Abstract
Muscarinic acetylcholine receptors (mAChR) play important roles in regulating complex behaviors such as cognition, movement, and reward, making them ideally situated as potential drug targets for the treatment of several brain disorders. Recent advances in the discovery of subtype-selective allosteric modulators for mAChRs has provided an unprecedented opportunity for highly specific modulation of signaling by individual mAChR subtypes in the brain. Recently, mAChR allosteric modulators have entered clinical development for Alzheimer’s disease (AD) and schizophrenia, and have potential utility for other brain disorders. However, mAChR allosteric modulators can display a diverse array of pharmacological properties, and a more nuanced understanding of the mAChR will be necessary to best translate preclinical findings into successful clinical treatments.
Targeting Muscarinic Acetylcholine Receptors
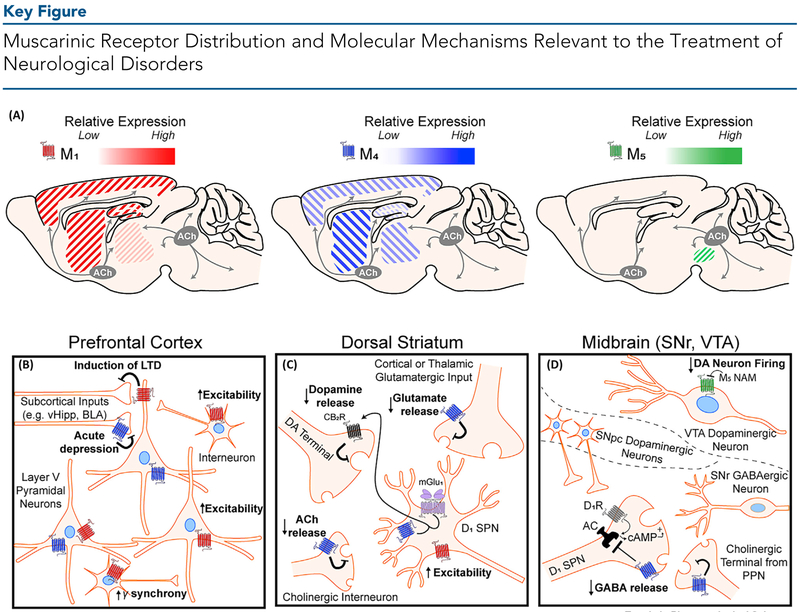
Acetylcholine (ACh) plays a major role as a neurotransmitter and neuromodulator throughout the central nervous system (CNS) as well as in multiple peripheral systems [1,2]. In the CNS, cholinergic sources include local interneurons that are present in multiple brain regions, and also projections originating from the brainstem pedunculopontine and lateral dorsal tegmental nuclei as well as from the basal forebrain nuclei [1]. The latter provides long-range cholinergic projections and is the major source of ACh in the neocortex, hippocampus, and amygdala (Figure 1A, Key Figure), brain regions important in learning and memory.
Figure 1.
(A) Distribution of M1, M4, and M5 muscarinic acetylcholine (ACh) receptors in brain regions implicated in neurological dysfunction. The relative expression of each receptor subtype is indicated by its respective color gradient. M2 and M3 (not shown) muscarinic ACh receptors (mAChRs) are also expressed widely throughout the brain. M1 mAChRs are highly expressed in the cortex, hippocampus, and dorsal and ventral striatum, and are expressed at low levels in thalamic areas. M4 mAChRs are highly expressed in striatal regions, moderately expressed across the cortex and thalamus, and are poorly expressed in the hippocampus. M5 mAChR expression is restricted to the midbrain. Cholinergic projection neurons produce and release ACh from two distinct clusters – the basal forebrain nuclei (grey circle, left) which innervates cortical, hippocampal, and thalamic areas, and the brain stem nuclei (grey circle, right) which innervates midbrain, hindbrain, thalamic, and cerebellar areas. Cholinergic tone in the dorsal and ventral striatum is primarily provided by large cholinergic interneurons (not depicted). (B) In the prefrontal cortex, M1 mAChR activation induces a form of long-term depression (LTD) of glutamatergic inputs from subcortical areas including the ventral hippocampus (vHipp) and basolateral amygdala (BLA). M1 mAChR activation also increases the excitability of pyramidal neurons and GABAergic interneurons. Activation of M1 via interneurons can also increase gamma oscillation synchrony in the cortex. M4 mAChRs can acutely inhibit neurotransmitter release. (C) In the dorsal striatum, M4 mAChRs expressed on direct pathway D1 receptor-positive spiny projection neurons (SPNs) interact with metabotropic glutamate receptor 1 (mGlu1) to produce endocannabinoids which then bind to cannabinoid type 2 (CB2) receptors to inhibit local dopamine release. In addition, M4 activation can reduce both ACh release from local cholinergic interneurons and act as a heteroreceptor on glutamatergic terminals from the cortex and thalamus to reduce glutamate release. M1 mAChRs expressed on D1-SPNs increase the excitability of these neurons. (D) In the midbrain, cholinergic modulation of dopaminergic (DA) neurons in the ventral tegmental area (VTA) and direct pathway input into the substantia nigra reticulata (SNr) are relevant to neurological disorders. In the VTA (top), M5 mAChRs are expressed on VTA DA neurons and M5 negative allosteric modulators (NAMs) are hypothesized to reduce DA neuron firing. In the SNr (bottom), M4 mAChR activation on direct pathway D1-SPN terminals directly opposes increased GABA release mediated through D1-receptor activation by DA released from the substantia nigra pars compacta (SNpc, middle). M4 can also act as an autoreceptor and reduce ACh release from cholinergic projection terminals.
ACh can signal through two distinct classes of receptors that include ligand-gated cation channels, termed nicotinic ACh receptors, and G protein-coupled muscarinic ACh receptors (mAChRs). Although both receptor classes play important roles in the central and peripheral nervous systems, in the CNS ACh acts primarily through mAChRs as a neuromodulator to shape ensembles of neurons and alter neuronal firing in response to changing environmental conditions [1,3], The five-member mAChR G protein-coupled receptor (GPCR) family consists of M1, M3, and M5, which primarily couple to Gq to activate phospholipase C, and M2 and M4, which primarily couple to Gi/o to inhibit adenylyl cyclase and modulate ion channels. Considerable evidence suggests that mAChRs are centrally involved in modulating complex behaviors such as cognition, motivation, and substance use disorder (SUD) [4,5], and their localization both pre- and postsynaptically throughout the CNS means that mAChRs are uniquely situated as potential targets for the treatment of multiple CNS disorders (Figure 1B–D).
Targeting mAChRs for the Treatment of AD and Schizophrenia
Cholinergic signaling is disrupted in AD, and several post-mortem studies have demonstrated a significant reduction in cholinergic projection neurons originating in the basal forebrain of patients with AD [6]. Current clinical strategies to combat the loss of cholinergic neurons and restore memory and cognition in AD include raising total cholinergic tone through systemic administration of acetylcholinesterase (AChE) inhibitors that block the breakdown of ACh [7]. Although AChE inhibitors such as tacrine and donepezil have demonstrated dose-dependent efficacy in improving cognition in patients with early-stage AD, they suffer from dose-limiting adverse effects attributed to generalized non-selective activation of cholinergic receptors in the CNS and periphery, thereby limiting their clinical utility [7]. Therefore, there is intense interest in developing more selective agents that activate specific receptor subtypes within the cholinergic system.
Robust preclinical and clinical evidence suggests that mAChRs are crucially involved in learning and memory [4], and significant investments have been made in developing ligands that engage mAChRs for the treatment of cognitive disruptions associated with AD, including the nonselective M1/M4 receptor-preferring agonist xanomeline. In a Phase III clinical study in patients with AD, xanomeline significantly reduced behavioral disturbances including vocal outbursts, suspiciousness, delusions, agitation, hallucinations, and had trending but not statistically significant improvements in cognition [8]. Although the clinical effects were promising, xanomeline has activity at all mAChR subtypes and induced severe dose-limiting gastrointestinal (GI) and other adverse effects that are mediated by activation of peripheral mAChRs [8,9]. Despite these peripheral effects, the promising reduction in behavioral disturbances and the trending effect on cognition prompted a small follow-up Phase II clinical trial in patients with schizophrenia [10]. Xanomeline produced significant improvements in the brief psychiatric rating scale (BPRS, see Glossary), positive and negative syndrome scale (PANSS), and the clinical global impression scale, compared to the placebo-controlled group [10]. Similar to the AD study, xanomeline produced GI disturbances, which halted further clinical development of xanomeline [9,10]. Follow-up preclinical studies suggest that activation of M2 and M3 in the periphery are responsible for the peripheral adverse effects of xanomeline [9]. Recently, Karuna pharmaceuticals renewed interest in xanomeline by advancing KarXT, a combination therapy of xanomeline with the peripherally restricted mAChR antagonist, trospium chloride [11], into a Phase II clinical trial for schizophrenia (Clinical Trial Numberi: ). Although this combination therapy may reduce the adverse effects of xanomeline in the periphery, and thereby increase the therapeutic window of xanomeline, a more targeted approach selectively activating specific mAChRs may provide the greatest clinical benefit.
Significant investment has been made to develop selective agonists devoid of M2 and M3 activation, including development of the M1 agonist HTL0018318 by Sosei Heptares, who recently partnered with Allergan to sponsor a Phase I clinical trial for AD (Clinical Trial Numberi:) and Phase II trial in patients with Lewy body dementia in Japan (JapicCTIii:183989) (Table 1). However, an unexpected HTL0018318 chronic dosing toxicology finding in nonhuman primates placed the clinical trial in Lewy body dementia patients on holdiii (Table 1). Unfortunately, despite major investments in medicinal chemistry to develop highly selective M1 or M4 orthosteric agonists, these efforts have largely failed owing to the highly conserved orthosteric site for ACh binding among the mAChRs.
Table 1.
Recent Compounds Targeting the mAChR in Clinical Developmenta
| mAChR | Compound | Ligand type | Therapeutic area | Refs | Preclinical or clinical stage | Clinical trial identifier |
|---|---|---|---|---|---|---|
| M1 | VU319 | PAM | AD | - | Phase I; ongoing | (USA) |
| MK-7622 | PAM | AD | [56] | Phase II; discontinued | (USA) | |
| TAK-071 | PAM | Mild cognitive impairment/AD | [39,88] | Phase I; concluded | (USA) | |
| HTL0018318 | Agonist | AD | - | Phase Ib; completed | (USA) | |
| Lewy body dementia | - | Phase II (Japan); withdrawn | JapicCTI-183989 (Japan), secondary ID: (USA) | |||
| NS | PAM | Schizophrenia | [37,38] | Preclinical | - | |
| M1/M4 | KarXT (xanomeline plus trospium chloride) | Nonselective M1/M4 preferring agonist plus peripherally restricted muscarinic antagonist | Schizophrenia | - | Phase II; ongoing | (USA) |
| M4 | HTL0016878 | Agonist | AD | - | Phase I; ongoing | (USA) |
| NS | PAM | Schizophrenia | [73,76,79] | Preclinical | - | |
| NS | NAM/antagonist | Parkinson’s disease [99] | [98] | Preclinical | - | |
| M5 | NS | NAM | Substance use disorder | [109,110] | Preclinical | - |
Abbreviations: AD, Alzheimer’s disease, mAChR, muscarinic acetylcholine receptor; NAM, negative allosteric modulator; NS, not specified; PAM, positive allosteric modulator.
Allosteric Modulators of mAChRs
To develop highly selective small-molecule ligands for specific mAChR subtypes, several groups have pursued the development of allosteric modulators that target less well conserved allosteric sites that are distinct from the orthosteric ACh binding site [12,13]. Significant progress has been made in understanding the structural basis of allosterism given the determination of the crystal structure for multiple mAChR subtypes [14–18], and the crystallization of several state-dependent receptor conformations [19]. Collectively, the insights into the exact nature of orthosteric and allosteric ligand interactions provided by these crystal structures, paired with state-of-the-art in silico docking of digital compound libraries, provide the exciting potential to screen large numbers of compounds in very little time and at low cost, thereby identifying new chemical scaffolds and novel selective ligands through rational drug design [20,21].
Positive allosteric modulators (PAMs) increase responses to orthosteric agonists, whereas negative allosteric modulators (NAMs) inhibit responses to orthosteric agonists [13]. PAMs and NAMs exert their effects by modulating the affinity of an orthosteric ligand to the receptor or by modulating coupling to intracellular signaling [13]. Follow-up functional studies, including work on receptor-knockout animals, have demonstrated that the procognitive and antipsychotic-like effects of xanomeline are likely mediated by M1 and M4 receptors, respectively [9,22,23]. Thus, multiple drug discovery efforts have focused on developing allosteric modulators for these two mAChR subtypes.
Potential Cognition-Enhancing Effects of M1 PAMs
The M1 mAChR is the most abundant of the five mAChR subtypes expressed in brain regions crucially involved in cognition, such as the prefrontal cortex (PFC) and hippocampus [24,25]. Pharmacological blockade [26–28] or genetic deletion [29] of M1 produces disturbances in learning and memory. Based on these studies, and extensive clinical studies implicating central mAChRs in cognitive processing [30–32], selective potentiation of M1 signaling in the CNS using highly selective M1 PAMs may hold promise to enhance cognition and reverse learning and memory disturbances.
Over the past decade, multiple studies have shown that M1 PAMs have robust efficacy in reversing cognitive disruptions in preclinical animal models relevant for AD [33–35] and schizophrenia [36–41]. M1 PAMs can potentiate a form of synaptic plasticity termed long-term depression (LTD) [37,42], enhance neuronal excitability [43], and reverse synaptic plasticity deficits in the PFC. In addition, several studies have demonstrated a role of the M1 mAChRs in hippocampal function since M1 PAMs can specifically potentiate LTD at the hippocampus–prefrontal cortex (PFC) synapse [44], and activation of M1 in the hippocampus can induce long-term potentiation (LTP) [45,46] as well as facilitate spatial reversal learning, an important hippocampus-dependent task [47].
Overactivation of the M1 mAChR May Be Detrimental to M1 PAM Efficacy
Although these findings are very promising, recent studies have revealed that some but not all M1 PAMs have adverse effects, including GI distress and behavioral convulsions in rodents and dogs [24,42,48]. It was previously demonstrated that the nonselective mAChR orthosteric agonist pilocarpine induced robust seizures in healthy adult mice and mice in which M2, M3, M4, or M5 receptors were genetically knocked out (KO), but produced no effect in M1-KO mice, suggesting that overactivation of the M1 receptor mediates these adverse effects [49,50]. Therefore, one possibility to account for the stark contrast between M1 PAMs that produce adverse effects and those that do not is the hypothesis that some M1 PAMs overactivate the M1 receptor and therefore lead to similar adverse effects as traditional orthosteric agonists [42,48,51,52]. This is reminiscent of studies from allosteric modulators for other GPCRs, such as the metabotropic glutamate receptor subtype 5 (mGlu5), which demonstrated that the allosteric agonist activity of mGlu5 PAMs can cause severe behavioral convulsions in rodents [53].
In agreement with this hypothesis, M1 PAMs such PF-06764427 and MK-7622 (Table 1) demonstrate robust agonist activity in addition to PAM activity (ago-PAM) and induce M1-dependent behavioral convulsions in rodents [42,48] that were absent in M1-KO mice. This contrasts with two structurally distinct M1 PAMs, VU0453595 and VU0550164, that were optimized to eliminate agonist activity [42]. Similarly to previously described M1 PAMs, VU0453595 and VU0550164 potentiate M1 responses to ACh [42]. However, in contrast to PF-06764427 and MK-7622, VU0453595 and VU0550164 lack agonist activity in all assays tested [42]. Furthermore, the severe adverse effects observed with the M1 ago-PAMs were not observed at any dose of VU0453595, an M1 PAM optimized to avoid allosteric agonist activity [42]. Finally, VU0453595 (but not MK-7622) has robust efficacy in improving object recognition memory in rats [42]. These studies suggest that the ability of MK-7622 to activate M1 mAChRs regardless of presynaptic ACh release may lead to aberrant receptor activity and may even disrupt cognition. These properties could therefore explain why MK-7622 did not meet clinical endpoints in a proof-of-concept clinical trial in AD patients [54]. Together, these studies have provided fundamental new insights into the impact of subtle differences in the modes of activity of different M1 PAMs and the need to strictly avoid allosteric agonist activity in these compounds.
Interestingly, a newer M1 PAM, PF-06827443, was reported to have minimal agonist activity in cell lines but still produced robust adverse effects in preclinical animal model studies [24]. However, allosteric agonist activity can vary dramatically depending on total receptor expression, and is much more evident in systems that contain high receptor reserve. A follow-up study demonstrated that PF-06827443 has robust agonist activity in moderate- and high-expressing cell lines, as well as in native brain tissue electrophysiological assays [52]. Thus, PF-06827443 is also an ago-PAM, and allosteric agonist activity likely contributes to the adverse effect liability of this compound.
M1 PAMs That Display Bias Can Have Differential Effects in the CNS
In addition to differences in allosteric agonist activity, M1 PAMs can also differ in their ability to confer bias to M1 signaling. Signal bias is the phenomenon by which different GPCR ligands induce distinct active receptor-complex states that are biased toward or away from specific signaling pathways (Figure 2A) [55]. To date, GPCR signal bias has been well characterized for μ opioid receptor agonists that can signal through G proteins, β-arrestin, or both [56]. Recent work suggests that μ opioid receptor agonists that avoid β-arrestin activity and preferentially signal through G proteins can induce analgesia while minimizing respiratory suppression. Therefore, these biased ligands could provide a larger therapeutic window than fentanyl, which preferentially signals through β-arrestin and produces robust respiratory depression [56,57]. Thus, characterization of potential signal bias in muscarinic ligands may provide opportunities to understand specific signaling pathways involved in efficacy, and potentially increase in vivo efficacy while minimizing adverse effect liability.
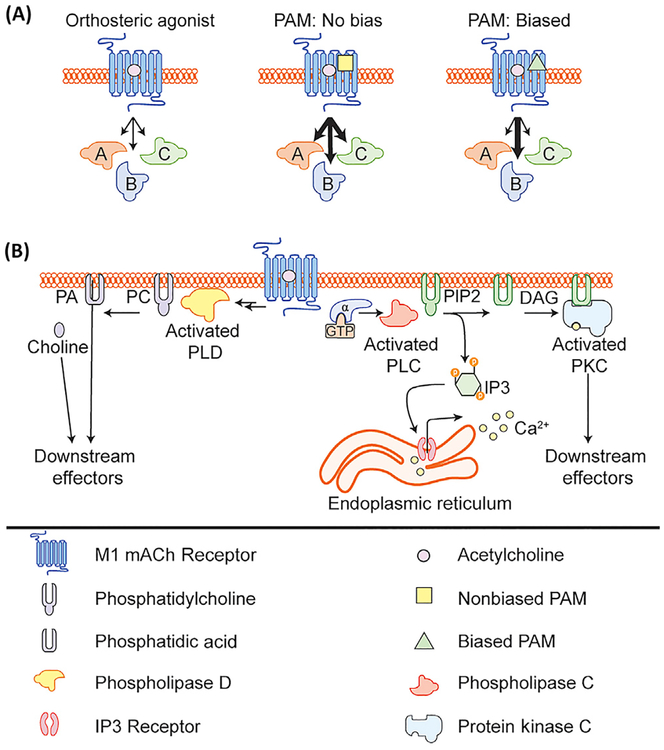
Figure 2. M1 Muscarinic Acetylcholine Receptor (mAChR) Allosteric Modulator-Induced Signal Bias.
(A) Schematic depicting the effects of acetylcholine (ACh) alone, ACh plus a non-biased M1 positive allosteric modulator (PAM), or ACh plus a biased M1 PAM on various downstream signaling cascades. (B) Activation of the M1 mAChR can lead to the activation of several downstream signaling pathways including canonical activation of Gaq signaling leading to activation of phospholipase C, release of calcium, and activation of PKC. M1 mAChR activation can also lead to activation of phospholipase D, through an unknown mechanism. Abbreviations: DAG, diacylglycerol; IP3, inositol trisphosphate; PA, phosphatidic acid; PC, phosphatidylcholine; PIP2, phosphatidylinositol bisphosphate.
Characterization of a broad range of structurally diverse M1 PAMs revealed that some M1 PAMs confer signal bias and potentiate receptor signaling through the canonical phospholipase C (PLC) pathway, but do not potentiate M1 receptor-mediated activation of phospholipase D (PLD) [58]. Little was known about the role of PLD in M1 signaling in the CNS or whether PLD is necessary for any M1-dependent signaling. Using brain slice electrophysiology, follow-up studies demonstrated that not all M1-dependent responses in the CNS are PLD-dependent, and biased M1 PAMs function similarly to nonbiased M1 PAMs in M1 signaling that was PLD-independent [59]. However, M1 PAMs that do not couple to PLD function dramatically differently from nonbiased M1 PAMs in their ability to potentiate PLD-dependent M1-mediated plasticity in the PFC [59]. These findings demonstrate that PLD plays a crucial role in the ability of M1 PAMs to modulate particular CNS functions, and that biased M1 PAMs function differently in synaptic plasticity in the cortex that is implicated in cognition. However, PLC and PLD are only two of many different signaling pathways downstream of the M1 mAChR, and future studies are necessary to identify M1 PAMs with favorable in vivo properties that may display signal bias for other signaling pathways, including ERK and β-arrestin, to fully dissect the downstream signaling pathways important for efficacy and adverse effect liability. Furthermore, additional studies are necessary to determine whether biased M1 mAChR ligands have a lower propensity for inducing seizures and could therefore provide a larger therapeutic window, as is the case with biased mGlu5 PAMs that avoid activation of NMDA receptors [60].
In conclusion, the high-profile failure of several experimental therapeutic approaches targeting the reduction of Aβ in patients with AD warrants the identification and development of novel therapeutic targets for the treatment of the cognitive disruptions in AD. Furthermore, current antipsychotics do not improve and may even worsen the cognitive deficits associated with schizophrenia [61]. The ability of M1 PAMs to improve cognition in multiple animal models [27,36,37,40–42] suggests strong potential for success in the clinic and may help to mitigate the crucial issue common to animal models – that they often fail to recapitulate the full range of disease symptoms and etiology. However, with the recent Phase II failure of MK-7622 to significantly improve cognitive endpoints in AD patients [54], there is an urgent need to fully characterize M1 PAMs with respect to agonist activity, signal bias, and other pharmacological properties (Box 1) so as to de-risk clinical candidates and move the M1 PAM with the highest chance of success forward into the clinic.
Box 1. M1 PAMs with Low a-Values May Minimize Adverse Effects.
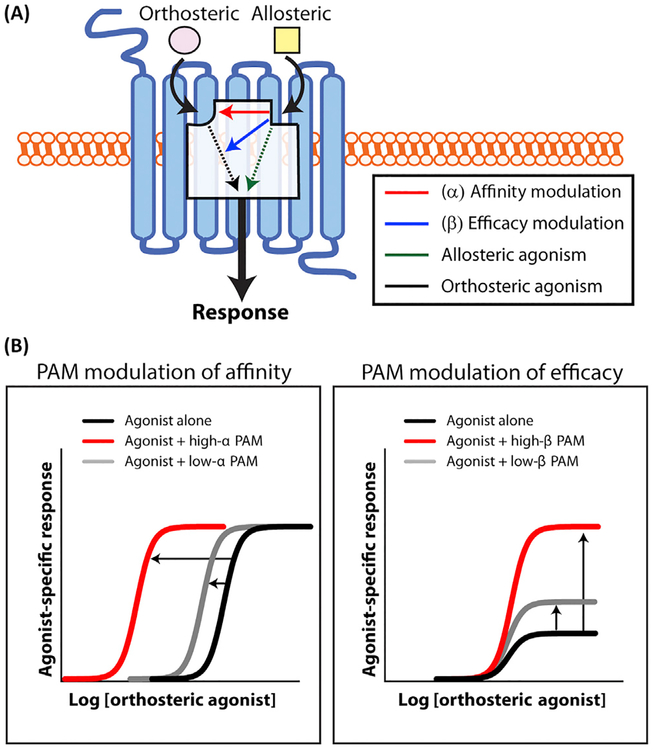
According to the operational allosteric ternary complex model [13,86], allosteric modulators can exert their effects by modulating the binding affinity of the orthosteric agonist (e.g., binding of ACh to the M1 mAChR) or by modulating receptor efficacy (e.g., the ACh response), termed α and b respectively (Figure IA). When α >1, the allosteric modulator increases the affinity of the orthosteric agonist, whereas α <1 demonstrates a decrease in receptor–agonist affinity. Conversely, when β >1, the allosteric modulator potentiates cellular activation, whereas when β <1 the modulator inhibits cellular activation (Figure IB). Importantly, α and β values are independent of each other and in theory can occur in every combination [86]. Several recent papers from Takeda Pharmaceutical Company suggest that M1 PAMs that possess low α values, such as TAK-071 (Table 1), have a wider therapeutic index in rodent models relevant for schizophrenia compared to M1 PAMs with high α values [38,87,88]. However, TAK-071 was not completely devoid of adverse effects, and demonstrated a concentration-dependent increase in spontaneous ileum motility [87]. Nonetheless, TAK-071 provides a much greater margin between doses leading to cognition-enhancing effects and those with adverse effects (e.g., diarrhea) compared to T-662, an M1 PAM with a high a value [38,87,89]. Furthermore, it is not known whether M1 PAMs with higher α and β values may be more beneficial in later stages of AD where there is greater loss of endogenous ACh [6,90]. Overall, although these results are promising, more extensive studies will be necessary to understand the exact relationship between α value, agonist activity, and signal bias to fully characterize the pharmacological profiles of M1 PAMs. Lastly, it is prudent to carefully consider the appropriate α and β values for a M1 PAM clinical candidate depending on the stage of AD (e.g., early or late) chosen for clinical intervention.
Figure I. Allosteric Modulator Modes of Action.
(A) Allosteric modulators (yellow squares) bind to a topographically and structurally distinct site on the muscarinic receptor to modulate orthosteric agonist (ACh, pink) affinity (red) and/or efficacy (blue). Binding of positive allosteric agonists (PAMs) or ago-PAMs can also directly induce receptor signaling in the absence of the orthosteric agonist (green). (B) (Left) Allosteric modulators that robustly modulate agonist affinity (high α value, red) will result in a large leftward shift in the orthosteric agonist concentration–response curve. By contrast, allosteric modulators that weakly enhance agonist affinity (low α value, grey) result in a modest leftward shift in the orthosteric agonist concentration–response curve. (Right) Allosteric modulators that strongly modulate agonist efficacy (high β value, red) may result in a large increase in the orthosteric agonist maximal response. By contrast, allosteric modulators that weakly enhance agonist efficacy (low β value, grey) result in a modest increase in the orthosteric agonist response. Sigmoidal curves were generated using Graphpad Prism8 (www.graphpad.com).
Selective M4 PAMs for the Treatment of Schizophrenia
The M4 mAChR is abundantly expressed in the dorsal striatum, nucleus accumbens, and the nigros-triatal and mesolimbic dopaminergic pathways [25,62], circuitry that has been implicated in the positive symptoms of schizophrenia that include hallucinations, delusions, and disorganized thought [63,64], as well as motivational deficits that contribute to the negative symptoms observed in schizophrenia patients. Given this localization and previous studies suggesting that mAChRs can reduce striatal dopamine signaling [65–68], it was hypothesized that activation of M4 mAChRs could reduce the hyperactivity of striatal dopaminergic pathways and exert antipsychotic-like efficacy. Consistent with this hypothesis, the antipsychotic-like effects of the M1/4-preferring agonist xanomeline were absent in M4-KO mice [23] as well as in mice in which M4 was specifically deleted from D1 dopamine receptor-expressing neurons [22]. Therefore, several research groups have aggressively pursued the development of highly selective, CNS-penetrant, M4 PAMs for the treatment of the psychotic symptoms of schizophrenia as well as of other brain disorders. Excitingly, several M4 PAMs including LY2033298 [69], VU0152099, and VU0152100 [70] have demonstrated robust antipsychotic-like efficacy in amphetamine- and apomorphine-induced models of psychosis including conditioned avoidance, hyperlocomotion, and disrupted prepulse inhibition (PPI). Moreover, M4 PAMs have displayed efficacy in other preclinical models relevant to neuropsychiatric and neurological disorders (Box 2).
Box 2. Potential Utility of M1 and M4 Allosteric Modulators for the Treatment of Other Schizophrenia Symptom Domains and Neurological Disorders.
In addition to potential efficacy in reversing cognitive deficits in AD and schizophrenia patients, recent studies suggest that M1 PAMs also improve social interactions in rodent models [37]. Previously, xanomeline demonstrated efficacy in reducing negative symptoms in schizophrenia patients [9,10], and it will therefore be important to fully evaluate the potential efficacy of M1 PAMs in animal models that are relevant for negative symptoms. To this same end, the wide variety of M1-and M4-selective tool compounds developed over the past decade have identified other psychiatric and neurological disorders in which subtype-selective muscarinic modulation may be effective. Consistent with procognitive efficacy, the M1 PAM BQCA improved learning and memory deficits in a rodent model of traumatic brain injury [91]. An M1 PAM was also able to enhance the consolidation and recall of fear extinction in a rodent model of post-traumatic stress disorder (PTSD) [44], suggesting that M1 PAMs could improve the efficacy of exposure therapy in the clinic for the treatment of PTSD and other anxiety disorders. In addition, M1 activation in combination with an M4 PAM accelerated the extinction of cocaine-seeking behavior [92], implying that potentiating M1 activation may broadly facilitate and/or enhance extinction learning across multiple behavioral paradigms.
Given their direct actions on dopamine release, M4 PAMs may provide benefit in other disorders that display exaggerated dopaminergic signaling. The M4 PAMs VU0467154 and VU0476406 reduced L-DOPA-induced dyskinesia in mice and nonhuman primate models, respectively [82]. VU0467154 also alleviated synaptic and motor deficits in the YAC128 model of Huntington’s disease [93], in part through effects on dopaminergic signaling but also via regulation of corticostriatal transmission [65]. Furthermore, M1 and M4 mAChRs may be viable targets for SUD because M1 activation reduced cocaine discrimination [94], and M4 PAMs effectively reduced cocaine self-administration and striatal dopamine release [95], facilitated extinction, and prevented reinstatement of cocaine-induced conditioned place preference [96].
Recent work suggests that M4 PAMs could be useful in the treatment of neurodevelopmental disorders such as fragile X and Rett syndrome. The M4 PAM VU0152100 normalized excessive protein synthesis and audiogenic seizures in Fmr1−/y mice, suggesting therapeutic potential in fragile X patients [97]. Further support for M4 PAMs as potential therapeutics for neurodevelopmental disorders is demonstrated by a recent RNA sequencing study that identified a reduction in total CHRM4 transcript levels (the mRNA encoding the M4 mAChR), in human Rett syndrome autopsy samples compared to controls, and found that an M4 PAM could rescue social and cognitive deficits in a mouse model of Rett syndrome [98]. The exact mechanisms underlying the procognitive effects of M4 PAMs and their efficacy in these neurodevelopmental models remain unknown, and future studies are necessary to fully understand these mechanisms.
Conversely, reducing M4 receptor signaling may be effective in disorders where dopamine is reduced, such as Parkinson’s disease (PD) [99]. Anticholinergic and pan-mAChR antagonists were some of the first clinically used treatments for PD [99] but, similarly to non-selective muscarinic agonists, they suffer from a lack of tolerability [99]. Important to PD treatment, we found that the M4 receptor may tonically inhibit D1-SPN transmission to the substantia nigra pars reticulata (SNr), an effect counteracted by D1 receptor signaling [66]. Therefore in states of reduced dopaminergic tone, such as in PD where the positive regulation of D1-SPN to SNr transmission via D1 is reduced or lost, M4 antagonism might alleviate cholinergic-mediated inhibition at this synapse, restore direct pathway function, and thereby restore normal motor function [66,99].
Of note, allosteric compounds targeting the M4 mAChRs exhibit species-specific pharmacology that must be taken into account when designing in vitro and in vivo assays [69,71,72]. LY2033298 was initially identified in a screen utilizing cell lines expressing human M4 receptors, and was later demonstrated to be significantly less potent at potentiating ACh responses in cell lines expressing rat M4 compared to human M4 [69]. Preclinical assessment of M4 PAM efficacy was further confounded because LY2033298 displays probe-dependence [71]. In these studies, LY2033298 failed to demonstrate a significant behavioral effect when dosed alone, but displayed antipsychotic-like effects when co-dosed with an ineffective dose of the synthetic mAChR agonist oxotremorine [69,71]. Overall, these pharmacodynamic challenges are not limited to LY2033298 and have been reported by other research groups [73,74], thereby producing challenges in determining the mechanism of action of these ligands as well as in developing clinical compounds. However, the findings from these early M4 mAChR studies demonstrate that detailed characterization of M4 mAChR pharmacology using cultured cells can be used to both predict and rationalize the subsequent design of in vivo studies to identify the mechanism by which M4 PAMs exert their antipsychotic efficacy.
To understand the biological mechanisms of M4 PAM antipsychotic efficacy in preclinical models, multiple rodent M4 PAMs have been developed, including VU0152100 [70,75] and VU0467154 [72,73]. Consistent with the proposed mechanism that the M4 mAChR is ideally localized to reduce hyperactivity of striatal dopamine signaling, VU0152100 attenuated amphetamine-induced activation of the dorsal striatum and nucleus accumbens as assessed by functional magnetic resonance imaging (fMRI) [75]. VU0152100 also attenuated amphetamine-induced striatal dopamine release [75], suggesting that M4 activation may have direct effects on dopaminergic signaling despite the lack of M4 expression on dopaminergic terminals in the striatum [76]. Further investigation into the biological mechanism of M4 PAM antipsychotic efficacy revealed a previously undescribed signaling pathway by which M4 activation of dopamine D1 receptor-expressing spiny projection neurons (D1-SPNs) in the dorsal striatum leads to the mobilization of endocannabinoids, which in turn activate cannabinoid CB2 receptors on dopaminergic terminals to locally reduce dopamine release [67]. In support of the importance of this mechanism for the in vivo antipsychotic efficacy of M4 PAMs, the ability of VU0467154 to reverse amphetamine-induced disruptions in PPI was lost in D1-SPN M4-KO mice and was blocked by the CB2 receptor antagonist AM630 [67]. These novel findings were intriguing because it is classically thought that Gq activation, not Gi/o, leads to the production of endocannabinoids. Subsequent studies revealed that coactivation of the metabotropic glutamate receptor subtype 1 (mGlu1) is required for both the M4 PAM-mediated reductions in dopamine release and the in vivo antipsychotic efficacy of M4 PAMs [68]. This finding has identified mGlu1 PAMs as novel potential antipsychotic treatments [68] and highlights the importance of fully characterizing the detailed mechanism of action of M4 PAMs in vivo because we could discover other druggable targets that act through similar pathways.
Finally, in addition to actions on dopamine release, it was recently demonstrated that M4 receptors at D1-SPN terminals in the substantia nigra pars reticulata (SNr) functionally antagonize D1 receptor-mediated increases in direct pathway transmission [66]. This is another mechanism by which M4 can counteract excessive dopamine signaling through D1 receptors that may be relevant to M4 antipsychotic-like efficacy. Together, the effects of M4 PAMs on dopamine release and dopamine D1 receptor signaling, and the fact that M4 PAMs lack any observable peripheral cholinergic effects seen with xanomeline [72], highlight the potential clinical advantages of M4 PAMs at reversing a hyperdo-paminergic state via a local, striatum-specific mechanism over the broad antagonism of dopamine receptors by current antipsychotic medications.
M4 PAMs Improve Cognition in Preclinical Studies
Although current antipsychotics can interfere with dopaminergic regulation of cognitive function in the hippocampus and PFC [77], M4 PAMs such as VU0467154 can improve cognitive function in multiple preclinical rodent models relevant to schizophrenia in addition to their well-established antipsychotic-like efficacy [72,78]. The cognition-enhancing and antipsychotic effects of VU0467154 persisted during chronic dosing, suggesting that it may be possible to clinically treat the pervasive cognitive deficits of schizophrenia patients while also preventing the relapse or induction of a psychotic episode with a single, chronically dosed M4 PAM. Therefore, the potential of M4 PAMs to treat multiple symptom clusters in schizophrenia patients may provide a substantial advantage over current antipsychotic medications that fail to effectively treat the cognitive disruptions of the disease.
In parallel to investigations of the mechanisms underlying the preclinical efficacy of M4 PAMs, efforts have been made to identify translatable biomarkers that could predict clinical efficacy. The use of quantitative electroencephalography (qEEG) provides a powerful approach that can discriminate between different behavioral states including arousal, sedation, and alertness [79]. Furthermore, dysfunctional qEEG measures have been correlated with psychotic symptoms and cognitive disruptions in schizophrenia [79]. In rodents, the M4 PAM VU0467154 increased arousal, as measured by qEEG during periods of wake, but importantly did not promote sedation, whereas the atypical antipsychotic clozapine increased arousal but exhibited sedative-like effects [80]. In addition, VU0467154 attenuated the elevation of gamma power induced by MK-801 [80], an electrophysiological correlate associated with positive symptoms and acute psychosis [79]. Overall, this study demonstrated that an M4 PAM may improve sleep, a considerable advantage over current antipsychotics, and that M4 target engagement can produce changes in qEEG signals that could be predictive of clinical efficacy. Therefore, qEEG may provide a quantifiable readout of target engagement in the clinic in the absence of a M4 mAChR specific radioligand.
Ultimately, the development of an M4 PAM clinical candidate relies on the optimization of a compound with activity at the human M4 receptor while also ideally retaining activity at multiple preclinical species (i.e., rat, dog, primate) to facilitate preclinical development and investigational new drug (IND)-enabling studies. Although the aforementioned species-specific pharmacology has complicated development, recent efforts have produced M4 PAMs with activity at M4 in multiple species including human and nonhuman primates [74,81]. The M4 PAM VU0476406 was recently developed that has similar potency at rat, human, dog, and cynomolgus M4 mAChR and favorable pharmacokinetic properties across species [81]. Unfortunately, suboptimal predicted human bioavailability and aqueous solubility prevented VU0476406 from being advanced into the clinic but allowed VU0476406 to become a useful tool compound for cross-species preclinical studies. Subsequently, it was shown that, similar to the effects of VU0467154 in mice, VU0476406 significantly reduced L-DOPA-induced dyskinesia in non-human primates [82], an effect related to excessive dopaminergic signaling in striatal circuitry [83]. Merck also recently disclosed the development of an M4 PAM with comparable potencies at rat and human M4 that was efficacious in reversing amphetamine-induced hyperlocomotion [74]. Finally, Sosei in collaboration with Allergan initiated a Phase I clinical trial of the purported human M agonist HTL0016878 (Clinical Trial Numberi: , Table 1); however, no preclinical pharmacology or efficacy data have so far been disclosed. Interestingly, the Merck M4 PAM exhibits moderate agonism at human M4 [74], and HTL0016878 has been publicly described as an M4 agonist. Altogether, these recent breakthroughs in developing compounds with favorable human pharmacodynamic properties are promising for the potential of M4 PAMs to treat multiple symptom domains of patients suffering from schizophrenia.
Concluding Remarks and Future Perspectives
A wealth of preclinical literature over the past decade suggest that allosteric modulators of several mAChRs hold great promise for the treatment of multiple devastating CNS disorders, including AD, schizophrenia, and SUD (Box 3), which have limited to no effective treatments. Recent advances in medicinal chemistry efforts to develop highly selective mAChR ligands have provided fundamental new insights into muscarinic receptor biology as well as key information for drug discovery efforts. As a consequence of these efforts, several allosteric modulators for M1, M4, and M5 mAChRs have already entered clinical trials or are quickly advancing toward the clinic (Table 1).
Box 3. Potential Utility of M5 NAMs for the Treatment of Substance Use Disorder.
Beyond dementia and schizophrenia, mAChRs are exciting targets for other CNS disorders such as SUD, a mental illness that afflicts >20 million people in the USAiv. SUD is a chronic, relapsing disorder characterized by compulsive drug-seeking behavior, continued use despite harmful consequences, and long-lasting changes in the brainv. Many current therapies for addiction (e.g., methadone) are inadequate because of their abuse liability or their inability to treat multiple distinct classes of addictive substances (e.g., naloxone or naloxone combination therapies) [100]. Many drugs of abuse alter mesolimbic dopamine reward circuitry, and targets that are exclusively found or highly enriched in this reward circuitry could therefore potentially provide treatments for multiple SUDs and thus have broad clinical efficacy [101].
The M5 mAChR is uniquely situated as a promising target for SUD because it is the only muscarinic receptor expressed on dopaminergic neurons of the substantia nigra pars compacta and ventral tegmental area [102,103]. Furthermore, the rewarding effects of drugs of abuse are diminished in M5-KO mice, including reduced cocaine self-administration, decreased cocaine- and morphine-induced place preference, and less severe cocaine and morphine withdrawal symptoms [104,105]. These M5-KO studies provide compelling evidence that reducing M5 receptor function could be a potential treatment for SUD. Importantly for human translation studies, the analgesic effects of morphine were completely unaltered in M5-KO mice [104]. Therefore, reduction of M5 receptor function through highly selective M5 NAMs provides great promise as a potential treatment for SUD in human patients.
Unfortunately, despite major investments in medicinal chemistry, little progress had been made towards the generation of a highly selective and brain-penetrant M5 NAM until the recent discovery of ML375, the first highly selective, potent, and brain-penetrant M5 NAM [106]. In preclinical rodent models relevant to SUD, ML375 was found to dramatically reduce self-administration of alcohol [107], cocaine [108], and opiates [109]. Furthermore, microinjections of ML375 into the dorsal lateral striatum decreased ethanol self-administration [107], suggesting that M5 mAChR expression on dopamine terminals may be important for M5 NAM efficacy in vivo [110,111].
Importantly, ML375 did not alter motor function nor did it reduce the natural rewarding effects of food [107,109]. Collectively, these studies suggest that M5 NAMs are poised to be a very promising treatment for SUD while avoiding unwanted effects on natural reward circuitry. Although the ability of M5 NAMs to reduce addiction-like behavior across multiple substances of abuse is promising, ML375 displays an unfavorably long half-life (80 h), and considerable medicinal chemistry work will therefore be necessary to generate a clinical candidate with more favorable ‘drug-like’ properties.
Although much progress has been made in developing allosteric modulators of the various mAChR subtypes for the potential treatment of several CNS disorders, there are still many outstanding questions that the muscarinic field is primed to address (see Outstanding Questions). This is best illustrated by the crucial need to understand which pharmacological properties are important for allosteric modulator efficacy and which, if any, are responsible for adverse effects. Overall, a better understanding of how these distinct pharmacological properties (e.g., bias, total brain exposure, partial agonism [84], differences in binding sites [85] etc.) drive efficacy and/or adverse effect liability could potentially explain how distinct mAChR ligands display differences in vivo.
Outstanding Questions.
Can biased ligands for other M1 signaling pathways (e.g., β-arrestin) and other mAChRs be developed?
Will M1 or M4 allosteric modulators have therapeutic potential for the negative symptoms (e.g., the disruptions in motivation) in patients with schizophrenia?
How do mAChRs function within key brain circuits that are important for complex behaviors such as learning, memory, motivation, and addiction?
How do allosteric modulators exert their effects at the brain-circuit level?
Recent characterization of biased allosteric ligands for the M1 mAChR have provided useful insight into the mechanism of action of these allosteric modulators. To date, however, we have only identified a limited number of biased ligands, and more focused drug discovery efforts will be necessary to identify biased ligands for other distinct signaling pathways as well as for the other mAChR subtypes. Therefore, dedicated medicinal chemistry paired with pathway-specific but still high-throughput pharmacological assays will be necessary to identify a wider range of biased ligands for all the mAChR subtypes. Information gleaned from these studies could greatly advance our collective knowledge of mAChR biology as well as help to inform drug discovery programs. Even modest investments into pharmacological characterization of the signaling pathways and pharmacological properties involved in allosteric modulator action in vivo could pay huge dividends for drug discovery efforts. Through better understanding of the pharmacological properties that are important for efficacy and adverse effects, we as a field can ultimately advance mAChR allosteric modulators forward into the clinic with the highest chance of success.
Highlights.
mAChR allosteric modulators demonstrate unparalleled subtype selectivity, can possess a wide array of distinct pharmacological properties, and are rapidly advancing into the clinic for the treatment of multiple central nervous system disorders.
M1 mAChR positive allosteric modulators (PAMs) may enhance cognition and reverse memory deficits in AD and schizophrenia, and may display a larger therapeutic window than acetylcholinesterase inhibitors.
M4 PAMs can reduce dopamine release and demonstrate antipsychotic-like effects in preclinical animal models.
Recent preclinical literature suggests that M5 negative allosteric modulators may effectively treat an array of substance use disorders without reducing the effects of natural rewards.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants F31 MH114368 and T32 MH64913 (to S.P.M.), Canadian Institutes of Health Research (CIHR) DFS146189 (J.M.), the Vanderbilt International Scholars Program (J.M.), and NIH R01 MH073676 (P.J.C.).
Disclaimer Statement
P.J.C. is an inventor on multiple patents protecting allosteric modulators for several classes of mAChRs. P.J.C. receives research support from Lundbeck, Boehringer Ingelheim, and Ancora Innovation.
Glossary
- Ago-PAM or PAM-agonists
these positive allosteric modulators (PAMs) can activate the receptor in the absence of the orthosteric agonist in addition to increasing the potency and/or efficacy of orthosteric agonists when present.
- Allosteric site
a binding site on a receptor that is topographically and structurally distinct from the orthosteric ligand binding site.
- Brief psychiatric rating scale (BPRS)
a rating scale which a clinician or researcher can use to measure psychiatric symptoms such as depression, anxiety, hallucinations, and unusual behavior.
- Clinical global impression
a brief clinician-administered scale that measures illness severity, global improvement or change, and therapeutic response in the patient.
- Investigational new drug (IND)-enabling studies
IND status is a key FDA milestone before clinical testing on humans. Studies include repeat-dose toxicology in rodents, nonclinical safety studies, and others to facilitate FDA submission before clinical testing.
- Orthosteric site
the binding site for the endogenous ligand on a receptor.
- Positive and negative syndrome scale (PANSS)
a medical scale for measuring symptom severity of patients with schizophrenia.
- Probe-dependence
the phenomenon whereby the ability of an allosteric ligand to enhance or reduce a response differs depending on the orthosteric ligand.
- Receptor reserve
the concept that a full pharmacological response can be induced at ligand concentrations that do not saturate the total receptor population.
- Synaptic plasticity
the biological process by which specific patterns of synaptic activity result in changes in synaptic strength; is thought to contribute to learning and memory.
- Therapeutic index
a quantitative measurement of the relative safety of a drug. It is a comparison of the amount of a therapeutic agent that causes the desired therapeutic effect to the amount that causes toxicity or undesired effects.
Footnotes
References
- 1.Picciotto MR et al. (2012) Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76, 116–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soreq H (2015) Checks and balances on cholinergic signaling in brain and body function. Trends Neurosci. 38, 448–458 [DOI] [PubMed] [Google Scholar]
- 3.Sarter M et al. (2014) Deterministic functions of cortical acetylcholine. Eur. J. Neurosci 39, 1912–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasselmo ME and Sarter M (2011) Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology 36, 52–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wess J et al. (2007) Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat. Rev. Drug Discov. 6, 721–733 [DOI] [PubMed] [Google Scholar]
- 6.Whitehouse PJ et al. (1981) Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol 10, 122–126 [DOI] [PubMed] [Google Scholar]
- 7.Birks J (2006) Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev, CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodick NC et al. (1997) Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch. Neurol 54, 465–473 [DOI] [PubMed] [Google Scholar]
- 9.Bender AM et al. (2017) Classics in chemical neuroscience: xanomeline. ACS Chem. Neurosci 8, 435–443 [DOI] [PubMed] [Google Scholar]
- 10.Shekhar A et al. (2008) Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry 165, 1033–1039 [DOI] [PubMed] [Google Scholar]
- 11.Callegari E et al. (2011) A comprehensive non-clinical evaluation of the CNS penetration potential of antimuscarinic agents for the treatment of overactive bladder. Br. J. Clin. Pharmacol 72, 235–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conn PJ et al. (2009) Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol. Sci 30, 148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conn PJ et al. (2009) Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 8, 41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thal DM et al. (2016) Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature 531, 335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haga K et al. (2012) Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature 482, 547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruse AC et al. (2012) Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 482, 552–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H et al. (2018) Structure-guided development of selective M3 muscarinic acetylcholine receptor antagonists. Proc. Natl. Acad. Sci. U. S. A 115, 12046–12050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vuckovic Z et al. (2019) Crystal structure of the M5 muscarinic acetylcholine receptor. bioRxiv Published online August 14, 2019 10.1101/730622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao Y et al. (2013) Activation and dynamic network of the M2 muscarinic receptor. Proc. Natl. Acad. Sci. U. S. A 110, 10982–10987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burger WAC et al. (2018) Toward an understanding of the structural basis of allostery in muscarinic acetylcholine receptors. J. Gen. Physiol 150, 1360–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macalino SJY et al. (2015) Role of computer-aided drug design in modern drug discovery. Arch. Pharm. Res 38, 1686–1701 [DOI] [PubMed] [Google Scholar]
- 22.Dencker D et al. (2011) Involvement of a subpopulation of neuronal M4 muscarinic acetylcholine receptors in the antipsychotic-like effects of the M1/M4 preferring muscarinic receptor agonist xanomeline. J. Neurosci 31, 5905–5908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolley ML et al. (2009) Attenuation of amphetamine-induced activity by the non-selective muscarinic receptor agonist, xanomeline, is absent in muscarinic M4 receptor knockout mice and attenuated in muscarinic M1 receptor knockout mice. Eur. J. Pharmacol 603, 147–149 [DOI] [PubMed] [Google Scholar]
- 24.Davoren JE et al. (2017) Design and synthesis of γ- and δ-lactam M1 positive allosteric modulators (PAMs): convulsion and cholinergic toxicity of an M1-selective PAM with weak agonist activity. J. Med. Chem 60, 6649–6663 [DOI] [PubMed] [Google Scholar]
- 25.Lein ES et al. (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 [DOI] [PubMed] [Google Scholar]
- 26.Miyauchi M et al. (2017) Muscarinic receptor signaling contributes to atypical antipsychotic drug reversal of the phencyclidine-induced deficit in novel object recognition in rats. J. Psychopharmacol. (Oxford) 31, 1588–1604 [DOI] [PubMed] [Google Scholar]
- 27.Gould RW et al. (2015) Role for the M1 muscarinic acetylcholine receptor in top-down cognitive processing using a touchscreen visual discrimination task in mice. ACS Chem. Neurosci 6, 1683–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheffler DJ et al. (2009) A novel selective muscarinic acetylcholine receptor subtype 1 antagonist reduces seizures without impairing hippocampus-dependent learning. Mol. Pharmacol 76, 356–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anagnostaras SG et al. (2003) Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat. Neurosci 6, 51–58 [DOI] [PubMed] [Google Scholar]
- 30.Lebois EP et al. (2018) Muscarinic receptor subtype distribution in the central nervous system and relevance to aging and Alzheimer’s disease. Neuropharmacology 136, 362–373 [DOI] [PubMed] [Google Scholar]
- 31.Foster DJ and Conn JP (2017) Allosteric modulation of GPCRs: new insights and potential utility for treatment of schizophrenia and other CNS disorders. Neuron 94, 431–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bubser M et al. (2012) Muscarinic receptor pharmacology and circuitry for the modulation of cognition. Handb. Exp. Pharmacol 208, 121–166 [DOI] [PubMed] [Google Scholar]
- 33.Ma L et al. (2009) Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc. Natl. Acad. Sci. U. S. A 106, 15950–15955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lebois EP et al. (2017) Disease-modifying effects of M1 muscarinic acetylcholine receptor activation in an Alzheimer’s disease mouse model. ACS Chem. Neurosci 8, 1177–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley SJ et al. (2017) M1 muscarinic allosteric modulators slow prion neurodegeneration and restore memory loss. J. Clin. Invest 127, 487–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grannan MD et al. (2016) Prefrontal cortex-mediated impairments in a genetic model of NMDA receptor hypofunction are reversed by the novel M1 PAM VU6004256. ACS Chem. Neurosci 7, 1706–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghoshal A et al. (2016) Potentiation of M1 muscarinic receptor reverses plasticity deficits and negative and cognitive symptoms in a schizophrenia mouse model. Neuropsychopharmacology 41, 598–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurimoto E et al. (2019) TAK-071, a muscarinic M1 receptor positive allosteric modulator, attenuates scopolamine-induced quantitative electroencephalogram power spectral changes in cynomolgus monkeys. PLoS One 14, e0207969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vardigan JD et al. (2015) Improved cognition without adverse effects: novel M1 muscarinic potentiator compares favorably to donepezil and xanomeline in rhesus monkey. Psychopharmacology 232, 1859–1866 [DOI] [PubMed] [Google Scholar]
- 40.Uslaner JM et al. (2013) The muscarinic M1 receptor positive allosteric modulator PQCA improves cognitive measures in rat, cynomolgus macaque, and rhesus macaque. Psychopharmacology 225, 21–30 [DOI] [PubMed] [Google Scholar]
- 41.Lange HS et al. (2015) The M1 muscarinic positive allosteric modulator PQCA improves performance on translatable tests of memory and attention in rhesus monkeys. J. Pharmacol. Exp. Ther 355, 442–450 [DOI] [PubMed] [Google Scholar]
- 42.Moran SP et al. (2018) M1-positive allosteric modulators lacking agonist activity provide the optimal profile for enhancing cognition. Neuropsychopharmacology 43, 1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirey JK et al. (2009) A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J. Neurosci 29, 14271–14286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maksymetz J et al. (2019) M1 muscarinic receptors modulate fear-related inputs to the prefrontal cortex: implications for novel treatments of posttraumatic stress disorder. Biol. Psychiatry 85, 989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dennis SH et al. (2016) Activation of muscarinic M1 acetylcholine receptors induces long-term potentiation in the hippocampus. Cereb. Cortex 26, 414–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui Y et al. (2008) Enhancement of memory function in aged mice by a novel derivative of xanomeline. Cell Res. 18, 1151–1153 [DOI] [PubMed] [Google Scholar]
- 47.Xiong C-H et al. (2019) M1 muscarinic receptors facilitate hippocampus-dependent cognitive flexibility via modulating GluA2 subunit of AMPA receptors. Neuropharmacology 146, 242–251 [DOI] [PubMed] [Google Scholar]
- 48.Rook JM et al. (2017) Diverse effects on M1 signaling and adverse effect liability within a series of M1 ago-PAMs. ACS Chem. Neurosci 8, 866–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamilton SE et al. (1997) Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proc. Natl. Acad. Sci. U. S. A 94, 13311–13316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bymaster FP et al. (2003) Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur. J. Neurosci 17, 1403–1410 [DOI] [PubMed] [Google Scholar]
- 51.Rook JM et al. (2018) A novel M1 PAM VU0486846 exerts efficacy in cognition models without displaying agonist activity or cholinergic toxicity. ACS Chem. Neurosci 9, 2274–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moran SP et al. (2018) PF-06827443 displays robust allosteric agonist and positive allosteric modulator activity in high receptor reserve and native systems. ACS Chem. Neurosci 9, 2218–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rook JM et al. (2013) Unique signaling profiles of positive allosteric modulators of metabotropic glutamate receptor subtype 5 determine differences in in vivo activity. Biol. Psychiatry 73, 501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voss T et al. (2018) Randomized, controlled, proof-of-concept trial of MK-7622 in Alzheimer’s disease. Alzheimers Dement. 4, 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wootten D et al. (2018) Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 19, 638–653 [DOI] [PubMed] [Google Scholar]
- 56.Conibear AE and Kelly E (2019) A biased view of μ-opioid receptors? Mol. Pharmacol 96, 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmid CL et al. (2017) Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 171, 1165–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marlo JE et al. (2009) Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol. Pharmacol 75, 577–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moran SP et al. (2019) Biased M1 positive allosteric modulators reveal novel role of phospholipase D in M1-dependent rodent cortical plasticity. bioRxiv Published online October 16, 2019 10.1101/806943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rook JM et al. (2015) Biased mGlu5-positive allosteric modulators provide in vivo efficacy without potentiating mGlu5 modulation of NMDAR currents. Neuron 86, 1029–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elie D et al. (2010) Cognitive effects of antipsychotic dosage and polypharmacy: a study with the BACS in patients with schizophrenia and schizoaffective disorder. J. Psychopharmacol. (Oxford) 24, 1037–1044 [DOI] [PubMed] [Google Scholar]
- 62.Hersch SM et al. (1994) Distribution of m1–m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J. Neurosci 14, 3351–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howes OD and Kapur S (2009) The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr. Bull 35, 549–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kesby JP et al. (2018) Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience. Transl. Psychiatry 8, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pancani T et al. (2014) M4 mAChR-mediated modulation of glutamatergic transmission at corticostriatal synapses. ACS Chem. Neurosci 5, 318–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moehle MS et al. (2017) Cholinergic projections to the substantia nigra pars reticulata inhibit dopamine modulation of basal ganglia through the M4 muscarinic receptor. Neuron 96, 1358–1372.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foster DJ et al. (2016) Antipsychotic-like effects of M4 positive allosteric modulators are mediated by CB2 receptor-dependent inhibition of dopamine release. Neuron 91, 1244–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yohn SE et al. (2018) Activation of the mGlu1 metabotropic glutamate receptor has antipsychotic-like effects and is required for efficacy of M4 muscarinic receptor allosteric modulators. Mol. Psychiatry Published online August 16, 2018 10.1038/s41380-018-0206-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan WY et al. (2008) Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc. Natl. Acad. Sci. U. S. A 105, 10978–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brady AE et al. (2008) Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats. J. Pharmacol. Exp. Ther 327, 941–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suratman S et al. (2011) Impact of species variability and ‘probe-dependence’ on the detection and in vivo validation of allosteric modulation at the M4 muscarinic acetylcholine receptor. Br. J. Pharmacol 162, 1659–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bubser M et al. (2014) Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents. ACS Chem. Neurosci 5, 920–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wood MR et al. (2017) Challenges in the development of an M4 PAM in vivo tool compound: the discovery of VU0467154 and unexpected DMPK profiles of close analogs. Bioorg. Med. Chem. Lett 27, 171–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schubert JW et al. (2019) Discovery, optimization, and biological characterization of 2,3,6-trisubstituted pyridine-containing M4 positive allosteric modulators. ChemMedChem 14, 943–951 [DOI] [PubMed] [Google Scholar]
- 75.Byun NE et al. (2014) Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU0152100. Neuropsychopharmacology 39, 1578–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weiner DM et al. (1990) Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc. Natl. Acad. Sci. U. S. A 87, 7050–7054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ott T and Nieder A (2019) Dopamine and cognitive control in prefrontal cortex. Trends Cogn. Sci 23, 213–234 [DOI] [PubMed] [Google Scholar]
- 78.Gould RW et al. (2018) Cognitive enhancement and antipsychotic-like activity following repeated dosing with the selective M4 PAM VU0467154. Neuropharmacology 128, 492–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uhlhaas PJ and Singer W (2015) Oscillations and neuronal dynamics in schizophrenia: the search for basic symptoms and translational opportunities. Biol. Psychiatry 77, 1001–1009 [DOI] [PubMed] [Google Scholar]
- 80.Gould RW et al. (2016) State-dependent alterations in sleep/wake architecture elicited by the M4 PAM VU0467154 – relation to antipsychotic-like drug effects. Neuropharmacology 102, 244–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Melancon BJ et al. (2017) Optimization of M4 positive allosteric modulators (PAMs): the discovery of VU0476406, a non-human primate in vivo tool compound for translational pharmacology. Bioorg. Med. Chem. Lett 27, 2296–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen W et al. (2015) M4 muscarinic receptor signaling ameliorates striatal plasticity deficits in models of L-DOPA-induced dyskinesia. Neuron 88, 762–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jenner P (2008) Molecular mechanisms of L-DOPA-induced dyskinesia. Nat. Rev. Neurosci 9, 665–677 [DOI] [PubMed] [Google Scholar]
- 84.Shannon HE et al. (1994) Xanomeline: a novel muscarinic receptor agonist with functional selectivity for M1 receptors. J. Pharmacol. Exp. Ther 269, 271–281 [PubMed] [Google Scholar]
- 85.Bradley SJ et al. (2018) Bitopic binding mode of an M1 muscarinic acetylcholine receptor agonist associated with adverse clinical trial outcomes. Mol. Pharmacol 93, 645–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kenakin T (2017) Theoretical aspects of GPCR–ligand complex pharmacology. Chem. Rev 117, 4–20 [DOI] [PubMed] [Google Scholar]
- 87.Sako Y et al. (2019) TAK-071, a novel M1 positive allosteric modulator with low cooperativity, improves cognitive function in rodents with few cholinergic side effects. Neuropsychopharmacology 44, 950–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mandai T et al. (2019) In vivo pharmacological comparison of TAK-071, a positive allosteric modulator of muscarinic M1 receptor, and xanomeline, an agonist of muscarinic M1/M4 receptor, in rodents. Neuroscience 414, 60–76 [DOI] [PubMed] [Google Scholar]
- 89.Kurimoto E et al. (2018) An approach to discovering novel muscarinic M1 receptor positive allosteric modulators with potent cognitive improvement and minimized gastrointestinal dysfunction. J. Pharmacol. Exp. Ther 364, 28–37 [DOI] [PubMed] [Google Scholar]
- 90.Coyle JT et al. (1983) Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science 219, 1184–1190 [DOI] [PubMed] [Google Scholar]
- 91.Holschneider DP et al. (2019) Positive allosteric modulation of cholinergic receptors improves spatial learning after cortical contusion injury in mice. J. Neurotrauma 36, 2233–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stoll K et al. (2018) Effects of muscarinic M1 and M4 acetylcholine receptor stimulation on extinction and reinstatement of cocaine seeking in male mice, independent of extinction learning. Psychopharmacology 235, 815–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pancani T et al. (2015) Allosteric activation of M4 muscarinic receptors improve behavioral and physiological alterations in early symptomatic YAC128 mice. Proc. Natl. Acad. Sci. U. S. A 112, 14078–14083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thomsen M et al. (2012) Contribution of both M1 and M4 receptors to muscarinic agonist-mediated attenuation of the cocaine discriminative stimulus in mice. Psychopharmacology 220, 673–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dencker D et al. (2012) An allosteric enhancer of M4 muscarinic acetylcholine receptor function inhibits behavioral and neurochemical effects of cocaine. Psychopharmacology 224, 277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dall C et al. (2017) Muscarinic receptor M4 positive allosteric modulators attenuate central effects of cocaine. Drug Alcohol Depend. 176, 154–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thomson SR et al. (2017) Cell-type-specific translation profiling reveals a novel strategy for treating fragile X syndrome. Neuron 95, 550–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gogliotti RG et al. (2018) Total RNA sequencing of rett syndrome autopsy samples identifies the M4 muscarinic receptor as a novel therapeutic target. J. Pharmacol. Exp. Ther 365, 291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moehle MS and Conn PJ (2019) Roles of the M4 acetylcholine receptor in the basal ganglia and the treatment of movement disorders. Mov. Disord 34, 1089–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bowman S et al. (2013) Reducing the health consequences of opioid addiction in primary care. Am. J. Med 126, 565–571 [DOI] [PubMed] [Google Scholar]
- 101.Joffe ME et al. (2014) Biological substrates of addiction. Wiley Interdiscip Rev Cogn Sci 5, 151–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yasuda RP et al. (1993) Development of antisera selective for m4 and m5 muscarinic cholinergic receptors: distribution of m4 and m5 receptors in rat brain. Mol. Pharmacol 43, 149–157 [PubMed] [Google Scholar]
- 103.Wei J et al. (1994) m1–m5 muscarinic receptor distribution in rat CNS by RT-PCR and HPLC. J. Neurochem 63, 815–821 [DOI] [PubMed] [Google Scholar]
- 104.Basile AS et al. (2002) Deletion of the M5 muscarinic acetylcholine receptor attenuates morphine reinforcement and withdrawal but not morphine analgesia. Proc. Natl. Acad. Sci. U. S. A 99, 11452–11457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thomsen M et al. (2005) Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J. Neurosci 25, 8141–8149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gentry PR et al. (2013) Discovery of the first M5-selective and CNS penetrant negative allosteric modulator (NAM) of a muscarinic acetylcholine receptor: (S)-9b-(4-chlorophenyl)-1-(3,4-difluorobenzoyl)-2,3-dihydro-1H-imidazo[2,1-a] isoindol-5(9bH)-one (ML375). J. Med. Chem 56, 9351–9355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Berizzi AE et al. (2018) Muscarinic M5 receptors modulate ethanol seeking in rats. Neuropsychopharmacology 43, 1510–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gunter BW et al. (2018) Selective inhibition of M5 muscarinic acetylcholine receptors attenuates cocaine self-administration in rats. Addict Biol. 23, 1106–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gould RW et al. (2019) Acute negative allosteric modulation of M5 muscarinic acetylcholine receptors inhibits oxycodone self-administration and cue-induced reactivity with no effect on antinociception. ACS Chem. Neurosci 10, 3740–3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shin JH et al. (2015) Muscarinic regulation of dopamine and glutamate transmission in the nucleus accumbens. Proc. Natl. Acad. Sci. U. S. A 112, 8124–8129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Foster DJ et al. (2014) M5 receptor activation produces opposing physiological outcomes in dopamine neurons depending on the receptor’s location. J. Neurosci 34, 3253–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]