Abstract
Purpose.
To compare patient/tumor characteristics and outcomes of Asians to Caucasian patients with epithelial ovarian cancer.
Methods.
Ancillary data were pooled and analyzed from ten prospective randomized front-line Gynecologic Oncology Group clinical trials from 1996 to 2011. Demographic, clinicopathologic features, disease-specific and all-cause survival were analyzed.
Results.
Of 7914 patients, 7641 were Caucasian and 273 Asian. When compared to Caucasians, Asians were younger at trial enrollment, had a better performance status, earlier-stage cancers (17.2% vs. 8.1% with stage I; p < 0.001), and were more likely to be of clear cell (15.8% vs. 6.2%, p < 0.001) and mucinous (3.3% vs. 1.9%, p < 0.001) histology. Asians had an improved 5-year disease-specific survival of 54.1% compared to 46.1% for Caucasians, p = 0.001. In multivariate analysis, the Asian race remained a significant prognostic factor for all-cause survival (HR: 0.84; 95% CI: 0.72–0.99; p = 0.04). Other factors predictive of improved survival included younger age, better performance status, optimal cytoreduction, earlier stage, non-clear cell histology, and lower grade tumors.
Conclusion.
Asians enrolled into phase III ovarian cancer clinical trials were younger,with better performance status, earlier-stage of disease, and have a greater number of clear cell andmucinous tumors. After adjusting for these prognostic factors, Asians have a better survival compared to Caucasians.
Keywords: Racial differences, Survival outcomes, Clear cell, Bevacizumab, Pharmacogenomics, Body mass index
1. Introduction
Epithelial ovarian carcinoma is the leading cause of gynecologic cancer mortality in the United States with an estimate of 14,080 deaths in 2017 [1]. Although significant survival gains have been reported for epithelial ovarian cancer in the general population, these improvements have not been observed in all ethnic groups. For example, the 5-year survival rates have increased in Caucasians from 37 to 45% but have decreased in African-Americans from 43 to 37% during the years 1975 to 2006 [2]. However, there are few studies that have evaluated survival outcomes of Asians.
In a large population-based study using the National Cancer Institute Surveillance, Epidemiology, and End Results, Asians were found to have a higher 5-year disease-specific survival compared to Caucasians (59.1% vs. 47.3%, p < 0.001) [3]. Recently, a Kaiser Permanente cancer registry evaluation found that there was a trend towards improved all-cause mortality for Asians compared to Caucasians (HR 0.93, 95% CI 0.76–1.13) adjusted for age at diagnosis, stage, grade and histology [4]. However, population analyses are limited by the lack of central pathology review and standardization for surgery, chemotherapy and follow-up methods. Furthermore, it appears that there are survival differences in the various racial groups after treatment when comparing populations treated with similar regimens in clinical trials based in Japan, Europe and the United States [5–7]. For example, response to weekly chemotherapy was variable on different racial groups in recent trials from the Japanese Gynecologic Oncology Group [5] and GOG [6].
The objective of the current study was to compare the patient/tumor parameters at presentation and outcomes between Asians and Caucasians with primary ovarian, fallopian tube and peritoneal cancers using data from NRG/GOG, members of the NCI National Cancer Trials Network, prospective phase III randomized clinical trials over the last 20 years.
2. Methods
2.1. Demographic information
We included patients with epithelial ovarian, fallopian tube or peritoneal carcinoma treated on front-line NRG/Gynecologic Oncology Group clinical trials from 1986 to 2009. De-identified data from the Statistical and Data Center were collected. The demographic and clinical data elements included age, race (Asian and Caucasian), body mass index, performance status, extent of residual disease following surgical cytoreduction, stage, cell type, tumor grade, date of enrollment, date of last follow up and vital status. All patients provided written informed consent prior to enrollment after the study had been approved by the appropriate research ethics board for each participating center.
2.2. Clinical trials evaluated
This ancillary analysis incorporated pooled historical data from all patients enrolled on ten prospective randomized front-line GOG trials as de-identified data. The treatment protocols included intravenous (IV) chemotherapy alone (GOG 111 [8], 157 [9], 158 [10], 175 [11], 182 [12]), IV chemotherapy plus intraperitoneal (IP) chemotherapy (GOG 95 [13], 104 [14] 114 [15], 172 [16]), and IV chemotherapy plus biologic therapy (GOG 218 [17]). GOG 95 [13] enrolled stage I and II patients comparing 32P vs. cyclophosphamide plus cisplatin. GOG 104 [14] enrolled stage III patients and compared either IP cisplatin or IV cisplatin either combined with IV cyclophosphamide. GOG 111 [8] enrolled stage III and IV patients and compared either cyclophosphamide or paclitaxel, combined with cisplatin. GOG 114 [15] enrolled randomized stage III patients and compared IP cisplatin vs. IV cisplatin combined with paclitaxel. GOG 157 [9] enrolled stage IA grade 3 to stage II patients and compared 3 vs. 6 cycles of carboplatin and paclitaxel. GOG 158 [10] enrolled randomized stage III patients and compared cisplatin vs. carboplatin combined with paclitaxel. GOG 172 [16] enrolled stage III patients and compared IP cisplatin vs. IV cisplatin combined with paclitaxel. GOG 175 [11] randomized stage I and II patients and compared paclitaxel maintenance vs. observation combined with carboplatin and paclitaxel. GOG 182 [12] enrolled randomized stage III and IV patients and compared gemcitabine, pegylated liposomal doxorubicin, and topotecan vs. vehicle combined with carboplatin and paclitaxel. GOG 218 [17] enrolled stage III and IV patients and compared paclitaxel and carboplatin with or without bevacizumab.
2.3. Statistical methods
Pearson chi-square test, Wilcoxon-Mann-Whitney test and Cox proportional hazards model were used. The co-primary endpoints of our analysis were disease-specific survival and all cause survival. Patients who were lost to follow-up were censored at time of last follow-up. Kaplan-Meier estimates were used in plotting the survival probability. All statistical tests were two-tailed with the significance level set at α = 0.05 and statistical analyses were performed using the R software (R Foundation for Statistical Computing, Vienna, Austria, 2013).
3. Results
3.1. Demographic and treatment information
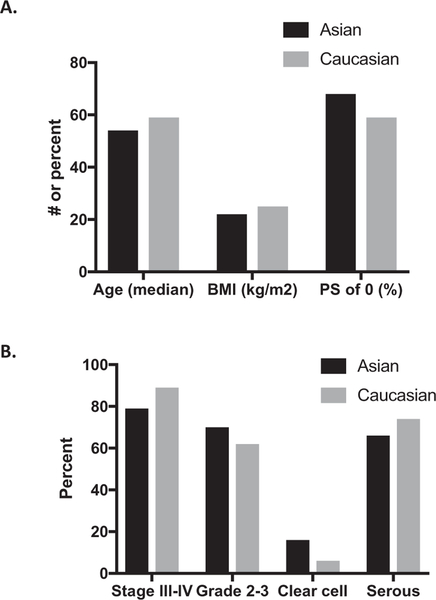
Of 7914 patients in the study population, 7641 (96.6%) were Caucasian and 273 (3.4%) were Asian. The demographic and clinical characteristics of the study population are shown in Table 1 and Fig. 1. Asians were younger compared to Caucasians (median age: 54 vs. 59 years; p < 0.001) and had a lower body-mass index (BMI) and better performance status. Both groups had similar rates of optimal cytoreduction (defined per individual GOG trial) at 53% vs. 51% (p = 0.63). Asians were more likely to present with stage I disease (17% vs. 8%; p < 0.001) and clear cell cancers (16% vs. 6%, p < 0.001).
Table 1.
Patient demographics and clinical characteristics (N = 7914).
| N | Asian | Caucasian | p-Value | |
|---|---|---|---|---|
|
N = 273 (%) |
N = 7641 (%) |
|||
| Age (mean years) (range) | 7914 | 54 (48–62) | 59 (51–67) | <0.001 |
| Age (years) | ||||
| <59 | 178 (65) | 3779 (50) | ||
| ≥59 | 95 (35) | 3862 (50) | ||
| BMI kg/m2 (range) | 6764 | 22 (20–25) | 25 (22–29) | <0.001 |
| Performance status | 7914 | =0.008 | ||
| 0 | 185 (68) | 4523 (59) | ||
| 1 | 72 (26) | 2708 (35) | ||
| 2/3 | 16 (6) | 410 (5) | ||
| FIGO stage | 7914 | <0.001 | ||
| I | 47 (17) | 617 (8) | ||
| II | 9 (3) | 266 (3) | ||
| III | 176 (65) | 5777 (76) | ||
| IV | 41 (15) | 981 (13) | ||
| Histology | 7914 | <0.001 | ||
| Serous | 179 (66) | 5630 (74) | ||
| Clear-cell | 43 (16) | 473 (6) | ||
| Endometrioid | 18 (7) | 613 (8) | ||
| Mucinous | 9 (3) | 147 (2) | ||
| Adenocarcarcinoma. NOS | 3 (1) | 119 (2) | ||
| Mixed epithelial | 15 (5) | 472 (6) | ||
| Other | 6 (2) | 187 (2) | ||
| Tumor grade (differentiation) | 7914 | <0.001 | ||
| 1 | 15 (5) | 579 (8) | ||
| 2 | 69 (25) | 2309 [30] | ||
| 3 | 146 (54) | 4355 (57) | ||
| Ungraded | 43 (16) | 398 (5) | ||
| Gross residual disease | 7914 | =0.63 | ||
| Yes | 129 (47) | 3725 (49) | ||
| No | 144 (53) | 3916 (51) | ||
| Treatment | 7914 | <0.001 | ||
| 32p | 0 (0) | 88(1) | ||
| CDDP + cyclophosphamide | 9 (3) | 377 (5) | ||
| CDDP (IP) + cyclophosphamide | 2 (1) | 61 (1) | ||
| CDDP + paclitaxel | 19 (7) | 910 (12) | ||
| CDDP (IP) + paclitaxel | 3 (1) | 185 (2) | ||
| Carboplatin + paclitaxel | 117 (43) | 2397 [31] | ||
| Carboplatin + paclitaxel + other | 50 (18) | 2676 (35) | ||
| Carboplatin + paclitaxel + bevacizumab | 73 (27) | 947 (12) | ||
| GOG Protocola | 7914 | <0.001 | ||
| GOG-0095 | 2 (0.7) | 182 (2.4) | ||
| GOG-0104 | 3 (1.1) | 120 (1.6) | ||
| GOG-O111 | 4 (1.5) | 343 (4.5) | ||
| GOG-0114 | 7 (2.6) | 467 (6.1) | ||
| GOG-0157 | 9 (3.3) | 270 (3.5) | ||
| GOG-0158 | 17 (6.2) | 681 (8.9) | ||
| GOG Protocol (Con’t) | ||||
| GOG-0172 | 12 (4.4) | 372 (4.9) | ||
| GOG-0175 | 45 (16.5) | 431 (5.6) | ||
| GOG-0182 | 63 (23.1) | 3337 (43.7) | ||
| GOG-0218 | 111 (40.7) | 1438 (18.8) | ||
BMI = Body Mass Index.
CDDP = Cisplatin.
IP = Intraperitoneal.
Study period GOG-0095: 1986–1994 GOG-0104: 6/1986–7/1992 GOG-111: - GOG-114: 8/1992–4/1995 GOG-157:3/1995–5/1998 GOG-158: - GOG-172:3/1998–1/ 2001 GOG-175:9/1998–12/2006 GOG-182:1/2001–9/2004 GOG-218: 10/2005–6/2009.
Fig. 1.
Clinical and demographic information on study population. A) Age, BMI, Performance status (PS), B) Stage, grade, clear cell, serous histology compared between Asians and Caucasians.
3.2. Survival based on race
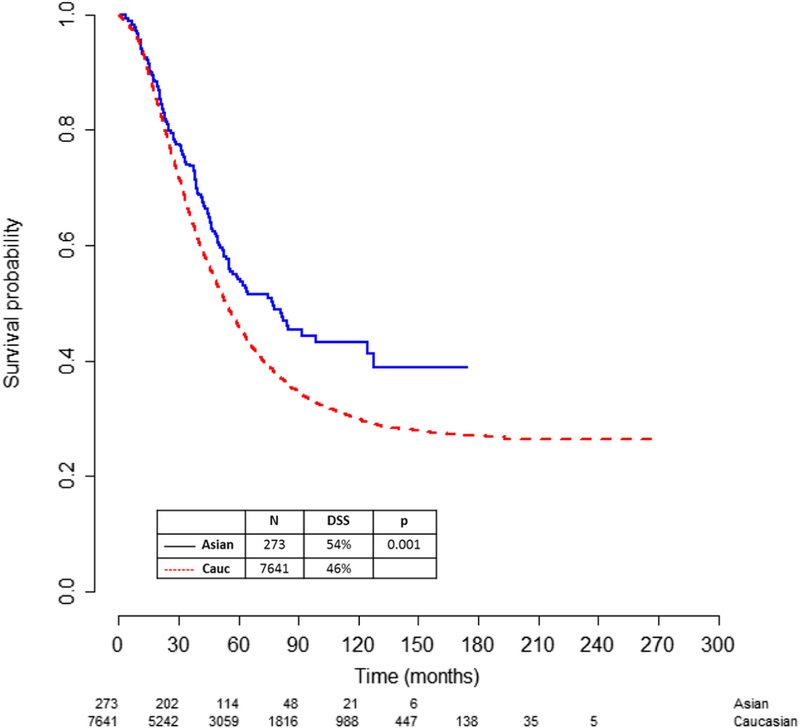
Asians had a higher 5-year disease-specific survival (DSS) of 54% compared to 46% for Caucasians (p = 0.001) (Fig. 2 and Table 2). The higher survival in Asians was demonstrated after stratifying for age, performance status, stage of disease, serous histology and presence of gross residual disease (Supplemental Figs. 1A–E). Asians also had an improved survival when stratified by BMI (Table 2) and an improved all-cause survival of 51% compared to 43% in Caucasians (p < 0.001) (Table 3). On multivariate analysis for disease-specific survival, younger age, Asian race, better performance status, lower BMI, early stage, lower grade, non-clear cell subtype and no residual disease were all independent predictors for better survival (Table 4).
Fig. 2.
5-year disease-specific survival (DSS) of Asians compared to Caucasians. Asians had an improved 5-year DSS of 54% compared to 46% for Caucasians (p = 0.001).
Table 2.
Kaplan-Meier 5-year disease-specific survival (N = 7914).
| Variable | Total (%) | Asian (%) | Caucasian (%) | Log rank |
|---|---|---|---|---|
| Overall | 46.4 | 54.1 | 46.1 | 0.001 |
| Age | ||||
| ≥59 | 42.3 | 47.3 | 42.2 | 0.050 |
| BMI (n = 6764) | ||||
| <25 | 44.5 | 49.4 | 44.2 | 0.024 |
| ≥25 | 43.6 | 65.6 | 43.2 | 0.004 |
| Performance status | ||||
| 0 | 51.4 | 55.9 | 51.3 | 0.047 |
| 1 | 40.5 | 51.8 | 40.2 | 0.02 |
| 2/3 | 27.8 | 40.7 | 27.4 | 0.77 |
| FIGO stage | ||||
| I | 89.0 | 89.1 | 89.0 | 0.69 |
| II | 80.7 | 63.5 | 81.2 | 0.48 |
| III | 43.4 | 47.9 | 43.2 | 0.18 |
| IV | 25.5 | 36.9 | 25.0 | 0.045 |
| Histology | ||||
| Serous | 41.6 | 50.5 | 41.3 | 0.006 |
| Clear-cell | 59.9 | 61.3 | 59.8 | 0.64 |
| Endometrioid | 67.3 | 64.9 | 67.3 | 0.77 |
| Mucinous | 55.8 | 77.8 | 54.4 | 0.34 |
| Adenocarcinoma | 66.7 | 100.0 | 65.8 | 0.25 |
| NOS mixed | 52.8 | 33.3 | 53.5 | 0.09 |
| Epithelial other | 49.3 | 66.7 | 48.8 | 0.21 |
| Tumor grade (differentiation) | ||||
| 1 | 61.7 | 73.3 | 61.4 | 0.38 |
| 2 | 43.1 | 41.3 | 43.2 | 0.98 |
| 3 | 44.5 | 53.8 | 44.2 | 0.007 |
| Ungraded | 64.1 | 68.3 | 63.6 | 0.42 |
| Gross residual disease | ||||
| Yes | 32.9 | 41.6 | 32.5 | 0.02 |
| No | 58.9 | 64.9 | 58.7 | 0.03 |
Table 3.
Kaplan-Meier 5-year all-cause survival (N = 7914).
| Variable | Total (%) | Asian (%) | Caucasian (%) | Log rank |
|---|---|---|---|---|
| Overall | 43.1 | 51.1 | 42.8 | <0.001 |
| Age | ||||
| <59 | 48.1 | 56.3 | 47.7 | 0.02 |
| ≥59 | 38.2 | 41.6% | 38.1 | 0.046 |
| BMI (n = 6764) | ||||
| <25.0 | 41.9 | 46.7 | 41.6 | 0.01 |
| ≥25.0 | 40.6 | 65.6 | 40.2 | 0.001 |
| Performance status | ||||
| 0 | 48.1 | 53.2 | 47.9 | 0.01 |
| 1 | 37.7 | 48.4 | 37.4 | 0.01 |
| 2/3 | 24.0 | 36.7 | 23.5 | 0.73 |
| I | 87.0 | 89.1 | 86.8 | 0.21 |
| II | 77.4 | 55.6 | 78.1 | 0.31 |
| III | 40.0 | 44.5 | 39.9 | 0.1 |
| IV | 23.6 | 34.4 | 23.1 | 0.04 |
| Histology | ||||
| Serous | 38.7 | 46.8 | 38.4 | 0.01 |
| Clear-cell | 56.8 | 61.3 | 56.4 | 0.34 |
| Endometrioid | 64.1 | 58.4 | 64.3 | 0.81 |
| Mucinous | 50.2 | 77.8 | 48.5 | 0.14 |
| Adenocarcinoma. | 47.0 | 100.0 | 45.8 | 0.13 |
| NOS mixed | 49.2 | 33.3 | 49.8 | 0.19 |
| Epithelial other | 47.8% | 66.7 | 47.2 | 0.16 |
| FIGO stage (range) | ||||
| I | 87.0 | 89.1 | 86.8 | 0.21 |
| II | 77.4 | 55.6 | 78.1 | 0.31 |
| III | 40.0 | 44.5 | 39.9 | 0.1 |
| IV | 23.6 | 34.4 | 23.1 | 0.04 |
| Tumor grade (differentiation) | ||||
| 1 | 59.0 | 73.3 | 58.7 | 0.25 |
| 2 | 40.8 | 39.7 | 40.8 | 0.95 |
| 3 | 41.6 | 51.3 | 41.2 | 0.003 |
| Ungraded | 50.3 | 60.9 | 49.2 | 0.03 |
| Yes | 29.8 | 37.4 | 29.6 | 0.014 |
| No | 55.8% | 62.3% | 55/5% | 0.009 |
Table 4.
Multivariate disease-specific survival analysis (N = 7914).
| HR | 95 CI | Log rank | ||
|---|---|---|---|---|
| Age ≥59 | 1.01 | 1.01 | −1.02 | 0.032 |
| Asian (Caucasian) | 0.78 | 0.64 | −0.96 | 0.017 |
| Body Mass Index (>25) | 1.01 | 1.002221g1.01 | −1.01 | <0.001 |
| Performance Status of2or3 (0) | 1.49 | 1.33 | −1.67 | <0.001 |
| Stage III (Stage 1) | 7.83 | 6.29 | −9.75 | <0.001 |
| Stage IV (Stage 1) | 11.46 | 9.09 | −14.4 | <0.001 |
| Clear-cell histology (serous) | 2.04 | 1.72 | −2.43 | <0.001 |
| Grade 3 (Grade 1) | 1.38 | 1.22 | −1.57 | <0.001 |
| No residual disease (residual) | 0.65 | 0.61 | −0.70 | <0.001 |
4. Discussion
In this current study, we found that Asians were younger, had lower BMIs, and better performance status when compared to Caucasians. Even after adjusting for these factors, Asians still had better survival outcomes. Prior studies have shown that patients from Asia, Europe and the United States have different survival outcomes despite similar treatments with weekly chemotherapies [5–7]. Nevertheless, there may be differences in the extent of surgery, relative dose intensity and post recurrence treatments across these clinical trials. In this current report, the extent of cytoreductive surgery was comparable between Asians and Caucasians. Asians have been found to have fewer polymorphisms in drug metabolizing enzymes that can lead to decreased activity for paclitaxel such as CYP2C8*3 (9.5–17% in Caucasians vs 0% in Asians), CYP3A5*3, *6, *7 (85–98% in Caucasians vs 60–77% in Asians) [18]. Additionally, Asians have been found to receive a higher relative dose intensity of chemotherapy which may contribute to the improved survival [4]. Further translational studies are needed to correlate the genomic data with chemotherapy tolerance of Asian and other racial groups to demonstrate whether these genetic variations may result in better survival for Asians.
Additionally, germlinemutations such as BRCA1 or BRCA2 have been found to be higher in certain Asian subgroups compared to Caucasians. For example, Wu et al. reported a 28.5% incidence of BRCA1 or BRCA2 germline mutations in Chinese ovarian cancer patients [19]. This is compared to an incidence of 15% of 1915 ovarian cancer patients identified from the University of Washington of gynecologic phase III clinical trials GOG 218 and 262, of which approximately 88% of the patients were non-Hispanic whites [21]. Higher response to cisplatin and overall survival has been reported in patients with BRCA mutations [22]. Similarly, improved outcomes have been reported for BRCA patients treated with PARP inhibitors [23]. These findings could, in part, help explain the better survival in Asians with ovarian cancer and can be studied in subsequent trials in which race/ethnicity and mutational status is controlled.
Similarly, improved overall survival in Asians compared to Caucasians has also been documented in lung cancer with the trend continuing to be seen when adjusting for risk factors such as surgical treatment (HR = 0.82; 95% CI; 0.79–0.86) [24] as well as in colorectal and breast cancer [25–27]. For example, the risk of death for patients with colorectal cancer was significantly lower for Asians than Caucasians (HR = 0.80; CI: 0.70–0.92) [25]. In the recent Annual Report to the Nation on the Status of Cancer, Jemal et al. noted that Asians had lower adjusted risk of cancer deaths for 12 out of 20 cancers [2]. The 5-year cause-specific survivals were 45.4% for the whites vs. 57.6% for Asian and Pacific Islanders. It is possible that social or economic differences, such as access to healthcare, may play a role in survival differences seen among ethnicities. These findings support our data showing that for cancer patients there may be underlying treatment and biologic differences that contribute to the better outcome with treatment.
Our current data analysis supports that, in a clinically well-annotated study, Asians continued to have an improved disease-specific survival compared with Caucasians. Bandera et al. [4] showed improved outcome for Asians vs Caucasians although, in their study, the differences were not statistically significant. The differences in surgery, varying chemotherapy regimens, lack of adjustment for extent of gross residual disease and potential differences in Asian subgroups in our GOG cohorts compared to those in the Bandera et al. may in part explain the differences in our findings [4]. Our prior study using the National Cancer Institute SEER data was limited by lack of standardized staging, extent of cytoreduction, type of treatment, surveillance and central pathology review. The use of prospectively collected data from cooperative group randomized trials on uniform protocols helps to minimize some of these deficits since enrollment for the GOG trials have standardized staging, performance status and comorbidity exclusion criteria. The strengths of the study are the following: offers the first large-scale study with central pathology review, standardized treatment by gynecologic oncologists and well-documented clinical follow-up. Unique to this study, we were able to address differences in Asians versus Caucasians on performance status, BMI, residual disease and treatment regimens particularly in all patients who received a platinum-based regimen.
Our study was limited by the small sample size of Asians for ovarian trials [30] and we did not undertake additional analyses based on Asian subgroups or on patients’ immigration status and duration of living in USA prior to diagnosis. We are also limited in knowing how many of the Asian women in this study were from Asia. The recommended dose and received dose by the patient were not evaluated. It is possible that Asians tolerated more cycles of chemotherapy, which may have biased their improved survival [4]. Given the small numbers of Asians receiving particular treatment regimens, such subsets for intraperitoneal chemotherapy versus bevacizumab-containing regimens, a subanalysis to determine whether Asians had an improved survival due to particular treatment regimens was not feasible. A prior study from our group showed that immigration affected survival [3]. Additionally, there were proportionally fewer Asians enrolling on clinical trials than based on the population, this may have been due to a cultural barrier and additional patient navigation resources could be useful.
In this first analysis evaluating clinico-demographic, clinical outcomes and survival differences in Asians and Caucasians from the clinically, well-annotated NRG/GOG database of 10 prospective, randomized clinical trials, we found that Asian patients were younger, had better GOG performance status and lower body-mass index at diagnosis. Asian race was found to be an independent predictor for disease-specific survival. Future international collaborative projects comparing demographic, socioeconomic, environment, genetic and pharmacogenomics of Asian vs. Caucasian patients treated with similar regimens may help us better understand racial differences in ovarian cancer survival.
Supplementary Material
HIGHLIGHTS.
Asians were younger at trial enrollment and had better performance status.
Asians had improved 5-year disease-specific survival than Caucasians.
Asian race remained significant prognostic factor in multivariate analysis.
Acknowledgements
This work supported by National Cancer Institute grants to Gynecologic Oncology Group Administrative Office (CA-27469), GOG Statistical Office (CA-37517), NRG Oncology (U10 CA 180822), NRG Operations (U10 CA180868) and UG1CA189867 (NCORP).
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: Ohio State University Comprehensive Cancer Center, University of Oklahoma Health Sciences Center, University of Minnesota Medical Center – Fairview, University of California Medical Center at Irvine – Orange Campus, University of Iowa Hospitals and Clinics, Duke University Medical Center, Washington University School of Medicine, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Abington Memorial Hospital, Fred Hutchinson Cancer Research Center, University of North Carolina at Chapel Hill, Southwest Oncology Group, Walter Reed National Military Medical Center, Tacoma General Hospital, Mayo Clinic, Moffitt Cancer Center and Research Institute, Cleveland Clinic Foundation, Australia New Zealand Gynaecological, University of Colorado Cancer Center – Anschutz Cancer Pavilion, Cancer Trials Support Unit, Wake Forest University Health Sciences, University of Kentucky, University of Mississippi Medical Center, University of Alabama at Birmingham, Abramson Cancer Center of the University of Pennsylvania, Rush University Medical Center, M D Anderson Cancer Center, Tufts-New England Medical Center, Women’s Cancer Center of Nevada, Stony Brook University Medical Center, Gynecologic Oncology Network/Brody School of Medicine, Penn State Milton S Hershey Medical Center, University of Massachusetts Memorial Health Care, Fox Chase Cancer Center, University of Cincinnati, Case Western Reserve University, Cooper Hospital University Medical Center, Roswell Park Cancer Institute, University of California at Los Angeles Health System, Thomas Jefferson University Hospital, Saitama Medical University International Medical Center, North Shore University Hospital, University of Chicago, Yale University, Community Clinical Oncology Program, University of Virginia, Albany Medical College, Metro-Minnesota CCOP, Johns Hopkins Oncology Center, Oregon Health Sciences University, Wayne State University/Karmanos Cancer Institute, University of Rochester Medical Center, University of Wisconsin Hospital and Clinics, Georgetown University Hospital, Eastern Pennsylvania GYN/ONC Center PC, Seoul National University Hospital, Medical University of South Carolina, University of Miami School of Medicine, Memorial Sloan Kettering Cancer Center, Ellis Fischel Cancer Center, Fletcher Allen Health Care, Cancer Research for the Ozarks NCORP, University of Pittsburgh Cancer Institute (UPCI), The Hospital of Central Connecticut, Stanford University Medical Center, Women and Infants Hospital, University of Hawaii, State University of New York at Syracuse, University of New Mexico, Michigan Cancer Research Consortium Community Clinical Oncology Program, Mount Sinai School of Medicine, Northern Indiana Cancer Research Consortium, Eastern Virginia Medical School, Northwestern University, University of Arizona, University of Texas – Galveston, Cancer Research Consortium of West Michigan NCORP, University of South California, ECOG Statistical Center, Long Island Jewish Medical Center, Saint Vincent Hospital, State University of New York Downstate Medical Center, North Central Cancer Treatment Group, Georgia Center for Oncology Research and Education (CORE), Scott and White Memorial Hospital, Wisconsin NCI Community Oncology Research Program, Missouri Valley Cancer Consortium CCOP, Carle Cancer Center, Gynecologic Oncology of West Michigan PLLC, Central Illinois CCOP, Delaware/ Christiana Care CCOP, Virginia Commonwealth University, Emory University Clinic, Kansas City CCOP, Kalamazoo CCOP, Southeast Gynecologic Oncology Associates, Evanston CCOP-NorthShore University Health System, Northern New Jersey CCOP, Virginia Mason CCOP, Western Regional CCOP, William Beaumont Hospital, Colorado Cancer Research Program NCORP, Saint Louis-Cape Girardeau CCOP, New York University Medical Center, Geisinger Medical Center, LDS Hospital, Aurora Women’s Pavilion of Aurora West Allis Medical Center, University of Illinois, Columbia River Oncology Program, Upstate Carolina CCOP, Dayton Clinical Oncology Program, Mainline Health CCOP, Heartland Cancer Research CCOP and Wichita CCOP. NCT00262847; NCT00006096; NCT00003644; NCT00003322.
Footnotes
Declaration of Competing Interest
The authors have no conflicts of interest with the exception of Dr. Java who reports an immediate family member serving as a consultant and receiving honoraria from Merck KGaA. Dr. Chan reports receiving honoraria from AstraZeneca, Clovis, Roche and Tesaro and serving as a consultant for these companies. Dr. Chan also participates in the Speakers’ bureau and has received travel, accommodations or reimbursement for such. Dr. Burger reports receiving personal fees from Genentech outside of the submitted work. Maurie Markman reports that he is employed as the President, Medicine and Science for Cancer Treatment Centers and he has received honoraria from Astra-Zeneca, Clovis, Genentech and Tesaro and participates in the Speakers bureau for these companies. Dr. Markman has served as a consultant for Celgene, Merck and Delmar Pharmaceuticals. Dr. Ozols serves as an advisor on DSMB’s and IDMC’s for Pfizer, Johnson & Johnson, Clovis, Astra Zeneca, Gradalis and Syndax.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2019.05.013.
This study was presented in part as a plenary presentation at SGO 2015 Annual Meeting, Chicago, IL.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2017, CA Cancer J. Clin 67 (2017) 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Jemal A, Ward EM, Johnson CJ, et al. , Annual report to the nation on the status of cancer, 1975–2014, featuring survival, J. Natl. Cancer Inst 109 (9) (2017) djx030, 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fuh KC, Shin JY, Kapp DS, et al. , Survival differences of Asian and Caucasian epithelial ovarian cancer patients in the United States, Gynecol. Oncol 136 (2015) 491–497. [DOI] [PubMed] [Google Scholar]
- [4].Bandera EV, Lee VS, Rodriguez-Rodriguez L, et al. , Racial/ethnic disparities in ovarian cancer treatment and survival, Clin. Cancer Res 22 (2016) 5909–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Katsumata N, Yasuda M, Isonishi S, et al. , Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial, Lancet Oncol 14 (2013) 1020–1026. [DOI] [PubMed] [Google Scholar]
- [6].Chan JK, Brady MF, Penson RT, et al. , Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer, N. Engl. J. Med 374 (2016) 738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pignata S, Scambia G, Katsaros D, et al. , Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial, Lancet Oncol 15 (2014) 396–405. [DOI] [PubMed] [Google Scholar]
- [8].McGuire WP, Hoskins WJ, Brady MF, et al. , Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer, N. Engl. J. Med 334 (1996) 1–6. [DOI] [PubMed] [Google Scholar]
- [9].Bell J, Brady MF, Young RC, et al. , Randomized phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: a Gynecologic Oncology Group Study, Gynecol. Oncol 102 (2006) 432–439. [DOI] [PubMed] [Google Scholar]
- [10].Ozols RF, Bundy BN, Greer BE, et al. , Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group Study, J. Clin. Oncol 21 (2003) 3194–3200. [DOI] [PubMed] [Google Scholar]
- [11].Mannel RS, Brady MF, Kohn EC, et al. , A randomized phase III trial of IV carboplatin and paclitaxel x 3 courses followed by observation versus weekly maintenance low-dose paclitaxel in patients with early-stage ovarian carcinoma: a Gynecologic Oncology Group Study, Gynecol. Oncol 122 (2011) 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bookman MA, Brady MF, McGuire WP, et al. , Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup, J. Clin. Oncol 27 (2009) 1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Young RC, Brady MF, Nieberg RK, et al. , Adjuvant treatment for early ovarian cancer: a randomized phase III trial of intraperitoneal 32P or intravenous cyclophosphamide and cisplatin–a gynecologic oncology group study, J. Clin. Oncol 21 (2003) 4350–4355. [DOI] [PubMed] [Google Scholar]
- [14].Alberts DS, Liu PY, Hannigan EV, et al. , Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer, N. Engl. J. Med 335 (1996) 1950–1955. [DOI] [PubMed] [Google Scholar]
- [15].Markman M, Bundy BN, Alberts DS, et al. , Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group, J. Clin. Oncol 19 (2001) 1001–1007. [DOI] [PubMed] [Google Scholar]
- [16].Armstrong DK, Bundy B, Wenzel L, et al. , Intraperitoneal cisplatin and paclitaxel in ovarian cancer, N. Engl. J. Med 354 (2006) 34–43. [DOI] [PubMed] [Google Scholar]
- [17].Burger RA, Brady MF, Bookman MA, et al. , Incorporation of bevacizumab in the primary treatment of ovarian cancer, N. Engl. J. Med 365 (2011) 2473–2483. [DOI] [PubMed] [Google Scholar]
- [18].Phan VH, Moore MM, McLachlan AJ, et al. , Ethnic differences in drug metabolism and toxicity from chemotherapy, Expert Opin. Drug Metab. Toxicol 5 (2009) 243–257. [DOI] [PubMed] [Google Scholar]
- [19].Wu X, Wu L, Kong B, et al. , The first nationwide multicenter prevalence study of germline BRCA1 and BRCA2 Mutations in Chinese ovarian cancer patients, Int. J. Gynecol. Cancer (8) (2017) 1650–1657 October. [DOI] [PubMed] [Google Scholar]
- [21].Norquist BM, Harrell MI, Brady MF, et al. , Inherited mutations in women with ovarian carcinoma, JAMA Oncol 2 (2016) 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sun C, Li N, Ding D, et al. , The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis, PLoS One 9 (2014), e95285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mirza MR, Monk BJ, Herrstedt J, et al. , Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer, N. Engl. J. Med 375 (2016) 2154–2164. [DOI] [PubMed] [Google Scholar]
- [24].Soneji S, Tanner NT, Silvestri GA, et al. , Racial and ethnic disparities in early-stage lung cancer survival, Chest 152 (2017) 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].White A, Vernon SW, Franzini L, et al. , Racial disparities in colorectal cancer survival: to what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer 116 (2010) 4622–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Warner ET, Tamimi RM, Hughes ME, et al. , Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors, J. Clin. Oncol 33 (2015) 2254–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hashiguchi Y, Hase K, Ueno H, et al. , Impact of race/ethnicity on prognosis in patients who underwent surgery for colon cancer: analysis for White, African, and East Asian Americans, Ann. Surg. Oncol 19 (2012) 1517–1528. [DOI] [PubMed] [Google Scholar]
- [30].Scalici J, Finan MA, Black J, et al. , Minority participation in Gynecologic Oncology Group (GOG) Studies, Gynecol. Oncol 138 (2015) 441–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Markman M, Liu PY, Moon J, et al. , Impact on survival of 12 versus 3 monthly cycles of paclitaxel (175 mg/m2) administered to patients with advanced ovarian cancer who attained a complete response to primary platinum-paclitaxel: follow-up of a Southwest Oncology Group and Gynecologic Oncology Group phase 3 trial, Gynecol. Oncol 114 (2009) 195–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.