see, the ligands with an equal number of atoms have higher catalytic activity if a nitrogen atom is present in the middle of the chain.
Figure 7.

Influence of the chain length of the pinch ligand in the catalytic activity of peroxidases models16,20 (with permission of Reyes-Ortega, Y. et al. Dalton Trans. 1998,4, 667–674), asterisks indicate when a nitrogen atom is present in the middle of the chain.
Conclusions
Six novel compounds of the pinch-porphyrin family were synthesized with a simple methodology. They have catalytic activity when tested as peroxidases models as well. However, the reaction rates were lower than the ones for native proteins such as the HRP and for the three porphyrin-Fe(III) used: proto, meso, and deuteroporphyrin, which according to our research group, is explained by the number of the atoms in the aliphatic chain of the ligand and the percentage of the Fe(III) species with s = 3/2. According to the UV–vis and ESR spectroscopic analyses, all compounds showed different spin states of Fe(III). These Fe species exhibit additionally to the high, intermediate, and low spin, the qms in the Fe(III), but the contribution in the species is different among the six complexes. Compounds 1 and 4 were the best peroxidase models with kcat = 2.00 × 104 and 4.58 × 102 M–1 s–1, respectively, and s = 3/2 percentage contribution of 85 and 92% in the species B and 80 and 90% in the species C. It is worth mentioning that both species are not necessarily the most abundant of all the three. This work helps us extend the study of the pinch-porphyrin compounds by our research group and adds to the interpretation that the percentage of the spin state in Fe(III) correlates with its catalytic activity; hence, we direct our research toward the synthesis of pinch porphyrin compounds with ligands that satisfy the s = 3/2 species to achieve better artificial peroxidases.
Materials and Methods
Titration and other spectrophotometric measurements were performed on a UV–vis/NIR Shimadzu 3100 spectrophotometer at 25 °C. The 1H NMR was measured using the CD3OD solvent in a Bruker 500 MHz frequency magnet in a range from −30 to 30 °C. ESR spectra were recorded using ultrapure quartz tubes of 3 mm on a JEOL JES-RE3X spectrometer at liquid-nitrogen temperature. The recording parameters in the microwave X-band (9.8 GHz) were as follows: sweep width 8000 G, power attenuation 20 mW, mode phase 100 kHz, and a time constant of 30 s. Magnetic measurements were performed in methanol at ca. 0.001 mM of 1–6 in a gelatin capsule using a Physical Property Measurement System (PPMS, Quantum Design, Inc., San Diego, CA, USA) from Quantum Design. Measurements were performed in small magnetic fields, from 20 to 200 G; the data were adjusted with the subtraction of the diamagnetic Pascal constant of methanol, the pinch ligand, and porphyrin.
Materials
Spectrophotometric and kinetic measurements were conducted in anhydrous methanol. The iron-porphyrins were prepared as described in previous studies.16,29 The pinch ligand 1,9-bis-(2-pyridyl)-2,8-diazanonane (picpenta) was prepared by the method of Ahmed et al., while the 1,12-bis-(2′-pyridil)-2,11-diazododecane (picocta) was obtained by the Sánchez-Sandoval methodology.16,30 The kinetic studies were conducted using aqueous 20.1 mM solution of guaiacol; porphyrin-iron methanolic solutions were in the range of 0.015–0.030 mM and the hydrogen peroxide solution was in the range of 1400–1600 mM.
Preparation of Iron-Pinch-Porphyrin Complexes
All pinch porphyrin complexes were synthesized using the method reported by Reyes-Ortega and co-workers. At 25 °C, 5 mL of Fe(III) porphyrin (0.01 mM) in methanol was added to 5 mL of 0.02 mM of the picpenta or picota ligand. The mixture was stirred for 6 h. The spectroscopic and kinetic studies were performed directly on the reaction solutions.
Kinetic Studies
Rate determinations of iron-porphyrin-catalyzed oxidation of guaiacol with hydrogen peroxide were carried out as previously described for horseradish peroxidase31 and other compounds. The used concentrations are reported in the Table S1. The oxidation product concentrations were determined by optical spectrophotometry. In order to observe the oxidation products (Scheme S1), the reaction (guaiacol with peroxide in the presence of each pinch-porphyrin complex) was observed by UV–vis spectroscopy with time intervals of 90 s. It is important to note that the complexes were stable throughout the studies. The pH range was 6.5–7.0, and the final concentrations were 0.017, 0.160, and 11.27 mM.
Acknowledgments
This research was funded by Vicerrectoría de Investigación y Estudios de Posgrado of BUAP projects REOY-NAT16-G, REOY-NAT17-G, and HEAS NAT-17 and Projects by Redes Temáticas PRODEP: Química de Coordinación con Aplicación al Magnetismo y Catálisis Homogénea 2015–2016 and PTC-463. CA-261-BUAP. U.A.A. thanks CONACyT for scholarship no. 202675 and Dr. Veronica Tello and Dr. Sandra E. Pineda for reviewing the paper.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03186.
1H NMR spectra at different temperatures of 1–6, UV–vis of 1–6, μeff versus T of 1–6, kinetic plots of 1–6, specific concentration for the kinetic analysis, and computational parameters (PDF)
Author Contributions
S.H.A., Y.R.O., and H.V.-L. integrated the data, wrote, and designed the manuscript. S.H.A., Y.R.O., D.R.R., and R.Z.U. performed and discussed the ESR measurements. U.A.A. performed the majority of experiments. A.P.B. edited, revised, and helped with the structure of the manuscript. H.V.-L. contributed with the DFT calculations and the discussion of the manuscript. The authors are grateful to CONACYT project 225115 for the acquisition of the ESR Bruker spectrophotometer.
The authors declare no competing financial interest.
Supplementary Material
References
- Albert L.; David L.; Nelson Y.; Cox M. M.. Lehninger Principles of Biochemistry, 5th ed.; WH Freemanand Company: New York, NY, USA, 2008. [Google Scholar]
- a Kalsoom U.; Bhatti H. N.; Asgher M. Characterization of Plant Peroxidases and Their Potential for Degradation of Dyes: a Review. Appl. Biochem. Biotechnol. 2015, 176, 1529–1550. 10.1007/s12010-015-1674-3. [DOI] [PubMed] [Google Scholar]; b Zucca P.; Neves C.; Simões M.; Neves M.; Cocco G.; Sanjust E. Immobilized Lignin Peroxidase-Like Metalloporphyrins as Reusable Catalysts in Oxidative Bleaching of Industrial Dyes. Molecules 2016, 21, 964–981. 10.3390/molecules21070964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistuzzi G.; Bellei M.; Bortolotti C. A.; Sola M. Redox properties of heme peroxidases. Arch. Biochem. Biophys. 2010, 500, 21–36. 10.1016/j.abb.2010.03.002. [DOI] [PubMed] [Google Scholar]
- a Gebicka L.; Gebicki J. L. Scavenging of oxygen radicals by heme peroxidases. Acta Biochim. Pol. 1996, 43, 673–678. [PubMed] [Google Scholar]; b Vlasova I. Peroxidase Activity of Human Hemoproteins: Keeping the Fire under Control. Molecules 2018, 23, 2561–2569. 10.3390/molecules23102561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydov R.; Osborne R. L.; Kim S. H.; Dawson J. H.; Hoffman B. M. EPR and ENDOR Studies of Cryoreduced Compounds II of Peroxidases and Myoglobin. Proton-Coupled Electron Transfer and Protonation Status of Ferryl Hemes. Biochemistry 2008, 47, 5147–5155. 10.1021/bi702514d. [DOI] [PubMed] [Google Scholar]
- Ayala M.; Roman R.; Vazquez-Duhalt R. A Catalytic Approach to Estimate the Redox Potential of Heme-Peroxidases. Biochem. Biophys. Res. Commun. 2007, 357, 804–808. 10.1016/j.bbrc.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Nakamura M.; Ikeue T.; Fujii H.; Yoshimura T.; Tajima K. Electron configuration and spin distribution in low-spin (meso -tetraalkylporphyrinato)iron(III) complexes carrying one or two orientationally fixed imidazole ligands 1. Inorg. Chem. 1998, 37, 2405–2414. 10.1021/ic9801241. [DOI] [Google Scholar]
- Hernández Anzaldo S.; Arroyo Abad U.; León García A.; Ramírez Rosales D.; Zamorano Ulloa R.; Reyes Ortega Y. Spectroscopic and kinetic characterization of peroxidase-like π-cation radical pinch-porphyrin-iron(III) reaction intermediate models of peroxidase enzymes. Molecules 2016, 21, 804. 10.3390/molecules21070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferranco A.; Basak S.; Lough A.; Kraatz H.-B. Metal Coordination of Ferrocene–Histidine Conjugates. Dalton Trans. 2017, 46, 4844–4859. 10.1039/c7dt00456g. [DOI] [PubMed] [Google Scholar]
- Hassani B. K.; Steunou A.-S.; Liotenberg S.; Reiss-Husson F.; Astier C.; Ouchane S. Adaptation to oxygen: Role of terminal oxidases in photosynthesis initiation in the purple photosynthetic bacterium, rubrivivax gelatinosus. J. Biol. Chem. 2010, 285, 19891–19899. 10.1074/jbc.m109.086066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Reed C. A.; Guiset F. A magnetochemical series. Ligand Field Strengths of Weakly Binding Anions Deduced from S = 3/2, 5/2 Spin State Mixing in Fe(III) Porphyrins. J. Am. Chem. Soc. 1996, 118, 3281–3282. 10.1021/ja954263i. [DOI] [Google Scholar]; b Sharma V. S.; Geibel J. F.; Ranney H. M. “Tension” on Heme by the Proximal Base and Ligand Reactivity: Conclusions Drawn from Model Compounds for the Reaction of Hemoglobin. Proc. Natl. Acad. Sci. U.S.A. 1978, 75, 3747–3750. 10.1073/pnas.75.8.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins P. W.Shriver & Atkins’ Inorganic Chemistry, 5th ed.; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- a Maltempo M. M.; Moss T. H. The Spin 3/2 State and Quantum Spin Mixtures in Haem Proteins. Q. Rev. Biophys. 1976, 9, 181–215. 10.1017/s0033583500002407. [DOI] [PubMed] [Google Scholar]; b Cammack R.; Cooper C. E. Electron Paramagnetic Resonance Spectroscopy of Iron Complexes and Iron-Containing Protein. Methods Enzymol. 1993, 227, 353–384. 10.1016/0076-6879(93)27014-8. [DOI] [PubMed] [Google Scholar]
- Maltempo M. M.; Moss T. H.; Cusanovich M. A. Magnetic Studies on the Changes in the Iron Environment in Chromatium Ferricytochrome, C.′. Biochim. Biophys. Acta, Protein Struct. 1974, 342, 290–305. 10.1016/0005-2795(74)90084-1. [DOI] [PubMed] [Google Scholar]
- Kirkland J. K.; Khan S. N.; Casale B.; Miliordos E.; Vogiatzis K. D. Ligand Field Effects on the Ground and Excited States of Reactive FeO 2+ Species. Phys. Chem. Chem. Phys. 2018, 20, 28786. 10.1039/c8cp05372c. [DOI] [PubMed] [Google Scholar]
- Reyes-Ortega Y.; Alvarez-Toledano C.; Ramírez-Rosales D.; Sánchez-Sandoval A.; González-Vergara E.; Zamorano-Ulloa R. Pinch-porphyrins, new spectroscopic and kinetic models of peroxidases. J. Chem. Soc., Dalton Trans. 1998, 4, 667–674. 10.1039/a704516f. [DOI] [Google Scholar]
- Montforts F.-P. The porphyrin handbook. Vols. 11-20. Edited by Karl, M. Kadish, Kevin, M. Smith and Roger Guilard. Angew. Chem., Int. Ed 2004, 43, 5431–5432. 10.1002/anie.200385127. [DOI] [Google Scholar]; Budd D. L.; La Mar G. N.; Langry K. C.; Smith K. M.; Nayyir-Mazhir R. Proton NMR Study of High-Spin Ferric Natural Porphyrin Derivatives as Models of Methemoproteins. J. Am. Chem. Soc. 1979, 101, 6091–6096. 10.1021/ja00514a036. [DOI] [Google Scholar]
- Hernández-Anzaldo S.; Sánchez-Morales N.; Zamorano-Ulloa R.; Escudero R.; de Jesús Rosales Hoz M.; Reyes-Ortega Y. Esr and magnetic studies of octahedral [Fe(III)(Cl)(pcd)(H2O)(dmso)] (pcd=pyridine-2,6-dicarboxylato) compound showing Fe(III) species with different spin states in solution. J. Mol. Struct. 2013, 1040, 39–46. 10.1016/j.molstruc.2013.02.021. [DOI] [Google Scholar]
- Traylor T. G.; Lee W. A.; Stynes D. V. Model compound studies related to peroxidases. Mechanisms of reactions of hemins with peracids. J. Am. Chem. Soc. 1984, 106, 755–764. 10.1021/ja00315a050. [DOI] [Google Scholar]
- Sánchez-Sandoval A.; Ramírez-Rosales D.; Zamorano-Ulloa R.; Álvarez-Toledano C.; Moya-Cabrera M.; Reyes-Ortega Y. New pinch-porphyrin complexes with quantum mixed spin ground state s=, of iron (III) and their catalytic activity as peroxidase. Biophys. Chem. 2003, 106, 253–265. 10.1016/s0301-4622(03)00186-8. [DOI] [PubMed] [Google Scholar]; b Gonzalez-Vergara E.; Meyer M.; Goff H. M. Proton nuclear magnetic resonance spectroscopy of horseradish peroxidase isoenzymes: Correlation of distinctive spectra with isoenzyme specific activities. Biochemistry 1985, 24, 6561–6567. 10.1021/bi00344a038. [DOI] [PubMed] [Google Scholar]
- Traylor T. G.; Popovitz-Biro R. Hydrogen bonding to the proximal imidazole in heme protein model compounds: Effects upon oxygen binding and peroxidase activity. J. Am. Chem. Soc. 1988, 110, 239–243. 10.1021/ja00209a039. [DOI] [Google Scholar]; b Dowsing R. D.; Gibson J. F. Electron spin resonance of high-spin d5 systems. J. Chem. Phys. 1969, 50, 294–303. 10.1063/1.1670791. [DOI] [Google Scholar]
- Maltempo M. M.; Ohlsson P. I.; Paul K. G.; Petersson L.; Ehrenberg A. Electron Paramagnetic Resonance Analyses of Horseradish Peroxidase in Situ and after Purification. Biochemistry 1979, 18, 2935–2941. 10.1021/bi00581a003. [DOI] [PubMed] [Google Scholar]
- Nesset M. J. M.; Cai S.; Shokhireva T. K.; Shokhirev N. V.; Jacobson S. E.; Jayaraj K.; Gold A.; Walker F. A. Electronic Effects in Transition Metal Porphyrins. Effect of Ortho Substituents on the Temperature Dependence of the NMR Spectra of a Series of Spin-Admixed Perchloratoiron(III) Tetrakis(2,6- or 2,4,6-Phenyl Substituted)Porphyrinates. Inorg. Chem. 2000, 39, 532–540. 10.1021/ic9907866. [DOI] [PubMed] [Google Scholar]
- Dhifaoui S.; Hajji M.; Nasri S.; Guerfel T.; Daran J. C.; Nasri H. A New High-Spin Iron(III) Bis(Aqua) Complex with the Meso-Tetra(Para-Chlorophenyl)Porphyrin: X-Ray Crystallography, Hirshfeld Surface Analysis, Magnetic, EPR and Electrochemical Properties. Res. Chem. Intermed. 2018, 44, 7259–7276. 10.1007/s11164-018-3555-1. [DOI] [Google Scholar]
- Drago R. S.Physical Methods for Chemists, 2nd ed.; Ft. Worth: Saunders College Pub: Florida, 1992; pp 470–475. [Google Scholar]
- Cleland W. W. Derivation of Rate Equations for Multisite Ping-Pong Mechanisms with Ping-Pong Reactions at One or More Sites. J. Biol. Chem. 1973, 248, 8353–8355. [PubMed] [Google Scholar]
- Fang M.; Wilson S. R.; Suslick K. S.; Suslick S. A Four-Coordinate Fe(III) Porphyrin Cation. J. Am. Chem. Soc. 2008, 130, 1134–1135. 10.1021/ja078061l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo J. D.; Pedreño E.; Garcia-Carmona F.; Garcia-Cánovas F.; Muñoz F. S.; Lozano J. A. Steady-State Study of the Mechanism of Dopa-Oxidase Activity of Tyrosinase. Int. J. Biochem. 1983, 15, 1455–1461. 10.1016/0020-711x(83)90078-2. [DOI] [PubMed] [Google Scholar]
- Falk A. D.The Porphyrins and Metalloporphyrins; Elsevier: New York, NY, USA, 1964. [Google Scholar]
- Ahmed E.; Chatterjee C.; Cooksey C. J.; Tobe M. L.; Williams G.; Humanes M. Base-catalysed aquation of αβ-syn- and αβ-anti-chloro- and bromo-[1,9-bis(2′-pyridyl)-2,5,8-triazanonane]-and-[1,11-bis(2′-pyridyl)-2,6,10-triazaundecane]-Co(III) cations. An unusually base-sensitive halogenopentamine Co(III) system. J. Chem. Soc., Dalton Trans. 1989, 645–654. 10.1039/dt9890000645. [DOI] [Google Scholar]
- Bergmeyer H. U.; Dermot H.; Williamson K. G.. Methods of Enzymatic Analysis; Elsevier: Amsterdan, 1974; pp 158–250. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





