Abstract
Background/Aim:
The prognosis of hepatocellular carcinoma (HCC) is very dismal and the targeted drugs of HCC are limited. Studies of HCC prognostic biomarkers have made little progress, though many new techniques such as high-throughput sequencing have been applied. FOS-like antigen 1 (FOSL1) is generally accepted as a proto-oncogene but its clinical significance in HCC has never been elucidated.
Materials and Methods:
In our study, we investigated the expression of FOSL1 in 114 paraffin-embedded HCC tissues, and detected FOSL1 mRNA levels in 20 pairs of fresh HCC tissues and their corresponding tumor adjacent tissues. The correlations between FOSL1 expression and clinicopathological factors were analyzed and the prognostic significance of FOSL1 was evaluated with univariate and multivariate analysis. Moreover, we detected the function of FOSL1 in HCC proliferation with experiments in vitro.
Results:
FOSL1 mRNAs in HCCs were significantly higher than those in tumor adjacent tissues. The percentage of high expression and low expression of FOSL1 accounted for 46% (53/114) and 54% (61/114), respectively. High expression of FOSL1 was significantly associated with larger tumor size (P = 0.021), hepatitis B virus infection (P = 0.014), advanced T stage (P = 0.014), and tumor necrosis metastasis stage (P = 0.014). Moreover, high expression of FOSL1 was significantly correlated with poor prognosis of HCC and could be identified as an independent prognostic biomarker of HCC (hazard ratio = 5.60, 95% confidence interval = 3.00–10.45, P < 0.001). With in vitro function assay, we demonstrated that FOSL1 played an essential role in HCC proliferation.
Conclusions:
High expression of FOSL1 is an independent risk factor of HCC predicting unfavorable prognosis, indicating that FOSL1 detection could stratify patients with high risk, and anti-FOSL1 therapy may be a promising way to treat HCC.
Keywords: FOS-like antigen 1, hepatocellular carcinoma, prognostic biomarker, proliferation
INTRODUCTION
Primary liver cancer is the second leading cause of cancer-related death worldwide, with increasing morbidity.[1] Primary liver cancer could be histopathologically divided into hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and other rare types, including fibrolamellar carcinoma and hepatoblastoma.[2] HCC accounts for 90% of liver cancer with nearly 800,000 new cases every year worldwide, while ICC takes up approximately 10%.[1] HCC is a severe public health burden in China because of the high incidence of hepatitis B virus (HBV) infection, which is a well-known etiological factor in the pathogenesis of HCC.[3] Although the treatments for HCC have been greatly improved, the outcome of HCC is still dismal. The overall 5-year survival rate of patients with HCC resection is only 30%.[4] One reason attributed to the unfavorable prognosis is that the effect of systemic treatments to HCC is usually unsatisfactory. The breakthrough drug, sorafenib, could only improve the median overall survival time of HCC patients in advanced-stage from 8 to 11 months.[5] Therefore, the discovery of a new target drug for HCC, usually based on prognostic biomarkers, is still an urgent need.
FOS family is a well-known component of activating protein-1 (AP-1) transcription factors, which also include JUN, ATF/CREB, and MAF families and are responsible for mis-regulation in carcinogenesis.[6] The FOS family consists of FOS, FOSB, FOS-like antigen 1 (FOSL1), and FOSL2. All of them are defined as immediate early genes. Their expression is increased in a few hours, induced by upstream stimulation such as Ras-ERK or PI-3K-AKT pathway, dramatically affecting the tumoral epigenetic landscape by promoting expression of scales of tumor-relevant genes.[7] FOSL1 was reported to promote epithelial-to-mesenchymal transition, tumor invasion, and metastasis in several types of malignancies such as colorectal cancer and breast cancer.[8,9] In HCC, FOSL1 demonstrated promotion of keratin 19 expression and leads to a more aggressive phenotype. However, the clinical and prognostic significance of FOSL1 in HCC is still unknown.
In this study we investigated the expression of FOSL1 in 20 pairs of fresh HCC tissues and 114 paraffin-embedded HCC tissues, and evaluated the clinical and prognostic significance of FOSL1 via analyzing the correlation between FOSL1 expression, clinicopathological factors, and survival rates. Moreover, we detected the function of FOSL1 in HCC proliferation with experiments in vitro.
MATERIALS AND METHODS
Our study is a consecutive cohort consisting of 220 patients who underwent radical resection of HCC in Yidu Central Hospital and the PLA 404 Hospital, China from 2006 to 2014. The final diagnoses were confirmed by routine pathology. A total of 114 patients were selected from the 220 patients according to the following criteria: (1) available tissue samples for immunohistochemistry (IHC) and follow-up; (2) no adjuvant therapy; (3) no severe operation-related complications. Another prospective cohort of 20 patients with HCC was established, and the fresh HCC tissues and adjacent tumor tissues of these patients were obtained from surgery and preserved in liquid nitrogen for mRNA extraction. The study was approved by the Ethics Committee of Yishui Central Hospital and the PLA 404 Hospital. All specimens were obtained with prior content of the patients. The tumor–necrosis–metastasis (TNM) stage was identified according to the guidelines of the Seventh American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) stage system.[10]
Cells and reagents
HCC cell line HepG2 was purchased from Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco's modified Eagle medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum and 100 U/ml penicillin and 100 μg/ml streptomycin (Thermo Fisher Scientific) in 5% CO2 resuscitation. All reagents were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA) without special illustration.
RNA interference and transfection
The siRNA of FOSL1 and scrambled siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The transfection of siRNA was realized with lipofectamine RNAiMAX transfection reagent (Invitrogen, Waltham, MA, USA) according to the manual. The sequence of siFOSL1 was: forward—GUCGAAGGCCUUGUGAACATT; reverse—UGUUCACAAGGCCUUCGCTT; and the sequence of scrambled siRNA was: forward—UUCUCCGAACGUGUCACGUTT; reverse—ACGUGACACGUUCGGAGAATT.
RNA extraction and quantitative real-time polymerase chain reaction
The mRNA level of FOSL1 in HCC tissues and their tumor adjacent tissues were analyzed with quantitative real-time polymerase chain reaction (qRT-PCR). The mRNAs were extracted with Trizol reagent (Thermo Fisher Scientific) according to the manufacturer's guidelines. The StepOnePlus RT-PCR system (Applied Biosystems, Waltham, MA, USA) with SYBR Green method was applied for the cDNA synthesis and qPCR. GAPDH was an internal control for the 2-ΔΔ CT equation. The average mRNA level of tumor adjacent tissues was set as 1.0 and other mRNA levels were standardized to this baseline. The sequences of primers of GAPDH and FOSL1 were as follows:
FOSL1 forward: 5′-CAGTGGATGGTACA GCCTCA-3′;
FOSL1 reverse: 5′-CAGTTTGTCAGTCTCCTGT TCAC-3′;
GAPDH forward: 5′-TGGAGAATGAGAGG TGGGATG-3′;
GAPDH reverse: 5′-GAGCTTCACGTTC TTGTATCTGT-3′.
IHC and evaluation
Streptavidin peroxidase complex method was applied to detect the expression of FOSL1 in HCC tissues. After deparaffinization and rehydration, the specimens were treated with 0.01 M citrate buffer (pH = 6.0) for better antigen retrieval and then incubated in 3% H2O2 to inactivate endogenous peroxidase. Unspecific binding was blocked by incubation of 5% bovine serum albumin. The primary antibody (AF4935, R and D Systems, Minneapolis, MN, USA) of FOSL1 was applied to incubate the tissues overnight at 4°C and secondary antibody (Sangon, Shanghai, China) labeled was used at room temperature for 1 h, and lastly, peroxidase complex reagent and 3,3′-diaminobenzidine solution were used for the visualization of antigen.
The IHC results were semi-quantified by calculating IHC score by two independent pathologists who were unaware of the clinical data. The IHC score comprised of two aspects: score of positive cell percentage and score of staining intensity. The scores for positive cell percentage were: 0 for <10% positive cells; 1 for 10–30% positive cells; 2 for 30–50% positive cells; and 3 for >50% positive cells. The scores for staining intensity were defined as: 0 for negative staining, 1 for weak staining, 2 for moderate staining, and 3 for strong staining. The total IHC score was the product of score of positive cell percentage multiplied by score of staining intensity, ranging from 0 to 9. The cohort was divided into low-expression and high-expression FOSL1 according to cut-offs identified by receiver operating characteristic (ROC) curve analysis in a previous study.[11] The cut-off in our test was 3.5 ascertained by ROC curve analysis.
MTT assay
MTT assay was used to detect HCC cell proliferation. In brief, 24 h after transfection of siRNA or scrambled RNA, HepG2 cells were passaged into 96-well plates about 4000 cells per well and incubated for indicated time. 10 μl MTT at 5 mg/ml was added into each well every 12 h to terminate cell proliferation. The supernatant was removed 4 h after MTT incubation and 100 μl DMSO was added to dissolve the crystals. Optical density (OD) at 570 nm was measured with a spectrophotometer (Molecular Devices Company, USA). Every group had at least six parallel wells. The OD of control group was set as 1.0 and proliferation ratio of other tested groups were standardized to this baseline. The data for analysis were taken from at least three independent experiments.
Statistical analysis
All data were analyzed with software SPSS 22.0 (IBM Cooperation, Chicago, IL, USA). The correlations between FOSL1 and clinicopathological factors were analyzed with Fisher's test. Differences of survival rate stratified into different subgroups were evaluated with log-rank test, and the independent prognostic factors were identified by Cox-regression hazard model and their hazard ratios (HRs) calculated. The difference between compared groups in proliferation test was analyzed by Student's t-test. P < 0.05 was considered as statistically significant.
RESULTS
Expression of FOSL1 in HCC and tumor adjacent tissue
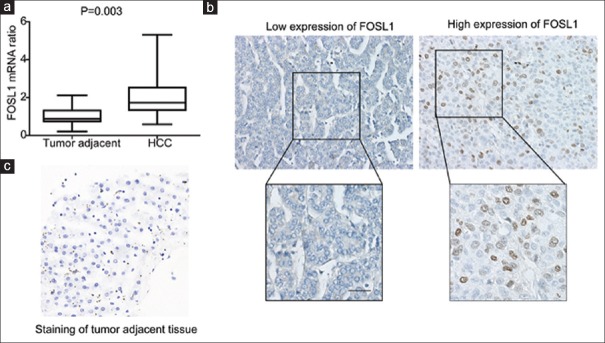
The expression of FOSL1 was first evaluated in 20 pairs of HCCs and their corresponding adjacent tissues by detecting FOSL1 mRNA with qRT-PCR [Figure 1a]. It turned out that FOSL1 mRNA in HCCs were significantly higher than those in tumor adjacent tissues, indicating the potential role of FOSL1 in HCC tumorigenesis. In addition, we investigated the expression and localization of FOSL1 in 114 formalin-fixed and paraffin-embedded HCC specimens. The percentage of high expression and low expression of FOSL1 accounted for 46% (53/114) and 54% (61/114), respectively [Table 1]. In HCC, FOSL1 expression was observed in nucleus in most cases with high-expression FOSL1 [Figure 1b]. The control staining of FOSL1 in tumor adjacent tissue was remarkably weaker than in HCC tissues [Figure 1c].
Figure 1.
Expression of FOSL1 in HCC and tumor adjacent tissues. (a) FOSL1 expression in HCC was significantly higher than tumor adjacent tissues. The mRNA levels of FOSL1 in 20 pairs of HCC and tumor adjacent tissues were detected with qRT-PCR. (b) Representative images of low or high expression of FOSL1 detected with IHC. Scale bar: 50 μm. In low FOSL1 expression, staining score was 0 and positive cell score was 0, so total score was 0. In high FOSL1 expression, staining score was 3 and positive cell score was 2, so total score was 6. (c) The control staining of FOSL1 in tumor adjacent tissue
Table 1.
Baseline characteristics of patients
| Factors | Number | Percentage |
|---|---|---|
| Sex | ||
| Female | 16 | 14.04 |
| Male | 98 | 85.96 |
| Age | ||
| <50 | 44 | 38.60 |
| ≥50 | 70 | 61.40 |
| Tumor size (cm) | ||
| ≤5 | 46 | 40.35 |
| >5 | 68 | 59.65 |
| Tumor number | ||
| Single | 104 | 91.23 |
| Multiple | 10 | 8.77 |
| AFP | ||
| ≥200 μg/ml | 30 | 26.32 |
| <200 μg/ml | 84 | 73.68 |
| Histopathological grade | ||
| I + II | 84 | 73.68 |
| III | 30 | 26.32 |
| HBsAg | ||
| Negative | 35 | 30.70 |
| Positive | 79 | 69.30 |
| HCV | ||
| Negative | 104 | 91.23 |
| Positive | 10 | 8.77 |
| Cirrhosis | ||
| Negative | 61 | 53.51 |
| Positive | 53 | 46.49 |
| T stage | ||
| I + II | 60 | 52.63 |
| III + IV | 54 | 47.37 |
| N stage | ||
| N0 | 112 | 98.25 |
| N1 | 2 | 1.75 |
| TNM stage | ||
| I | 15 | 13.16 |
| II | 45 | 39.47 |
| III | 52 | 45.61 |
| IV | 2 | 1.75 |
| FOSL 1 | ||
| Low | 61 | 53.51 |
| High | 53 | 46.49 |
FOSL1: FOS-like antigen 1
FOSL1 expression is associated with tumor size and T stage
The correlation between FOSL1 and the clinicopathological factors in 114 HCC cases is shown in Table 2. High-expression FOSL1 was significantly associated with larger tumor size (P = 0.021). Moreover, expression of FOSL1 correlated with HCC T stage (P = 0.014), and TNM stage (P = 0.014), suggesting that tumor size was an important determinant to T and TNM stage in HCC according to the AJCC/UICC tumor stage. It was interesting to note that FOSL1 expression was associated with HBV infection (P = 0.014). In addition, male patients appeared to be more likelier to have high expression of FOSL1 (P = 0.057), although there was no evidence supporting that FOSL1 was related to sex hormone.
Table 2.
Correlation between FOSL1 expression and clinicopathologic parameters
| Factors | FOSL1 |
P* | |
|---|---|---|---|
| Low | High | ||
| Sex | |||
| Female | 12 | 4 | 0.057 |
| Male | 49 | 49 | |
| Age | |||
| <50 | 25 | 19 | 0.700 |
| ≥50 | 36 | 34 | |
| Tumor size (cm) | |||
| ≤5 | 31 | 15 | 0.021 |
| >5 | 30 | 38 | |
| Tumor number | |||
| Single | 54 | 50 | 0.334 |
| Multiple | 7 | 3 | |
| AFP | |||
| ≥200 μg/ml | 15 | 15 | 0.675 |
| <200 μg/ml | 46 | 38 | |
| Histopathological grade | |||
| I + II | 45 | 39 | 0.982 |
| III | 16 | 14 | |
| HBsAg | |||
| Negative | 25 | 10 | 0.014 |
| Positive | 36 | 43 | |
| HCV | |||
| Negative | 55 | 49 | 0.749 |
| Positive | 6 | 4 | |
| Cirrhosis | |||
| Negative | 30 | 31 | 0.351 |
| Positive | 31 | 22 | |
| T stage | |||
| I + II | 39 | 21 | 0.014 |
| III + IV | 22 | 32 | |
| N stage | |||
| N0 | 60 | 52 | 0.92 |
| N1 | 1 | 1 | |
| TNM stage | |||
| I + II | 39 | 21 | 0.014 |
| III + IV | 22 | 32 | |
FOSL1: FOS-like antigen 1. *Fisher’s test
FOSL1 is an independent prognostic biomarker of HCC
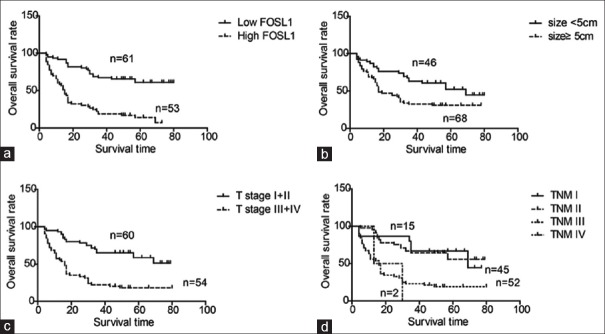
The relationship between the overall survival rates and the clinicopathological variables including FOSL1 expression was explored [Table 3]. Patients with high FOSL1 expression had a remarkably poorer prognosis than those with low expression of FOSL1 (P < 0.001, 5-year survival rate: 60.9 vs. 14.2%) [Figure 2a]. In addition, the tumor size was also identified as a prognostic factor of HCC patients (P < 0.001, 5-year survival rate: 52.2 vs. 30.9%) [Figure 2b]. Advanced T stage (P < 0.001, 5-year survival rate: 8.3 vs. 18.3%) and TNM stage (P < 0.001, 5-year survival rate: 66.7 vs. 55.7% vs. 19.0 vs. 0.0%) also could predict unfavorable prognosis of HCC [Figure 2c and d].
Table 3.
Correlation between clinicopathologic features and overall survival rate
| Factors | 5-year survival rate (%) | P* | HR | 95% CI | P# |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 48.2 | 0.295 | 1 | ||
| Male | 37.8 | 0.76 | 0.34-1.71 | 0.762 | |
| Age | |||||
| <50 | 35.7 | 0.467 | 1 | ||
| ≥50 | 41.4 | 0.81 | 0.46-1.43 | 0.470 | |
| Tumor size (cm) | |||||
| ≤5 | 52.2 | 0.004 | 1 | ||
| >5 | 30.9 | 0.77 | 0.38-1.58 | 0.481 | |
| Tumor number | |||||
| Single | 37.9 | 0.695 | 1 | ||
| Multiple | 50.0 | 1.49 | 0.54-4.05 | 0.440 | |
| AFP | |||||
| ≥200 μg/ml | 37.9 | 0.822 | 1 | ||
| <200 μg/ml | 39.5 | 1.28 | 0.73-2.27 | 0.393 | |
| Histopathological grade | |||||
| I + II | 40.4 | 0.593 | 1 | ||
| III | 39.7 | 1.21 | 0.66-2.20 | 0.533 | |
| HBsAg | |||||
| Negative | 48.0 | 0.441 | 1 | ||
| Positive | 35.0 | 0.68 | 0.34-1.35 | 0.267 | |
| HCV | |||||
| Negative | 39.0 | 0.733 | 1 | ||
| Positive | 40.0 | 1.66 | 0.67-4.09 | 0.270 | |
| Cirrhosis | |||||
| Negative | 40.8 | 0.990 | 1 | ||
| Positive | 38.5 | 1.74 | 1.02-2.97 | 0.042 | |
| T stage | |||||
| I + II | 8.3 | <0.001 | 1 | ||
| III + IV | 18.3 | 4.50 | 2.18-9.33 | <0.001 | |
| N stage | |||||
| N0 | 39.9 | 0.258 | 1 | ||
| N1 | 0.0 | 1.92 | 0.39-9.36 | 0.419 | |
| TNM stage | |||||
| I | 66.7 | <0.001 | |||
| II | 55.7 | ||||
| III | 19.0 | ||||
| IV | 0.0 | ||||
| FOSL1 | |||||
| Low | 60.9 | <0.001 | 1 | ||
| High | 14.2 | 5.60 | 3.00-10.45 | <0.001 |
FOSL1: FOS-like antigen 1; HR: Hazard ratio; CI: Confidence interval; HDGF: Hepatoma derived growth factor. *Log-rank test. #Cox proportional hazards regression
Figure 2.
High expression of FOSL1, large size, advanced T and TNM stage correlated with poor prognosis of HCC. Overall survival curves of HCC were stratified with FOSL1 expression (a), tumor size (b), T stage (c), and TNM stage (d). The curves were displayed by Kaplan–Meier method and statistical differences of subgroups were analyzed with log-rank test
Independent prognostic biomarkers of HCC were further identified with Cox-regression hazard model [Table 3]. All the clinicopathological factors were enrolled to the multivariate analysis except TNM stage because of its natural interaction with T stage. In the Cox-regression model, high expression of FOSL1 was identified as an independent risk for poor prognosis [hazard ratio (HR) = 5.60, 95% confidence interval (CI) =3.00–10.45, P < 0.001]. Moreover, the T stage (HR = 4.50, 95% CI = 2.18–9.33, P < 0.001) and cirrhosis (HR = 1.74, 95% CI = 1.02–2.97, P = 0.042) were also defined as independent prognostic factors of HCC.
FOSL1 knockdown impairs proliferation of HCC cells
In the clinical observation, we demonstrated that FOSL1 was significantly associated with tumor size. Previous studies also proved that FOSL1 could improve cell survival and promote proliferation,[12] so we further investigated the function of FOSL1 in HCC proliferation. The expression of FOSL1 in HCC cell line hepG2 was silenced by siRNA with a scrambled siRNA as control. The successful FOSL1 knockdown was verified by Western blotting and qRT-PCR [Figure 3a and b]. Moreover, the proliferation of HepG2 cells was detected with MTT assay after FOSL1 knockdown. The cells with FOSL1 knockdown had a remarkably slower proliferation after 36 h compared with the control group. This suggests that FOSL1 knockdown could impair proliferation of HCC cells and indicate the essential role of FOSL1 in HCC proliferation.
Figure 3.
FOSL1 was essential in HCC proliferation. (a and b) FOSL1 knockdown was verified by Western blotting (a) and qRT-PCR (b). (c) Silencing FOSL1 significantly impaired HepG2 proliferation after 36 h. Cell proliferation was detected with MTT assay. The statistical significance was generated with Student'st-test and displayed with ± SEM
DISCUSSION
The poor response to systemic therapy is one important reason attributed to the poor outcome of HCC patients. For a long time from 2007 to 2016, the multitarget tyrosine kinase inhibitor, sorafenib, was the only systemic therapy approved for HCC treatment.[13] Although sorafenib has anti-angiogenic and anti-proliferative effects, it could only extend the median overall survival time from 8 to 11 months for patients with advanced-stage HCC.[5] Fortunately, new target drugs of HCC are emerging. Lenvatinib was demonstrated to prolong survival time of HCC and is now an approved frontline drug in the market.[14] Regorafenib, cabozantinib, and ramucirumab are second line drugs after disease progression on sorafenib.[15,16] Moreover, only a minority of HCC patients benefit from the systemic therapies because HCC is still highly therapy-resistant. Thus, effective target drug development for HCC is still in urgent need. However, the landscape of molecular alterations of HCC has been revealed, thanks to the breakthroughs of techniques such as high-throughput sequencing. Still, most mutations are not applicable as drug targets, and only 25% of HCCs have targetable drivers.[13] New prognostic biomarkers are still rare because they could provide new insights to the discovery of target drugs. Our findings demonstrated that FOSL1 is an independent prognostic biomarker of HCC. This could expand our understanding of HCC prognostic biomarkers and provide more insight to the HCC targeted therapy. FOSL1 may be an effective drug target, and anti-FOSL1 therapy may be a potential way to treat HCC.
The main risk factors associated with HCC are well-defined and include viral hepatitis (B and/or C), alcohol abuse, and nonalcoholic fatty liver disease in patients with metabolic syndrome and diabetes.[17] Other co-factors of HCC development, such as aflatoxin B1 and tobacco, increase the incidence of the disease if other common risk factors are present.[18] HBV is the leading cause of HCC, especially in Asian countries. In our study, it was interesting to note that FOSL1 expression was significantly associated with HBV infection but not cirrhosis or HCV. It is to be noted that this association demonstrated by χ2 was raw, and many possible explanations. It is feasible that HBV virus has interaction with FOSL1, or that FOSL1 expression was influenced by infection process. Nevertheless, this interesting observation deserves further experiments to verify.
Although most studies demonstrated the oncogenic role of AP-1, the effect is still context-dependent and tissue-specific. Several studies reported the tumor suppressor role of AP-1. For example, FOSB was reported to have apoptosis-promoting function in breast cancer.[19] In FOS family, FOSL1 is usually considered as a proto-oncogene, and its overexpression was observed in multiple human carcinomas, such as colon adenocarcinoma, ovarian cancer, breast cancer, head and neck, lung and esophageal squamous cell carcinoma, etc.[20] Previous studies revealed that the oncogenic function of FOSL1 was linked to KRAS and ectopic activation of ERK. Constitutive activation of ERK and FOSL1 could modulate different tumor progression in different tumor types, such as expedite cancer cell survival, facilitate tumor proliferation, or promote EMT.[21,22,23] In our study, we observed that FOSL1 expression was mainly associated with tumor size of HCC, and FOSL1 knockdown could notably decrease HCC proliferation, indicating that FOSL1 may play an oncogenic role by promoting tumor proliferation rather than accelerating invasion in HCC. However, this hypothesis would require further experimentation to confirm he findings. We hope this report could initiate more interest on the clinical and biological significance of FOSL1 in HCC and help elucidate the underlying mechanisms of its oncogenic role.
In conclusion, in this study, we investigated the expression of FOSL1 in 20 pairs of fresh HCC tissues and 114 paraffin-embedded HCC tissues in our study, and demonstrated that high expression of FOSL1 was significantly associated with larger tumor size, advanced T stage, and lower survival rates. Moreover, we identified FOSL1 as an independent prognostic biomarker of HCC. With experiments in vitro, we proved that FOSL1 played an essential role in HCC proliferation.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 2.Sia D, Villanueva A, Friedman SL, Llovet JM. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology. 2017;152:745–61. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Xiao S, Chang RM, Yang MY, Lei X, Liu X, Gao WB, et al. Actin-like 6A predicts poor prognosis of hepatocellular carcinoma and promotes metastasis and epithelial-mesenchymal transition. Hepatology. 2016;63:1256–71. doi: 10.1002/hep.28417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chau I, Peck-Radosavljevic M, Borg C, Malfertheiner P, Seitz JF, Park JO, et al. Corrigendum to 'Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib: Patient-focused outcome results from the randomised phase III REACH study' [Eur J Canc 81 (2017) 17-25] Eur J Cancer. 2018;100:135–6. doi: 10.1016/j.ejca.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Vogt PK. Fortuitous convergences: The beginnings of JUN. Nat Rev Cancer. 2002;2:465–9. doi: 10.1038/nrc818. [DOI] [PubMed] [Google Scholar]
- 7.Tice DA, Soloviev I, Polakis P. Activation of the Wnt pathway interferes with serum response element-driven transcription of immediate early genes. J Biol Chem. 2002;277:6118–23. doi: 10.1074/jbc.M111255200. [DOI] [PubMed] [Google Scholar]
- 8.Desmet CJ, Gallenne T, Prieur A, Reyal F, Visser NL, Wittner BS, et al. Identification of a pharmacologically tractable Fra-1/ADORA2B axis promoting breast cancer metastasis. Proc Natl Acad Sci U S A. 2013;110:5139–44. doi: 10.1073/pnas.1222085110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang S, Li Y, Gao J, Zhang T, Li S, Luo A, et al. MicroRNA-34 suppresses breast cancer invasion and metastasis by directly targeting Fra-1. Oncogene. 2013;32:4294–303. doi: 10.1038/onc.2012.432. [DOI] [PubMed] [Google Scholar]
- 10.Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: Assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245:909–22. doi: 10.1097/01.sla.0000254368.65878.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu YF, Liu ZL, Pan C, Yang XQ, Ning SL, Liu HD, et al. HMGB1 correlates with angiogenesis and poor prognosis of perihilar cholangiocarcinoma via elevating VEGFR2 of vessel endothelium. Oncogene. 2019;38:868–80. doi: 10.1038/s41388-018-0485-8. [DOI] [PubMed] [Google Scholar]
- 12.Vallejo A, Perurena N, Guruceaga E, Mazur PK, Martinez-Canarias S, Zandueta C, et al. An integrative approach unveils FOSL1 as an oncogene vulnerability in KRAS-driven lung and pancreatic cancer. Nat Commun. 2017;8:14294. doi: 10.1038/ncomms14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PubMed] [Google Scholar]
- 14.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–73. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 15.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 17.Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477–91 e1. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chuang SC, La Vecchia C, Boffetta P. Liver cancer: Descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer Lett. 2009;286:9–14. doi: 10.1016/j.canlet.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Ting CH, Chen YC, Wu CJ, Chen JY. Targeting FOSB with a cationic antimicrobial peptide, TP4, for treatment of triple-negative breast cancer. Oncotarget. 2016;7:40329–47. doi: 10.18632/oncotarget.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young MR, Colburn NH. Fra-1 a target for cancer prevention or intervention. Gene. 2006;379:1–11. doi: 10.1016/j.gene.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Elangovan IM, Vaz M, Tamatam CR, Potteti HR, Reddy NM, Reddy SP. FOSL1 Promotes Kras-induced Lung Cancer through Amphiregulin and Cell Survival Gene Regulation. Am J Respir Cell Mol Biol. 2018;58:625–35. doi: 10.1165/rcmb.2017-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang K, Myllymaki SM, Gao P, Devarajan R, Kytola V, Nykter M, et al. Oncogenic K-Ras upregulates ITGA6 expression via FOSL1 to induce anoikis resistance and synergizes with alphaV-Class integrins to promote EMT. Oncogene. 2017;36:5681–94. doi: 10.1038/onc.2017.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maurus K, Hufnagel A, Geiger F, Graf S, Berking C, Heinemann A, et al. The AP-1 transcription factor FOSL1 causes melanocyte reprogramming and transformation. Oncogene. 2017;36:5110–21. doi: 10.1038/onc.2017.135. [DOI] [PubMed] [Google Scholar]