Abstract
Background:
Poorer health outcomes and lower survival rates have been well documented among African American/Black (Black) women diagnosed with breast cancer. Black women are 41% more likely to die from breast cancer than White women despite a lower incidence rate. Apart from pharmacotherapy, psychosocial interventions are recommended by the Institute of Medicine as standard medical care for breast cancer patients at all phases of treatment. The current review is the first attempt to systematically evaluate the literature on the influence of psychosocial interventions for Black women diagnosed with breast cancer.
Methods:
This systematic review aimed to adhere to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. A comprehensive computerized literature search of CINAHL, PsycINFO, PubMed, and Web of Science was conducted to obtain relevant studies.
Results:
Interventions demonstrated improved mood, decreased distress, increased ability to cope with intrusive thoughts and cancer-related stress, personal growth, and improved social well-being. However, aspects unique to this population require additional scientific inquiry. Over 80% of empirical interventions focused on Black women diagnosed with breast cancer have been concentrated on the posttreatment phase. There is a paucity of work at the time of diagnosis and during treatment.
Conclusions:
To address gaps in the scientific literature, more work is needed to better understand how psychosocial interventions can improve the health trajectory for Black women diagnosed with breast cancer particularly in the areas of seeking help and support, identifying culturally acceptable methods for engaging support networks, and identifying best practices for enhancing coping skills.
Background
Poorer health outcomes and lower survival rates have been well documented among African American/Black (Black) women diagnosed with breast cancer. Black women are 41% more likely to die from breast cancer than White women despite a lower incidence rate [1]. Additionally, they are more likely to suffer a greater number of complications during treatment and more posttreatment morbidities [2–5]. Apart from pharmacotherapy, psychosocial interventions are recommended by the Institute of Medicine as standard medical care for breast cancer patients at all phases of treatment [6]. The purpose of the current review is to synthesize the state of knowledge regarding the influence of psychosocial interventions that address the needs of Black women diagnosed with breast cancer.
Unique stressors for Black women
Black women face unique stressors such as economic challenges, lack of health information, poor patient–provider communication, fearful perceptions of treatment, and distrust of the healthcare system [7–12]. Although these stressors are often confounded by socioeconomic status (SES), a unique phenomenon observed in this population is that many Black breast cancer patients express a reluctance to share their illness with others [7]. Extant literature suggests that in general, Black women are more likely to report lack of social support, greater cancer-related stigma, and poorer breast cancer-related quality of life than White women [7,8]. Some Black women report that even their close female relatives express discomfort when they attempt to discuss their illness, and others note that close friends and partners withdrew support after learning of their diagnosis [13]. Work and family demands also cause some women to feel that they cannot allow themselves to take on a sick role [14]. By trying to appear healthy and conceal their illness, Black women may hinder their own treatment process. Growing evidence suggests a positive influence of psychosocial intervention on health outcomes among those affected by breast cancer; however, less is known about the role of psychosocial interventions on the health outcomes of Black women. Although Black women face unique stressors during the diagnosis, treatment, and survivorship process, it may be possible to implement psychosocial interventions that positively influence prognosis. A small but growing number of interventions have specifically addressed psychosocial outcomes among Black women diagnosed with breast cancer. To better address the health disparities facing Black women diagnosed with breast cancer, understanding the impact of psychosocial interventions in this population is imperative.
Psychosocial interventions and health outcomes among breast cancer patients
In 1989, Spiegel et al. [15] published a landmark study showing that women with metastatic breast cancer who participated in weekly supportive group therapy with self-hypnosis lived longer than those in the control condition. In a 2002 review, Luecken and Compas [16] found that psychosocial interventions yield positive benefits for psychological adjustment among breast cancer survivors. Since then, numerous studies have shown that psychosocial interventions can improve psychological adaptation among breast cancer patients and lead to improved health outcomes [17–21].
Although evidence suggests that psychosocial interventions are beneficial for breast cancer patients at all phases of treatment, historically, most studies assessing the effectiveness of psychosocial interventions included few or no minorities. Black women face multiple stressors that may increase distress during breast cancer treatment [7–9,22,23]. These stressors have also been associated with poor prognosis [3,10]. Among Black women, effective psychosocial interventions may facilitate improved health outcomes through improved psychological functioning and adaptation to the disease process. Therefore, an understanding of the best ways to address the unique psychosocial needs of Black women diagnosed with breast cancer is critical to improving morbidity and mortality outcomes in this population.
Objective
The current review is the first attempt to systematically evaluate the literature on psychosocial interventions for Black women diagnosed with breast cancer. The primary aims of the present study were as follows: (1) to evaluate present knowledge regarding the influence of psychosocial interventions on health outcomes among Black breast cancer patients and (2) to outline future research priorities for this population.
Methods
This systematic review aimed to adhere to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines [24]. A comprehensive computerized literature search of CINAHL, PsycINFO, PubMed, and Web of Science was conducted from June to September 2013 with the following search terms: breast cancer intervention and Black women (i.e., ‘Black women’, ‘African American women’, and ‘women of color’). The search did not restrict by publication date. Criteria for manuscript eligibility included the following: the study should be peer reviewed, conducted in the USA, reported the intervention results or reported a description of the development and other important process-related components of the intervention, and specifically recruited Black women diagnosed with breast cancer or, in studies with mixed-race samples, included a large enough number of Black participants to permit examination of the intervention’s effects on Black participants. The reasons for article exclusion were as follows: there were duplicate search results, the interventions addressed screening or prevention, there were an insufficient number of Black participants, the intervention did not target psychosocial factors, and the article did not describe the development, implementation, or outcome of an intervention (e.g., the article highlighted a problem or need for intervention).
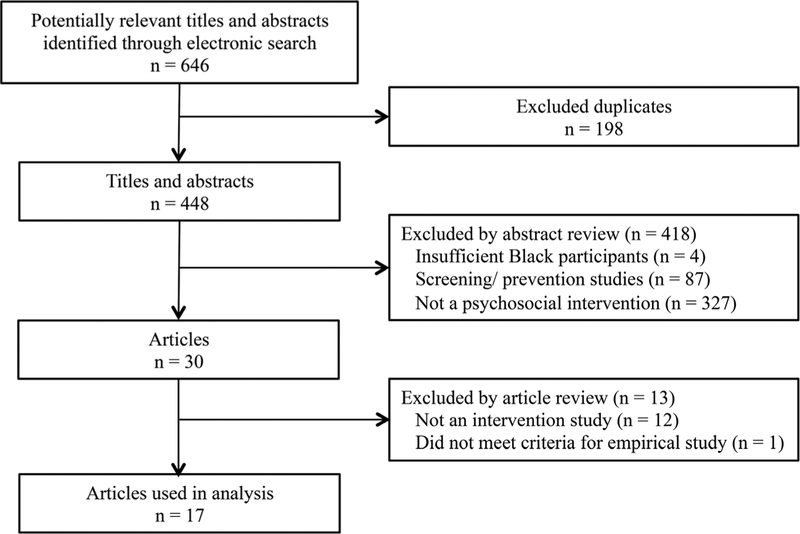
PsycINFO was the primary search engine and yielded 87 peer-reviewed articles. One study author screened each article for inclusion in the study, and the second author verified each selected article’s acceptability for inclusion. The final article list was arrived at by discussion and agreement between the authors. Figure 1 presents a flow chart of study selection. Microsoft Excel was used to organize the information.
Figure 1.
Flow chart of study selection for the current analysis
Terminology
Time of diagnosis is the period from the patients being told that they have breast cancer until active treatment such as surgery, chemotherapy, or radiation begins.
Treatment phase is defined as the period of active treatment (which varies on the basis of stage), which includes surgery, chemotherapy, or radiation.
Posttreatment phase is the period after active treatment has been completed in which patients, often referred to as ‘cancer survivors’, may still be on hormone therapy such as tamoxifen or herceptin.
Results
Seventeen articles that focused on issues influencing outcomes among Black women diagnosed with breast cancer were reviewed. All articles were published within the last 10 years (2003–2013) [25–27,29–41,43]. Twelve articles focused only on Black women as the population of interest (i.e., 100% of the sample identified as Black), whereas the other five specifically oversampled Black women. Twelve articles were empirical pilot or randomized controlled trial tests of 10 unique interventions, four were descriptive studies addressing process-related issues specific to intervention development and population engagement, and one was a dismantling study. Table 1 presents the study characteristics.
Table 1.
Description of intervention studies (N = 12)
| Unique interventions (N) | 10 |
| Participants per study | |
| ≤100 | 7 |
| 101–249 | 1 |
| 250–509 | 4 |
| Type of study | |
| Pilot | 4 |
| RCT | 7 |
| Other | 1 |
| Control group type | |
| Waitlist control | 2 |
| TAU | 4 |
| Comparative effectiveness | 1 |
| Attention matched | 1 |
| None | 4 |
| Intervention period | |
| In treatment | 3 |
| Posttreatment | 9 |
| Used community partner | |
| Yes | 5 |
| No | 7 |
| Sample demographics | |
| African American/Black only | 8 |
| Mixed sample | 4 |
| Mean age ranges | 48.6–65.19 |
| Year published | |
| 2013 | 4 |
| 2012 | 2 |
| 2011 | 1 |
| 2009 | 1 |
| 2006 | 2 |
| 2005 | 3 |
| 2003 | 1 |
| Journal published | |
| J. Cancer Ed. | 1 |
| Obesity | 1 |
| Cancer | 2 |
| J. Clinical Oncology | 1 |
| Psycho-oncology | 1 |
| Prev. of Chronic Disease | 2 |
| Oncology Nursing Forum | 1 |
| Inter J. of Behavioral Medicine | 1 |
| Health Psychology | 1 |
| Health and Nutrition | 1 |
| Funding source | |
| NIH | |
| Mixed: NIH and Foundation | 7 |
| Principal Investigator Institution | 3 |
| Unknown | 1 |
| 1 |
TAU, treatment as usual; RCT, randomized controlled trial; NIH, National Institutes of Health.
Empirical studies
Seven articles were classified as empirical studies [25–27,29,33,35,41]. These studies used randomized control groups and substantive follow-up period (4 months–1 year), and all but one study reported retention rates [27]. Two additional articles included in this section [34,40] provided supporting evidence for empirical studies discussed (Table 2). Altogether, the empirical studies addressed a broad range of issues related to coping and quality of life and used a variety of methods to implement their intervention. Interventions included cognitive behavior strategies to manage uncertainty [26,27,41], enhanced culturally relevant social support [25,29], culturally relevant peer support [34,35], and culturally engaged weight management [33]. Interventions were delivered in group and individual format using both in-person and telehealth delivery methods. The interventions demonstrated improved mood [25], decreased distress [26,27,29,40], increased ability to cope with intrusive thoughts and cancer-related stress [25–27,40], personal growth [26], and improved social well-being [25,29].
Table 2.
Characteristics of studies included in the review (N = 17)
| Study | Study design | Inclusion criteria | Sample | Group comparison | Intervention (modification specific to the needs of Black women) | Measures | Results | Limitations |
|---|---|---|---|---|---|---|---|---|
| Taylor et al. [25] | RCT. 12-month follow-up. | Black women with stages 0–IIIA BC who had undergone surgery within the past 10 months. No psychotic illness. No drug or alcohol abuse. No severe cognitive impairment or previous systemic cancer diagnosis. | N = 73. Black (100%). Stage 0 BC (4.1%); I (41%); II (50.5%); IIIA (4.1%). Hormonal therapy (39.7%). Mean age: 54 years (SD = 11). Education: ≥13 years (50%). Monthly income: ≥$2500 (43%). Married (44%). Consent (62.8%). Attrition (50%). | Support group intervention versus usual care control group. | Eight weekly, 2-h meetings: relaxation training, spirituality, recurrence fears, social support, cancer education, nutrition, treatment side effects, and breast prostheses. (Supportive cultural context and spirituality.) | Cancer-related QoL (CARES-SF); mood over past week (POMS); psychological distress (MHI); cancer-related distress (IES); breast cancer-related knowledge. | At 12 months, intervention improved mood and reduced intrusive thoughts among those with greater baseline distress. Low income women maintained baseline functioning. | High attrition rate may reduce generalizability of findings. |
| Gil et al. [26] | 2 (intervention, control) × 2 (Black and White) randomized block. 20-month follow-up. | Recurrence-free women 5–9 years of posttreatment for BC. English speaking. Have telephone access. No major cognitive impairments. | N = 483. Black (29.2%); White (70.8%). Mean age: 64 years. Mean education: 13 years. Monthly income: ≥$2000 (61.2%). Married (58.6%). Consent (55%). Attrition (5%). | Intervention versus usual care control group. | Four weekly telephone sessions. Nurses guided participants in the use of audiotaped cognitive-behavioral strategies and a self-help manual. (None specified.) | Illness uncertainty (MUIS-S); cancer knowledge (Cancer Survivor Knowledge Scale); social support satisfaction (Social Support Questionnaire); patient–provider communication (Likert scale); cognitive reframing and problem solving (Self-control Schedule); coping strategies (CSQ); negative mood state (POMS-SF); positive life change due to serious illness (GTUS). | Intervention increased cognitive reframing and cancer knowledge and decreased illness uncertainty for Black and White women. Black women in the intervention showed stable personal growth and flexibility (versus decline in control group). | No control for nonspecific effects of attention by nurse and time spent in a structured activity. |
| Germino et al. [27] | 2 (intervention, control) × 2 (Black and White) randomized block. 8- to 10-month follow-up. | Black and White women at least 1–4 years of posttreatment for stages I–IV BC. | N = 313. Black (37.4%); White (62.6%). Mean age: 44 years. | YS-UMI versus attention control. | CD of cognitive-behavioral strategies for uncertainty management and guidebook of young survivor topics plus four, 20-min phone calls by nurses to reinforce skill learning. (None specified.) | Cognitive reframing and problem solving (Self-control Schedule); knowledge (Cancer Survivor Knowledge Scale); uncertainty (Mishel Uncertainty in Illness Scale–Survivor Version); fears of recurrence (Concerns About Recurrence Scale); Intrusive thoughts (IES); Affect (PANAS). | Intervention group reported decreased uncertainty, improved knowledge, and increased cognitive coping strategies. Black women in intervention reported decreased negative affect at time of a cancer stress event. | Limited sample demographic information. |
| Heiney et al. [29] | Randomized trial design. 16-week follow-up. | US-born. English speaking. Black female. ≥21 years old. Diagnosed with invasive ductal cancer within the past 6 months and underwent lumpectomy. No metastatic disease. No psychosis or major cognitive impairment. No past or current diagnosis of cancer (except skin basal or squamous cell). | N = 185. Black (100%). Chemo (26.5%). Radiation (35.1%). Combined chemo and radiation (38.4%). Mean age: 56 years (SD = 11.06). Education: ≥high school (83.8%). Income: <$19,000 (30%). Married (37.8%). Consent (68.3%). Attrition (7%). | STORY intervention versus usual psychosocial care control. | Eight weekly, 90-min group teleconference sessions plus two biweekly sessions aimed to increase social connection and cancer knowledge and decrease fear and fatalism. (Use of oral story telling tradition and larger kin context to provide support.) | Social connection (SSQ and SWB subscale of FACT-B); breast cancer knowledge (Breast Cancer Knowledge Scale); fear (POMS-B); loneliness (UCLA Loneliness Scale); fatalism (Powe Fatalism Inventory Revised). | Intervention group reported increased social connection and decreased fear and fatalism. | Study only included participants who underwent lumpectomy. |
| Sheppard et al. [30] | QE. Single group. Pre-post. | Female. Self-identified Black. Current patients had histologically confirmed BC of any stage. >21 years old. No recurrent cancer. No secondary cancers. | N = 76. Black (100%). Mean age: 51.9 years (SD = 10.60). BC stage I (39.6%); stages II and III (60.4%). >High-school education (71.6%). Full-time employment (45.1%). Currently single (65.8%). | — | One-on-one, in-person session with a BC survivor coach using a patient guidebook and TALK Back!© model incorporating sharing stories, decision support, and communication skills training. (Health literacy and patient empowerment.) | Self-efficacy in communication with providers; self-efficacy in shared decision-making; self-efficacy in treatment knowledge; patient-centered communication PICS. | Post-intervention, patients reported 77% increase in decision-making self-efficacy. | No control group (although pilot study). |
| Wilson et al. [31] | QE. Single group. Pre-post. | Female. Black. Prior BC diagnosis. Completed BC treatment ≥3 months ago. Physically mobile. Less than 70 years old. | N = 24. Black (100%). Diagnosis: <1 year ago (14%); 1–3 years (32%); 4–6 years (18%); ≥7 years (37%). Chemo and radiation (46%). Taking tamoxifen (23%). Mean age: 55 years (range 47–66 years). Married (50%). Education: ≥high school (96%). | — | Eight, 75-min weekly sessions held at a local church. Curriculum on health benefits of exercise and problem-solving barriers to exercise. (Access through community-based locations.) | BMI, steps per day (measured by pedometer); body fat percentage (measured using Futrex); attitudes toward exercise (exercise decisional balance instrument). | Post-intervention, significant increase in mean daily steps; significant BMI and body weight decrease; significant body fat decrease; significantly increased positive perception of exercise. | No control group. Small sample size (although pilot study). |
| Stolley et al. [32] | QE. Single group. Pre–post. | Self-identified Black. Age: ≥ 18 years old. Stages I–III BC. BMI: ≥25 kg/m2. Completed BC treatment (except endocrine treatment) ≥6 months earlier. Physician cleared to engage in moderate physical activity. No prescription weight-loss medications. Not in organized weight-loss program. | N = 23. Black (100%). Mean age: 51.4 years (SD = 8.9). Education: >high school (87.0%). Income: ≥$50,000 (47.83%). Married (34.78%). Full-time employment (69.57%). Consent (unknown). Attrition (13%). | — | Moving forward: culturally relevant weight-loss program for Black BC survivors. Two weekly classes: one, 2-h class with 1 h devoted to information on diet, exercise, and weight and 1 h for an exercise class. The second weekly meeting was an exercise class. (Food preference, kin networks, and spirituality.) | Food intake (FFQ); physical activity (long IPAQ); friend and family support for eating and dietary changes (Social Support for Eating and Exercise Questionnaire); QoL (FACT-B). | At 6-month post-intervention, significant increase in vegetable and fiber consumption; significant decrease in weight and BMI; significant increase in social support for healthy eating and exercise. | No control group. Small sample size (although pilot study). |
| Greenlee et al. [33] | RCT. 12-month follow-up. | Female. 21–70 years old. Self-identified Black/Hispanic. Stages 0–IIIA BC. Completed treatment ≥6 months ago. BMI >25 kg/m2. <20 min of weekly vigorous physical activity. No weight-loss program. Nonsmoker. A1c: <8%. BP: <140/90. LDL: <150 mg/dl. | N = 42. Black (21%). Hispanic (79%). Mean age: 50.7 years (SD = 8.9). Postmenopausal (81%). Average BMI: 33.2 (SD = 5.9) kg/m2. Mean VO2 max 18.4 ml/kg/min (SD = 3.6 ml/kg/min). Consent (55.36%). Attrition (9.5%). | Commercial curves weight-loss program versus waitlist control. | Six months of Curves Weight Management Program: encouragement to use Curves fitness centers five times weekly and attend six, 1-h nutrition sessions at Curves. (None specified.) | Height; weight; body composition (measured by densitometer); VO2 max; serum metabolic markers; physical activity (self-administered Kaiser Physical Activity Survey); diet (Block questionnaire). | At 6 months, intervention group lost significantly more weight than control group. Intervention group regained most of lost weight during 12-month follow-up but weighed significantly less than at baseline. | Small sample size (although pilot study). |
| Schover et al. [34] | Randomized trial. 3-month follow-up. | Female. Self-identified Black. ≥1 year of post-diagnosis of stages 0–IIIA BC. Completed BC treatment except hormonal therapy. Not currently undergoing breast reconstruction. | N = 48. Mean age: 49.29 years (SD = 8.39). Mean time since BC diagnosis: 4.52 years (SD = 3.84). Married (50%). Education: ≥high school (92%). Annual income: ≤$25,000 (19%). Tamoxifen currently (25%). Consent (65%). Attrition (10% of intervention group; 29% of control group). | SPIRIT peer-counseled group versus waitlist control. | SPIRIT. An informational workbook on survivor sexual health concerns. Peer counselors met individually three times over 6 weeks for 60–90 min focusing on a workbook chapter. (Supportive cultural context.) | Spirituality (Spiritual Well Being Scale of FACIT-Sp); emotional distress (BSI-18); sexual dysfunction (FSFI); menopausal symptoms (Breast Cancer Prevention Trial Symptom Checklist). | Knowledge of reproductive issues, distress, and menopausal symptoms significantly improved baseline to 3 months. | Small sample size. |
| Schover et al. [35] | Comparative effectiveness. 12-month follow-up. | Female. Self-identified Black. ≥1 year post-BC diagnosis. English speaking. | N = 300. Black (100%). Mean age: 54 years (SD = 9.8). Married (40.11%). Education: ≥high school (94.92%). Family income: ≤$25,000 (15.49%). Regular menses (7.6%). Postmenopausal (44.7%). Irregular menses (7.6%). Hysterectomy (38.1%). Consent (unknown). Attrition (38%). | SPIRIT peer-counseled group versus SPIRIT telephone-counseling group. | SPIRIT. informational workbook on survivor sexual health concerns. Peer counselors met individually three times over 6 weeks for 60–90 min focusing on one workbook chapter. Telephone-counseling participants received counselor’s contact information and calling card and were encouraged to call counselor to discuss workbook. (Supportive cultural context.) | Spirituality (Spiritual Well Being Scale of FACIT-Sp); emotional distress (BSI-18); sexual dysfunction (FSFI); menopausal symptoms (Breast Cancer Prevention Trial Symptom Checklist). | Both in-person peer counseling and telephone peer counseling improved reproductive knowledge and reported decreased distress and hot flashes. No significant differences between groups. | High attrition rate, high educational attainment, and high income may reduce generalizability of the findings. |
| Germino et al. [36] | Descriptive. Recruitment and retention strategies. | Black and White women at least 1–4 years of posttreatment for stages I–IV BC. | N = 104. Attrition (13%) for both Black and White women. | Younger Breast Cancer Survivors: Managing Uncertainty Intervention versus control. | CD of cognitive-behavioral strategies for uncertainty management and guidebook of young survivor topics plus four, 20-min phone calls by nurses to reinforce skill learning. (Community engagement through churches) | — | Implementation of culturally relevant recruitment and retention strategies increased enrollment by 373% in 11 months. | Lack of participant demographic information. |
| Sheppard et al. [37] | Qualitative design. Intervention development. | Female. Self-identified Black. Current patients had histologically confirmed BC of any stage. >21 years old. | N = 34. Cancer patients, survivors, and cancer providers. Aged 38–69 years. Attrition (38%). | Semistructured, individual interviews. | TALK Back!© communication and decision-making intervention. | — | Themes of interviews: importance of patient–provider relationship, cultural values of collectivism, religious faith, and oral tradition. | |
| Chung et al. [38] | Qualitative design. | Self-identified Black. ≥25 years old. Confirmed BC diagnosis. Completed primary treatment 2–12 months earlier. | N = 13. Aged 41–72 years. Consent (86. 7%). | Focus groups. | Taking CHARGE self-management program aimed to improve prevention and resolution of problems related to life after BC treatment. | Relevance of program to Black BC survivors. | Three content areas identified as needing enhancement: spirituality, strength, and body image. | No quantitative measures. |
| Lechner et al. [39] | Descriptive. Intervention development, recruitment, and retention. | Female. English speaking. Self-identified Black. Any stage of BC diagnosis. Received ≥1 medical BC treatment. No previous cancer history. Self-reported life expectancy: ≥12 months. No inpatient psychiatric treatment or substance dependence within the past year. No active suicidality. | N = 55. Black (100%). Mean age: 49.45 years (SD = 8.82). Stage 0 BC (9.1%); I (23.6%); II (41.8%); III (23.6%); IV (1.8%). Mean time since BC diagnosis: 14 months. Employed full time or part time (49%). Mean educational attainment: 13 years. Mean income: $31,000. Married (30.9%). Christian affiliation (100%). Consent (62.5%). Attrition (9.5%). | CBSM intervention versus enhanced breast cancer education control. | Ten-week CBSM intervention delivered in group format: cognitive-behavioral skills and relaxation training. (Use of kin context, spiritual coping, and supportive cultural context.) | — | High program acceptability ranging from 81% to 94% for those in the intervention condition and from 71% to 90% for the control condition. | High educational attainment of sample may reduce generalizability of the findings. |
| Gil et al. [40] | Descriptive. Intervention development and dismantling. | Recurrence-free women 5–9 years of posttreatment for BC. English speaking. Have telephone access. No major cognitive impairments. | N = 509. Black (29.27%); White (70.73%). Stage I/II BC (84%). Tamoxifen currently (36.3%). Mean age: 64 years. Monthly income: ≥$2000 (61.2%). Married (58%); Widowed (21%). Living alone (34%). Consent (55%). Attrition (5%). | Uncertainty management intervention versus usual care control. | Four weekly telephone sessions. Nurses guided participants in the use of audiotaped cognitive-behavioral strategies and a self-help manual. (None specified.) | Use and helpfulness of intervention components to deal with potential triggers of fear of recurrence (e.g., pain). | Calming self-talk was most commonly used strategy, followed by distraction. Black women used skills less frequently than White women and found them less helpful. | No control for nonspecific effects of attention by nurse and time spent in a structured activity. |
| Mishel et al. [41] | 2 (intervention, control) × 2 (Black and White) randomized block. 10-month follow-up. | Recurrence-free women 5–9 years of posttreatment for BC. English speaking. Have telephone access. No major cognitive impairments. | N = 509. Black (29.27%); White (70.73%). Stage I/II BC (84%). Tamoxifen currently (36.3%). Mean age: 64 years (SD = 8.9). Monthly income: ≥$2000 (61.2%). Married (58%). Widowed (21%). Living alone (34%). Consent (55%). Attrition (5%). | Uncertainty management intervention versus usual care control. | Four weekly telephone sessions. Nurses guided participants in the use of audiotaped cognitive-behavioral strategies and a self-help manual. (None specified.) | Cancer knowledge (Cancer Survivor Knowledge Scale); social support satisfaction (Social Support Questionnaire); patient–provider communication; cognitive reframing and problem solving (Self-control Schedule); coping strategies (CSQ); negative mood state (POMS-SF). | Among Black women at 10-month follow-up, the intervention improved cognitive reframing, increased communication with providers, and decreased catastrophizing. | No control for nonspecific effects of attention by nurse and time spent in a structured activity. |
| Griffith et al. [43] | QE. Single group. Pre-post. | Self-identified Black. Aged Between 30 and 70 years. Stages 0–IIIA BC and completed surgery, chemotherapy, and radiation 3–8 months prior. | N = 8. Black (100%). Mean age: 61.1 years (SD = 3.1). Stage 0 BC (25%); I (12.5%); II (50%). Annual income: <$20,000 (37.5%). Current/previous hormonal therapy (75%). Married (37.5%). Mean BMI: 30.7 kg/m2. Consent (40.9%). Attrition (11.2%). | — | Eight, 45- to 60-min individual nutritional counseling sessions: education, review of food records, goal setting, problem solving, and cognitive strategies. (Food preference.) | Fasting insulin and glucose levels; daily consumption of: fat, calcium, vitamin D, vegetables, and fruit. | High adherence (100%). Intervention significantly reduced triglycerides and daily fat consumption and increased mean daily fruit and vegetable consumption. | No control group. Small sample size (although pilot study). |
BC, breast cancer; BMI, Body mass index; BP, Blood pressure; BSI-18, Brief Symptom Inventory-18; CARES–SF, Cancer Rehabilitation Evaluation System – Short Form; CD, compact disc; Chemo, chemotherapy; CSQ, Cognitive Coping Strategies Questionnaire; FACIT, Functional Assessment of Chronic Illness Therapy; FACIT-Sp, Functional Assessment of Chronic Illness Therapy Spiritual Well Being subscale; FACT-B, Functional Assessment of Cancer Therapy – Breast; FFQ, Food Frequency Questionnaire; FSFI, Female Sexual Functioning Inventory; GTUS, Growth Through Uncertainty Scale; IES, Impact of Events Scale; long IPAQ; International Physical Activity Scale, Long Format; LDL, Low-density lipoprotein cholesterol; MHI, Mental Health Inventory; MUIS-S, Mishel Uncertainty in Illness Scale-Survivor; PANAS, Positive and Negative Affect Scale; PICS, Patient Perceived Involvement in Care; POMS, Profile of Mood States; POMS-B, Profile of Mood States Brief; POMS-SF, Profile of Mood States Short Form; QE, quasi-experimental; QoL, quality of life; SSQ, Northouse Social Support Questionnaire; SWB, Social Well Being subscale; UCLA, University of California Los Angeles.
Three articles that aimed to elucidate how Black and White women’s needs differ had a rigorous factorial design with adequate control groups. Although Black women had improved psychological outcomes [27], enhanced cognitive reframing skills [41], increased informational support seeking [41], greater personal growth, and greater flexibility [26], differences were found in the way that Black women utilized portions of the interventions and the benefits that they derived compared with White women. In dismantling their uncertainty management intervention, Gil et al. [40] noted that calming self-statements and distraction were the most frequently used strategies and rated as the most helpful by Black women; however, Black women reported using these skills less often than did White women. In addition, they also found that Black women reported a greater reduction in negative affect following the uncertainty management intervention than did White women [27]. All except two [25,29] of the empirical studies focused on populations posttreatment; this suggests a significant gap in psychosocial interventions focused on addressing the needs of women who are newly diagnosed and in active treatment.
Replication studies are still needed along with further exploration on the most effective intervention approaches for Black women. Existing studies have found cognitive-behavioral strategies to be beneficial [26,27,41], although certain cognitive-behavioral strategies appear to benefit Black women more than others [40]. In addition, culturally sensitive approaches and enhanced support have been found to be beneficial [25,29,33,35]. Although it is an emerging area of research, empirical studies demonstrate that Black women benefit from psychosocial interventions and cognitive-behavioral strategies to cope with cancer-related stressors. In addition, culturally engaged approaches and improving access to social resources and connection appear beneficial.
Quality of evidence
Of the seven empirical studies evaluated, the most formative work included a rigorous 2 × 2 factorial design study that examined differences in how Black and White women responded to an uncertainty management intervention [26,27,41]. Most other studies had adequate sample size with only two studies reporting 60 or fewer participants [25,33]. However, samples were not well described, with only a few studies addressing the confounding factors of race and SES (i.e., [25,26,29,34,35,41]). All longitudinal studies reported moderate rates (7–15%) of loss to follow-up, which could introduce attrition bias. Heterogeneity of the sample designs, lack of consistency in measured outcomes, and heterogeneity in treatment phase limit the utility of meta-analytical techniques to provide a summarized measure of the effects of the interventions [42].
All seven empirical studies included in our analyses specified eligibility criteria, randomly allocated participants to groups, obtained measures of at least one key outcome from more than 85% of original participants, reported results of between-group comparisons for at least one key outcome, and provided point measures and measures of variability for at least one key outcome. Although an empirically designed study, one article was excluded from our analysis as an empirical study because it did not meet these criteria [28].
Emerging interventions
Four emerging interventions that are currently in the pilot phase were identified [31–33,43]. All were weight-loss interventions tailored specifically for Black women diagnosed with breast cancer in the posttreatment phase. Although all interventions demonstrate promise, there are numerous limitations that restrict their scientific contribution. First, small sample size, ranging from 8 to 42, was a limitation of all of these pilot studies. Second, the lack of a control group in these studies limits rigorous testing of the interventions’ internal validity. Finally, limited follow-up periods did not permit an examination of the robustness of the intervention effects. Nonetheless, these interventions all provide valuable data regarding the feasibility, acceptability, and implementation of culturally tailored interventions to address posttreatment weight management, an issue that is a significant concern.
Intervention development
Four articles addressed the development or improvement of interventions. Articles in the intervention development domain focused on engaging Black women in intervention studies through community engagement [36], using a cultural framework based on qualitative data to develop interventions [37], and adapting existing interventions to Black women using focus group data with this population [38,39]. These descriptive studies provide valuable approaches for best practices in how to do work in this population. Community engagement resulted in a 373% increase in participants enrolled in an uncertainty management intervention [36]. Community engagement consisted of increasing familiarity of the study within the communities of interest, making study information easily available, and using community partnerships as cultural brokers. Qualitative work found that communication was a significant issue identified by patients, and a framework that included sharing personal stories, communication skills training, and decision-making support to create a culturally relevant protocol was developed [37]. Other culturally sensitive changes were made to existing protocols such as Taking CHARGE, a theory-based self-management program developed to assist women with survivorship concerns and adapted on the basis of participants’ feedback to include spirituality, coping with persistent fatigue, competing demands, disclosure, and age-specific concerns about body image/sexuality in order to better meet the needs of Black women [38]. A cognitive-behavioral stress management intervention was adapted to improve women’s access to the program, increase the cultural relevance of the intervention materials, and provide needed support in a culturally sensitive manner [39].
Conclusions
A diagnosis of breast cancer and its treatment are associated with significant psychological distress, which negatively impacts psychosocial adjustment to the disease process and health outcomes. Greater distress among breast cancer patients has been associated with poorer physical and mental health during treatment. Women diagnosed with breast cancer experience higher levels of stress during and after treatment [44–49]. The purpose of this investigation was to evaluate the present knowledge regarding the influence of both culturally sensitive and culturally inclusive psychosocial interventions on outcomes affecting Black women diagnosed with breast cancer. Interventions were classified as empirical, emerging, and in development. Although this is an emerging body of literature, the current review found scientific evidence that suggests that Black women diagnosed with breast cancer benefit from psychosocial interventions. However, many aspects unique to this population require additional scientific inquiry. Although diagnosis represents a time when women seek information and connection with others with whom they can relate, little work to date has been performed with newly diagnosed women in this population.
It is important to note that the aspects of uncertainty management functioned differently for Black women than for White women, although Black women’s coping skills were improved through cognitive reframing and increased knowledge. These skills are critical to posttreatment survivorship, and clinicians can deliver brief interventions that address patients’ anxiety in a manner that is relevant to their needs. Similarly, the decision-making intervention showed improved self-efficacy, enabling Black women to better engage with their healthcare team on treatment decisions such as chemotherapy [30]. Follow-up work is needed to better understand the impact that this intervention has on treatment decisions and the impact of those decisions on health outcomes.
Qualitative work has demonstrated that the intervention process is a significant contributing factor to success with this population. It appears that engaging women in a cultural context in which they feel comfortable promotes trust and may improve intervention uptake. Although having a community partner was reported to promote participant engagement, it appears that creating a culturally welcoming environment was the most relevant factor for participant engagement. Women need to be engaged in a manner in which they feel comfortable and using resources that they value. Psychosocial intervention research among Black women diagnosed with breast cancer has begun to address the unique needs of this population. Interventions that appear to have the greatest success have created a cultural environment in which Black women feel welcome, have established trust, and use resources valued in the community such as spirituality, kin networks, and oral storytelling. However, this work has only begun, and more work needs to be carried out to address the unique stressors that Black women face related to engaging support networks, communication, self-care management, depression, and anxiety.
Limitations
Bias at the data extraction stage of the review process was reduced by developing a set of criteria that each reviewer applied independently. In addition, only peer-reviewed articles were included; therefore, dissertations that may have informed this area were not included. Further, we included studies that were exclusively Black and studies that specifically oversampled Black populations; therefore, we do not account for potential cultural biases in how interventions were delivered.
Future directions
Over 80% of empirical intervention work focused on Black women diagnosed with breast cancer has been concentrated on the posttreatment phase. Although posttreatment is a crucial time in need of further study, there is a paucity of work at the time of diagnosis and during treatment. Previous research demonstrates that how women approach cancer treatment and self-care is key opportunities to improve health outcomes. To address the gaps in the scientific literature, additional work will be needed to understand how psychosocial interventions can improve the health trajectory for Black women, particularly in the areas of seeking help and support, identifying culturally acceptable methods for engaging support networks, and identifying best practices for enhancing coping skills. In addition, although cultural context, community partnerships, and spirituality have been woven into the fabric of many interventions, future studies should address the mechanisms that make culturally sensitive interventions effective.
Acknowledgements
This research was supported by the Department of Clinical and Health Psychology at the University of Florida and through funding from the Dean’s Office in the College of Public Health and Health Professions at the University of Florida.
The funders played no role in the study design, collection, analysis, interpretation of data, writing of the report, or the decision to submit the paper for publication. They accept no responsibility for the contents.
References
- 1.American Cancer Society. 2013 Cancer Facts & Figs. American Cancer Society, Inc.: Atlanta, 2013. [Google Scholar]
- 2.Gorey KM, Luginaah IN, Schwartz KL et al. Increased racial differences on breast cancer care and survival in America: historical evidence consistent with a health insurance hypothesis, 1975–2001. Breast Cancer Res Treat 2009;113:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis E, Quale C, Haggstrom D, Smith-Bindman R. Racial and ethnic differences in breast cancer survival: how much is explained by screening, tumor severity, biology, treatment, comorbidities, and demographics? Cancer 2008;112:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat 2011;127:729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cross CK, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the US – what have we learned from clinical studies? Cancer 2002;95:1988–1999. [DOI] [PubMed] [Google Scholar]
- 6.Adler N, Page AEK (eds). Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. The National Academies Press: Washington, DC; 2008. [PubMed] [Google Scholar]
- 7.Gerend MA, Pai M. Social determinants of Black–White disparities in breast cancer mortality: a review. Cancer Epidem Biomar 2008;17:2913–2923. [DOI] [PubMed] [Google Scholar]
- 8.Blackman DJ, Masi CM. Racial and ethnic disparities in breast cancer mortality: are we doing enough to address the root causes? J Clin Oncol 2006;24:2170–2178. [DOI] [PubMed] [Google Scholar]
- 9.Halbert CH, Armstrong K, Gandy OH, Shaker L. Racial differences in trust in health care providers. Arch Intern Med 2006;166:896–901. [DOI] [PubMed] [Google Scholar]
- 10.Mandelblatt J, Andrews H, Kerner J, Zauber A, Burnett W. Determinants of late stage diagnosis of breast and cervical cancer: the impact of age, race, social class, and hospital type. Am J Public Health 1991;81:646–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silber JH, Rosenbaum PR, Clark AS, et al. Characteristics associated with differences in survival among Black and White women with breast cancer. JAMA – J Am Med Assoc 2013; 310:389–397. [DOI] [PubMed] [Google Scholar]
- 12.Yabroff KR, Breen N, Vernon SW, Meissner HI, Freedman AN, Ballard-Barbash R. What factors are associated with diagnostic follow-up after abnormal mammograms? Findings from a US national survey. Cancer Epidem Biomar 2004;13:723–732. [PubMed] [Google Scholar]
- 13.Wilmoth MC, Sanders LD. Accept me for myself: African American women’s issues after breast cancer. Oncol Nurs Forum 2001;28:875–879. [PubMed] [Google Scholar]
- 14.Moore RJ. African American women and breast cancer: notes from a study of narrative. Cancer Nurs 2001;24:35–42. [DOI] [PubMed] [Google Scholar]
- 15.Spiegel D, Bloom JR, Kraemer HC, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet 1989;2:888–891. [DOI] [PubMed] [Google Scholar]
- 16.Luecken LJ, Compas BE. Stress, coping, and immune function in breast cancer. Ann Behav Med 2002;24:336–344. [DOI] [PubMed] [Google Scholar]
- 17.Birnie K, Garland SN, Carlson LE. Psychological benefits for cancer patients and their partners participating in mindfulness-based stress reduction (MBSR). Psycho-Oncol 2010;19:1004–1009. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann T, Heinrichs N, Baucom DH. “Does one size fit all?” Moderators in psychosocial interventions for breast cancer patients: a meta-analysis. Ann Behav Med 2007;34:225–239. [DOI] [PubMed] [Google Scholar]
- 19.Lerman R, Jarski R, Rea H, Gellish R, Vicini F. Improving symptoms and quality of life of female cancer survivors: a randomized controlled study. Ann Surg Oncol 2012;19:373–378. [DOI] [PubMed] [Google Scholar]
- 20.Branstrom R, Kvillemo P, Moskowitz JT. A randomized study of the effects of mindfulness training on psychological well-being and symptoms of stress in patients treated for cancer at 6-month follow-up. Int J Behav Med 2012;19:535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matchim Y, Armer JM, Stewart BR. Mindfulness-based stress reduction among breast cancer survivors: a literature review and discussion. Onc Nurs Forum 2011;38:E61–E71. [DOI] [PubMed] [Google Scholar]
- 22.Ashton CM, Haidet P, Paterniti DA, et al. Racial and ethnic disparities in the use of health services – bias, preference, or poor communication? J Gen Intern Med 2003;18(2):146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiatt RA, Breen N. The social determinants of cancer – a challenge for transdisciplinary science. Am J Prev Med 2008;35:S141–S150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med 2009;3:e123–130. [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor KL, Lamdan RM, Siegel JE, Shelby R, Moran-Klimi K, Hrywna M. Psychological adjustment among African American breast cancer patients: one-year follow-up results of a randomized psychoeducational group intervention. Health Psychol 2003;22:316–323. [DOI] [PubMed] [Google Scholar]
- 26.Gil KM, Mishel MH, Belyea M, Germino B, Porter LS, Clayton M. Benefits of the uncertainty management intervention for African American and White older breast cancer survivors: 20-month outcomes. Int J Behav Med 2006;13:286–294. [DOI] [PubMed] [Google Scholar]
- 27.Germino BB, Mishel MH, Crandell J, et al. Outcomes of an uncertainty management intervention in younger African American and Caucasian breast cancer survivors. Oncol Nurs Forum 2013;40:82–92. [DOI] [PubMed] [Google Scholar]
- 28.Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol 2007;25:4387–4395. [DOI] [PubMed] [Google Scholar]
- 29.Heiney SP, Millon Underwood S, Tavakoli A, Arp Adams S, Wells LM, Bryant LH. Randomized trial of therapeutic group by teleconference: African American women with breast cancer. Cancer 2012;118:3822–3832. [DOI] [PubMed] [Google Scholar]
- 30.Sheppard VB, Wallington SF, Willey SC, et al. A peer-led decision support intervention improves decision outcomes in Black women with breast cancer. J Cancer Educ 2013;28:262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson DB, Porter JS, Parker G, Kilpatrick J. Anthropometric changes using a walking intervention in African American breast cancer survivors: a pilot study. Prev Chronic Dis 2005;2:1–7. [PMC free article] [PubMed] [Google Scholar]
- 32.Stolley MR, Sharp LK, Oh A, Schiffer L. A weight loss intervention for African American breast cancer survivors, 2006. Prev Chronic Dis 2009;6:A22. [PMC free article] [PubMed] [Google Scholar]
- 33.Greenlee HA, Crew KD, Mata JM, et al. A pilot randomized controlled trial of a commercial diet and exercise weight loss program in minority breast cancer survivors. Obesity (Silver Spring) 2013;21:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schover LR, Jenkins R, Sui DW, Adams JH, Marion MS, Jackson KE. Randomized trial of peer counseling on reproductive health in African American breast cancer survivors. J Clin Oncol 2006;24:1620–1626. [DOI] [PubMed] [Google Scholar]
- 35.Schover LR, Rhodes MM, Baum G, et al. Sisters Peer Counseling in Reproductive Issues After Treatment (SPIRIT): a peer counseling program to improve reproductive health among African American breast cancer survivors. Cancer 2011;117:4983–4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Germino BB, Mishel MH, Alexander GR, et al. Engaging African American breast cancer survivors in an intervention trial: culture, responsiveness and community. J Cancer Surviv 2011;5:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheppard VB, Williams KP, Harrison TM, et al. Development of decision-support intervention for Black women with breast cancer. Psycho-Oncol 2010;19:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung LK, Cimprich B, Janz NK, Mills-Wisneski SM. Breast cancer survivorship program: testing for cross-cultural relevance. Cancer Nurs 2009;32:236–245. [DOI] [PubMed] [Google Scholar]
- 39.Lechner SC, Ennis-Whitehead N, Robertson BR, et al. Adaptation of a psycho-oncology intervention for Black breast cancer survivors: project CARE. Couns Psychol 2013;41:286–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gil KM, Mishel MH, Germino B, Porter LS, Carlton-LaNey I, Belyea M. Uncertainty management intervention for older African American and Caucasian long-term breast cancer survivors. J Psychosoc Oncol 2005; 23:3–21. [DOI] [PubMed] [Google Scholar]
- 41.Mishel MH, Germino BB, Gil KM, et al. Benefits from an uncertainty management intervention for African-American and Caucasian older long-term breast cancer survivors. Psycho-Oncol 2005;14:962–978. [DOI] [PubMed] [Google Scholar]
- 42.Hedges LV, Pigott TD. The power of statistical tests in meta-analysis. Psychol Methods 2001;6:203–217. [PubMed] [Google Scholar]
- 43.Griffith KA, Royak-Schaler R, Nesbitt K, et al. A culturally specific dietary plan to manage weight gain among African American breast cancer survivors: a feasibility study. Nutr Health 2012;21:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cordova MJ, Andrykowski MA, Kenady DE, McGrath PC, Sloan DA, Redd WH. Frequency and correlates of posttraumatic-stress-disorder-like symptoms after treatment for breast cancer. J Consult Clin Psychol 1995;63:981–986. [DOI] [PubMed] [Google Scholar]
- 45.Epping-Jordan JE, Compas BE, Osowiecki DM, Oppedisano G, Gerhardt C, Primo K, Krag DN Psychological adjustment in breast cancer: processes of emotional distress. Health Psychol 1999;18:315–326. [DOI] [PubMed] [Google Scholar]
- 46.Montgomery M, McCrone SH. Psychological distress associated with the diagnostic phase for suspected breast cancer: systematic review. J Adv Nurs 2010;66:2372–2390. [DOI] [PubMed] [Google Scholar]
- 47.Ng CG, Boks MP, Zainal NZ, de Wit NJ. The prevalence and pharmacotherapy of depression in cancer patients. J Affect Disord 2011;131:1–7. [DOI] [PubMed] [Google Scholar]
- 48.Reyes-Gibby CC, Anderson KO, Morrow PK, Shete S, Hassan S. Depressive symptoms and health-related quality of life in breast cancer survivors. J Womens Health 2012;21:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanton AL Psychosocial concerns and interventions for cancer survivors. J Clin Oncol 2006;24:5132–5137. [DOI] [PubMed] [Google Scholar]