Abstract
BACKGROUND
Secondary surgical cytoreduction in women with platinum-sensitive, recurrent epithelial ovarian, primary peritoneal, or fallopian-tube (“ovarian”) cancer is widely practiced but has not been evaluated in phase 3 investigation.
METHODS
We randomly assigned patients with recurrent ovarian cancer who had received one previous therapy, had an interval during which no platinum-based chemotherapy was used (platinum-free interval) of 6 months or more, and had investigator-determined resectable disease (to no macroscopic residual disease) to undergo secondary surgical cytoreduction and then receive platinum-based chemotherapy or to receive platinum-based chemotherapy alone. Adjuvant chemotherapy (paclitaxel–carboplatin or gemcitabine–carboplatin) and use of bevacizumab were at the discretion of the investigator. The primary end point was overall survival.
RESULTS
A total of 485 patients underwent randomization, 240 to secondary cytoreduction before chemotherapy and 245 to chemotherapy alone. The median follow-up was 48.1 months. Complete gross resection was achieved in 67% of the patients assigned to surgery who underwent the procedure. Platinum-based chemotherapy with bevacizumab followed by bevacizumab maintenance was administered to 84% of the patients overall and was equally distributed between the two groups. The hazard ratio for death (surgery vs. no surgery) was 1.29 (95% confidence interval [CI], 0.97 to 1.72; P=0.08), which corresponded to a median overall survival of 50.6 month and 64.7 months, respectively. Adjustment for platinum-free interval and chemotherapy choice did not alter the effect. The hazard ratio for disease progression or death (surgery vs. no surgery) was 0.82 (95% CI, 0.66 to 1.01; median progression-free survival, 18.9 months and 16.2 months, respectively). Surgical morbidity at 30 days was 9%; 1 patient (0.4%) died from postoperative complications. Patient-reported quality of life decreased significantly after surgery but did not differ significantly between the two groups after recovery.
CONCLUSIONS
In this trial involving patients with platinum-sensitive, recurrent ovarian cancer, secondary surgical cytoreduction followed by chemotherapy did not result in longer overall survival than chemotherapy alone. (Funded by the National Cancer Institute and others; GOG-0213 ClinicalTrials.gov number, NCT00565851.)
THE AMERICAN CANCER SOCIETY HAS EStimated that in 2019 approximately 22,500 women would be diagnosed with epithelial ovarian, primary peritoneal, or fallopian-tube (“ovarian”) cancer, and 14,000 would die.1 Despite the absence of randomized data showing a survival benefit conferred by primary cytoreductive surgery, meta-analyses support the approach.2–4 Theoretically, maximal surgical effort may help overcome intrinsic drug resistance, increase drug perfusion, enhance host immunologic response, increase the growth fraction of tumor cells, and circumvent acquired drug resistance after adjuvant platinum-based and taxane-based systemic therapy.5–7 Unfortunately, recurrent disease develops in more than 80% of women. The 10-year rates of disease-free survival among patients with recurrent disease are abysmal and are below 15%.8
Given the widespread adoption of primary surgical cytoreduction, it is not surprising that the approach is also strongly considered for patients with recurrent disease — particularly those who are considered to be candidates for platinum reinduction (e.g., a prolonged treatment-free interval after platinum therapy) and those with isolated or limited-volume recurrent disease. Numerous single-institution and multi-institution retrospective reviews and meta-analyses have bolstered support for the procedure, showing that patients who had the greatest benefit were those with little or no postoperative residual disease and those considered to be platinum-sensitive.3,9–12 Current guidelines from the National Comprehensive Cancer Network list secondary cytoreduction as a treatment option for patients with a treatment-free interval of 6 months or more after a complete remission from previous chemotherapy.13
A clear limitation of this body of evidence is bias in patient selection, which is not easily controlled without a randomized clinical trial. Furthermore, with the availability of bevacizumab and poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors as maintenance medical treatments with proven progression-free survival benefit among patients with platinum-sensitive, recurrent ovarian cancer who have a response to salvage therapy, it is important to clarify the role of secondary cytoreductive surgery in this disease.14–18 Therefore, we designed the Gynecologic Oncology Group (GOG)–0213 trial to assess whether secondary cytoreduction would increase overall survival among women with platinum-sensitive, recurrent ovarian cancer who otherwise were considered to be surgical candidates.
METHODS
TRIAL DESIGN
The GOG-0213 trial is an open-label, phase 3, multicenter, international, randomized clinical trial designed to assess two clinically relevant hypotheses: that bevacizumab added to paclitaxel and carboplatin chemotherapy followed by maintenance bevacizumab improves overall survival (chemotherapy objective) and that secondary surgical cytoreduction in platinum-sensitive, surgically amenable patients improves overall survival (surgical objective). The trial design and patient characteristics have been reported previously, as has the successful assessment of the chemotherapy objective.14 The protocol is available with the full text of this article at NEJM.org. At trial activation (December 10, 2007), patients were eligible to participate in both the chemotherapy and surgical components of the trial if they met the criteria to be considered for secondary cytoreduction. Patients who were not eligible for the surgical component were enrolled in the chemotherapy component only. Once the required number of patients was enrolled (674, on August 28, 2011), only patients meeting surgical eligibility were allowed to enroll. At that point, 107 women had undergone randomization (in a 1:1 ratio) to surgery or no surgery.
The trial was designed by the authors and supported by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI). The data were collected and analyzed and the manuscript was written by the authors, who vouch for the completeness and accuracy of the data and for the adherence of the trial to the protocol. All the patients provided written informed consent. Genentech provided bevacizumab and supplemental funding support to the GOG through a cooperative research and development agreement with the NCI but played no role in the collection or analysis of data or its interpretation.
INCLUSION AND EXCLUSION CRITERIA
Patients eligible for the surgical component of the trial had measurable (according to the Response Evaluation Criteria in Solid Tumors, version 1.0; see the Supplementary Appendix, available at NEJM.org), platinum-sensitive, recurrent epithelial ovarian cancer that was deemed by the investigator to be amenable to complete gross resection. All eligible patients were women 18 years of age or older who had had a complete clinical response to at least three cycles of primary platinum-based chemotherapy, as assessed by a normal physical examination, a normal serum cancer antigen 125 (CA-125) value, and, if obtained, negative imaging studies. Platinum sensitivity was defined as disease-free survival of 6 months or more after the infusion of the last cycle of chemotherapy. If maintenance chemotherapy (e.g., paclitaxel) was administered, a minimum of 6 months since the last infusion was also required. Similarly, a treatment-free window of 4 weeks or more was required if biologic therapy (e.g., bevacizumab) or hormonal maintenance therapy was used. Patients were also required to have adequate renal, hepatic, and bone marrow function, as well as a GOG performance-status score of 0 to 2 (on a scale from 0 to 4, with higher scores indicating greater disability). Women who were not medically fit for surgery and those with diffuse carcinomatosis, ascites, or extraabdominal disease were excluded.
TREATMENT
Patients who were assigned to the surgery group underwent their procedure within 4 weeks after registration. The objective at surgery was resection of tumor to no gross residuum. During the assessment of the chemotherapy objective, surgically amenable patients also underwent randomization (in a 1:1 ratio) to paclitaxel (175 mg per square meter of body-surface area) and carboplatin (area under the curve [AUC] of 5 mg per milliliter per minute) intravenously every 3 weeks for six cycles or to paclitaxel–carboplatin (same doses as above) and bevacizumab (15 mg per kilogram of body weight) every 3 weeks for six cycles. CTEP approved an amendment on August 29, 2011, to allow the physician’s choice of either paclitaxel–carboplatin or gemcitabine (1000 mg per square meter on days 1 and 8 every 3 weeks) plus carboplatin (AUC of 4 mg per milliliter per minute, administered every 3 weeks), without or with bevacizumab for six cycles. Two additional cycles were allowed for patients having a partial response. Patients who were assigned to the bevacizumab group also received maintenance therapy with bevacizumab (15 mg per kilogram) every 3 weeks until progression, death, unacceptable toxic effects, or patient voluntary withdrawal. Safety was monitored during each cycle. The choice of chemotherapy was made at surgical randomization, and chemotherapy was administered within 6 weeks after registration or 6 weeks postoperatively.
ASSESSMENTS
Baseline imaging with the use of abdominopelvic computed tomography or magnetic resonance imaging was to be completed during a 28-day window in which chemotherapy was initiated. Disease was also assessed after cycles 3 and 6 of trial treatment (and after cycle 8, if administered), every 3 months for 2 years, and then every 6 months thereafter. Surgical patients underwent radiographic assessment during a 2-week window in which chemotherapy was initiated. Physical examinations were performed and serum CA-125 levels measured at the beginning of each cycle of chemotherapy; in maintenance, these procedures were performed every 6 weeks.
Trial treatments were assigned sequentially from lists composed of randomly permuted blocks. The list of treatments was prepared by the GOG Statistical and Data Center (Buffalo, New York) and remained concealed during the conduct of the trial. During the chemotherapy component of the trial, treatment assignments were stratified according to treatment-free interval (6 to 12 months or >12 months after the last platinum infusion) and participation in the surgical component of the trial (yes or no). After closure of the chemotherapy component, treatment assignments were stratified according to previous treatment-free interval (6 to 12 months or >12 months after the last platinum infusion) and chemotherapy regimen (paclitaxel–carboplatin, paclitaxel–carboplatin plus bevacizumab, gemcitabine–carboplatin, or gemcitabine–carboplatin plus bevacizumab).
Patient-reported outcomes included quality of life (score on the Trial Outcome Index of the Functional Assessment of Cancer Therapy–Ovary; range, 0 to 100, with higher scores indicating a better quality of life), physical functioning (score on the physical functioning subscale of the RAND 36-item Short Form Survey; range, 0 to 100, with higher scores indicating better physical functioning), and surgery-related symptoms (score on a 6-item subscale assessing surgery-related side effects; range, 0 to 24, with higher scores indicating less severe symptoms). The assessments took place at six time points: before surgery (for those patients assigned to cytoreductive surgery), before initiation of chemotherapy, before cycle 3 (6 weeks after starting chemotherapy), before cycle 6 (15 weeks after starting chemotherapy), 6 months after starting chemotherapy, and 12 months after starting chemotherapy. A 5-point difference in score between trial groups was considered to be clinically meaningful.19
END POINTS
The primary end point, overall survival, was measured from randomization to death from any cause on an intention-to-treat basis. Data on patients alive at their last follow-up visit were censored at that time. Investigator-assessed progression-free survival, defined as the time from randomization to disease progression or death from any cause, was a secondary end point. Safety analyses, including 30-day surgical morbidity and mortality and treatment-related adverse events, were reported for patients who received any assigned trial treatment.
STATISTICAL ANALYSIS
The statistical analysis plan for the primary analysis and assessment of patient-reported outcomes is available with the protocol at NEJM.org.14 During the chemotherapy component of the trial, in which two-way randomization took place for the subgroup of surgical candidates, we assumed no interaction between the two randomized treatments (chemotherapy and surgery). The target enrollment for the surgical component of the trial was 485 patients and was considered mature for analysis when at least 250 deaths (events) were reported. The design provided 81% power to assess a true hazard ratio for death of 0.70. This would be associated with a between-group difference in the percentage of patients alive at 22 months of 11.5 percentage points (61.5% in the surgery group and 50% in the no-surgery group). A log-rank test, stratified according to the same factors that were used to balance the treatment assignments, was used to compare the treatment groups with regard to overall survival. The relative hazard of death was estimated under the assumption of proportional hazards. All proportional-hazards models were stratified according to the factors used to balance the treatment assignments (interval during which no platinum-based chemotherapy was received [platinum-free interval] and previous bevacizumab use) unless otherwise noted. The percentages of patients surviving at a particular time point were estimated with the Kaplan–Meier procedure.
An interim analysis of overall survival was prespecified when at least 125 deaths (events) (50% maturity) were documented. The guideline for monitoring efficacy was determined by an alpha-spending function, proposed by Lan and DeMets, and harmonizes with the O’Brien and Fleming group-sequential boundary.20,21 The overall type I error was set at 0.025 (one-tailed), including the type I error spent for interim analyses. Similarly, the design included an O’Brien–Fleming–like type II error spending function for monitoring futility. On May 2, 2017, the trial data were locked for the scheduled interim analysis. At this point, the trial was fully enrolled, and 130 events were recorded. The interim results indicated that the boundary for futility had been crossed; subsequently, the data and safety monitoring committee recommended that the NCI and the trial team release the trial results ahead of the scheduled maturity of the trial (250 events). For the purpose of this presentation, the trial data were frozen and locked on April 24, 2019, with 197 events (79% maturity).
RESULTS
PATIENTS
From December 6, 2007, through June 9, 2017, a total of 485 patients were randomly assigned to secondary surgical cytoreduction followed by platinum-based chemotherapy (240 patients) or to chemotherapy alone (245 patients). All enrolled patients (Fig. 1) were included in the intention-to-treat analyses for overall and progression-free survival. Demographic and clinical characteristics of the trial sample and specific disease-distribution characteristics are presented in Tables S1 and S4. These features were well balanced between the two groups. Previous bevacizumab exposure was recorded in 55 patients (11%). The median platinum-free interval was 20.4 months (interquartile range, 13.2 to 34.0) in the surgery group and 18.8 months (interquartile range, 12.5 to 28.4) in the no-surgery group.
Figure 1. Randomization and Follow-up.
Patients who were included in the intention-to-treat analysis were evaluated for overall survival and progression-free survival.
SURGICAL OUTCOMES
Of 240 patients assigned to surgery, 225 eligible patients (94%; 15 declined surgery) underwent secondary cytoreduction (per-protocol cohort) and 221 had operative reports confirming the postoperative tumor residual (4 were excluded for pending data). With the inclusion of the 15 eligible patients with measurable disease who were assigned to secondary cytoreductive surgery but did not undergo the procedure, the percentage of patients who had complete gross resection was 63% (150 of 239, excluding 1 without surgical results). Among women who were assigned to surgery, underwent the procedure, and had information on residual disease, the percentage who had complete gross resection was 67% (150 of 224). Characteristics of surgical procedures and surgical patients are presented in Table 1 and are noteworthy for a low incidence of aborted procedures (8 patients, 4%), low 30-day morbidity (20 patients, 9%), and low 30-day mortality (1 patient, 0.4%).
Table 1.
Characteristics of Surgical Procedure and Surgical Patients.
| Characteristic | Value |
|---|---|
| Estimated blood loss | |
| Patients included in analysis — no. | 192 |
| Median (interquartile range) — ml | 200 (100–498) |
| Range — ml | 5–3900 |
| Transfusion — no./total no. (%)* | 17/215 (8) |
| Bowel resection — no./total no. (%) | 62/221 (28) |
| Stoma — no./total no. (%) | 4/221 (2) |
| Perioperative thrombosis — no./total no. (%)† | 3/225 (1) |
| Procedure aborted — no./total no. (%) | 8/221 (4) |
| Surgery-related adverse events at 30 days — no./total no. (%)† | 20/225 (9) |
| Death at 30 days — no./total no. (%) | 1/225 (0.4) |
| Repeat laparotomy — no./total no. (%) | 0/225 |
The amount of blood that was transfused ranged from 1 to 14 units.
Data are for cases that were considered by the investigator to be at least possibly due to surgery, that were of grade 3 or higher, and that occurred before the onset of chemotherapy.
ADJUVANT CHEMOTHERAPY
The median time to chemotherapy initiation in the surgery group was 40 days (interquartile range, 28 to 51), as compared with 7 days (interquartile range, 4 to 11) in the no-surgery group. Most patients (408 of 485, 84%) received concomitant and maintenance bevacizumab; the paclitaxel–platinum combination was preferred (337 of 485, 69%). The use of each regimen was similar within each randomized cohort. Adverse events and adverse events of special interest were consistent with previous reports related to the chosen chemotherapy and bevacizumab.14,15 No new safety signals were seen. Three deaths were considered by the investigator to be “at least possibly” related to the trial treatment (either surgery or chemotherapy). Two deaths in the no-surgery group were due to cardiopulmonary arrest, and one death in the surgery group was due to a pulmonary embolus (postoperative, pre-chemotherapy). Three additional deaths were not considered to be treatment-related. (For details on chemotherapy regimens and adverse events of special interest, see Tables S2 and S3.)
PATIENT-REPORTED OUTCOMES
Patients in the surgery group reported a significant decrease in quality of life (Fig. 2) and physical functioning and an increase in surgery-related symptoms immediately after secondary cytoreduction. However, there was no significant interaction between treatment group and time point and no significant difference between the groups, on average, over assessment time points, which indicates that the surgery group and the no-surgery group did not differ overall in any of the patient-reported outcomes.
Figure 2. Quality of Life.
The plot lines represent the patient-reported scores on the Trial Outcome Index of the Functional Assessment of Cancer Therapy–Ovary (FACT-O TOI). Scores range from 0 to 100, with higher scores indicating a better quality of life. The estimates of least-squares mean scores were obtained from a fitted mixed model that was adjusted for pre-treatment score (baseline score), chemotherapy received, the country in which the treatment was given, the interval during which no platinum-based chemotherapy was used (platinum-free interval) before recurrence (6 to 12 months vs. >2 months), and the patient’s age at enrollment. Surgery was associated with significantly lower postoperative FACT-O TOI scores. The scores were similar in the two groups at 6 weeks after treatment and at every subsequent assessment.
EFFICACY RESULTS
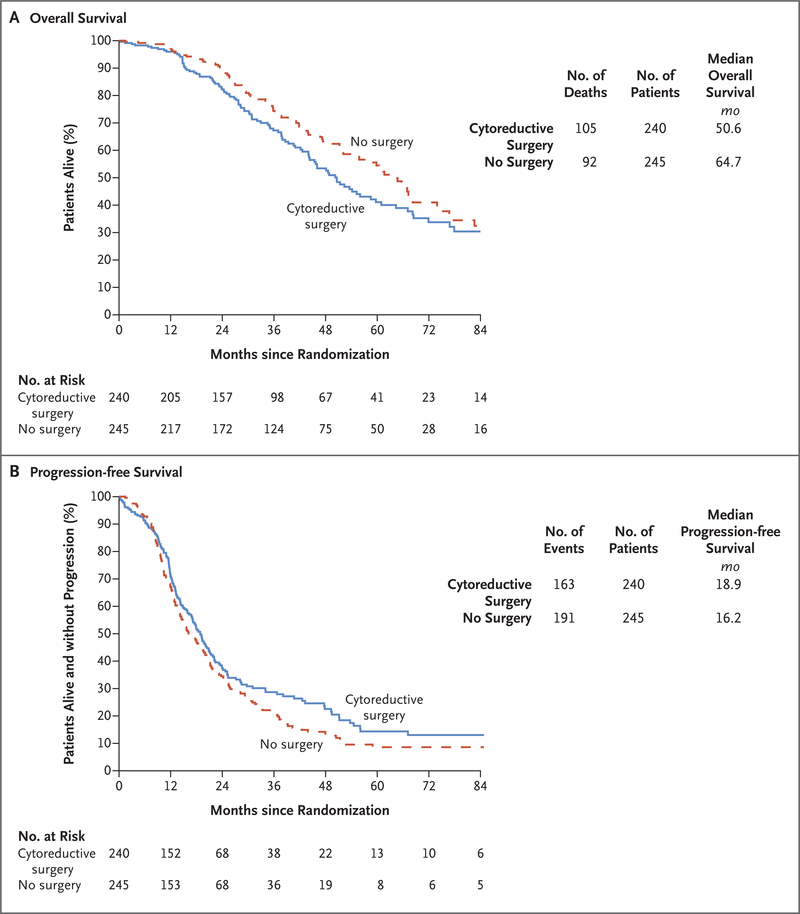
As of April 24, 2019, a total of 197 deaths had occurred, and 135 patients (56%) in the surgery group were still alive, as compared with 153 (62%) in the no-surgery group. The median follow-up at data lock was 48.1 months. The adjusted hazard ratio for death (surgery vs. no surgery) was 1.29 (95% confidence interval [CI], 0.97 to 1.72; P=0.08), which corresponded to a median overall survival of 50.6 months and 64.7 months, respectively (Fig. 3A). The percentage of patients surviving at 3 years was 67% (95% CI, 60 to 74) in the surgery group and 74% (95% CI, 68 to 81) in the no-surgery group.
Figure 3. Overall Survival and Progression-free Survival.
Shown are overall survival (primary outcome; Panel A) and progression-free survival (Panel B) among patients randomly assigned to surgical cytoreduction and chemotherapy (cytoreductive-surgery group) or chemotherapy alone (no-surgery group).
The adjusted hazard ratio for disease progression or death (surgery vs. no surgery) was 0.82 (95% CI, 0.66 to 1.01), which corresponded to a median progression-free survival of 18.9 months and 16.2 months, respectively (Fig. 3B). The percentage surviving without progression at 3 years was 29% (95% CI, 22 to 35) in the surgery group and 20% (95% CI, 15 to 26) in the no-surgery group.
Figure 4 presents the subgroup analysis for overall survival among the stratification variables and patient demographic characteristics. (See Fig. S1 for progression-free survival.)
Figure 4. Subgroup Analysis for Overall Survival.
Shown are hazard ratios for the effect of cytoreductive surgery on overall survival according to stratification variables (chemotherapy regimen and platinum-free interval) and patient characteristics. The results of statistical testing of the interaction between treatment and subgroup factors suggest reasonable consistency of the treatment effect within randomization strata as well as demographic and prognostic subgroups. Ethnic group was reported by the patients.
An ad hoc, exploratory analysis evaluating the effect of complete gross resection on overall and progression-free survival was conducted within the surgical population (239 patients), with adjustment for platinum-free interval and previous bevacizumab use and number of metastatic sites. Complete gross resection (in 150 patients), as compared with incomplete resection (in 89 patients), was associated with longer overall survival (hazard ratio for death, 0.61; 95% CI, 0.40 to 0.93; median overall survival, 56.0 months vs. 37.8 months) and longer progression-free survival (hazard ratio for disease progression or death, 0.51; 95% CI, 0.36 to 0.71; median progression-free survival, 22.4 months vs. 13.1 months) (Fig. S2).
However, a comparison of the complete gross resection subpopulation (150 patients) with the entire no-surgery group (245 patients) did not show a benefit with respect to overall survival (hazard ratio for death, 1.03, 95% CI, 0.74 to 1.46; median overall survival, 56.0 months and 64.7 months, respectively), although there was a benefit with respect to progression-free survival (hazard ratio for disease progression or death, 0.62; 95% CI, 0.48 to 0.80; median progression-free survival, 22.4 months and 16.2 months, respectively) (Fig. S3). Similarly, patients from South Korea accounted for nearly half the trial population, and their aggregate incidence of complete gross resection of 65% did not have a meaningful effect on overall survival (hazard ratio for death excluding the South Korean cohort, 0.92; 95% CI, 0.33 to 2.52).
DISCUSSION
In this prospective, randomized, multinational, phase 3 clinical trial of secondary surgical cytoreduction in women with resectable, platinum-sensitive, recurrent ovarian cancer, we found that the procedure followed by chemotherapy did not result in longer overall survival than chemotherapy alone. The median progression-free survival was numerically longer in the surgery group than in the no-surgery group, but the hazard ratio for disease progression or death did not indicate that surgery plus chemotherapy was superior to chemotherapy alone. Although patients who underwent surgery appeared to benefit from complete gross resection, we did not find that surgery itself had an overall survival benefit over chemotherapy alone, even when restricting the analysis to patients who had complete gross resection (67% of patients undergoing surgery). As expected, patients who underwent cytoreductive surgery reported a significant decline in quality of life and patient-reported outcomes immediately after the procedure; by 6 weeks, they reached parity with patients who did not undergo surgery, and they maintained parity at subsequent assessments. Our findings call into question the merit of surgical cytoreduction in this population, and the shorter overall survival in the surgery group than in the no-surgery group (range in relative hazard for death, 3% lower risk to 72% higher risk) among the intention-to-treat population underscores the importance of formally assessing the value of the procedure in clinical care.
Three key items should be considered with these findings. First, clinical trials evaluating a surgical intervention are extremely difficult to perform owing to inherent bias in patient selection and investigator interpretation of preexisting data. The issue is compounded when no data from prospective, randomized trials that control for relevant prognostic factors are available to properly inform decision making. Evidence of patient selection for randomization was evident in the current trial, because enrollees had relatively limited tumor volume. More than half the patients who were considered for this trial had two or fewer sites of recurrent disease (data not shown), which reflects the investigator intent of achieving complete gross resection. In addition, patients were substantially platinum-sensitive, with a median platinum-free interval of 20.4 months. The effect of these two factors may have diluted an independent surgical effect.
Second, previous results from this trial (chemotherapy component) and others have validated the clinical effect of adjuvant and maintenance bevacizumab among women treated with second-line, platinum-based chemotherapy (with respect to response, progression-free survival, and overall survival).14,15 Besides the 53 patients assigned to receive no bevacizumab in the chemotherapy component, only 24 of the 432 additionally enrolled patients (6%) did not receive bevacizumab by choice at the time of randomization. The effect of bevacizumab use on overall survival among the randomized population is presented in Figures S4A and S4B. Although a detriment in overall survival was observed among those undergoing surgery but not receiving bevacizumab, it is unknown whether patients not receiving bevacizumab at the time of either randomization were less likely to receive it in subsequent lines of therapy; such an imbalance could affect long-term outcomes. Furthermore, selection of highly active chemotherapy may have masked an incremental benefit from surgery, similar to the evaluation of bevacizumab combinations in frontline therapy.22,23 Although a slight difference in the time to the first dose of chemotherapy was seen between the surgery group and the no-surgery group, a landmark analysis assessing the delay in initiation of chemotherapy in the surgery group was not associated with an increase in the hazard of death (data not shown).
Third, the median overall survival for the entire trial cohort was nearly three times longer than expected when the trial was designed. The precise reasons for this are unknown but are probably related to improvements in clinical care and the availability of more effective treatments, particularly those in selected populations (e.g., use of PARP inhibitors in patients with tumors with alterations in BRCA1 or BRCA2). Extended postprogression survival can dilute the treatment effect measured according to progression-free survival by reducing statistical power to assess overall survival and enabling a higher likelihood of intervening treatment.24,25
At least three other ongoing phase 3 trials are comparing surgery and chemotherapy with surgery alone: DESKTOP-III (ClinicalTrials.gov number, NCT01166737), Surgery for Ovarian Cancer Recurrence (SOCceR; Netherlands Trial Register number, NL3137), and Surgery or Chemotherapy in Recurrent Ovarian Cancer (SOC 1; NCT01611766). The DESKTOP-III trial, now fully enrolled, has a design similar to that of the GOG-0213 trial, with overall survival as its primary end point.
The trials differ with respect to patient-selection criteria and adjuvant therapy. The DESKTOP-III trial uses a selection criteria algorithm (Arbeitsgemeinschaft Gynaekologische Onkologie [AGO] score) based on a performance status of 0 on the Eastern Cooperative Oncology Group scale (range, 0 [no disability] to 5 [death]), ascites of less than 500 ml, and complete gross resection at primary surgery, which was shown to predict complete resection in more than 66% of patients undergoing secondary cytoreduction.26,27 The SOC-1 trial is using the iMODEL scoring algorithm to select patients for randomization. The use of the DESKTOP selection criteria algorithm in the DESKTOP-III trial has led to complete gross resection in 68% of the patients, a percentage similar to that in the GOG-0213 trial (67% in the per-protocol cohort).28 Maturity of the DESKTOP-III trial and other trials will shape the debate on the value or merit of surgery in this patient population.
In conclusion, secondary surgical cytoreduction in patients with platinum-sensitive, recurrent epithelial ovarian cancer selected according to these criteria appears to be feasible, with acceptable postoperative morbidity, but did not result in longer overall survival than no surgery. The hazard ratio for disease progression or death did not indicate that surgery plus chemotherapy was superior to chemotherapy alone.
Supplementary Material
Acknowledgments
Supported by National Cancer Institute (NCI) grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469), the GOG Statistical Office (CA 37517), NRG Oncology (1U10 CA180822), and the NRG Network Operations Center (U10CA180868) and in part by the National Institutes of Health/NCI under award number P30CA016672 (the investigators used the clinical research shared resource). Roche/Genentech supported the NCI cooperative research and development agreement enabling this trial. Dr. Coleman is supported in part by the Ann Rife Cox Chair in Gynecology and the Judy Reis/Albert Pisani, M.D., Ovarian Cancer Research Fund.
Footnotes
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69: 7–34. [DOI] [PubMed] [Google Scholar]
- 2.Al Rawahi T, Lopes AD, Bristow RE, et al. Surgical cytoreduction for recurrent epithelial ovarian cancer. Cochrane Database Syst Rev 2013;2:CD008765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bristow RE, Puri I, Chi DS. Cytoreductive surgery for recurrent ovarian cancer: a meta-analysis. Gynecol Oncol 2009;112: 265–74. [DOI] [PubMed] [Google Scholar]
- 4.Bristow RE, Lagasse LD, Karlan BY. Secondary surgical cytoreduction for advanced epithelial ovarian cancer: patient selection and review of the literature. Cancer 1996;78:2049–62. [PubMed] [Google Scholar]
- 5.Skipper HE. Adjuvant chemotherapy. Cancer 1978;41:936–40. [DOI] [PubMed] [Google Scholar]
- 6.Goldie JH, Coldman AJ. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep 1979;63:1727–33. [PubMed] [Google Scholar]
- 7.Norton L, Simon R. Tumor size, sensitivity to therapy, and design of treatment schedules. Cancer Treat Rep 1977;61:1307–17. [PubMed] [Google Scholar]
- 8.Dood RL, Zhao Y, Armbruster SD, et al. Defining survivorship trajectories across patients with solid tumors: an evidence-based approach. JAMA Oncol 2018; 4:1519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bommert M, Harter P, Heitz F, du Bois A. When should surgery be used for recurrent ovarian carcinoma? Clin Oncol (R Coll Radiol) 2018;30:493–7. [DOI] [PubMed] [Google Scholar]
- 10.Santillan A, Karam AK, Li AJ, et al. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol Oncol 2007;104:686–90. [DOI] [PubMed] [Google Scholar]
- 11.Zang RY, Harter P, Chi DS, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer 2011;105: 890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer 2006;106: 1933–9. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer (version 2.2018). 2019. (https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf). [DOI] [PMC free article] [PubMed]
- 14.Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2017;18:779–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 2012;30:2039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18:1274–84. [DOI] [PubMed] [Google Scholar]
- 17.Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 2016;375:2154–64. [DOI] [PubMed] [Google Scholar]
- 18.Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:1949–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes 2003;1:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeMets DL, Lan G. The alpha spending function approach to interim data analyses. Cancer Treat Res 1995;75:1–27. [DOI] [PubMed] [Google Scholar]
- 21.DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med 1994;13:1341–52. [DOI] [PubMed] [Google Scholar]
- 22.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011;365:2473–83. [DOI] [PubMed] [Google Scholar]
- 23.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011;365:2484–96. [DOI] [PubMed] [Google Scholar]
- 24.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst 2009;101:1642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morita S, Sakamaki K, Yin G. Detecting overall survival benefit derived from survival postprogression rather than progression-free survival. J Natl Cancer Inst 2015;107(8):djv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harter P, du Bois A, Hahmann M, et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol 2006;13:1702–10. [DOI] [PubMed] [Google Scholar]
- 27.Harter P, Sehouli J, Reuss A, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II: a project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer 2011;21:289–95. [DOI] [PubMed] [Google Scholar]
- 28.Du Bois A, Vergote I, Ferron G, et al. Randomized controlled phase III study evaluating the impact of secondary cytoreductive surgery in recurrent ovarian cancer: AGO DESKTOP III/ENGOT ov20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.