Abstract
Background/Aims:
We aimed to investigate incidence, characteristics, and possible risk factors of pancreatic cancer in patients under observation for hepatocellular carcinoma (HCC) because the association of hepatitis virus B infection and pancreatic cancer has been reported.
Patients and Methods:
We performed a retrospective cohort study in the Gastroenterology Department of a University Hospital in Japan between 2004 and 2012. A total of 1848 patients who underwent treatment for HCC were included at the initiation of treatment for HCC (mean follow-up period, 33.6 months). The patients received trimonthly radiological follow-ups. Newly developed cases of pancreatic cancer during follow-up for HCC were compared with that of an age- and sex-matched theoretical cohort from national statistics. Possible predisposing factors for pancreatic cancer related to HCC were assessed. Cumulative probabilities of developing a pancreatic cancer were compared using log-rank test.
Results:
About 13 of 1848 patients developed pancreatic cancer (mean follow-up period, 45.2 months). The risk ratio for all patients was 3.02 (log-rank test: P =0.01). Statistical analyses showed no effects of the following factors on the development of pancreatic cancer: age, sex, follow-up period, alcohol intake, laboratory data, presence of hepatitis virus, characteristics of HCC, type of treatment, number of radiological examinations, and cumulative effective dose.
Conclusions:
Increased incidence of pancreatic cancer was found in patients under observation for HCC in a relatively small cohort. HCC or other common underlying conditions might be a risk factor for development of pancreatic cancer.
Keywords: Hepatitis virus, liver cirrhosis, metachronous cancer, pancreas, radiation-induced cancer, synchronous cancer
INTRODUCTION
Pancreatic adenocarcinoma is the fourth to fifth leading cause of death from cancer in both males and females in the United States,[1] Japan,[2] and other developed countries.[3] The disease has a very poor prognosis, with 6% of patients surviving for 5 years[1] and only 20% of patients undergoing surgery, which is the only curative treatment for the disease.[4] Known risk factors for pancreatic adenocarcinoma include smoking, long-standing diabetes mellitus, obesity, nonhereditary and hereditary chronic pancreatitis, and a few genetic syndromes.[5] Some previous studies have suggested that chronic hepatitis B virus (HBV) infection is also associated with an increased incidence of pancreatic cancer, but hepatocellular carcinoma (HCC) has not been reported as a risk factor until now.[6,7,8,9] Repeated computed tomography (CT) has also been reported to increase the rates of solid cancers because diagnostic X-rays have the potential to break double-stranded DNA;[10,11] however, CT has not been reported to increase the risk of pancreatic cancer.[12] The present preliminary study aimed to explore incidence, characteristics, and possible risk factors of pancreatic cancer in patients with HCC in light of viral hepatitis infection, HCC, and radiation exposure due to repeated upper-abdominal CT performed in follow-up of HCC.
PATIENTS AND METHODS
Our Institutional Review Board approved this STROBE-compliant single-center, retrospective cohort study and waived informed consent. However, written informed consent for prospective data collection was obtained from all patients. All experiments and methods in this study were performed in accordance with the Declaration of Helsinki.
Patients
The cohort group included all patients who were referred to the Gastroenterology Department of our University Hospital between January 2004 and May 2012 and who underwent treatment for HCC (initial or secondary treatment). They were selected as the cohort because they underwent trimonthly periodic dynamic CT examinations and had a better prognosis (average survival at our institution is 6.4 years)[13] than patients with pancreatic adenocarcinoma. This cohort has been reported in our previous study on precancerous findings of pancreatic adenocarcinoma on CT, which did not investigate the same hypothesis as the present study.[14]
Clinical data collection
The following patient data were collected before treatment of HCC at our institution: age, sex, body mass index, amount of daily alcohol intake, laboratory data, background liver condition, presence or absence of ascites and encephalopathy at the initial assessment for HCC, type of subsequent HCC treatment, follow-up period, and time and amount of radiation exposure (CT, angiography). The presence of diabetes mellitus was not strictly assessed because collection of data on diabetes was not performed in patients being treated for HCC. The patients received trimonthly radiographic follow-up examinations for HCC; most underwent four-phase liver CT, and others underwent magnetic resonance imaging (MRI), noncontrast CT, and ultrasonography. Board-certified staff radiologists assessed the images for HCC and screened the pancreas for the presence of pancreatic adenocarcinoma. A diagnosis of pancreatic cancer was made when a specimen of pancreatic adenocarcinoma was obtained surgically or by image-guided biopsy (pathological diagnosis) or a combination of typical image findings was observed (clinical diagnosis). The patients who developed pancreatic cancer were included in the case group. The date of diagnosis was determined by the date on which the specimen of pancreatic cancer was obtained for cases with pathological diagnosis and by the date on which the pathological diagnosis was abandoned when the diagnosis of pancreatic cancer was considered nearly definitive on clinical basis. Staging was based on the AJCC Cancer Staging Manual, 6th and 8th editions, by the American Joint Committee on Cancer,[15,16] and the 7th edition of the General Rules for the Study of Pancreatic Cancer, by the Japan Pancreas Society (JPS2016).[17]
Expected rate of pancreatic cancer for an age- and sex-matched theoretical cohort
The expected rate per year of development of pancreatic cancer for each patient in the cohort adjusted by age and sex was calculated on the basis of the pancreatic cancer incidence by 5-year age groups from the National Cancer Statistics retrieved from the Center for Cancer Control and Information Services in Japan, multiplied by the follow-up period and then integrated for the cohort and subgroup.[2] Data from the American National Cancer Data Base (1992–1998) were retrieved for reference.[18]
Estimation of radiation dose
For estimation of the radiation dose, the whole database of CT examinations in our institution was retrieved. The reference radiation dose was determined by averaging the radiation doses in 20 sample studies for each body CT protocol that included the pancreas. The number of CT studies, the CT dose index, and the effective dose (dose length product × normalized effective dose) were calculated for each patient in the cohort and case groups.[19] Assessment of radiation exposure caused by angiography and radioactive scans was abandoned because the data were incomplete.
Statistical analyses
The incidence of pancreatic cancer and the amount of radiation dose due to radiological examinations were calculated. For univariate analyses, nominal data were analyzed by Fisher's exact test and numerical data were analyzed by the t-test. Multiple regression analysis was performed to assess the effect of radiological examinations on the development of pancreatic cancer. Significant variables in univariate analyses were used in this multivariate analysis. All P values were two-sided, and P values <0.05 were considered as indicative of statistical significance. Family-wise error was corrected by the Bonferroni method. All statistical analyses were performed by using EZR (Saitama Medical Center, Saitama, Japan), which is a graphical user interface for R version 3.2.2 (The R Foundation for Statistical Computing).[20]
RESULTS
Patient characteristics
A total of 1848 patients with HCC were included in the study and were evaluated before treatment of HCC. No pancreatic cancer was observed at inclusion. During treatment for HCC, 13 patients developed pancreatic cancer. About 9 of the 13 patients were histologically confirmed to have pancreatic adenocarcinoma, and the other four patients were clinically diagnosed as having exocrine pancreatic cancer by typical findings of pancreatic mass on CT, MRI, ultrasonography, laboratory data, and clinical course and were subsequently treated by chemotherapy, radiation, or best supportive care. About 3 of the 13 patients had possible synchronous intraductal mucinous neoplasms at sites different from that of the pancreatic cancer. Specimens of the pathologically diagnosed cases of pancreatic cancer had unexceptional findings of pancreatic adenocarcinoma. The demographics of the patients in the cohort and case groups are presented in Table 1. A comparison of the clinical data is also presented in Table 1, which includes factors associated with obesity, alcohol intake, hepatitis viral infection, HCC, and portal hypertension, between the cohort and case groups and shows no predisposition to pancreatic cancer. No hereditary or genetic syndrome was known for these 13 patients.
Table 1.
Demographics of the patients in the cohort and case groups
| Characteristic | Cohort group (n=1848) | Case group (n=13) | P |
|---|---|---|---|
| Age (year) | 68.5 (9.4) | 71.2 (5.5) | 0.12 |
| Male | 1225 [67%] | 8 [62%] | 0.77 |
| Observation period (month) | 34.4 (24.3) | 50.0 (27.9) | 0.78 |
| Body mass index (kg/m2) | 23.5 (3.6)† | 23.0 (2.3) | 0.47 |
| Alcohol intake (>50 g/day) | 482 [26] | 4 [31] | 0.75 |
| Initial therapy at our institution | 1121 [60%] | 8 [62%] | 0.78 |
| Initial regimen | |||
| PEIT/RFA | 1450 [78%] | 12 [92%] | 0.32 |
| TACE | 315 [17%] | 1 [8%] | 0.71 |
| Chemotherapy | 52 [3%] | 0 [0%] | 1 |
| Best supportive care | 40 [2%] | 0 [0%] | 1 |
| Radiation | 3 [0%] | 0 [0%] | 1 |
| Molecular targeted drug therapy | 1 [0%] | 0 [0%] | 1 |
| Maximum diameter of HCC (mm) | 27 (17)‡ | 24 (10) | 0.33 |
| No. of HCC lesions | 2.4 (2.9)§ | 1.9 (1.0) | 0.11 |
| Aspartate aminotransferase (IU) | 60 (40) | 70 (42) | 0.44 |
| Alanine aminotransferase (IU) | 53 (41) | 61 (44) | 0.50 |
| Total bilirubin (mg/dL) | 1.1 (1.3) | 0.9 (0.6) | 0.34 |
| Albumin (g/L) | 3.6 (0.5) | 3.6 (0.4) | 0.58 |
| HBs antigen positive | 245 [13%] | 1 [8%] | 1 |
| HCV antibody positive | 1290 [70%] | 10 [77%] | 0.77 |
| Non-B, non-C hepatitis | 313 [17%] | 1 [8%] | 0.71 |
| Ascites | 379 [21%] | 1 [8%] | 0.47 |
| Encephalopathy | 51 [3%] | 0 [0%] | 1 |
Results are given as the mean (standard deviation) or n [%]. HBs: Hepatitis B surface, HCC: Hepatocellular carcinoma, HCV: Hepatitis C virus, PEIT: Percutaneous ethanol injection therapy, RFA: Radiofrequency ablation, TACE: Transcatheter arterial chemoembolization. †Data unavailable in 30 cases (not included). ‡Unmeasurable in 27 cases with diffuse-type HCC (not included). §Uncountable in 38 cases and data unavailable in four cases (not included)
Incidence of pancreatic cancer
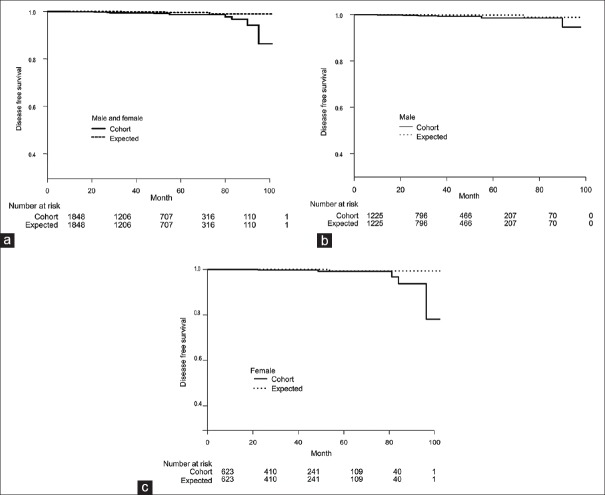
The expected and observed numbers of patients with pancreatic cancer in the cohort adjusted by age, sex, and follow-up period are shown in Table 2. Compared with the general population, a three-fold greater incidence of pancreatic cancer was observed in the cohort of patients receiving HCC treatment. The male-to-female ratio in the cohort was similar to that in the population. Kaplan–Meier curves show cumulative increased probabilities of developing pancreatic cancer in the cohort compared with a population model matched for age, sex, and follow-up period derived from the National Cancer Statistics in Japan [Figure 1]. The P values according to the log-rank test for all patients, male patients and female patients were 0.01, 0.06 and 0.10, respectively.
Table 2.
Expected and observed number of patients with pancreatic cancer (adenocarcinoma) in the cohort adjusted by age, sex, and follow-up period
| Patients | Expected No. (/105) | Observed No. (/105) | Relative risk (95% CI) |
|---|---|---|---|
| All | 4.30 (232.7) | 13 (802.6) | 2.62* (1.30-5.29) |
| Male | 3.03 (247.3) | 8 (703.5) | 3.94* (1.63-9.52) |
| Female | 1.27 (203.9) | 5 (653.1) | 3.02* (1.72-5.25) |
CI: Confidence interval. *Statistically significant
Figure 1.
Kaplan–Meier curves show the cumulative probabilities of developing pancreatic cancer during observation for hepatocellular carcinoma (HCC) in a cohort of patients who underwent treatment for HCC in a Department of Gastroenterology (solid lines in a-c) and in a theoretical model of an age-, sex, and follow-up period-matched population that included the same number of subjects calculated from the National Cancer Statistics in Japan (dotted lines in a-c). Therefore, the numbers at risk are the same for the two groups. Disease-free survival of pancreatic cancer was shorter in the cohort than in the theoretical model. ThePvalues according to the log-rank test for (a) all patients (male and female combined), (b) male patients, and (c) female patients were 0.01, 0.06, and 0.10, respectively
Effect of radiological examinations
The calculated reference radiation dose indexes (mGy) for pancreas determined by using CT were as follows: chest protocol, 13.0; abdomen to pelvis, 20.0; chest to pelvis, 20.8, and multiphasic abdomen, 98.1. A summary of radiological examinations of the cohort and case groups is shown in Table 3. The number of exposures and the cumulative dose of radiation exposure were larger in the case group than in the cohort group. Multivariate analyses found no effects of cumulative radiation exposure on the development of pancreatic cancer by CT, time of CT examination, or follow-up period, even after multicollinearity was considered. The forest plot of the potential risk factors for pancreatic cancer is depicted in Figure 2.
Table 3.
Summary of radiological examinations of the cohort and case groups
| Variable | Whole database | Cohort group (n=1,848) | Case group (n=13) |
|---|---|---|---|
| No. of patients (dose data available) | 69,640 | 1,741 | 13 |
| No. of CT studies (dose data available) | 283,158 | 26,546 | 282 |
| No. of CT studies per patient | 4.1 | 14.4 | 21.7* |
| No. of abdominal angiographies per patient | NA | 0.43† | 1.69 |
| No. of radioactive scans per patient | NA | 0.15† | 1.07* |
| Cumulative CTDI per patient (mGy) | 144 | 1,031 | 1,680* |
| Cumulative effective dose per patient (mSv) | 65 | 351 | 560* |
| Follow-up period (mo.) | NA | 33.6 | 50* |
CT: Computed tomography, CTDI: Computed tomography dose index, NA: Not available. *Statistically significant before and statistically nonsignificant after family-wise correction compared with the cohort group (P=0.02-0.03). †Average of randomly chosen data from 112 subjects
Figure 2.
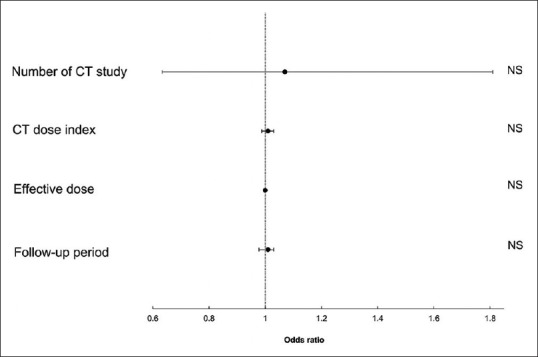
Forest plot shows the odds ratios for the development of pancreatic cancer in the cohort group for the four top potential risks in terms of total radiation exposure during the observation period and length of follow-up period calculated by comparison of the cohort and case group. There were no significant increased risks for development of pancreatic cancer according to the number of computed tomography (CT) studies per patient, CT dose index per patient, effective dose per patient, and follow-up period, even after multicollinearity was considered. NS, not significant
Clinical course of pancreatic cancer coexisting with HCC in the case group
Pancreatic cancer developed in 13 patients in the case group 50 months after enrolment in the study on average (range, 10–95 months) [Table 2]. The clinical stage was lower in the case group than in the reported National Cancer Statistics of pancreatic cancer [Figure 3]. The treatment targeting pancreatic cancer was as follows: surgery alone, two patients; surgery and chemotherapy, four; chemotherapy alone, three patients; chemotherapy and proton radiation therapy one, and best supportive care, three. Margin-negative resection was successful in all resected cases. Most patients died of pancreatic cancer; overall survival was 22.3 months after diagnosis of pancreatic cancer (for four patients diagnosed without pathology, 9.7 months) (updated 9 January 2017).
Figure 3.
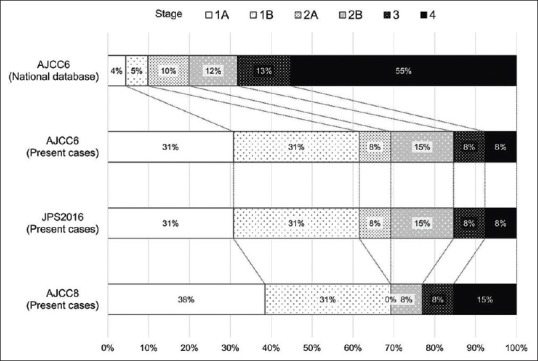
Distribution of pancreatic cancer by clinical stage is depicted in the case group and in the National Cancer Database of the United States (1992–1998) classified according to theCancer Staging Manual, 6th edition (AJCC6, 2002), 8th edition (AJCC8, 2016) by the American Joint Committee on Cancer and the 7th edition of theGeneral Rules for the Study of Pancreatic Cancerby the Japan Pancreas Society (JPS2016, 2016). The clinical stage of pancreatic cancer was lower in the case group than in the reported national statistics, which means that pancreatic cancers in the patients with hepatocellular carcinoma were detected at an early stage
DISCUSSION
In this study, the incidence of pancreatic cancer was increased three-fold in a cohort of patients undergoing treatment for HCC compared with that in the general population.
We found that most of the pancreatic cancers in patients with HCC occurred at an earlier stage than that in the pancreatic cancers in the population. This finding may be because the patients in our study were periodically followed up by CT, and thus, cancers were more likely to be detected. The patients in our study had a better prognosis because they had a greater chance to receive a curative operation at an earlier stage than that of the patients reported in the statistics. Otherwise, our patients had pathologically unexceptional common pancreatic adenocarcinomas and unexceptional clinical courses when clinical stages were matched. Lead time bias should be considered when early diagnosis of cancers is made but lead time bias would not be a consideration with pancreatic cancer because of its very poor prognosis.
Known risk factors and established genetic syndromes associated with pancreatic adenocarcinoma include smoking, long-standing diabetes mellitus, nonhereditary and chronic pancreatitis, obesity, and genetic syndromes, such as hereditary pancreatitis, hereditary breast and ovarian cancer syndromes, and Peutz–Jeghers syndrome, among others.[5] In this study, we assumed several reasons for the increased risk of pancreatic cancer: history of infection with hepatitis virus, chronic inflammation associated with viral hepatitis infection, radiological findings associated with cirrhosis and portal hypertension, and repeated radiation exposure due to CT examinations for HCC.
A Taiwanese cohort study, REVEAL-HBV, found that HBV infection was associated with a two-fold increased risk of pancreatic cancer, most notably in women, people ≤50 years of age, nonsmokers, and nondrinkers. An increased risk of pancreatic cancer was observed for hepatitis B surface antigen-seropositive persons with active HBV replication.[6] The mechanism is speculated to be a result of acute or chronic inflammation due to infection by HBV[6] because intrapancreatic HBV viral particles and elevated pancreatic enzymes are found in patients with HBV,[21,22,23] although there is insufficient evidence on this question. A population-based study revealed that the risk of pancreatic cancer was slightly elevated in people with hepatitis C virus (HCV) (hazard ratio, 1.23–1.32) but was attenuated after adjustment for alcohol use, pancreatitis, and other variables.[7] In this study, >10 other factors, including HBV and HCV infection and findings associated with cirrhosis, were compared between the cohort and case groups, but no predisposition to pancreatic cancer was detected. However, the prevalence rates of viral hepatitis infection, HCC, and other HCC-associated factors were higher in the cohort group than in the Japanese population: approximately 3% for HBV and HCV infections and 30.7 per 100,000 person-years for HCC.[24] Therefore, the statistically negative results in this study neither support nor deny the effect of viral hepatitis infection or HCC on the development of pancreatic cancer.
Chronic pancreatitis is one of the major factors that increases the incidence of pancreatic cancer.[25,26] The mechanism is speculated to involve accumulation of mutations and clonal expansion that are indirectly caused by recurrent pancreatitis and chronic inflammation.[27] However, in this study, no relationship was found between the development of pancreatic cancer and the amount of alcohol intake or radiographic signs of chronic pancreatitis. Chronic Helicobacter pylori infection can affect both the stomach and pancreas and may be a potential risk factor for chronic pancreatitis and, therefore, also a risk factor for pancreatic cancer,[26,28] but H. pylori infection was not assessed in this study.
At the beginning of the study, we hypothesized that radiation exposure due to repeated radiological examinations for HCC might increase the incidence of pancreatic cancer. The calculated reference radiation dose at our institution was similar to that in a previous report.[10] There is strong epidemiological evidence that the relationship between radiation exposure and solid cancer induction is approximately linear for intermediate doses of approximately 0.15–1.5 Gy, the range of doses received by the patients in this study.[11] CT scans are reported to increase the risk of solid cancers in children and adolescents, and the risk decreases with age at first exposure.[10,29] CT-induced cancer reportedly has a variable incubation period ranging from 1 to 30 years.[10,29,30] However, there is no reported evidence that radiation exposure increases the risk of pancreatic cancer, and no increase has yet been observed in death from pancreatic cancer among atomic bomb survivors in Hiroshima and Nagasaki.[12] In this study, the patients in the cohort received a larger number of CT examinations than that of the Japanese population (10.3/10−6 times per year).[31] There was no significant relationship between pancreatic cancer and radiation. Hence, it is unlikely that pancreatic cancer was induced by CT examinations.
The study has some other inherent limitations. This study was not designed to determine the reasons for the increase in pancreatic cancer and did not include full check-ups for smoking history and diabetes mellitus because the patients in the cohort were treated for HCC. The study was performed in a single high-volume center, and a multicenter approach is required to further investigate and confirm our results.
In conclusion, increased incidence of pancreatic cancer was found in patients under observation for HCC in a relatively small cohort. History of HCC or common underlying conditions might be a risk factor for development of pancreatic cancer, which needs further investigation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors thank Drs. Yoko Mise, Takeyuki Watadani, Jiro Sato, and Naoki Okura for supporting this study.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Takayama S ; 2015 [] Tsukiji, Japan: Foundation for Promotion of Cancer Research. 2015. [Last updated 2015 May 21; Last cited on 2015 Jul 03]. Available from: http://ganjoho.jp/data/reg_stat/statistics/brochure/2014/cancer_statistics_2014.pdf .
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Brennan MF, Moccia RD, Klimstra D. Management of adenocarcinoma of the body and tail of the pancreas. Ann Surg. 1996;223:506–11. doi: 10.1097/00000658-199605000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–49. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 6.Iloeje UH, Yang HI, Chen CJ. Natural history of chronic hepatitis B: What exactly has REVEAL revealed? Liver Int. 2012;32:1333–41. doi: 10.1111/j.1478-3231.2012.02805.x. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, Engels EA, Landgren O, Chiao E, Henderson L, Amaratunge HC, et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49:116–23. doi: 10.1002/hep.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Liu S, Guo C, Zong J, Sun MZ. The association of annexin A2 and cancers. Clin Transl Oncol. 2012;14:634–40. doi: 10.1007/s12094-012-0855-6. [DOI] [PubMed] [Google Scholar]
- 9.Hassan MM, Li D, El-Deeb AS, Wolff RA, Bondy ML, Davila M, et al. Association between hepatitis B virus and pancreatic cancer. J Clin Oncol. 2008;26:4557–62. doi: 10.1200/JCO.2008.17.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sodickson A, Baeyens PF, Andriole KP, Prevedello LM, Nawfel RD, Hanson R, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251:175–84. doi: 10.1148/radiol.2511081296. [DOI] [PubMed] [Google Scholar]
- 11.Shah DJ, Sachs RK, Wilson DJ. Radiation-induced cancer: A modern view. Br J Radiol. 2012;85:e1166–73. doi: 10.1259/bjr/25026140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu Y KH, Schull WJ. Risk of cancer among atomic bomb survivors. J Radiat Res. 1991;32(Supple 2):10. doi: 10.1269/jrr.32.supplement2_54. [DOI] [PubMed] [Google Scholar]
- 13.Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569–77. doi: 10.1038/ajg.2011.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonoi W, Hayashi TY, Okuma H, Akahane M, Nakai Y, Mizuno S, et al. Development of pancreatic cancer is predictable well in advance using contrast-enhanced CT: A case-cohort study. Eur Radiol. 2017;27:4941–50. doi: 10.1007/s00330-017-4895-8. [DOI] [PubMed] [Google Scholar]
- 15.Cancer AJCo. AJCC Cancer Staging Manual. 6th ed. Chicago: Springer; 2002. [Google Scholar]
- 16.Kakar S, Pawlik TM, Allen PJ, Vauthey JA. AJCC Cancer Staging Manual. 8th ed. New York: Springer-Verlag; 2016. Exocrine Pancreas. Pancreatic Adenocarcinoma. [Google Scholar]
- 17.Society JP. The 7th Edition of General Rules for the Study of Pancreatic Cancer by Japan Pancreas Society. 7th ed. Tokyo: Kanehara; 2016. [Google Scholar]
- 18.Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, et al. Validation of the 6th ed. AJCC pancreatic cancer staging system: Report from the national cancer database. Cancer. 2007;110:738–44. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 19.Valentin J. International Commission on Radiation P. Managing patient dose in multi-detector computed tomography (MDCT).ICRP Publication 102. Ann ICRP. 2007;37:1–79. doi: 10.1016/j.icrp.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–8. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hohenberger P. Detection of HBs-Ag in the pancreas in cases of pancreatic carcinoma. Hepatogastroenterology. 1984;31:239–41. [PubMed] [Google Scholar]
- 22.Katakura Y, Yotsuyanagi H, Hashizume K, Okuse C, Okuse N, Nishikawa K, et al. Pancreatic involvement in chronic viral hepatitis. World J Gastroenterol. 2005;11:3508–13. doi: 10.3748/wjg.v11.i23.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain P, Nijhawan S. Acute viral hepatitis with pancreatitis: Is it due to the viruses or sludge? Pancreatology. 2007;7:544–5. doi: 10.1159/000108974. [DOI] [PubMed] [Google Scholar]
- 24.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ragulin-Coyne E, Smith JK, Ng S, McDade TP, Shah SA, Tseng JF. Potential predictors of pancreatic cancer: A population-based screen. J Clin Oncol. 2011;29(4_suppl):164. [Google Scholar]
- 26.Lowenfels AB, Maisonneuve P, Lankisch PG. Chronic pancreatitis and other risk factors for pancreatic cancer. Gastroenterol Clin North Am. 1999;28:673–85. doi: 10.1016/s0889-8553(05)70080-7. [DOI] [PubMed] [Google Scholar]
- 27.Whitcomb DC. Inflammation and Cancer V. Chronic pancreatitis and pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2004;287:G315–9. doi: 10.1152/ajpgi.00115.2004. [DOI] [PubMed] [Google Scholar]
- 28.Rabelo-Goncalves EM, Roesler BM, Zeitune JM. Extragastric manifestations of Helicobacter pylori infection: Possible role of bacterium in liver and pancreas diseases. World J Hepatol. 2015;7:2968–79. doi: 10.4254/wjh.v7.i30.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: Data linkage study of 11 million Australians. BMJ. 2013;346:f2360. doi: 10.1136/bmj.f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Micke O, Schafer U, Glashorster M, Prott FJ, Willich N. Radiation-induced esophageal carcinoma 30 years after mediastinal irradiation: Case report and review of the literature. Jpn J Clin Oncol. 1999;29:164–70. doi: 10.1093/jjco/29.3.164. [DOI] [PubMed] [Google Scholar]
- 31.Tsushima Y, Taketomi-Takahashi A, Takei H, Otake H, Endo K. Radiation exposure from CT examinations in Japan. BMC Med Imaging. 2010;10:24. doi: 10.1186/1471-2342-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]