Abstract
Polycomb Repressive Complex 2 (PRC2) catalyzes mono-, di-, and trimethylation of lysine 27 on histone H3 (H3K27me1–3) to control expression of genes important for differentiation and maintenance of cell identity. PRC2 activity is regulated by a number of different inputs, including allosteric activation by its product, H3K27me3. This positive feedback loop is thought to be important for the establishment of large domains of condensed heterochromatin. In addition to other chromatin modifications, ancillary subunits of PRC2, foremost JARID2, affect the rate of H3K27 methylation. Many gaps remain in our understanding of how PRC2 integrates these various signals to determine where and when to deposit H3K27 methyl marks. In this study, we utilize designer chromatin substrates to demonstrate that propagation of H3K27 methylation by the PRC2 core complex has geometrically defined preferences that are overridden by the presence of JARID2. Our studies also show that phosphorylation of JARID2 can partially regulate its ability to stimulate PRC2 activity. Collectively, these biochemical insights further our understanding of the mechanisms that govern PRC2 activity, and highlight a role for JARID2 in de novo deposition of H3K27me3-containing repressive domains.
Graphical Abstract

INTRODUCTION
Gene regulation in eukaryotes occurs at the level of chromatin, the nucleic acid-protein complex that is the physiologically relevant form of the genome. The fundamental repeating unit of chromatin is the nucleosome core particle in which DNA is wrapped around a disc-shaped molecular scaffold composed of an octamer of basic histone proteins.1, 2 Histones are subject to myriad covalent posttranslational modifications (PTMs) that are installed, removed, and recognized by specific chromatin-associated proteins.3 These histone PTMs (or “marks”) serve as one level of epigenetic control, either by directly modulating chromatin structure or by recruiting epigenetic effectors that mediate downstream biochemical and/or structural processes that operate on the chromatin polymer.3, 4
The functional interplay between histone marks, in terms of their combinatorial co-occurrence and distribution on chromatin, remains one of the most important areas of study in the field of epigenetics.5, 6 The complexity of this problem is exemplified by trimethylation of lysine 27 on histone H3 (H3K27me3). This PTM is associated with transcriptionally silenced regions of the genome.7 The mark is installed and interpreted by the polycomb-group of proteins, which are critical for cell differentiation and cellular memory.8 Thus, H3K27me3 (along with H3K27me and H3K27me2) is deposited by the PRC2 methyltransferase (Figure 1a), while the PRC1 silencing complex binds to the mark and is ultimately thought to establish domains of transcriptionally repressed heterochromatin that are maintained over successive cell divisions.8 In normal healthy cells, PRC2 activity is controlled by multiple, inter-connected mechanisms.9, 10 Indeed, the intricacy of this spatial-temporal regulation is highlighted by the many ways PRC2 activity can become dys-regulated in disease; aberrant enzyme activity is associated with mutations in the histone substrate11–14 and in several of its component subunits,15 as well as mutations that alter the levels of other histone PTMs.16 While it clear that PRC2 is sensitive to multiple features on the chromatin polymer, exactly how the enzyme integrates these inputs to ensure proper deposition of the H3K27me1–3 remains poorly understood (Figure 1b,c).
Figure 1.
Current understanding and open questions regarding PRC2. (a) Domain architecture and major features of PRC2 core complex subunits and ancillary subunit JARID2 (left). SRM = stimulation response motif (gold). ncRBD = noncoding RNA binding domain (yellow). CXC domain (blue). SET = catalytic domain (pink). Zn = zinc finger (blue). VEFS domain (green). WD 40 repeats (purple). HTH = helix-turn-helix motif (green). JmjN = Jumonji N domain (blue). ARID = AT-rich interaction domain (yellow). JmjC = Jumonji C domain (blue). Cartoon representations of different PRC2 compositions used in this study (right). (b) PRC2 is known to engage chromatin though multivalent interactions with DNA and histone PTMs. The EED subunit of PRC2 tightly binds H3K27me3 to further stimulate EZH2 methylation of H3K27. The presence of euchromatic marks H3K4me3 and H3K36me3 are known to inhibit PRC2 methyltransferase activity. (c) Open questions. What is the geometry of PRC2 propagation of H3K27me3? How do ancillary subunits such as JARID2 affect PRC2 sensing of chromatin states? Can modifications on these cofactors themselves modulate PRC2 activity?
PRC2 is composed of four core subunits: enhancer of zeste homologue 2 (EZH2), embryonic ectoderm development (EED), suppressor of zeste 12 (SUZ12), and retinoblastoma-binding protein p48 (RBBP4) (Figure 1a). The catalytic subunit, EZH2, contains a su(var)3–9, enhancer of zeste, and trithorax (SET) domain that utilizes the cofactor S-adenosyl methionine to deposit H3K27me1–3.7 SUZ12 plays an important structural role within the complex,17 but has also been implicated in chromatin recognition.18 EED binds the H3K27me3 catalytic product, allosterically activating EZH2 in a positive feedback loop,19 while the RBBP4 subunit preferentially binds the unmodified H3 tail, and is thought to play a role in sensing marks associated with actively transcribed genes such as H3K4me3.20 Various auxiliary subunits have also been shown to associate with the PRC2 core complex and modulate its substrate recognition or activity.21 The best studied of these is Jumonji/ARID domain-containing protein 2 (JARID2).22 This protein is expressed predominantly in embryonic stem cells, where it has been shown to colocalize with the PRC2 core complex and to play an important role in cell differentiation.23 In addition to DNA binding domains, JARID2 contains a Jumonji histone demethylase domain that is catalytically inactive due to mutations in key iron-binding residues.24, 25 The JARID2 protein is also a substrate of PRC2, and when di- or trimethylated on Lys-116 (JARID2-K116me2/3) stimulates the enzyme by binding EED in a manner analogous to H3K27me3.26 Intriguingly, there are three serine phosphorylation sites close to K116 in JARID2.27–30 The function of these PTMs remains unclear, though their proximity to K116me3 suggests that they may influence the stimulatory effect on PRC2.
Histone marks, especially on the H3 N-terminal tail, serve as a platform for signal integration by PRC2, whereby PTM inputs are recognized and processed by the enzyme to result in a single directed output.31 Examples include the inhibition of PRC2 enzyme activity by transcriptionally activating marks such as H3K4me3 and H3K36me3, as well as the stimulation of PRC2 activity by H2A ubiquitylation installed by PRC1.20, 32, 33 The most extensively studied example of PRC2 signal integration is the propagation of the H3K27me3 mark itself, a phenomenon referred to as “spreading”. This behavior is thought to be the result of the aforementioned positive feedback loop in which EZH2 catalysis is stimulated by the presence of H3K27me3.19, 34, 35 The structural basis of this auto-stimulation is well understood; binding of H3K27me3 to an aromatic cage within the WD40 beta propeller domain in the EED subunit leads to movement of a mobile alpha-helical stimulation response motif (SRM) within EZH2 that is coupled to a reorganization of the SET domain.36 This allosteric autostimulation offers an attractive mechanistic explanation for how large, multi-kilobase stretches of H3K27me3, which are necessary for proper gene silencing, are established.
Previous studies have provided structural, biochemical, and cell-based evidence for PRC2-mediated spreading of the H3K27 methyl mark. A recent cryo-EM structure of PRC2 bound to a dinucleosome template illustrates the ability of the enzyme complex to simultaneously engage H3 tails on two distinct nucleosomes, with one histone tail serving as an activator, and the other as a substrate.37 Biochemical investigations using reconstituted nucleosome arrays, containing stochastic mixtures of unmodified nucleosomes and either H3K27A or H3K27me3 nucleosomes, show that PRC2 most effectively methylates arrays containing pre-installed H3K27me3, and that this substrate preference is abolished upon disrupting the ability of H3K27me3 to bind the aromatic cage in EED.38 Knock-down of the EED subunit in mammalian cell lines, as well as more targeted mutations in the EED aromatic cage, lead to a decrease in global levels of H3K27me3 and to attenuated occupancy of PRC2 subunits at polycomb target genes.35, 38, 39 Similarly, mutations that impair the allosteric movements of the SRM within EZH2 leads to reduced levels of H3K27me2/3 in mouse embryonic stem cells.38
Though much as been learned about the allosteric mechanism underlying stimulation of PRC2 by H3K27me3, and there is general agreement that propagation of the mark is critical for polycomb-mediated gene silencing,8, 10, 40 questions still remain regarding the extent to which the enzyme can install the PTM from a defined nucleation site, i.e. whether there are the geometric constraints on spreading (Figure 1c). Thus, it is unclear whether spreading is restricted to adjacent nucleosomes, as might be predicted by the recent cryo-EM structural data,37 or if long-range stimulation traversing multiple nucleosomes can also occur. It is also unclear to what degree the spreading behavior of PRC2 is influenced by the presence of JARID2 and whether the stimulatory activity of this auxiliary subunit itself is subject to any form of regulation. In this study, we employ protein-engineering methods in conjunction with various designer chromatin templates to address these questions. Our studies reveal that PRC2-mediated spreading preferentially occurs proximal to the site of nucleation, but that the stimulated enzyme is able to methylate more distal regions, albeit to a lesser extent. Further, we find that the JARID2 subunit, like the H3K27me3 product, activates PRC2 primarily through an increase in kcat,app for the reaction. Our data are consistent with the proposed role for JARID2 in the establishment of H3K27me3 nucleation sites from which the mark is then propagated via the positive feedback mechanism ultimately leading to the generation of large polycomb silenced domains.38, 41
RESULTS
Stimulation of PRC2 activity occurs in a predominantly intra-array fashion
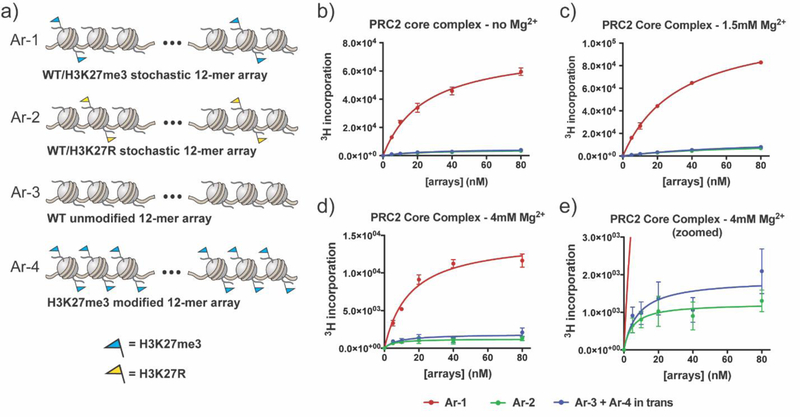
A biochemical approach, employing reconstituted chromatin, was used to study the geometric constraints on the PRC2 spreading phenomenon. To this end, we generated a series of reconstituted 12mer nucleosome arrays containing unmodified and/or modified (i.e., PTM-bearing) histones (Figure 2a, Supplemental Figures 1, 2). The first designer chromatin array (Ar-1) contains a stochastic mix of 67% unmodified nucleosomes (substrates of PRC2) alongside 33% H3K27me3-modified nucleosomes (stimulators of PRC2) to interrogate intra-array propagation. The second array (Ar-2) contains a stochastic mix of unmodified nucleosomes and H3K27R nucleosomes that cannot be modified, to control for substrate concentration, and to evaluate initiation of K27 methylation. The third and fourth arrays (Ar-3 and Ar-4) contain either unmodified or H3K27me3-modified nucleosomes respectively. Mixing Ar-3 and Ar-4 allows for evaluation of inter-array stimulation. PRC2 activity on these chromatin substrates was assessed using a scintillation-based histone methyltransferase (HMT) radio-assay employing 3H-labeled S-adenosyl-L-methionine ([3H]SAM) cofactor. Recombinant PRC2 complexes were generated using the Multibac platform42 and isolated from an insect cell expression system (Supplemental Figure 3).
Figure 2.
HMT activity of PRC2 core on stochastic 12mer arrays. (a) Cartoon representation of homogeneous and heterogeneous “scrambled” 12mer nucleosome array substrates containing unmodified, mutant, and methylated nucleosomes. (b) PRC2 core complex HMT activity as measured by incorporation of 3H (from [3H]SAM cofactor) on array substrates at 0 mM Mg2+. Error bars represent standard error measurements, n = 3. (c) PRC2 core complex activity measured on array substrates at 1.5 mM Mg2+. Errors = s.e.m. (n = 3). (d) PRC2 core complex activity measured on array substrates at 4 mM Mg2+. Errors = s.e.m. (n = 3). (e) Zoomed in view of the same data as panel (d) highlighting the activity on Ar-2 versus Ar-3 in combination with Ar-4. Note (b−e): Differences in the absolute HMT activity reflect sensitivity of PRC2 to Mg2+ concentration as well as the effect of Mg2+ on substrate solubility.
PRC2 core complex was found to be substantially more active on Ar-1 compared to Ar-2 (Figure 2b), a finding that is consistent with previous studies showing that pre-installed H3K27me3 on a chromatin substrate can stimulate PRC2 HMT activity.38 These initial assays were performed in the absence of any added divalent metal ions such as Mg2+, conditions under which 12mer nucleosome arrays are largely unstructured and dynamic.43 To explore the impact of higher-order chromatin folding on PRC2 activity, we repeated the HMT assays in the presence of 1.5 mM and 4 mM Mg2+ (Figure 2c, d, Supplemental Figure 4).44 In both cases, we again observed higher PRC2 activity on Ar-1 versus Ar-2. Importantly, this elevated HMT activity was not observed using a 2:1 mixture of Ar-3 and Ar-4 compared to Ar-2, irrespective of the Mg2+ concentration present in the assay (Figure 2b-e, Supplemental Figure 4). From this we conclude that, under the conditions of our assays, the enhanced activity of PRC2 on Ar-1 operates primarily in an intra-array fashion, i.e., the stimulation operates in cis.
PRC2 stimulation by H3K27me3 occurs preferentially on neighboring nucleosomes
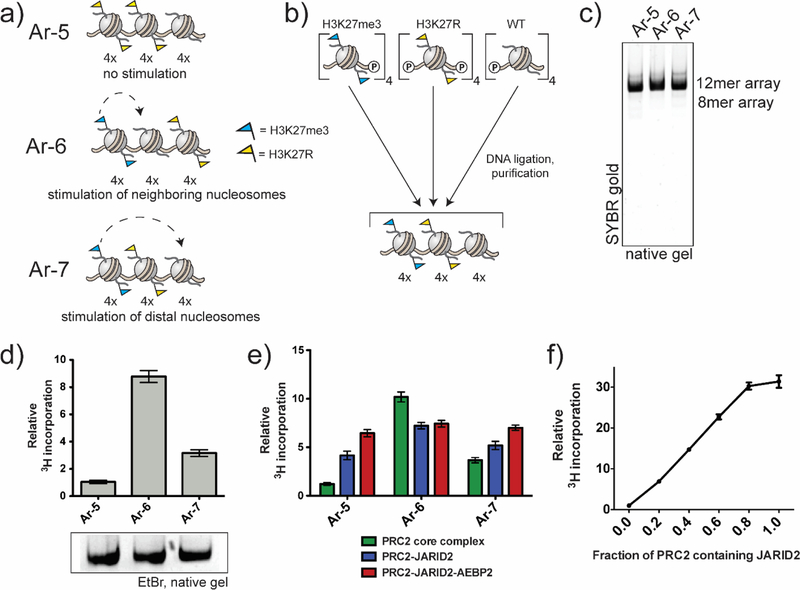
We next asked whether PRC2-mediated spreading of the H3K37 methyl mark within a nucleosome array has any geometrically defined preferences. To explore this, we designed a series of regio-defined nucleosome arrays (Figure 3a). These chromatin substrates have well-defined boundaries between tetranucleosome blocks bearing different histone modifications/mutants. Recent publications have provided evidence for a tetranucleosome motif in chromatin fiber folding in vivo.45–47 Within our arrays, H3K27me3-containing blocks serve as local or global activators of PRC2, unmodified nucleosomes serve as substrates for the enzyme, and H3K27R-containing nucleosomes, which cannot be modified, act as potential barriers to spreading. In Ar-7, the substrate block is distal to the stimulatory block, whereas in Ar-6 they are in a proximal arrangement. Consequently, comparison of PRC2 activity on Ar-6 and Ar-7 will probe short-range (0.1–0.7 kbp) versus medium-range (≥1 kbp) spreading of the mark. We also designed control array, Ar-5, containing two H3K27R tetranucleosome blocks adjacent to a block of unmodified nucleosomes. We anticipated this array would allow us to evaluate the extent of medium-range spreading versus initiation of K27me domains.
Figure 3.
HMT activity of PRC2 complexes on designer chromatin substrates. (a) Cartoon representations of heterotypic designer chromatin arrays containing homogeneously modified tetranucleosome units. (b) Assembly of heterotypic designer 12mer arrays by one-pot DNA ligation of three purified tetranucleosome building blocks and subsequent sucrose gradient purification of the assembled 12mers. (c) Native (APAGE) gel electrophoresis of final purified products. (d) HMT activity of PRC2 core complex on various designer chromatin substrates. Data from radioHMT assays are plotted relative PRC2 core on Ar-5. Errors = s.e.m. (n = 3). Array substrate loading is shown below. (e) Comparison of HMT activity of different PRC2 compositions on indicated designer chromatin substrates. PRC2 core complex data (green) is the same as in panel d. Data from radio-HMT assays are plotted relative to PRC2 core complex on Ar-5. Errors = s.e.m. (n = 3−4). (f) Titration of full-length JARID2 into a HMT assay of PRC2 core complex on Ar-3 (WT array substrates). Data from radio-HMT assays are plotted relative to PRC2 core in the absence of JARID2. Errors = s.e.m. (n = 3−4).
Designer 12mer arrays were assembled from homogenous tetranucleosome building blocks using a regio-specific, tandem DNA ligation process (Figure 3b, Supplemental Figure 5). The 4mer building blocks were prepared by depositing refolded histone protein octamers (containing the desired H3 variant) onto a ds-DNA template containing four repeats of the Widom 601 nucleosome positioning sequence48, with each repeat separated by a 30-bp DNA linker and containing a non-palindromic overhang49. A one-pot DNA ligation reaction was then used to generate the designer 12mer arrays, each of which was purified by Mg2+ precipitation followed by preparative sucrose gradient centrifugation (Figure 3c).
HMT assays on the designer chromatin substrates revealed a clear geometric preference for PRC2-mediated spreading of the H3K27 methyl mark (Figure 3d). Thus, PRC2 core complex activity was substantially elevated on nucleosomes proximal to the stimulatory H3K27me3-containing nucleosomes over those at a distal location, as demonstrated by the higher HMT activity on Ar-6 compared to Ar-7. Nonetheless, some longer-range stimulation does appear to occur, given the higher PRC2 activity on Ar-7 substrates compared to Ar-5. To further explore this, we generated an additional substrate, Ar-8, containing only a single H3K27R-containing nucleosome spacer between the stimulatory and substrate blocks (Supplemental Figure 6). In this case, we observed intermediate behavior between Ar-6 and Ar-7 (Supplemental Figure 6), indicating that the level of this distal-stimulation is distance dependent.
We next asked whether this geometric constraint on spreading was influenced by the presence of ancillary PRC2 subunits. Thus, we generated a version of the enzyme containing JARID2, as well as an additional subunit, AEBP2, which has been suggested to play a role in PRC2 targeting through its DNA binding activity (Supplemental Figure 3).34, 50 In this case, we observed high levels of HMT activity on all three designer array substrates (Figure 3e), such that the stimulating effect of having preinstalled blocks of H3K27me3 is largely lost. We imagined that this override effect was primarily the result of JARID2, since when methylated on Lys-116, this subunit can stimulate PRC2 in a similar manner to H3K27me3.26 Consistent with this idea, a similar override effect was observed using a PRC2-JARID2 complex lacking AEBP2 (Figure 3e). No additional stimulation of the PRC2-JARID2 complex was observed in trans, i.e., using a mixture of arrays 3 and 4 (Supplemental Figure 4). Titration of JARID2 subunit into the PRC2 complex led to a gradual increase in HMT activity on Ar-3 that plateaued out at a stoichiometry of around 1 (Figure 3f, Supplemental Figure 7). Note, mass spectrometric analysis of JARID2 in the complex revealed high levels of K116me2/3 (Supplemental Figure 8, Supplemental Table 1).
Kinetic analysis of H3K27 methylation by PRC2-JARID2
Since PRC2 catalyzes mono-, di-, and trimethylation of H3K27, we wondered whether the presence of ancillary subunits affects substrate preference of the enzyme. With this in mind, 12mer nucleosome arrays containing semisynthetic H3K27me (Ar-9) and H3K27me2 (Ar-10) were generated (Supplemental Figures 1, 2). These substrates (along with unmodified Ar-3) allow us compare deposition of each successive methyl mark under HMT assay conditions in which only single methylation state turnover (unmodified to mono-methylation, mono- to di-methylation, di- to trimethylation) is observed (Supplemental Figure 9).
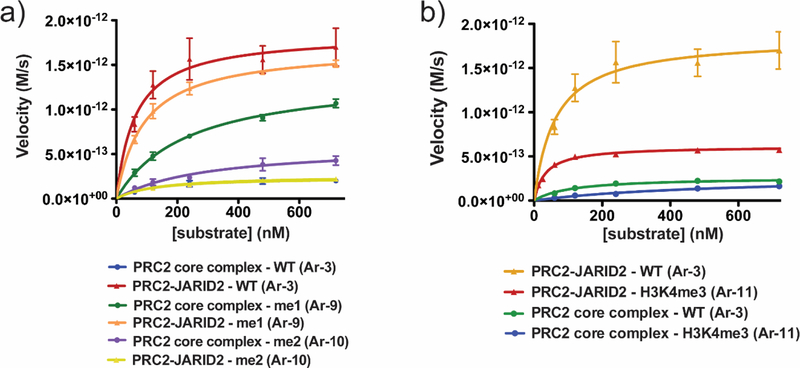
Use of the 12mer array substrates afforded us the opportunity to perform Michaelis-Menten kinetic analysis of successive H3K27 methylation by different PRC2 complexes as a function of substrate concentration (Figure 4a, Supplemental Figure 9, Table 1). For unmodified substrates Ar-3, the catalytic efficiency, as defined by ratio of the apparent kcat (kcat, app) and the Km of the substrate (kcat, app/Km, substrate), was found to be an order of magnitude greater for PRC2-JARID2 than that for the core complex, an effect that is largely driven by an increase in the apparent kcat for the former. Thus, PRC2-JARID2 is a more effective monomethyltransferase than the core enzyme and highly active on unmodified substrates. Notably, the monomethylation activity of PRC2-JARID2 was fully inhibited by the allosteric EED inhibitor, EED22651 as well as by the orthosteric EZH2 inhibitor, EPZ6438.52 Though the orthosteric inhibitor was equipotent for the PRC2 core complex and PRC2-JARID2, the allosteric inhibitor which has been shown to bind the same pocket of the EED subunit as methylated JARID2, showed a 10-fold increase in IC50 for PRC2-JARID2 relative to the PRC2 core complex (Supplemental Figure 10). Interestingly, we found that the PRC2 core complex was more active on H3K27me-containing substrates (Ar-9) compared to unmodified substrates, whereas the activity of PRC2-JARID2 was more or less unaltered in this context. While we were unable to achieve substrate saturation for the H3K27me2 substrates, and thus reliably determine individual kcat, app and Km, substrate values, by using the slope of a plot of initial rate vs. initial substrate concentration, kcat, app/Km, substrate values were determined.53 The two enzyme complexes had lower, and indeed comparable, activities on H3K27me2-containing substrates (Ar-10), which reflects the fact that PRC2-JARID2 has steeply reduced (~100 fold) catalytic activity on these substrates compared to Ar-3 or Ar-9.
Figure 4.
Kinetic analysis of PRC2 core complex and PRC2-JARID2. (a) Michaelis−Menten kinetic analysis of PRC2 core complex and PRC2- JARID2 on unmodified (Ar-3), H3K27me1 (Ar-9), and H3K27me2 (Ar-10) 12mer array substrates. Errors = s.e.m. (n = 3−4). (b) Michaelis− Menten kinetic analysis of PRC2 core complex and PRC2-JARID2 on unmodified (Ar-3) or H3K4me3 (Ar-11) 12mer array substrates. Errors = s.e.m. (n = 3). kcat,app and Km, substrate values are reported in Table 1. Additional kinetic data can be found in Supplemental Figure 9.
Table 1.
Kinetic Constants of PRC2 Core Complex and PRC2-JARID2 on Various 12mer Nucleosome Array Substratesa,b
| PRC2 Core Complexa | PRC2-JARID2b | |||||||
|---|---|---|---|---|---|---|---|---|
| Substrate | Ar-3 (WT) |
Ar-9 (H3K27mel) |
Ar-10 (H3K27me2) |
Ar-11 (H3K4me3) |
Ar-3 (WT) |
Ar-9 (H3K27mel) |
Ar-10 (H3K27me2) |
Ar-11 (H3K4me3) |
| kcat, app(s−1) | (1.6 ±0.2) ×l0−5 | (9.3 ±0.5) ×l0−5 | — | — | (1.2 ± 0.1) ×l0−4 | (1.13 ±0.04) ×l0−4 | — | (4.0 ±0.1) ×l0−5 |
| km,substrate(M) | (1.2 ±0.6) ×l0−7 | (2.3 ± 0.3) ×l0−7 | — | — | (6.2 ± 2.3) ×l0−8 | (9.1 ± 1.2) ×l0−8 | — | (3.0 ±0.6) ×l0−8 |
| kcat, appKm,substrate(s−1M−1) | (1.4 ±0.7) ×l02 | (4.0 ±0.6) ×l02 | (3.6 ± 1.1) ×l01 | (1.6 ± 0.1) ×l01 | (2.0± 0.8) ×l03 | (1.3 ±0.2) ×l03 | (1.5 ±0.6) ×l01 | (1.3 ±0.2) ×l03 |
Measurements were collected using 15 nM PRC2 core complex in a 10 μL reaction with 0.66 μM 3H-SAM, and varying array substrate concentrations at 30 °C for 40 min.
Measurements were collected using 15 nM PRC2-JARID2 under analogous reaction conditions as described above.
We also examined the effect of H3K4me3 on JARID2 stimulation – this mark is associated with active transcription and has previously been shown to inhibit PRC2 activity.23 We generated 12mer arrays containing semisynthetic H3K4me3 (Ar-11, Supplemental Figures 1, 2), and compared them with unmodified arrays, Ar-3, in HMT assays employing PRC2 core and PRC2-JARID. As expected, the presence of the H3K4me3 mark led to a reduction in HMT activity for the PRC2 core enzyme as reflected by a ~8.5-fold reduction in the kcat, app/Km, substrate (Figure 4b, Table 1). The H3K4me3 mark also led to a reduction in HMT activity for PRC2-JARID2, although in this case the effect was much more modest (~1.5-fold reduction in kcat, app/Km, substrate), suggesting this version of the enzyme is less sensitive to the presence of the PTM, at least for the installation of the H3K27 monomethyl mark.
Phosphorylation of JARID2 Ser120 abrogates the stimulatory effect of K116me3
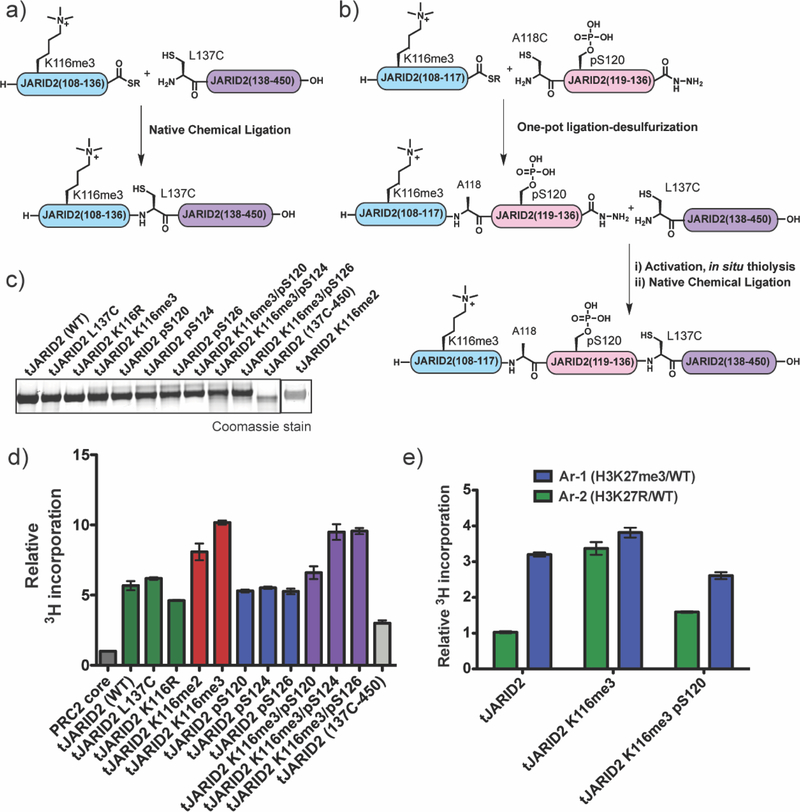
Recent reports have shown that JARID2 bearing the K116me3 mark can also be phosphorylated on nearby serine residues S120, S124, and S126.27–30 While the function of these phospho-serine (pSer) marks remains unknown, the proximity of the modifications to the EED-stimulatory region within JARID2 suggests they could have an impact on PRC2 activation. To explore this, we generated a series of semisynthetic JARID2 constructs containing various combinations of these phospho- and methyl-marks, as well as relevant controls (Figure 5 a–c Supplemental Figure 11). Two different semisynthetic routes were employed in the preparation of these proteins, both based on generation of a truncated version of JARID2 containing residues 108–450, hereafter referred to as tJARID2, that retains the ability to bind the PRC2 core complex and stimulate HMT activity when trimethylated at lysine 116.26 For the tJARID2 constructs containing single PTM modifications, we adopted a previously described two-piece native chemical ligation strategy to connect a 28 amino acid synthetic peptide (residues 108–136, containing the relevant PTM) to a recombinant protein fragment corresponding to residues 137–450.26 The two-piece ligation strategy proved inefficient for the generation of the dually modified proteins containing K116me3 and pSer120. Thus, we developed a three-piece route for these targets involving the sequential ligation of two shorter synthetic peptides to the recombinant fragment (Figure 5a,b, Supplemental Figure 11).
Figure 5.
Semisynthesis and activity of modified tJARID2 proteins. (a) Schematic of the two-piece ligation strategy used to prepare tJARID2 K116me2, tJARID2 K116me3, tJARID2 pS120, tJARID2 pS124, and tJARID2 pS126. For experimental details, see Supporting Information. (b) Schematic of the three-piece ligation strategy used to prepare tJARID2 K116me3/pS120, tJARID2 K116me3/pS124, and tJARID2 K116me3/ pS126. For experimental details, see Supporting Information. (c) Coomassie stain of SDS-PAGE analysis of all semisynthetic and recombinant tJARID2 constructs used in this study. (d) Relative activity of either PRC2 core complex or PRC2 core complex with an equimolar amount of the indicated tJARID2 construct on an unmodified 12mer nucleosome array substrate (Ar-3). Data from radio-HMT assays are plotted relative PRC2 core on Ar-3. Errors = s.e.m. (n = 3−4). (e) Relative activity of PRC2 with tJARID2 K116me3 or PRC2 with tJARID2 K116me3/pS120 on Ar-1 (containing H3K27me3 stimulating nucleosomes) or Ar-2 (containing H3K27R nonsubstrate nucleosomes). Data from radio-HMT assays are plotted relative PRC2- tJARID2 on Ar-2.
Consistent with previous studies,26 semisynthetic tJARID2 containing the K116me3 mark led to a substantial increase (~10-fold) in HMT activity on unmodified 12mer arrays (Ar-3) when added to the PRC2 core (Figure 5d). Semisynthetic tJARID2 containing the dimethylated lysine (K116me2) also stimulated PRC2 activity, albeit to a slightly lesser degree than tJARID2-K116me3 (~8-fold vs. ~10 fold). Interestingly, non-modified versions of tJARID2 also stimulated PCR2 core activity, albeit to about 50% the level of tJARID2-K116me2/3. This remaining activation was not, however, due to in situ methylation of lysine-116 during the reaction since a tJARID2-K116R mutant had comparable activity (Figure 5d). Removal of the stimulatory motif entirely, tJARID2(137C-450), further reduced the ability of the protein to activate PRC2 core activity, although we note that this truncated protein still retained some residual stimulatory activity (Figure 5d). Thus, tJARID2 contains additional functional motifs that stimulate the PRC2 core complex in manner that is independent of K116me3 engagement with the canonical EED allosteric site.
Next, we assessed the impact of the phospho-serine modifications on the ability of tJARID2 to stimulate the HMT activity of PRC2 core on the Ar-3 substrate. The basal activity associated with unmodified tJARID2 was unaffected by the presence of the individual pSer modifications (Figure 5d). Of note, the phospho-tJARID2 variants were comparable substrates of the PRC2 core complex compared to the unmodified control protein (Supplemental Figure 12). Introduction of one of the three reported pSer marks in the context of tJARID2-K116me3 led to an inhibition of PRC2 core stimulation (Figure 5d). Phosphorylation of Ser-120, the site closest to the methylation mark, reduced HMT activity back nearly to the level associated with the addition of the non-methylated version of the protein, tJARID2-K116R. By contrast, phosphorylation at residues Ser-124 and Ser-126 did not have such an inhibitory effect (Figure 5d). These results indicate that phosphorylation of Ser-120 largely overrides the additional stimulation associated with JARID2 upon trimethylation of K116me3, likely because the stimulatory motif in JARID2 disengages from the allosteric binding pocket in the EED subunit. Consistent with the idea that Ser-120 phosphorylation leads to EED disengagement, a PRC2 complex containing the dual modified version of tJARID2 was stimulated by the presence of H3K27me3 in cis – as expected, such re-sensitization is not observed for tJARID2-K116me3 ( Figure 5e).
DISCUSSION AND CONCLUSIONS
In this study, we have employed biochemically-defined systems, including the use of heterotypic designer chromatin substrates, to derive a better mechanistic understanding of how the H3K27 methylation mark is propagated by PRC2. Our data are consistent with the idea that allosteric stimulation of PRC2 catalytic activity can serve to both establish and maintain chromatin domains containing H3K27me1–3, with JARID2-mediated activation important for the former and H3K27me3 autostimulation driving the latter.10, 26, 38 We demonstrate that PRC2 spreading behavior primarily occurs in cis within a nucleosome array in vitro, even at high divalent metal ion concentrations known to promote chromatin compaction. More specifically, PRC2 core complex prefers to methylate substrates adjacent to nucleosomes pre-modified with H3K27me3, and the addition of auxiliary subunits such as JARID2 can override such preferences.
Collectively, our data indicate that spreading of H3K27 methylation preferentially occurs when a stimulatory nucleosome (i.e. containing H3K27me3) is close in space to a substrate nucleosome. Our finding that higher HMT activity occurs on designer array Ar-6 than on Ar-7 suggests spreading optimally occurs locally at the boundary region between the product and substrate – we stress that spreading is restricted to between 1–4 nucleosomes in our experimental system. Such a model is consistent with a recent cryo-EM structure of PRC2 bound to a dinucleosome.37 Nonetheless, our data indicates that some longer-range stimulation can occur: PRC2 has higher activity on Ar-7 and Ar-8 substrates compared to Ar-5. Importantly, this longer-range stimulation seems to operate in cis (i.e. within the nucleosome array) since control studies with arrays, Ar-3 and Ar-4, indicate that stimulation in trans does not occur under similar conditions. We note that these HMT assays were performed in the presence of 0.5 mM Mg2+, which is known to drive nucleosome arrays towards a condensed 30 nm fiber structure.54 Thus, the longer-range stimulation may simply reflect the ability of PRC2 to bridge intervening nucleosomes within such a structure. Alternatively, this mode of stimulation could involve ‘looping’ of the chromatin substrate in which the distal region of the array bends back to engage PCR2 bound to the stimulatory region. Our data indicates that transient interactions between stimulatory and substrate nucleosomes, as mimicked by mixing Ar-3 and Ar-4 in the presence of 1.5 mM Mg2+, does not lead elevated HMT activity. However, use of conditions that lead to longer lived inter-array interactions (4 mM Mg2+) did result in slightly increased HMT activity over substrate Ar-2 which contains no H3K27me3 nucleosomes, suggesting that PRC2 can act upon a chromatin substrate that adopts a more stable condensed structure.
Our studies help shed new light on the impact of JARID2 on PRC2 activity towards chromatin substrates. The presence of this subunit largely overrides the stimulation associated with having preinstalled H3K27me3 within the substrate, a result that stems from the elevated HMT activity of PRC2 when associated with this subunit. Kinetic experiments indicate that this boost in activity is largely the result of the superior ability of PCR2-JARID2 to catalyze the installation of the first and second methyl groups on H3K27. This difference is largely driven by an increased apparent kcat for the PRC2-JARID2 enzyme (compared to PRC2 core complex), consistent with an allosteric activation mechanism. In agreement with previous biochemical studies on PRC2 core complex,55 we find that both the PRC2 core complex and PRC2-JARID2 are significantly less active at installing the trimethyl mark on H3K27 in vitro. By contrast, we observed no decrease in activity, for either version of the enzyme, going from unmodified substrates to those with preinstalled H3K27me, a result that differs from the earlier work of Orkin and co-workers who reported reduced activity on H3K27me-containing peptide, histone, and mononucleosome substrates.23 A possible explanation for this difference might relate to our using more physiological chromatin substrates, versus free histones or mononucleosomes. We also note that, in contrast to the Orkin study, we did not see any inhibition of PRC2 activity in the presence JARID2. Rather, we observed a gradual increase in activity as a function of JARID2 in the PRC2 complex.
Proteomics-ms analyses of various mammalian cell lines,29 including embryonic stem cells,30 have identified three sites of serine phosphorylation (S120, S124 and S126) on JARID2 that are close in primary sequence to the known allosteric motif centered on trimethylated Lys-116.27 The kinase(s) responsible for installing these PTMs is currently unknown (an analysis of kinase consensus motifs suggest ATM, GSK3, and CDK family members as potential candidates – see Supplemental Figure 1356) as is the physiological role of these modifications. Our biochemical studies, employing semisynthetic versions of JARID2, point to a possible regulatory role for at least one of these phosphorylation events. We find that phosphorylation at Ser-120, inhibits the PRC2 stimulatory activity of JARID2-K116me3. As serine phosphorylation is generally a much more dynamic modification than lysine methylation,57 it is tempting to speculate that the observed methylation/phosphorylation crosstalk could be a quick way to drive the transition between JARID2-driven initiation of H3K27 methylation, and PRC2 propagation. Further studies will be needed to explore this intriguing idea. Our data also show that unmodified tJARID2 that lacks K116 methylation retains the ability to activate PRC2 methyltransferase activity, albeit at ~50% the level of the methylated version of the protein. We note that this result is broadly in line with previous work from Reinberg and coworkers using truncated versions of JARID2 in in vitro assays.58 The origins of this basal stimulation are unclear. Conceivably, the non-methylated motif centered on K116 could still engage and partially activate the EED allosteric site when JARID2 is otherwise bound to the PRC2 core. In support of this idea, deletion of the motif entirely (in the context of tJARID2 (137–450)) led to a reduction of this basal activity. However, that some residual stimulation remained in this truncated protein suggests that additional mechanisms make also be involved,
PRC2 is known to sense modifications along the H3 tail, and is inhibited by transcriptionally activating marks, including H3K4me3.20 The presence of domains containing both H3K4me3 and H3K27me3, termed bivalent domains, has been shown to decorate developmentally important genes in embryonic stem cells, and is thought to strike a balance between silencing genes for lineage regulation and poising them for activation upon differentiation.59 However, given the antagonistic roles of these PTMs, how these domains are established has been cryptic.60 Our observation that PRC2-JARID2 remains highly active on H3K4me3-modified 12mer arrays suggests that JARID2, which is expressed in undifferentiated stem cells, may play a role in establishment of H3K4me3/H3K27me3 dually modified chromatin.
Collectively, our investigations into the regulation of PRC2 spreading add to the growing body of evidence supporting a model wherein PRC2-JARID2 is optimized for H3K27me3 establishment, while the PRC2 core complex efficiently propagates the H3K27me3 marks from nucleation sites10, 26. Our studies also highlight the value of performing biochemical studies on reconstituted designer chromatin substrates. In the present example, this allowed us to extract information on the geometric preferences of PRC2 activity. More generally, we envision that systems of this type will be useful in studying the mechanism of many chromatin-modifying proteins.
Supplementary Material
Acknowledgements
The authors thank Dr. Robert Thompson, Dr. Zack Brown, Dr. Galia Debelouchina, Rasmus Pihl, and members of the Muir laboratory for valuable discussions. This work was supported by the U.S. National Institutes of Health (NIH grants R37-GM086868, and P01- CA196539).
References
- 1.Kornberg RD, Chromatin structure: a repeating unit of histones and DNA. Science 1974, 184 (4139), 868–71. [DOI] [PubMed] [Google Scholar]
- 2.Luger K; Mader AW; Richmond RK; Sargent DF; Richmond TJ, Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 1997, 389 (6648), 251–60. [DOI] [PubMed] [Google Scholar]
- 3.Jenuwein T; Allis CD, Translating the histone code. Science 2001, 293 (5532), 1074–80. [DOI] [PubMed] [Google Scholar]
- 4.Kouzarides T, Chromatin modifications and their function. Cell 2007, 128 (4), 693–705. [DOI] [PubMed] [Google Scholar]
- 5.Strahl BD; Allis CD, The language of covalent histone modifications. Nature 2000, 403 (6765), 41–5. [DOI] [PubMed] [Google Scholar]
- 6.Du JM; Patel DJ, Structural biology-based insights into combinatorial readout and crosstalk among epigenetic marks. Biochim. Biophys. Acta - Gene. Regul. Mech. 2014, 1839 (8), 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuzmichev A; Nishioka K; Erdjument-Bromage H; Tempst P; Reinberg D, Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev 2002, 16 (22), 2893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossniklaus U; Paro R, Transcriptional Silencing by Polycomb-Group Proteins. Cold Spring Harb. Perspect. Biol. 2014, 6 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comet I; Riising EM; Leblanc B; Helin K, Maintaining cell identity: PRC2-mediated regulation of transcription and cancer. Nat. Rev. Cancer 2016, 16 (12), 803–810. [DOI] [PubMed] [Google Scholar]
- 10.Holoch D; Margueron R, Mechanisms Regulating PRC2 Recruitment and Enzymatic Activity. Trends Biochem. Sci. 2017, 42 (7), 531–542. [DOI] [PubMed] [Google Scholar]
- 11.Wu G; Broniscer A; McEachron TA; Lu C; Paugh BS; Becksfort J; Qu C; Ding L; Huether R; Parker M; Zhang J; Gajjar A; Dyer MA; Mullighan CG; Gilbertson RJ; Mardis ER; Wilson RK; Downing JR; Ellison DW; Zhang J; Baker SJ; St. Jude Children’s Research Hospital-Washington University Pediatric Cancer Genome, P., Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 2012, 44 (3), 251–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khuong-Quang DA; Buczkowicz P; Rakopoulos P; Liu XY; Fontebasso AM; Bouffet E; Bartels U; Albrecht S; Schwartzentruber J; Letourneau L; Bourgey M; Bourque G; Montpetit A; Bourret G; Lepage P; Fleming A; Lichter P; Kool M; von Deimling A; Sturm D; Korshunov A; Faury D; Jones DT; Majewski J; Pfister SM; Jabado N; Hawkins C, K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012, 124 (3), 439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartzentruber J; Korshunov A; Liu XY; Jones DT; Pfaff E; Jacob K; Sturm D; Fontebasso AM; Quang DA; Tonjes M; Hovestadt V; Albrecht S; Kool M; Nantel A; Konermann C; Lindroth A; Jager N; Rausch T; Ryzhova M; Korbel JO; Hielscher T; Hauser P; Garami M; Klekner A; Bognar L; Ebinger M; Schuhmann MU; Scheurlen W; Pekrun A; Fruhwald MC; Roggendorf W; Kramm C; Durken M; Atkinson J; Lepage P; Montpetit A; Zakrzewska M; Zakrzewski K; Liberski PP; Dong Z; Siegel P; Kulozik AE; Zapatka M; Guha A; Malkin D; Felsberg J; Reifenberger G; von Deimling A; Ichimura K; Collins VP; Witt H; Milde T; Witt O; Zhang C; Castelo-Branco P; Lichter P; Faury D; Tabori U; Plass C; Majewski J; Pfister SM; Jabado N, Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 2012, 482 (7384), 226–31. [DOI] [PubMed] [Google Scholar]
- 14.Lewis PW; Muller MM; Koletsky MS; Cordero F; Lin S; Banaszynski LA; Garcia BA; Muir TW; Becher OJ; Allis CD, Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science 2013, 340 (6134), 857–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wassef M; Margueron R, The Multiple Facets of PRC2 Alterations in Cancers. J. Mol. Biol. 2017, 429 (13), 1978–1993. [DOI] [PubMed] [Google Scholar]
- 16.Lu C; Jain SU; Hoelper D; Bechet D; Molden RC; Ran LL; Murphy D; Venneti S; Hameed M; Pawel BR; Wunder JS; Dickson BC; Lundgren SM; Jani KS; De Jay N; Papillon-Cavanagh S; Andrulis IL; Sawyer SL; Grynspan D; Turcotte RE; Nadaf J; Fahiminiyah S; Muir TW; Majewski J; Thompson CB; Chi P; Garcia BA; Allis CD; Jabado N; Lewis PW, Histone H3K36 mutations promote sarcomagenesis through altered histone methylation landscape. Science 2016, 352 (6287), 844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciferri C; Lander GC; Maiolica A; Herzog F; Aebersold R; Nogales E, Molecular architecture of human polycomb repressive complex 2. eLife 2012, 1, e00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao R; Zhang Y, SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex. Mol. Cell 2004, 15 (1), 57–67. [DOI] [PubMed] [Google Scholar]
- 19.Margueron R; Justin N; Ohno K; Sharpe ML; Son J; Drury WJ 3rd; Voigt P; Martin SR; Taylor WR; De Marco V; Pirrotta V; Reinberg D; Gamblin SJ, Role of the polycomb protein EED in the propagation of repressive histone marks. Nature 2009, 461 (7265), 762–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitges FW; Prusty AB; Faty M; Stutzer A; Lingaraju GM; Aiwazian J; Sack R; Hess D; Li L; Zhou S; Bunker RD; Wirth U; Bouwmeester T; Bauer A; Ly-Hartig N; Zhao K; Chan H; Gu J; Gut H; Fischle W; Muller J; Thoma NH, Histone methylation by PRC2 is inhibited by active chromatin marks. Mol. Cell 2011, 42 (3), 330–41. [DOI] [PubMed] [Google Scholar]
- 21.Kloet SL; Makowski MM; Baymaz HI; van Voorthuijsen L; Karemaker ID; Santanach A; Jansen P; Di Croce L; Vermeulen M, The dynamic interactome and genomic targets of Polycomb complexes during stem-cell differentiation. Nat. Struct. Mol. Biol. 2016, 23 (7), 682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G; Margueron R; Ku M; Chambon P; Bernstein BE; Reinberg D, Jarid2 and PRC2, partners in regulating gene expression. Genes Dev. 2010, 24 (4), 368–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen X; Kim W; Fujiwara Y; Simon MD; Liu Y; Mysliwiec MR; Yuan GC; Lee Y; Orkin SH, Jumonji modulates polycomb activity and self-renewal versus differentiation of stem cells. Cell 2009, 139 (7), 1303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landeira D; Fisher AG, Inactive yet indispensable: the tale of Jarid2. Trends Cell Biol. 2011, 21 (2), 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klose RJ; Kallin EM; Zhang Y, JmjC-domain-containing proteins and histone demethylation. Nat. Rev. Genet. 2006, 7 (9), 715–27. [DOI] [PubMed] [Google Scholar]
- 26.Sanulli S; Justin N; Teissandier A; Ancelin K; Portoso M; Caron M; Michaud A; Lombard B; da Rocha ST; Offer J; Loew D; Servant N; Wassef M; Burlina F; Gamblin SJ; Heard E; Margueron R, Jarid2 Methylation via the PRC2 Complex Regulates H3K27me3 Deposition during Cell Differentiation. Mol. Cell 2015, 57 (5), 769–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasinath V; Faini M; Poepsel S; Reif D; Feng XA; Stjepanovic G; Aebersold R; Nogales E, Structures of human PRC2 with its cofactors AEBP2 and JARID2. Science 2018, 359 (6378), 940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beli P; Lukashchuk N; Wagner SA; Weinert BT; Olsen JV; Baskcomb L; Mann M; Jackson SP; Choudhary C, Proteomic investigations reveal a role for RNA processing factor THRAP3 in the DNA damage response. Mol. Cell 2012, 46 (2), 212–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber C; Schreiber TB; Daub H, Dual phosphoproteomics and chemical proteomics analysis of erlotinib and gefitinib interference in acute myeloid leukemia cells. J. Proteomics 2012, 75 (4), 1343–56. [DOI] [PubMed] [Google Scholar]
- 30.Van Hoof D; Munoz J; Braam SR; Pinkse MW; Linding R; Heck AJ; Mummery CL; Krijgsveld J, Phosphorylation dynamics during early differentiation of human embryonic stem cells. Cell Stem Cell 2009, 5 (2), 214–26. [DOI] [PubMed] [Google Scholar]
- 31.Badeaux AI; Shi Y, Emerging roles for chromatin as a signal integration and storage platform. Nat. Rev. Mol. Cell Biol. 2013, 14 (4), 211–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalb R; Latwiel S; Baymaz HI; Jansen PW; Muller CW; Vermeulen M; Muller J, Histone H2A monoubiquitination promotes histone H3 methylation in Polycomb repression. Nat. Struct. Mol. Biol. 2014, 21 (6), 569–71. [DOI] [PubMed] [Google Scholar]
- 33.Yuan W; Xu M; Huang C; Liu N; Chen S; Zhu B, H3K36 methylation antagonizes PRC2-mediated H3K27 methylation. J. Biol. Chem. 2011, 286 (10), 7983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee CH; Holder M; Grau D; Saldana-Meyer R; Yu JR; Ganai RA; Zhang J; Wang M; LeRoy G; Dobenecker MW; Reinberg D; Armache KJ, Distinct Stimulatory Mechanisms Regulate the Catalytic Activity of Polycomb Repressive Complex 2. Mol. Cell 2018, 70 (3), 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CH; Yu JR; Kumar S; Jin Y; LeRoy G; Bhanu N; Kaneko S; Garcia BA; Hamilton AD; Reinberg D, Allosteric Activation Dictates PRC2 Activity Independent of Its Recruitment to Chromatin. Mol. Cell 2018, 70 (3), 422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiao L; Liu X, Structural basis of histone H3K27 trimethylation by an active polycomb repressive complex 2. Science 2015, 350 (6258), aac4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poepsel S; Kasinath V; Nogales E, Cryo-EM structures of PRC2 simultaneously engaged with two functionally distinct nucleosomes. Nat. Struct. Mol. Biol. 2018, 25 (2), 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oksuz O; Narendra V; Lee CH; Descostes N; LeRoy G; Raviram R; Blumenberg L; Karch K; Rocha PP; Garcia BA; Skok JA; Reinberg D, Capturing the Onset of PRC2-Mediated Repressive Domain Formation. Mol. Cell 2018, 70 (6), 1149–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao Q; Wang X; Zhao M; Yang R; Malik R; Qiao Y; Poliakov A; Yocum AK; Li Y; Chen W; Cao X; Jiang X; Dahiya A; Harris C; Feng FY; Kalantry S; Qin ZS; Dhanasekaran SM; Chinnaiyan AM, The central role of EED in the orchestration of polycomb group complexes. Nat. Commun. 2014, 5, 3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao R; Wang L; Wang H; Xia L; Erdjument-Bromage H; Tempst P; Jones RS; Zhang Y, Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 2002, 298 (5595), 1039–43. [DOI] [PubMed] [Google Scholar]
- 41.Boyer LA; Plath K; Zeitlinger J; Brambrink T; Medeiros LA; Lee TI; Levine SS; Wernig M; Tajonar A; Ray MK; Bell GW; Otte AP; Vidal M; Gifford DK; Young RA; Jaenisch R, Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 2006, 441 (7091), 349–53. [DOI] [PubMed] [Google Scholar]
- 42.Bieniossek C; Richmond TJ; Berger I, MultiBac: multigene baculovirus-based eukaryotic protein complex production. Curr. Protoc. Protein Sci. 2008, Chapter 5, Unit 5 20. [DOI] [PubMed] [Google Scholar]
- 43.Kilic S; Felekyan S; Doroshenko O; Boichenko I; Dimura M; Vardanyan H; Bryan LC; Arya G; Seidel CAM; Fierz B, Single-molecule FRET reveals multiscale chromatin dynamics modulated by HP1alpha. Nat. Commun. 2018, 9 (1), 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen JC, Conformational dynamics of the chromatin fiber in solution: determinants, mechanisms, and functions. Annu. Rev. Biophys. Biomol. Struct. 2002, 31, 361–92. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh TH; Weiner A; Lajoie B; Dekker J; Friedman N; Rando OJ, Mapping Nucleosome Resolution Chromosome Folding in Yeast by Micro-C. Cell 2015, 162 (1), 108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohno M; Ando T; Priest DG; Kumar V; Yoshida Y; Taniguchi Y, Sub-nucleosomal Genome Structure Reveals Distinct Nucleosome Folding Motifs. Cell 2019, 176 (3), 520–534. [DOI] [PubMed] [Google Scholar]
- 47.Ricci MA; Manzo C; Garcia-Parajo MF; Lakadamyali M; Cosma MP, Chromatin fibers are formed by heterogeneous groups of nucleosomes in vivo. Cell 2015, 160 (6), 1145–58. [DOI] [PubMed] [Google Scholar]
- 48.Lowary PT; Widom J, New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J. Mol. Biol 1998, 276 (1), 19–42. [DOI] [PubMed] [Google Scholar]
- 49.Muller MM; Fierz B; Bittova L; Liszczak G; Muir TW, A two-state activation mechanism controls the histone methyltransferase Suv39h1. Nat Chem Biol 2016, 12 (3), 188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim H; Kang K; Kim J, AEBP2 as a potential targeting protein for Polycomb Repression Complex PRC2. Nucleic Acids Res. 2009, 37 (9), 2940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi W; Zhao K; Gu J; Huang Y; Wang Y; Zhang H; Zhang M; Zhang J; Yu Z; Li L; Teng L; Chuai S; Zhang C; Zhao M; Chan H; Chen Z; Fang D; Fei Q; Feng L; Feng L; Gao Y; Ge H; Ge X; Li G; Lingel A; Lin Y; Liu Y; Luo F; Shi M; Wang L; Wang Z; Yu Y; Zeng J; Zeng C; Zhang L; Zhang Q; Zhou S; Oyang C; Atadja P; Li E, An allosteric PRC2 inhibitor targeting the H3K27me3 binding pocket of EED. Nat. Chem. Biol. 2017, 13 (4), 381–388. [DOI] [PubMed] [Google Scholar]
- 52.Knutson SK; Kawano S; Minoshima Y; Warholic NM; Huang KC; Xiao Y; Kadowaki T; Uesugi M; Kuznetsov G; Kumar N; Wigle TJ; Klaus CR; Allain CJ; Raimondi A; Waters NJ; Smith JJ; Porter-Scott M; Chesworth R; Moyer MP; Copeland RA; Richon VM; Uenaka T; Pollock RM; Kuntz KW; Yokoi A; Keilhack H, Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-Hodgkin lymphoma. Mol. Cancer Ther. 2014, 13 (4), 842–54. [DOI] [PubMed] [Google Scholar]
- 53.Privett HK; Kiss G; Lee TM; Blomberg R; Chica RA; Thomas LM; Hilvert D; Houk KN; Mayo SL, Iterative approach to computational enzyme design. Proc. Natl. Acad. Sci. U.S.A. 2012, 109 (10), 3790–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allahverdi A; Chen Q; Korolev N; Nordenskiold L, Chromatin compaction under mixed salt conditions: opposite effects of sodium and potassium ions on nucleosome array folding. Sci. Rep. 2015, 5, 8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCabe MT; Graves AP; Ganji G; Diaz E; Halsey WS; Jiang Y; Smitheman KN; Ott HM; Pappalardi MB; Allen KE; Chen SB; Della Pietra A 3rd; Dul E; Hughes AM; Gilbert SA; Thrall SH; Tummino PJ; Kruger RG; Brandt M; Schwartz B; Creasy CL, Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27 (H3K27). Proc. Natl. Acad. Sci. U.S.A. 2012, 109 (8), 2989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim ST; Lim DS; Canman CE; Kastan MB, Substrate specificities and identification of putative substrates of ATM kinase family members. J. Biol. Chem. 1999, 274 (53), 37538–43. [DOI] [PubMed] [Google Scholar]
- 57.Walsh CT; Garneau-Tsodikova S; Gatto GJ Jr., Protein posttranslational modifications: the chemistry of proteome diversifications. Angew. Chem., Int. Ed. 2005, 44 (45), 7342–72. [DOI] [PubMed] [Google Scholar]
- 58.Vastenhouw NL; Schier AF, Bivalent histone modifications in early embryogenesis. Curr. Opin. Cell Biol. 2012, 24 (3), 374–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Voigt P; Tee WW; Reinberg D, A double take on bivalent promoters. Genes. Dev. 2013, 27 (12), 1318–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.