Abstract
Increasing evidence suggests that long-term opioids and pain induce similar adaptive changes in the brain’s reward circuits, however, how pain alters the addictive properties of opioids remains poorly understood. In this study using a rat model of morphine self-administration (MSA), we found that short-term pain, induced by an intraplantar injection of complete Freund’s adjuvant, acutely decreased voluntary morphine intake, but not food intake, only at a morphine dose that did not affect pain itself. Pre-treatment with indomethacin, a non-opioid inhibitor of pain, before the pain induction blocked the decrease in morphine intake. In rats with steady MSA, the protein level of GluA1 subunits of glutamate AMPA receptors (AMPARs) was significantly increased, but that of GluA2 was decreased, resulting in an increased GluA1/GluA2 ratio in central nucleus of the amygdala (CeA). In contrast, pain decreased the GluA1/GluA2 ratio in the CeA of rats with MSA. Microinjection of NASPM, a selective inhibitor of homomeric GluA1-AMPARs, into CeA inhibited morphine intake. Furthermore, viral overexpression of GluA1 protein in CeA maintained morphine intake at a higher level than controls and reversed the pain-induced reduction in morphine intake. These findings suggest that CeA GluA1 promotes opioid use and its upregulation is sufficient to increase opioid consumption, which counteracts the acute inhibitory effect of pain on opioid intake. These results demonstrate that the CeA GluA1 is a shared target of opioid and pain in regulation of opioid use, which may aid in future development of therapeutic applications in opioid abuse.
Keywords: morphine self-administration, AMPA receptors, opioid addiction, pain, amygdala
INTRODUCTION
Opioids are currently the most prescribed analgesics for the treatment of various pain conditions. However, long-term use of opioids may cause opioid abuse and addiction due to opioids’ strong rewarding effects. In fact, there is currently an alarming opioid epidemic that involves prevailing prescription opioid abuse and addiction, which could lead to overdose and death (Hedegaard et al., 2017). Epidemiological surveys demonstrate that a significant increase in opioid prescription to treat chronic pain is accompanied with an escalating rate of opioid abuse (Ballantyne and LaForge, 2007; Denisco et al., 2008; Passik and Kirsh, 2011). However, our understanding of how pain conditions may impact opioid use is still rather limited. Previous studies in animal models of pain have reported inconsistent results, as pain is shown to reduce opioid intake (Martin et al., 2007; Woller et al., 2012; Wade et al., 2013) or to promote opioid reward, the driving force for opioid use and abuse (Cahill et al., 2013; Zhang et al., 2014a; Zhang et al., 2014b). Furthermore, little is known about the molecular mechanisms underlying the pain–opioid interactions in opioid use.
Pain and reward are traditionally thought as two opposing emotional and motivational events that interact and influence each other on overlapping brain circuits (Leknes and Tracey, 2008; Volkow and McLellan, 2016). Pain is an unpleasant and aversive experience involving sensory perception of a harmful stimulus, negative emotions of affect and behavioral responses of avoidance (Fields, 2004; Baliki and Apkarian, 2015). Thus, many cortical, mesolimbic and brainstem structures are involved in processing, evaluating and modulating pain, including the anterior cingulate cortex, medial thalamic nuclei, the amygdala complex, and a descending pain-modulating system consisting of the amygdala–periaqueductal grey–rostral ventromedial medulla–spinal dorsal horn pathway (Price, 2000; Fields, 2004; Vogt, 2005; Baliki and Apkarian, 2015; Neugebauer, 2015). On the other hand, reward is a euphoric and motivational experience with positive emotions, which drives compulsive behavior of drug use and abuse (Fields and Margolis, 2015; Koob, 2019). Prominent brain structures involved in the processing, decision making, and behavioral output of reward include prefrontal cortex, the nucleus accumbens, the ventral tegmental area, the amygdala, and the dopamine transmitter system (Baxter and Murray, 2002; Volkow et al., 2012; Fields and Margolis, 2015; Koob, 2019). Interestingly, recent studies increasingly suggest that overlapping brain structures and common mechanisms are involved in the development of pain and drug addiction (Elman and Borsook, 2016).
The central nucleus of amygdala (CeA) is a key brain structure that regulates emotional responses to both positive (such as reward) and negative (such as pain and fear) environmental stimulation (Baxter and Murray, 2002; Murray, 2007; Neugebauer, 2015). CeA is actively involved in the rewarding effects of psychostimulants such as morphine, cocaine and alcohol, including cue-induced craving and relapse behavior during abstinence (Zhu et al., 2007; Li et al., 2008; Cai et al., 2013; Kallupi et al., 2013). CeA also plays a critical role in regulation of both sensory and affective/emotional components of pain (Price, 2000; Neugebauer, 2015). Neuropathic and inflammatory pain potentiates excitatory synaptic transmission in CeA neurons (Neugebauer et al., 2003; Li and Neugebauer, 2004; Ikeda et al., 2007). While human imaging studies show that noxious stimuli can activate the brain’s reward circuits including amygdala (Price, 2000; Vogt, 2005), systemic morphine administration suppresses CeA neuronal activation in response to noxious stimulus (Huang et al., 1993). Moreover, excitotoxic lesion of CeA impairs morphine-induced inhibition of sensory pain (Manning and Mayer, 1995b, a; Manning, 1998) and diminishes pain-induced avoidance behavior of conditioned place aversion (Tanimoto et al., 2003; Gao et al., 2004). Thus, CeA is an important conjunctive brain site for regulation of pain and opioid reward.
Glutamate synaptic transmission plays a central role in the neuroplasticity involved in the development of opioid addiction and pain conditions (Kauer and Malenka, 2007; Kuner, 2010; von Hehn et al., 2012; Luo et al., 2014). Particularly, glutamate AMPA receptors (AMPARs) are crucial for emotional events of learning and memory by activity-dependent subunit re-composition and resultant synaptic strengthening (Malinow and Malenka, 2002; Derkach et al., 2007; Bowers et al., 2010), including those AMPARs in the amygdala (Maren, 2005; McCool et al., 2010). AMPARs are heteromeric tetramers predominantly consisting of GluA1 and GluA2 subunits (Wenthold et al., 1996). Dynamic membrane insertion of GluA2-lacking, homomeric GluA1 AMPARs that have higher calcium permeability and channel conductance is a major mechanism underlying activity-induced synaptic strengthening and long-term potentiation (LTP) (Mahanty and Sah, 1998; Hayashi et al., 2000). Previous studies have shown that pain changes GluA1 and GluA2 levels in several brain areas, resulting in enhanced synaptic responses to noxious stimuli (Guan et al., 2003; Xu et al., 2008; Goffer et al., 2013). Similarly, exposure to opioids and other psychostimulants increases AMPAR-mediated synaptic response through upregulated function of GluA2-lacking AMPARs, which prompts addictive drug intake and craving (Carlezon et al., 1997; Conrad et al., 2008; Choi et al., 2011; McCutcheon et al., 2011).
Despite the important role of GluA1 and adaptive function of AMPARs in pain processing and in opioid use and addiction, it remains poorly understood how GluA1 AMAPRs act in voluntary opioid use and how it may be affected by pain conditions. Therefore, in the present study, we investigated effects of persistent pain on opioid consumption using a rat model of morphine self-administration (MSA). To control for the pain-inhibiting effect of morphine in these experiments, we used a non-opioid pain inhibitor indomethacin to isolate the effect of pain on morphine intake. Prominently, we determined the role of CeA GluA1 in operant behavior of opioid intake and in the pain-opioid interaction.
EXPERIMENTAL PROCEDURES
Animals
Male Wistar rats weighting 250–300 g, and a total of 219 rats were used in this study. The rats were housed 2–3 per cage and maintained on a 12-h light/dark cycle with access to food and water ad libitum. All procedures involving the use of animals conformed to the guidelines by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center.
Experimental designs
We employed a simple experimental design by statistically comparing the difference between two animal groups (one control and one treatment group) or three groups (two control groups and one treatment group). Data from all animals were included in the final analyses of results. In the MSA experiments, a morphine-yoked control group, in addition to the control group of saline self-administration, was used to assess the motivational effect of morphine and its pharmacological effect. In the experiments of pain tests, GluA1/GluA2 expression and staining, and viral manipulation of GluA1, a blind design was used where the experimenter was unaware of the treatment or control groups for the animals and tissues examined.
Food reward training
Experiments were performed in operant chambers (Med Associates) with two levers above a smooth floor. One lever was paired with reinforcement delivery (active lever) while the other lever had no programmed consequence (inactive lever). Each chamber was also equipped with a house light illuminating throughout each training session. To facilitate the acquisition of lever pressing for subsequent MSA, food was initially restricted to 15 g/day/rat and rats were trained to press a lever on a fixed ratio 1 (FR1) schedule during a daily 30-min session. Pressing the active lever resulted in the delivery of a food pellet (45 mg) and illumination of a cue light above the lever, followed by a 5-sec timeout period. Daily food training continued until the rat self-administered 100 pellets per session for three consecutive sessions. After this acquisition training, rats were freely fed for at least 1 d before catheterization surgery and MSA experiments.
Intravenous Catheterization
Under isoflurane anesthesia, a catheter (SAI, 11 cm length, 0.1 cm outer diameter, 0.06 cm inner diameter) was inserted into the right jugular vein and the other end of the catheter was routed subcutaneously to the back where it exited just posterior to the scapula. The catheter was flushed daily with heparin (0.2 ml of 50 units) to prevent infection and maintain catheter patency.
Morphine self-administration (MSA)
All self-administration sessions occurred during the light cycle at the same time of the day every day. After at least 6-day postoperative recovery, the rat was placed in the operant chamber during daily 3 h sessions and a syringe pump was connected to the catheter by the quick connection systems (Strategic Applications Inc). Pressing the active lever caused an intravenous infusion of morphine (0.2 or 1 mg/kg/infusion) or saline in 0.05 ml for about 4 s on the FR1 schedule with the cue light illuminated. Each infusion was followed by a 30 s timeout during which lever presses did not result in additional infusions. To separate the pharmacological effects of morphine from its motivational effects, a yoked paradigm was used. The rats in the yoked group received intravenous injections of morphine at the same dose and same injection numbers as those in the matching rats of MSA group. Lever presses in the yoked group was of no consequence.
Inflammatory pain and pain test
Following 10 d MSA with steady morphine infusions of reinforcing response to morphine, the rats were randomly assigned to pain group treated with complete Freund’s adjuvant (CFA, Sigma) and control group treated with saline. Under isoflurane anesthesia, 50 μl CFA (pain group) or saline (control group) was injected into the planter of left hind paw of the rats 1 h after the completion of self-administration sessions. About 20 h after CFA/saline injection on the next day, next self-administration sessions started. Paw withdrawal latency to a noxious thermal stimulus was recorded to measure CFA-induced inflammatory pain responses (hyperalgesia) with a Hargreaves analgesic instrument (Stoelting). After 20 min habituation, a radiant beam of light was positioned under the hind paw and the time for the rat to remove the paw from the thermal stimulus was automatically recorded as the paw withdrawal latency. The pain test was performed 3 h before a daily self-administration session.
Intracranial cannulation and microinjection
For intracranial microinjection, a rat was anesthetized with isoflurane and restrained in a stereotaxic apparatus. A 26-gauge guide cannula (Plastic One) was implanted bilaterally into the CeA (anteroposterior, −2.3 mm from the bregma; lateral, 4.0 mm; dorsoventral, −7.0 mm from the skull) (Paxinos and Watson, 1986). The rats were allowed to recover for 3 d before intravenous catheterization. Following 10 d MSA sessions, the homomeric GluA1 AMPAR inhibitor NASPM was microinjected bilaterally into the CeA 15 min before MSA through a 33-gauge injection cannula (1.0 mm beyond the tip of the guide cannula) for 2 min and an additional 2 min was allowed for drug diffusion.
GluA1 overexpression in CeA
The construction of AAV-GluA1 vector was described in our earlier study (Cai et al., 2013). After completion of food reward training, the rat was microinjected bilaterally (1 μl/side) with AAV-GluA1 (5×109 GC/μl) or AAV-GFP (2.5 × 109 GC/μl) into CeA using the following coordinates: anteroposterior, −2.3 from the bregma; lateral, 4.0; dorsoventral, −8.0 from the skull. The rats were allowed to recover for 3 d before intravenous catheterization. After 6 d postoperative recovery, the rats underwent 10 d MSA sessions, followed by intraplantar CFA injection.
Western blots
The rat was deeply anesthetized by isoflurane and decapitated within 2–4 h after last self-administration session, and the brain was harvested and cut in a vibratome in cold (4 °C) artificial CSF to obtain brain slices (0.5 mm thick). Both sides of the CeA were punched from the brain slices using a blunt-end, 18-gauge syringe needle (0.8 mm inner diameter). The CeA tissues were frozen in liquid nitrogen and stored in a −80 °C freezer. CeA tissues were prepared as we described previously (Cai et al., 2013). Protein concentration was determined by BCA analysis (Thermo). Equal protein amount (10 μg) of samples was used to detect immunoreactivity of GluA1 (1:500, Santa Cruz Biotechnology, cat# SC-13152), GluA2 (1:6001000, Millipore, cat# MAB397) and actin (1:5000, Sigma, cat# A3853). The immuno-positive signals were quantified by Quantity One software (Bio-Rad). The protein level of GluA1 and GluA2 was normalized to that of actin.
Immunohistochemistry
A rat was deeply anesthetized with pentobarbital and transcardially perfused with heparinized saline and subsequently with ice-cold 4% paraformaldehyde in 1 × PBS (pH 7.4). The brain was removed and post-fixed in 4% paraformaldehyde overnight at 4°C, followed by dehydration with 30% sucrose in 1 × PBS. Tissues were sectioned into 30-μm thick coronal sections with a cryostat at −20°C. Sections were blocked with 5% normal goat serum in PBS containing 0.3% Triton X-100 and incubated overnight with primary antibodies: rabbit anti-GluA1 antibody, 1:500 dilution (Millipore, Cat#05–855R), mouse anti-GluA2 antibody, 1:500 (Millipore, Cat# MAB397). Sections were then rinsed and incubated with the goat anti-rabbit or goat anti-mouse Alexa Fluor-conjugated secondary antibody, 1:500 (Molecular Probes Cat# A-11008 and RRID:AB_141371, Cat# A-11004), and were mounted on slides, dried and coverslipped with ProLong Gold anti-fade reagent for staining with the fluorescent reporter 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen). The stained sections were examined with an Olympus BX51 fluorescence microscope.
Drugs
Morphine sulfate was kindly supplied by the National Institute on Drug Abuse Drug Program. All other drugs were purchased from Sigma-Aldrich. NASPM (1-naphthyl acetyl spermine), a selective channel blocker of GluA2-lacking AMPARs, was used to inhibit homomeric GluA1 AMPARs. Morphine and NASPM were dissolved in 0.9 % saline and PBS, respectively. Indomethacin was dissolved in Tris buffer (0.1 M, pH = 8.0) with 5% tween 20.
Statistical analysis
Data samples were tested and had normal distributions and equal variances, and were analyzed with parametric tests of Student’s t tests, one- or two-way ANOVA, followed by Bonferroni’ post hoc tests. Some behavioral data from self-administration experiments were normalized to baseline defined by the average value of infusion numbers for the three consecutive sessions before CFA or saline injection. Differences with p < 0.05 were considered statistically significant. All statistical analyses were performed with the Prism software version 8 (GraphPad Software). All data are presented as means ± SEM.
RESULTS
Persistent pain acutely decreases morphine intake
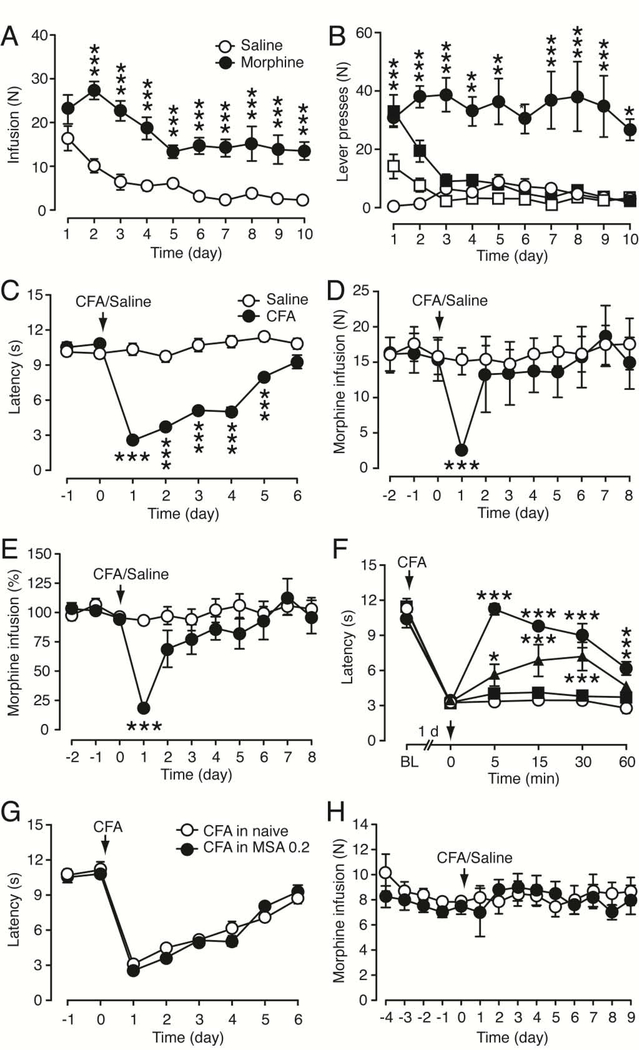
To determine the effect of pain on behavior of voluntary opioid intake, we used the rat model of morphine self-administration (MSA), as we reported before (Hou et al., 2015). Rats were trained to press a lever before undergoing the surgery of catheter implantation into the jugular vein for intravenous infusion of morphine. In daily 3 h MSA sessions, the number of daily morphine infusions reached a relatively steady level on the 5th d in both rat groups of MSA (0.2 mg/kg/infusion, i.v.) and saline self-administration (SSA); however, the steady level of infusion numbers in MSA group was significantly higher than that in SSA group (morphine: F1,93 = 60.45, p < 0.0001, Fig. 1A). As shown in Figure 1B, rats reliably discriminated between the two levers for morphine (F1,12 = 34.24, p < 0.0001), pressing significantly more on the active lever than on the inactive lever. However, rats with SSA failed to discriminate the active and inactive levers after the initial positive response to the active lever. These results demonstrate the positively reinforcing effect of morphine at this dose. Then, rats with stable MSA were randomly assigned to two groups of control and pain, with no significant difference in their baseline infusion numbers (control, 16.4 ± 2.4, pain, 16.8 ± 2.4, p > 0.05). After 10 d MSA with steady morphine infusion, the rat received an intraplantar injection of saline in control group and complete Freund’s adjuvant (CFA, 50 μl) in pain group to induce persistent inflammatory pain sensitization (hyperalgesia) on d 10 (d 0 for the intraplantar injection). In these rats of pain group, injection of CFA induced typical thermal pain sensitization that peaked 1 d after CFA injection and lasted several days before recovery (CFA: F1,12 = 271.4, p < 0.0001, Fig. 1C). In those rats with steady MSA for 10 d, morphine intake in pain group was dramatically decreased 1 d after CFA injection when compared to saline-injected control group (CFA: F1,12 = 5.253, p = 0.0408), as reflected both in morphine infusion numbers (Fig. 1D) and in percent of morphine infusion normalized to the 3-d average of infusion numbers prior to the CFA or saline injection (Fig. 1E). The pain-induced decrease in morphine intake was acute, peaking 1 d after CFA injection and returning to baseline in the following days when significant amount of CFA-induced pain was still present.
Figure 1. Persistent pain acutely decreases daily intake of morphine at a low dose.
(A) persistent inflammatory pain by in rats (top), and Operant behavior of morphine self-administration (MSA) at a low dose (0.2 mg/kg/infusion, i.v., n = 7) and saline self-administration (SSA, n = 8) measured as daily infusion numbers in rats. (B) Numbers of active (filled symbols) and inactive (open symbols) lever presses for MSA (0.2 mg/kg/infusion, circles) and SSA (squares) in rats as in A. Comparisons were made between active and inactive lever presses for MSA. (C) Persistent thermal hyperalgesia induced by an intraplantar injection of complete Freund’s adjuvant (CFA, 50 μl, n = 6) compared to saline-injected rats (n = 8) on day 0 in rats after 10 d MSA. The latency of paw withdrawal was measured daily 3 h before MSA sessions. (D,E) Daily morphine infusion as infusion numbers (D) and after normalized to the averaged infusion numbers of the 3 MSA sessions before CFA or saline injection (E) in rats (n = 7, each group) after 10 d MSA sessions. (F) Effects of morphine (filled symbols) and saline (open circles) on CFA-induced hyperalgesia by an injection (i.v.) of morphine or saline on day 0 (arrow) in naïve rats (n = 5, each group). Morphine doses: squares, 0.2 mg/kg, triangles, 0.5 mg/kg, and circles, 1 mg/kg). Latency was measured before (BL, baseline) and 1 d after CFA injection. (G) CFA-induced hyperalgesia measured as paw withdrawal latency in naïve rats and in rats after 10 d MSA sessions (n = 6, each group). (H) MSA at a high dose (1 mg/kg/infusion) in saline- (n = 6) and CFA- (n = 5) injected rats. * p < 0.05, ** p < 0.01, *** p < 0.001 (Two-way ANOVA and Bonferroni post hoc test).
To assess the effect of self-administered morphine on CFA-induced pain, we first examined dose dependence of the morphine effect in naïve rats. As shown in Fig. 1F, a single injection of morphine at the same dose as in MSA (0.2 mg/kg, i.v.) had no significant effect on CFA-induced pain sensitization, but higher morphine doses inhibited the CFA pain in a dose-dependent manner. We then compared the CFA effects in naïve rats and in rats with 10-d MSA at this morphine dose (0.2 mg/kg/infusion) to determine the effect of accumulating morphine through 10-d MSA. Injected >20 h after a prior MSA session, CFA induced pain sensitization of comparable amplitude in rats with continuous daily MSA when compared with that in naïve rats (MSA: F1,10 = 0.6191, p = 0.4496, Fig. 1G). Furthermore, in rats self-administering a more commonly used high morphine dose (1 mg/kg/infusion), which, in a single dose, significantly inhibited CFA-induced hyperalgesia in naïve rats (Fig. 1F), CFA failed to alter the infusion number of MSA (CFA: F1,8 = 0.2672, p = 0.6192, Fig. 1H). These results suggest that short-term pain can acutely decrease morphine intake, which is blocked by higher doses of self-administered morphine that inhibits the pain per se.
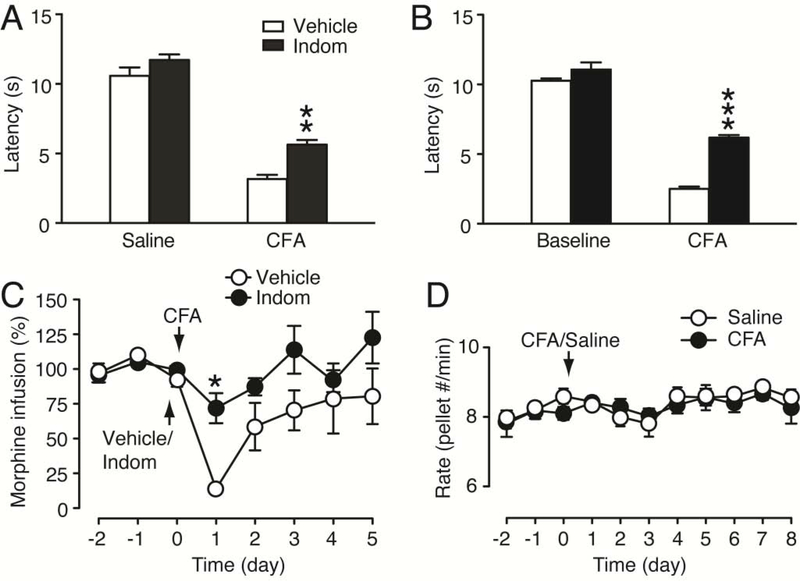
To further validate that the decrease in morphine intake was indeed caused by CFA-induced hyperalgesia, we treated the rats with indomethacin, a non-opioid, non-steroidal anti-inflammatory drug that inhibits CFA-induced pain sensitization (Cunha et al., 2003; Zhang et al., 2014b). Treatment with indomethacin (10 mg/kg, i.p.) 30 min before CFA injection (pre-treatment), having no effect by itself on pain threshold in saline-injected rats, significantly reduced CFA-induced hyperalgesia measured 1 d after CFA injection in naïve rats (CFA: F1,17 = 261.9, p < 0.0001; indomethacin: F1,17 = 18.26, p = 0.0005, Fig. 2A). In rats after 10 d MSA (0.2 mg/kg/infusion), the indomethacin pre-treatment also effectively alleviated CFA-induced hyperalgesia measured 1 d after CFA injection (indomethacin: F1,11 = 117.1, p < 0.0001, Fig. 2B). In the MSA rats, the same indomethacin pre-treatment nearly blocked the CFA-induced decrease in morphine infusions 1 d after CFA injection (indomethacin: F1,11 = 5.704, p = 0.0360, Fig. 2C). These results suggest that the reduction in morphine intake is indeed caused by CFA-induced hyperalgesia. Next, we determined whether the pain effect on morphine intake was due to a generalized impact on the brain’s natural reward mechanisms or impaired response capability of the rats. In naïve rats with a steady level of lever-pressing behavior for food pellets, CFA injection failed to alter the operant behavior and the rats continued the normal pressing behavior of food intake at a steady daily rate (CFA: F1,9 = 0.1079, p = 0.7500, Fig. 2D). The normal operant behavior of food reward after CFA suggests that the CFA inhibition of morphine intake is not due to pain-induced impairment in general lever-pressing ability or locomotor activity of the rats, which is consistent with the results of previous reports under similar conditions (Urban et al., 2011; Wade et al., 2013).
Figure 2. Inhibition of CFA-induced pain reverses pain-induced decrease in morphine intake.
(A,B) Effects of indomethacin (Indom, 10 mg/kg, i.p.) given 30 min before CFA injection (pre-treatment) on CFA-induced pain sensitization in naive rats (saline, n = 5, CFA, n = 6, A) and in rats after 10 d MSA sessions (vehicle, n = 7, indomethacin, n = 6, B). Pain tests were performed right before (baseline) and 1 d after CFA/vehicle injection (3 h before MSA in B). (C) Normalized daily intake of morphine in MSA rats with the pre-treatment (30 min before) of indomethacin (n = 6) or vehicle (n = 7) on day 0 after 10 d MSA. (D) Daily food intake in saline- (n = 6) and CFA- (n = 5) injected rats. (One-way or two-way ANOVA and Bonferroni post hoc test).
GluA1 in CeA promotes morphine intake
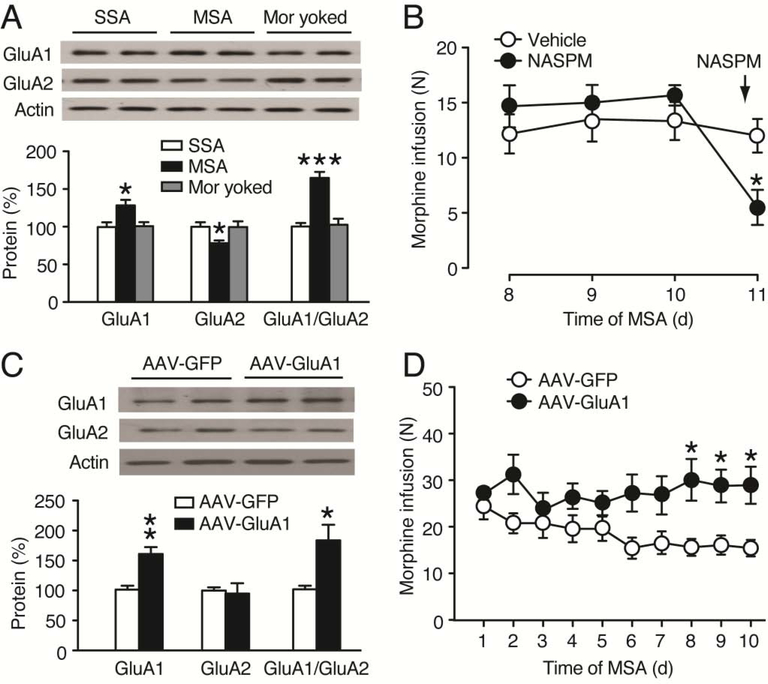
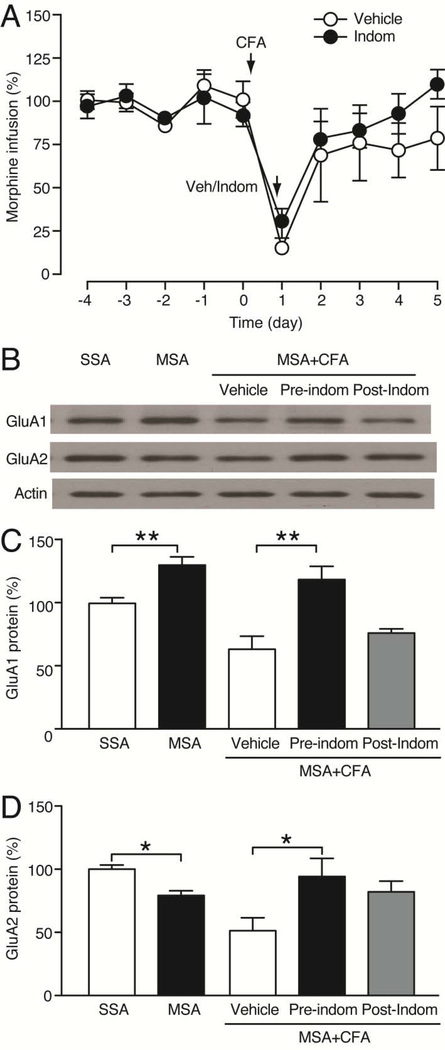
Given the important role of glutamate AMPARs in drug reward and addiction (Carlezon et al., 1997; Conrad et al., 2008; Choi et al., 2011; Cai et al., 2013), we examined the effect of MSA on the protein level of GluA1 and GluA2 subunits of AMPARs in CeA. In rats with stable morphine intake after 10 d MSA sessions, we found that the CeA level of GluA1 protein was significantly increased when compared to that in SSA rats; in contrast, the CeA level of GluA2 protein was decreased in these rats, resulting in a significant increase in the GluA1/GluA2 ratio (GluA1: F2,12 = 5.766, p = 0.0176; GluA2: F2,12 = 5.689, p = 0.0183; GluA1/GluA2: F2,12 = 18.47, p < 0.001, Fig. 3A). There was no change in either GluA1 or GluA2 protein level in the CeA of rats that received morphine by passive yoked intravenous injections, indicative of a response-contingent (reinforcement) change in regulation of the AMPAR subunits in CeA.
Figure 3. GluA1 in CeA promotes morphine intake.
(A) Western blots (top) and normalized results (bottom) of GluA1 and GluA2 proteins in the CeA of SSA rats (n = 5), MSA rats (n = 6) and morphine (mor)-yoked rats (n = 4) after 10 d operant sessions. CeA tissues were taken 2–4 h after the last operant session. (B) Morphine intake in MSA rats (n = 6, each group) before and after bilateral CeA microinjection of vehicle or NASPM (40 μg/0.5μl/side) 15 min before the MSA session on day 11. (C) Western blots (top) and normalized results (bottom) of GluA1 and GluA2 proteins in the CeA of rats (n = 4, each group) 1 week after bilateral microinjection of AAV-GFP or AAV-GluA1 into the CeA. (D) Daily morphine intake in rats (n = 6, each group) after bilateral CeA microinjection of AAV-GFP or AAV-GluA1 7 d before the 1st MSA session. (Student’s t test and one-way or two-way ANOVA with Bonferroni post hoc test).
Our results of increased GluA1/GluA2 ratio in MSA rats suggest that increased function of GluA2-lacking, homomeric GluA1 AMPARs with enhanced signaling in CeA may be important to maintain MSA behavior. To test this hypothesis, we first used local CeA microinjection of NASPM, a selective inhibitor of GluA2-lacking AMPARs (Conrad et al., 2008). Following the stabilization of MSA for 10 d, bilateral microinjection of NASPM (40 μg/0.5μl/side) into CeA 15 min before an MSA session markedly attenuated morphine infusion when compared to vehicle-injected control group (NASPM: F1,10 = 14.90, p = 0.0032, Fig. 3B). Next, we used the adeno-associated virus (AAV) vector AAV-GluA1 we generated previously to overexpress GluA1 in CeA as we demonstrated in a previous study (Cai et al., 2013). Western blots analysis showed that, one week after single, bilateral microinjection of AAV-GluA1 (1 μl/side) into CeA, the CeA level of GluA1 protein was significantly elevated when compared with that in rats that received similar microinjection of AAV-GFP vector as control (GluA1: t6 = 4.540, p = 0.0039; GluA2: t6 = 0.2728, p = 0.7941, Fig. 3C). The CeA level of GluA2 protein was not changed in the CeA of these AAV-GluA1-injected rats, resulting in a significant increase in the GluA1/GluA2 ratio (GluA1/GluA2: t6 = 3.039, p = 0.0228). In rats with MSA, we found that, one week after the vector microinjection into CeA, rats that received AAV-GulA1 maintained MSA at a level that was significantly higher than that in rats that received AAV-GFP during 10 d MSA sessions (AAV-GluA1: F1,10 = 8.341, p = 0.0162, Fig. 3D). These findings suggest that upregulation of GluA1 and consequently enhanced synaptic function of homomeric GluA1 AMPARs in CeA could promote behavior of opioid intake.
Upregulation of CeA GluA1 reverses pain effect on morphine intake
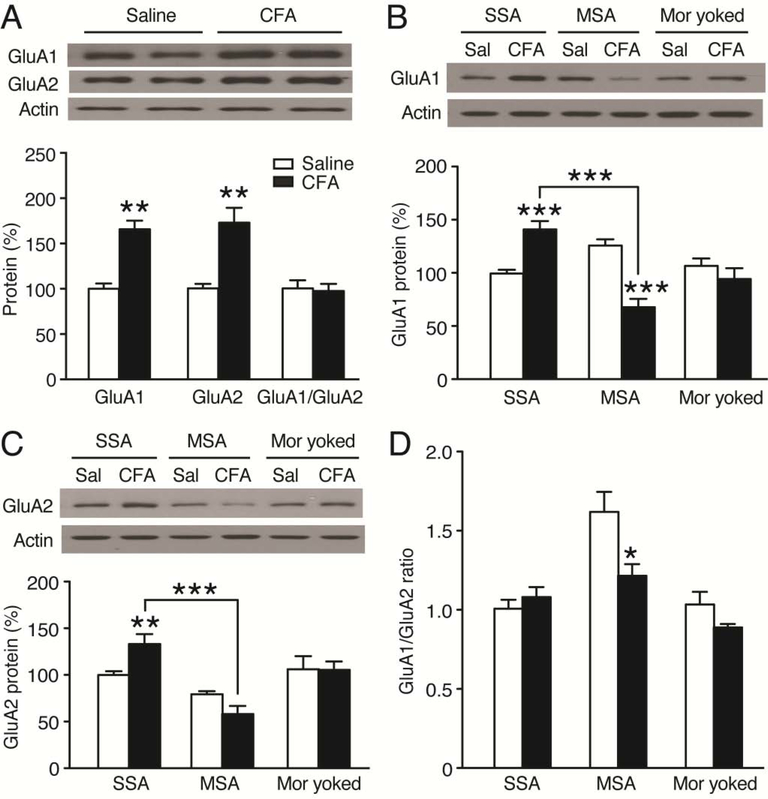
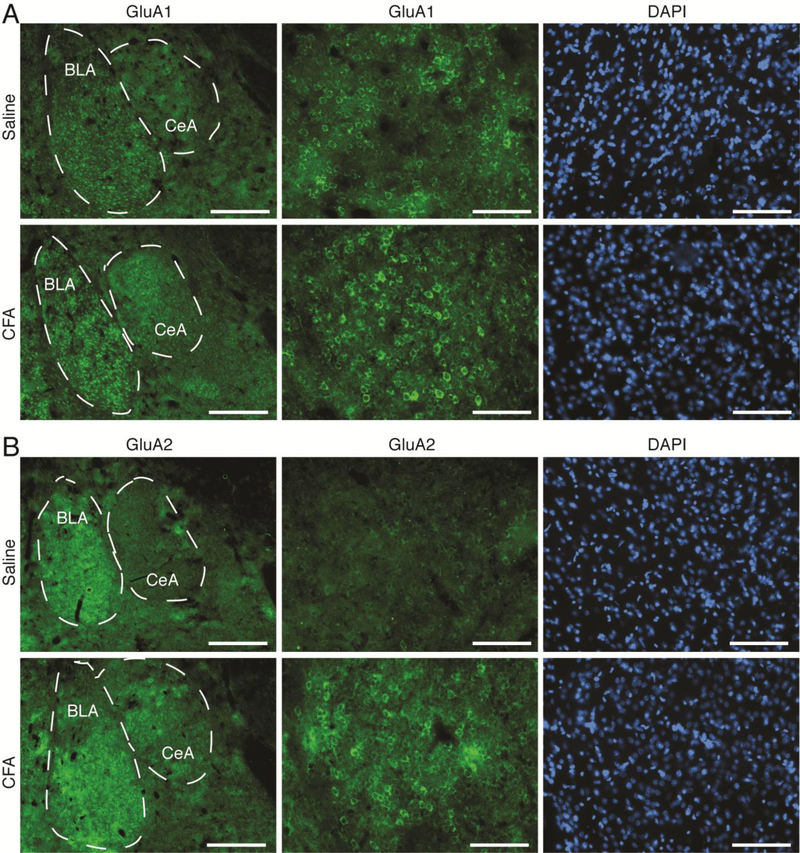
Based on the important role of CeA GluA1 AMPARs in MSA behavior, we were wondering whether the pain-induced decrease in morphine intake described above involved altered changes in subunit composition of CeA AMPARs. We first examined changes in CeA levels of GluA1 and GluA2 proteins in naïve rats under the pain condition. We found that, in contrast to the effect of MSA (Fig. 3A), both GluA1 and GluA2 protein levels were significantly increased in the CeA of CFA-injected rats 1 d after CFA injection when compared to saline-injected rats; as such, the GluA1/GluA2 ratio remained unchanged (GluA1: F1,6 = 5.802, p = 0.0011; GluA2: F1,6 = 4.209, p = 0.0056; GluA1/GluA2: F1,6 = 0.2563, p = 0.8063, Fig. 4A). We then determined pain effect on the GluA1 and GluA2 proteins in rats with steady MSA for 10 d. In control SSA rats for 10 d, CFA increased both GluA1 and GluA2 protein levels in CeA 1 d after CFA injection (GluA1: treatment, F2,32 = 7.547, p = 0.0021; CFA, F1,32 = 2.731, p = 0.1082, Fig. 4B; GluA2: treatment, F2,32 = 22.82, p < 0.0001; CFA, F1,32 = 0.3076, p = 0.5830, Fig. 4C), an effect similar to the CFA-induced changes in rats without the operant behavior (Fig. 4A). This indicates that daily operant behavior of SSA did not alter the effect of pain on the subunit composition of AMPARs in CeA. However, in MSA rats, CFA instead significantly decreased GluA1 and caused a small, non-significant decrease in GluA2 in CeA when compared with saline-injected MSA rats 1 d post CFA injection (Fig. 4B,C). Further analysis showed that the amount of CFA-induced decrease was significantly larger in GluA1 (46.2 ± 6.28%) than in GluA2 (26.8 ± 10.9%), resulting in a significantly reduced GluA1/GluA2 ratio by CFA in these MSA rats (treatment: F2,32 = 15.33, p < 0.0001; CFA: F1,32 = 4.489, p = 0.0420, Fig. 4D). CFA in rats with yoked injections of morphine at the same dose had no effect on the GluA1 or GluA2 level, or on the GluA1/GluA2 ratio in CeA, further supporting the notion that pain-induced down-regulation of CeA GluA1 is related to altered morphine reinforcement, but not to the pharmacological effect of morphine per se. Figure 5 illustrates increased expression of GluA1 and GluA2 in CeA cells of naïve rats 1 day after CFA induction of persistent pain.
Figure 4. Persistent pain decreases GluA1 and GluA1/GluA2 ratio in the CeA of MSA rats.
(A) Western blots (top) and normalized results (bottom) of GluA1 and GluA2 proteins in the CeA of naïve rats (n = 4) 1 d after CFA or saline injection. (B–D) Similar data of GluA1 (B) and GluA2 (C) proteins, and GluA1/GluA2 ratio (D) 1 d after CFA or saline (Sal) injection in 3 groups of SSA, MSA and morphine-yoked rats (n = 4–8, each group). CFA or saline was injected after 10 d operant sessions. (Student’s t test and two-way ANOVA with Bonferroni post hoc test).
Figure 5. Persistent pain increases expression of GluA1 and GluA2 in the CeA of naïve rats.
Representative immunohistochemical images of GluA1 (A) and GluA2 (B) and DAPI staining of cells in the CeA from naïve rats 1 d after an intraplantar injection of saline (n = 4 rats) or CFA (n = 4 rats). Scale bars = 500 μM (left panels) and 100 μM (middle and right panels). BLA, basolateral amygdala.
We further examined the role of CeA GluA1 in the pain inhibition of morphine intake by determining the pain impact on MSA and CeA GluA1 level at a different time point of pain inhibition during MSA. Interestingly, when indomethacin was injected 1 d after CFA injection (post-treatment) but 30 min before an MSA session, CFA decreased MSA to a similar extent when compared to vehicle-injected control rats (indomethacin: F1,9 = 0.5172, p = 0.4903, Fig. 6A), in strong contrast to the blockade of MSA by indomethacin pre-treatment (given 30 min before CFA, Fig. 2C). This lack of MSA reduction by acute pain inhibition 1 d after pain induction suggests that it is the pain-induced adaptive changes (such as CeA GluA1) already developed one day after CFA injection, but not acute removal of pain itself, that may underlie the pain reduction of morphine intake. When pain tests were performed 1 d after CFA injection in naïve rats, both the pre- and post-treatment with indomethacin inhibited pain by a similar amount (inhibition amount: pre-treatment, 40.25 ± 3.19, post-treatment, 50.40 ± 4.55, t9 = 2.26, p = 0.09), excluding the influence of the amount of pain inhibition by the pre- and post-indomethacin treatments. To determine the GluA1 role in this pain effect, we analyzed CeA GluA1 levels in CeA tissues from these indomethacin-treated rats. CeA GluA1 was upregulated while GluA2 down-regulated after 10 d MSA when compared to SSA rats (GluA1: t10 = 3.827, p = 0.0033; GluA2: t10 = 4.915, p = 0.0006, Fig. 6B–D), similar to the results in Fig. 3A under similar conditions. In the MSA rats, CFA attenuated the CeA level of GluA1 protein, and it was largely reversed by the pre-treatment with indomethacin (p = 0.003; one-way ANOVA: F2,10 = 9.876, p = 0.0043), but not by the post-treatment (p = 0.6738) (Fig. 6B, C). Similar results were obtained for GluA2, with the CFA-induced GluA2 reduction reversed by indomethacin pre-treatment (p = 0.0416), but not by indomethacin post-treatment (p = 0.1562) (Fig. 6B, D). Taken together, these results indicate that the decreased GluA1/GluA2 ratio plays an important role in pain-induced reduction in behavior of morphine intake.
Figure 6. Acute pain inhibition does not alter pain-induced reduction in morphine intake and GluA1 or GluA2 level.
(A) Normalized daily morphine infusions in CFA-injected rats with systemic treatment of vehicle (Veh, n = 6) or indomethacin (10 mg/kg, i.p., n = 5) 1 d after (post-treatment) CFA injection (30 min before the MSA session). (B–D) Western blots (B) and normalized results (C,D) of CeA levels of GluA1 and GluA2 proteins in rats after 10 d SSA or MSA sessions, and in CFA-injected MSA rats treated with vehicle, pre-treatment and post-treatment of indomethacin (n = 4, each group). (One-way or two-way ANOVA and Bonferroni post hoc test).
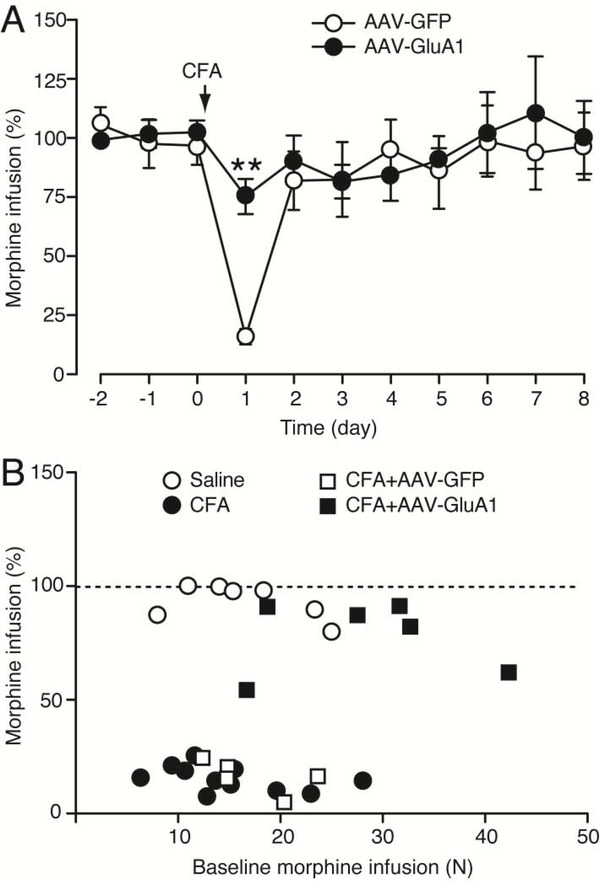
To determine a causal role of CeA GluA1 in the pain reduction of MSA, we overexpressed CeA GluA1 and examined how it affected the pain impact on MSA. Rats received CeA microinjection of AAV-GluA1 to overexpress GluA1 or the control vector AAV-GFP, followed one week later by 10 d steady MSA. We found that, in the AAV-GFP-injected control rats, CFA significantly reduced MSA 1 d after CFA injection (Fig. 7A), similar to the CFA effect in naïve rats (Fig. 1E); however, in AAV-GluA1-injected rats, the CFA-induced MSA reduction was completely reversed (AAV-GluA1: F1,8 = 0.0197, p = 0.8918, Fig. 7A), indicating a causal role of CeA GluA1 in promoting MSA behavior and its involvement in the pain reduction of MSA. This GluA1 role is also supported by our result that rats with overexpression of CeA GluA1 displayed a higher level of baseline MSA than control rats with CeA microinjection of AAV-GFP (Fig. 3D). Excluding a potential influence of different MSA baseline levels on the GluA1 effect, our correlation analysis showed that there was no correlative relationship between CFA-induced changes in morphine infusion numbers and the baseline level of MSA before CFA injection (AAV-GluA1: r < 0.001, p = 0.9767, AAV-GFP: r = 0.2810, p = 0.3582; naïve rats without virus injection: r = 0.2299, p = 0.1147, Fig. 7B). Therefore, it is unlikely that the AAV-GluA1 overexpression-induced reversal of the pain effect was due to the higher baseline level of MSA in AAV-GluA1-injected rats.
Figure 7. Overexpression of CeA GluA1 reverses pain-induced reduction in morphine intake.
(A) Daily morphine infusions in CFA-injected rats (n = 5, each group) after bilateral CeA microinjection of AAV-GFP or AAV-GluA1. CFA was injected on day 0 after 10 d MSA. (B) Correlation analysis of the relationship between CFA-induced changes in morphine infusion numbers and the baseline MSA levels before CFA. (Two-way ANOVA and Bonferroni post hoc test).
DISCUSSION
In the present study, we have presented molecular and behavioral evidence suggesting that GluA1 of AMPARs in CeA promotes behavior of opioid intake and its functional upregulation reverses short-term pain-induced reduction in opioid intake. These findings reveal a causal role of GluA1 and dynamic function of homomeric GluA1 AMPARs in CeA in promoting voluntary behavior of opioid consumption, which may act as a pivot in the mechanisms for interaction of pain and opioid use.
Dynamic regulation of subunit re-composition of central AMPARs has been implicated in brain processes of normal functions, such as learning and memory, and chronic neurologic diseases such as opioid addiction (Zhang and Abdullah, 2013). Homomeric GluA1 AMPARs are recruited to synapses during the early phase of LTP, a synaptic process thought to be the cellular basis for learning and memory as well as for the pathogenesis of many neurological diseases including opioid addiction and chronic pain (Mahanty and Sah, 1998; Hayashi et al., 2000; Hyman et al., 2006; Kauer and Malenka, 2007; Fortin et al., 2010; Zhuo, 2016). The importance of GluA1 incorporation into AMPARs has been documented in fear conditioning, a form of associative learning in which negative experience of animals is associated with specific contextual setting (Rumpel et al., 2005; Matsuo et al., 2008; Clem and Huganir, 2010). On the other hand, our previous study has shown that CeA GluA1 is also involved in associative learning of morphine reward as positive environmental context (Cai et al., 2013). These findings suggest that GluA1 adaptation plays a critical role in both negative and positive associative learning. Moreover, GluA1 AMPARs are required for behavioral sensitization to psychostimulants including locomotor sensitization and increased craving during abstinence (Carlezon et al., 1997; Conrad et al., 2008; Choi et al., 2011; Gipson et al., 2013). Taken together, it indicates that upregulated function of homomeric GluA1 AMPARs in CeA may promote voluntary opioid use likely through enhanced associative learning of opioid reward and sensitized response to the rewarding effect of opioids.
Dynamic adaptation of GluA1 AMPARs is also involved in the cellular mechanisms of pain sensitization. Persistent nociceptive stimuli lead to the increased abundance of GluA1 AMPARs in central synapses of pain-processing circuits, which results in central sensitization and consequently pain hypersensitization (Tao, 2010; Qiu et al., 2014). In the CeA, neuropathic or inflammatory pain has been shown to potentiate AMPAR synaptic activity (Li and Neugebauer, 2004; Ikeda et al., 2007). Consistently, our current results show that inflammatory pain non-selectively increases protein levels of both GluA1 and GluA2.
How pain affects opioid use has been a central and heated issue especially in the current environment of ongoing opioid epidemic. Unfortunately, previous basic research on animal models of pain and opioid use provides elusive and inconsistent results, and particularly lacking are basic studies looking into the mechanisms for pain-opioid interactions. Pain is known as an aversive stimulus and is associated with negative emotion (Gao et al., 2004; Hummel et al., 2008; Bushnell et al., 2013). As such, pain has been traditionally thought to inhibit opioid reward that promotes positive emotion and reinforcement (Leknes and Tracey, 2008; Volkow and McLellan, 2016). Indeed, several studies show that pain reduces opioid consumption and decreases the reinforcing effect of opioids (Martin et al., 2007; Woller et al., 2012; Wade et al., 2013). Consistent in this regard, our current results show that persistent pain (days) acutely decreases intake of morphine that has no effect on the sensory pain itself. Interestingly, treatment with indomethacin, which inhibited CFA-induced pain, blocked CFA-induced decrease in morphine intake only when indomethacin was given prior to CFA injection, but not 1 day after CFA injection before MSA sessions. These results suggest that short-term pain reduces opioid use likely by causing pain-induced adaptive changes (such as GluA1 decrease in CeA) that require some time to develop, but not by the pain sensation itself, as acute relief of the pain failed to affect CFA-induced reduction in morphine intake.
Nevertheless, pain-opioid interactions involve many other contributing factors that affect the ultimate outcomes of the pain effect, including, most prominently, opioid doses, learning and memory of opioid reward, pain-induced negative emotion, and positive reinforcement induced by opioid removal of aversive pain. For example, we and others have shown that persistent pain facilitates the learning and acquisition of opioid reward, promoting potential opioid use (Cahill et al., 2013; Zhang et al., 2014a; Zhang et al., 2014b). It is also important to note that, in clinical cases of opioid use or abuse, high opioid doses are used to relieve pain. Although individuals with or without pain consume similar amount of opioids at analgesic doses, as shown in this study and in a previous study (Martin et al., 2007), it does not necessarily indicate that pain is no longer effective on opioid consumption and underlying mechanisms. First, as removal of aversive pain is rewarding (Fields, 2004), consumption of analgesic opioids under pain is reinforcing and motivational for sustained and prolonged opioid use. Supporting this notion, our pervious study has demonstrated that persistent pain maintains opioid-seeking behavior in opioid-withdrawn rats (Hou et al., 2015). And second, while pain can be inhibited by consumed analgesic opioids, pain-induced adaptive changes and their interacting effects on the reward circuits likely persist, altering the sensitivity and perpetuation of responses to the rewarding effect of opioids as seen in drug craving, relapse and overdose.
The molecular mechanisms underlying the pain-opioid interaction are still poorly understood. Increasing evidence suggests that both long-term opioids and persistent pain induce overlapping adaptations and involve common brain mechanisms (Elman and Borsook, 2016). Specifically, we have shown that long-term opioids and persistent pain activate the same epigenetic regulation pathway in CeA and the pain effect could prime the shared mechanism and contribute to the heightened response to opioid reward under pain conditions (Zhang et al., 2014a). The current study reveals a causal role of CeA GluA1 in promoting opioid use and its involvement in both effects of opioids and pain. The neuronal circuits and associated cell types in CeA involved in the pain-opioid interaction are largely unknown at present. Over 95% of CeA cells are GABAergic (Tye et al., 2011). Thus, the GluA1 and GluA2 changes induced by self-administered morphine and by pain would overwhelmingly occurred in these GABAergic cells. However, increasing evidence suggests that there are functionally distinct subtypes of cells in CeA. For example, CeA cells expressing calcitonin gene-related peptide receive affective pain signal for establishing a threat memory (Han et al., 2015). We have recently shown that CeA cells receiving glutamate input conveying pain signal and CeA cells receiving glutamate input carrying corticolimbic modulatory signal have opposing effects on pain-induced negative emotion and behavior of reward (Cai et al., 2018).
It is interesting to note that persistent pain increased CeA levels of both GluA1 and GluA2 in naïve rats, but instead decreased their levels in rats under steady MSA (Fig. 4). The molecular mechanism underlying this dramatic change in the pain effect is unclear. The pain effect appears unselective, affecting both GluA1 and GluA2, unlike self-administered morphine that specifically increases GluA1 but decreases GluA2. This may reflect the fact that self-administered morphine is a voluntary operant behavior involving active associative learning of opioids and reward, the known function of GluA1, while pain is a passive environmental stimulus activating glutamate synaptic transmission non-selectively along the pain-processing pathways. The differential pain effects may suggest that the pain effect is state-dependent, increasing GluA1 at basal GluA1 level and decreasing GluA1 at elevated GluA1 level. Nevertheless, the GluA1/GluA2 ratio in CeA appears positively correlated to opioid intake under MSA and pain conditions in rats. It is important to note that negative emotion as the affective dimension of pain would develop under chronic pain conditions (Neugebauer, 2015; Wang et al., 2017; Cai et al., 2018), which would be another important factor for motivational opioid use to mitigate the negative emotion. This will be an interesting topic for future studies.
In conclusion, the current study demonstrates that GluA1 subunits of AMPARs in CeA promotes voluntary opioid intake and GluA1 upregulation reverses pain-induced acute reduction in opioid intake. It highlights CeA GluA1 as the common target of self-administered opioids and pain, and as a key protein in the mechanisms of pain impact on opioid use. It indicates that GluA1 antagonism may be useful for future development of therapeutic strategies in the treatment of opioid addiction under pain conditions.
Highlights.
Short-term pain acutely decreases voluntary morphine intake.
Morphine self-administration increases GluA1, but decreases GluA2, subunits of AMPA receptors in central amygdala.
Viral overexpression of GluA1 in central amygdala increases morphine self-administration.
Viral overexpression of GluA1 in central amygdala reverses the inhibitory pain effect on morphine intake.
ACNOWLEDGEMENTS
The authors would like to thank Drs. Zhi Zhang and Wei Wang for their technical support and helpful academic discussions during the course of this study.
FUNDING
This work was supported by NIH National Institute of Dental and Craniofacial Research grant DE025943 and National Institute of Neurological Disorders and Stroke grant NS113256.
Footnotes
CONFLICT OF INTEREST
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baliki MN, Apkarian AV (2015) Nociception, Pain, Negative Moods, and Behavior Selection. Neuron 87:474–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne JC, LaForge KS (2007) Opioid dependence and addiction during opioid treatment of chronic pain. Pain 129:235–255. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA (2002) The amygdala and reward. Nat Rev Neurosci 3:563–573. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Chen BT, Bonci A (2010) AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron 67:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Ceko M, Low LA (2013) Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Xue L, Grenier P, Magnussen C, Lecour S, Olmstead MC (2013) Changes in morphine reward in a model of neuropathic pain. Behav Pharmacol 24:207–213. [DOI] [PubMed] [Google Scholar]
- Cai YQ, Wang W, Paulucci-Holthauzen A, Pan ZZ (2018) Brain Circuits Mediating Opposing Effects on Emotion and Pain. J Neurosci 38:6340–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai YQ, Wang W, Hou YY, Zhang Z, Xie J, Pan ZZ (2013) Central amygdala GluA1 facilitates associative learning of opioid reward. J Neurosci 33:1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA Jr., Boundy VA, Haile CN, Lane SB, Kalb RG, Neve RL, Nestler EJ (1997) Sensitization to morphine induced by viral-mediated gene transfer. Science 277:812–814. [DOI] [PubMed] [Google Scholar]
- Choi KH, Edwards S, Graham DL, Larson EB, Whisler KN, Simmons D, Friedman AK, Walsh JJ, Rahman Z, Monteggia LM, Eisch AJ, Neve RL, Nestler EJ, Han MH, Self DW (2011) Reinforcement-related regulation of AMPA glutamate receptor subunits in the ventral tegmental area enhances motivation for cocaine. J Neurosci 31:7927–7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Huganir RL (2010) Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 330:1108–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME (2008) Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha JM, Sachs D, Canetti CA, Poole S, Ferreira SH, Cunha FQ (2003) The critical role of leukotriene B4 in antigen-induced mechanical hyperalgesia in immunised rats. Brit J Pharmacol 139:1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisco RA, Chandler RK, Compton WM (2008) Addressing the intersecting problems of opioid misuse and chronic pain treatment. Exper Clin Psychopharm 16:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR (2007) Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci 8:101–113. [DOI] [PubMed] [Google Scholar]
- Elman I, Borsook D (2016) Common Brain Mechanisms of Chronic Pain and Addiction. Neuron 89:11–36. [DOI] [PubMed] [Google Scholar]
- Fields H (2004) State-dependent opioid control of pain. Nat Rev Neurosci 5:565–575. [DOI] [PubMed] [Google Scholar]
- Fields HL, Margolis EB (2015) Understanding opioid reward. Trends Neurosci 38:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DA, Davare MA, Srivastava T, Brady JD, Nygaard S, Derkach VA, Soderling TR (2010) Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I. J Neurosci 30:11565–11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Ren WH, Zhang YQ, Zhao ZQ (2004) Contributions of the anterior cingulate cortex and amygdala to pain- and fear-conditioned place avoidance in rats. Pain 110:343–353. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW (2013) Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A 110:9124–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffer Y, Xu D, Eberle SE, D’Amour J, Lee M, Tukey D, Froemke RC, Ziff EB, Wang J (2013) Calcium-permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. J Neurosci 33:19034–19044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Guo W, Zou SP, Dubner R, Ren K (2003) Inflammation-induced upregulation of AMPA receptor subunit expression in brain stem pain modulatory circuitry. Pain 104:401–413. [DOI] [PubMed] [Google Scholar]
- Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD (2015) Elucidating an Affective Pain Circuit that Creates a Threat Memory. Cell 162:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287:2262–2267. [DOI] [PubMed] [Google Scholar]
- Hedegaard H, Warner M, Minino AM (2017) Drug overdose deaths in the United States, 1999–2016 In: National Center for Health Statistics. (Hyattsville MD, ed). [Google Scholar]
- Hou YY, Cai YQ, Pan ZZ (2015) Persistent pain maintains morphine-seeking behavior after morphine withdrawal through reduced MeCP2 repression of GluA1 in rat central amygdala. J Neurosci 35:3689–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GF, Besson JM, Bernard JF (1993) Intravenous morphine depresses the transmission of noxious messages to the nucleus centralis of the amygdala. Eur J Pharmacol 236:449–456. [DOI] [PubMed] [Google Scholar]
- Hummel M, Lu P, Cummons TA, Whiteside GT (2008) The persistence of a long-term negative affective state following the induction of either acute or chronic pain. Pain 140:436–445. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565–598. [DOI] [PubMed] [Google Scholar]
- Ikeda R, Takahashi Y, Inoue K, Kato F (2007) NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain 127:161–172. [DOI] [PubMed] [Google Scholar]
- Kallupi M, Wee S, Edwards S, Whitfield TW Jr., Oleata CS, Luu G, Schmeichel BE, Koob GF, Roberto M (2013) Kappa opioid receptor-mediated dysregulation of gamma-aminobutyric acidergic transmission in the central amygdala in cocaine addiction. Biol Psychi 74:520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC (2007) Synaptic plasticity and addiction. Nat Rev Neurosci 8:844–858. [DOI] [PubMed] [Google Scholar]
- Koob GF (2019) Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biol Psychi (PMID: 31400808. DOI: 10.1016/j.biopsych.2019.05.023). [DOI] [PubMed] [Google Scholar]
- Kuner R (2010) Central mechanisms of pathological pain. Nat Med 16:1258–1266. [DOI] [PubMed] [Google Scholar]
- Leknes S, Tracey I (2008) A common neurobiology for pain and pleasure. Nat Rev Neurosci 9:314–320. [DOI] [PubMed] [Google Scholar]
- Li W, Neugebauer V (2004) Block of NMDA and non-NMDA receptor activation results in reduced background and evoked activity of central amygdala neurons in a model of arthritic pain. Pain 110:112–122. [DOI] [PubMed] [Google Scholar]
- Li YQ, Li FQ, Wang XY, Wu P, Zhao M, Xu CM, Shaham Y, Lu L (2008) Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci 28:13248–13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Kuner T, Kuner R (2014) Synaptic plasticity in pathological pain. Trends Neurosci 37:343–355. [DOI] [PubMed] [Google Scholar]
- Mahanty NK, Sah P (1998) Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature 394:683–687. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25:103–126. [DOI] [PubMed] [Google Scholar]
- Manning BH (1998) A lateralized deficit in morphine antinociception after unilateral inactivation of the central amygdala. J Neurosci 18:9453–9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BH, Mayer DJ (1995a) The central nucleus of the amygdala contributes to the production of morphine antinociception in the formalin test. Pain 63:141–152. [DOI] [PubMed] [Google Scholar]
- Manning BH, Mayer DJ (1995b) The central nucleus of the amygdala contributes to the production of morphine antinociception in the rat tail-flick test. J Neurosci 15:8199–8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S (2005) Synaptic mechanisms of associative memory in the amygdala. Neuron 47:783–786. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC (2007) Opioid self-administration in the nerve-injured rat: relevance of antiallodynic effects to drug consumption and effects of intrathecal analgesics. Anesthesiology 106:312–322. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Reijmers L, Mayford M (2008) Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science 319:1104–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Christian DT, Diaz MR, Lack AK (2010) Glutamate plasticity in the drunken amygdala: the making of an anxious synapse. Int Rev Neurobiol 91:205–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M (2011) Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci 31:5737–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA (2007) The amygdala, reward and emotion. Trends Cogn Sci 11:489–497. [DOI] [PubMed] [Google Scholar]
- Neugebauer V (2015) Amygdala pain mechanisms. Handb Exp Pharmacol 227:261–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Bhave G, Gereau RWt (2003) Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci 23:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passik SD, Kirsh KL (2011) Addictions in pain clinics and pain treatment. Ann New York Acad Sci 1216:138–143. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd Edition. Sydney: Academic Press. [Google Scholar]
- Price DD (2000) Psychological and neural mechanisms of the affective dimension of pain. Science 288:1769–1772. [DOI] [PubMed] [Google Scholar]
- Qiu S, Zhang M, Liu Y, Guo Y, Zhao H, Song Q, Zhao M, Huganir RL, Luo J, Xu H, Zhuo M (2014) GluA1 phosphorylation contributes to postsynaptic amplification of neuropathic pain in the insular cortex. J Neurosci 34:13505–13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R (2005) Postsynaptic receptor trafficking underlying a form of associative learning. Science 308:83–88. [DOI] [PubMed] [Google Scholar]
- Tanimoto S, Nakagawa T, Yamauchi Y, Minami M, Satoh M (2003) Differential contributions of the basolateral and central nuclei of the amygdala in the negative affective component of chemical somatic and visceral pains in rats. Eur J Neurosci 18:2343–2350. [DOI] [PubMed] [Google Scholar]
- Tao YX (2010) Dorsal horn alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking in inflammatory pain. Anesthesiology 112:1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K (2011) Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471:358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban R, Scherrer G, Goulding EH, Tecott LH, Basbaum AI (2011) Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain 152:990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA (2005) Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, McLellan AT (2016) Opioid Abuse in Chronic Pain--Misconceptions and Mitigation Strategies. N Engl J Med 374:1253–1263. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D (2012) Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol 52:321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hehn CA, Baron R, Woolf CJ (2012) Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73:638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CL, Krumenacher P, Kitto KF, Peterson CD, Wilcox GL, Fairbanks CA (2013) Effect of chronic pain on fentanyl self-administration in mice. PLoS One 8:e79239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li C, Cai Y, Pan ZZ (2017) Pain vulnerability and DNA methyltransferase 3a involved in the affective dimension of chronic pain. Mol Pain 13:1744806917726713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J II, Niedzielski AS (1996) Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci 16:1982–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woller SA, Moreno GL, Hart N, Wellman PJ, Grau JW, Hook MA (2012) Analgesia or addiction?: implications for morphine use after spinal cord injury. J Neurotrauma 29:1650–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M (2008) Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci 28:7445–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Abdullah JM (2013) The role of GluA1 in central nervous system disorders. Rev Neurosci 24:499–505. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tao W, Hou YY, Wang W, Kenny PJ, Pan ZZ (2014a) MeCP2 Repression of G9a in Regulation of Pain and Morphine Reward. J Neurosci 34:9076–9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Tao W, Hou YY, Wang W, Lu YG, Pan ZZ (2014b) Persistent Pain Facilitates Response to Morphine Reward by Downregulation of Central Amygdala GABAergic Function. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Bie B, Pan ZZ (2007) Involvement of non-NMDA glutamate receptors in central amygdala in synaptic actions of ethanol and ethanol-induced reward behavior. J Neurosci 27:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M (2016) Neural Mechanisms Underlying Anxiety-Chronic Pain Interactions. Trends Neurosci 39:136–145. [DOI] [PubMed] [Google Scholar]