Abstract
Objective:
Cervical cancer rates in the United States have declined since the 1940’s, however, cervical cancer incidence remains elevated in medically-underserved areas, especially in the Rio Grande Valley (RGV) along the Texas-Mexico border. High-resolution microendoscopy (HRME) is a low-cost, in vivo imaging technique that can identify high-grade precancerous cervical lesions (CIN2+) at the point-of-care. The goal of this study was to evaluate the performance of HRME in medically-underserved areas in Texas, comparing results to a tertiary academic medical center.
Methods:
HRME was evaluated in five different outpatient clinical settings, two in Houston and three in the RGV, with medical providers of varying skill and training. Colposcopy, followed by HRME imaging, was performed on eligible women. The sensitivity and specificity of traditional colposcopy and colposcopy followed by HRME to detect CIN2+ were compared and HRME image quality was evaluated.
Results:
174 women (227 cervical sites) were included in the final analysis, with 12% (11% of cervical sites) diagnosed with CIN2+ on histopathology. On a per-site basis, a colposcopic impression of low-grade precancer or greater had a sensitivity of 84% and a specificity of 45% to detect CIN2+. While there was no significant difference in sensitivity (76%, p=0.62), the specificity when using HRME was significantly higher than that of traditional colposcopy (56%, p=0.01). There was no significant difference in HRME image quality between clinical sites (p=0.77) or medical providers (p=0.33).
Conclusions:
HRME imaging increased the specificity for detecting CIN2+ when compared to traditional colposcopy. HRME image quality remained consistent across different clinical settings.
Keywords: cervical cancer, cervical cancer prevention, cervical dysplasia, colposcopy, high-resolution cervical imaging
Introduction
Cervical cancer remains a significant global health problem, especially in medically-undeserved areas (MUAs) of the world [1]. Most cases of cervical cancer and related deaths occur in low- and middle-income countries where access to cervical cancer screening and prevention is limited. In contrast, the incidence and mortality of cervical cancer in the United States (U.S.) and other high-income countries have significantly decreased due to the implementation of cervical cancer screening with cytology and/or human papillomavirus (HPV) testing as a standard part of routine women’s health care [2]. However, cervical cancer incidence is higher among medically-underserved women living in poverty in the U.S. [3–5].
The incidence and mortality of cervical cancer in Texas along the Texas-Mexico border are among the highest in the United States, with rates of 12.3 per 100,000 and 4.1 per 100,000 respectively; in contrast, the overall incidence and mortality of cervical cancer in the U.S. are 8 per 100,000 and 2 per 100,000 respectively [6,7]. The Rio Grande Valley of Texas (RGV) is a region along the Texas-Mexico border that is made up of some of the poorest counties in the state of Texas: Hidalgo, Cameron, Willacy, and Starr counties [8]. Over 20% of people under the age of 65 years living in the RGV have no health insurance, severely limiting their access to health care, including services for cervical cancer prevention [4,9]. Another factor contributing to limited health care access is the lack of trained medical providers who are able to provide follow-up diagnostic and treatment services for women who have a positive screening test (abnormal cytology and/or positive HPV test) [10].
The standard of care in the U.S. for a woman with a positive cervical screening test is to undergo colposcopy, a procedure in which acetic acid is placed on the cervix and a medical provider examines the cervix with a colposcope (a magnifying instrument) to identify any potential precancerous lesions [11]. Abnormal lesions identified by the provider as potential precancer are biopsied and submitted for pathologic evaluation, the results of which take days or weeks to return depending on the type and availability of pathology services required. Any diagnosis of high-grade precancer is followed-up with ablative or excisional treatment to remove the dysplastic cells and prevent the development of cancer.
The ability to identify precancerous lesions during colposcopy is a skill that requires training and practice, and providers with this expertise are not always available in MUAs [12]. In vivo microscopy has the potential to assist medical providers in the accurate detection of precancerous lesions at the point-of-care. We developed a high-resolution microendoscope (HRME), a low-cost fiber-optic device that images cervical epithelial cell nuclei in real time, providing morphologic information associated with high-grade dysplasia [13–15]. The HRME system is equipped with real time image analysis software to provide decision-making support at the point-of-care. Recent studies conducted in Brazil at a central hospital and mobile clinic found the HRME to have similar sensitivity and specificity to detect high-grade cervical precancer and cancer as traditional colposcopy performed by an experienced colposcopist [16]. In this study, we aimed to evaluate the feasibility and clinical performance of the HRME in five clinical settings in Texas: an out-patient clinic at a tertiary academic cancer center in Houston, TX, an out-patient clinic at a large county hospital in Houston, TX, two federally-qualified heath care clinics in the RGV, and one mobile clinic in the RGV. Furthermore, the study was conducted with medical providers of different skills and training to investigate the potential for HRME to support the detection of cervical high-grade precancer in a variety of clinical settings in the U.S.
Materials and Methods
Study Design
We conducted a single-arm prospective study to evaluate the use of colposcopy followed by HRME imaging in MUAs in Texas to diagnose high-grade cervical precancerous lesions. The study conducted in Houston, TX (Clinical Trial ID: ) was approved by the institutional review boards at The University of Texas MD Anderson Cancer Center (MD Anderson) (IRB# 2014-0368) and Rice University (IRB# 2017-385). The study conducted in the RGV (Clinical Trial ID: ) was approved by the institutional review boards of The University of Texas Medical Branch in Galveston (UTMB) (IRB# 14-0302), Rice University (IRB# 2017-436), MD Anderson (IRB# 2015-0477), and The University of Texas Health Science Center at Houston School of Public Health (UTHealth) (IRB# HSC-SPH-16-0569).
Clinical Settings
The study was conducted at five clinical sites in Houston and the RGV of Texas: MD Anderson outpatient colposcopy clinic (Houston), Lyndon B. Johnson County Hospital (LBJ) outpatient colposcopy clinic (Houston), the UTMB Dysplasia and Cancer Stop Clinic located in McAllen, TX (RGV), Su Clinica Brownsville in Brownsville, TX (RGV), and the UTHealth Mobile Health Clinic (RGV). All of the clinical sites primarily serve uninsured and underinsured populations, with the exception of the clinic at MD Anderson.
MD Anderson is a tertiary care hospital specializing in cancer care and prevention. LBJ is a public county hospital that primarily serves uninsured or underinsured patients in the Houston area. The LBJ Hospital colposcopy clinic is staffed by physicians and nurse practitioners from MD Anderson. For this study, patients seen at either the MD Anderson or LBJ colposcopy clinics were examined by a gynecologic oncologist or general gynecologist from MD Anderson.
The UTMB Dysplasia Cancer Stop Clinic and Su Clinica Brownsville are both federally-qualified health care clinics in the RGV. For the study, colposcopy examinations at the UTMB clinic were conducted by a general gynecologist from Galveston who travels to the clinic in the RGV once per month, while the colposcopies at Su Clinica were conducted by a local general gynecologist and nurse practitioner who typically provide colposcopy examinations at that site.
The UTHealth Mobile Health Clinic is a mobile clinic supported by UTHealth that rotates between different sites in the RGV region on a monthly basis to provide health care to those without health insurance. Screening with cytology/HPV testing and colposcopy are provided by one physician assistant who staffs the mobile clinic.
Patient Enrollment
Patients were eligible to participate in the study if they were visiting the clinic for a colposcopy examination due to an abnormal Pap test, positive HPV test, and/or had a history of cervical dysplasia and met the following criteria: 1) had an intact cervix (patients who had undergone a previous LEEP, cone and/or cryotherapy were eligible); 2) were not pregnant or breastfeeding; 3) were at least 21 years of age; 4) had no known allergy to proflavine, acriflavine, or iodine; and 5) were willing and able to provide written informed consent. Women of childbearing potential were required to have a negative pregnancy test within the past 14 days in order to be eligible.
High-Resolution Microendoscopy
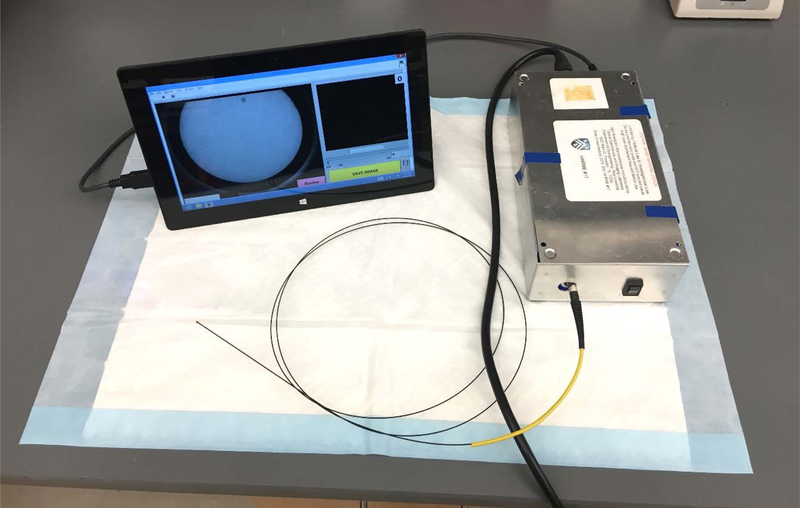
The HRME is an in vivo imaging device that allows medical providers to further interrogate cervical lesions noted during a clinical exam (Figure 1). The HRME consists of a portable, low-cost fluorescence microscope attached to a flexible fiber-optic probe [17]. The probe images a 790 µm field-of-view with a transverse resolution of 4 µm.
Figure 1:
Photo of the HRME
Photo of the high-resolution microendoscope (HRME).
HRME imaging is performed following the topical application of 0.01% proflavine, a contrast agent used to fluorescently stain the cell nuclei in the superficial epithelium. Proflavine is a topical antiseptic, with a long history of safe clinical use, including for cervical imaging [16,18]. Lugol’s iodine is applied after the application of 0.01% proflavine; Lugol’s iodine helps highlight abnormal lesions on the cervix and improves the optical contrast of HRME images. Proflavine is then reapplied to ensure staining of the cervical nuclei during HRME imaging.
When the tip of the fiber-optic probe is placed in contact with the cervix, the HRME displays a live image of the tissue site, with epithelial cell nuclei visible on a tablet computer screen. To save and analyze an image of interest, the clinician presses a foot pedal. Once the image is saved, image analysis software provides analysis of the image within 5 seconds [19,20]. The automatic image analysis software identifies the fraction of the field-of-view that contains quality image data (in focus, not saturated, free of debris). In this region, the algorithm then segments nuclei and identifies abnormal nuclei as those that exceed the preset size and shape criteria determined from past studies [16]. The tablet image is updated to show all segmented nuclei with those identified as abnormal outlined in red. If the number of abnormal nuclei per mm2 exceeds 120 abnormal nuclei/mm2, the image is classified as being HRME positive for high-grade cervical precancer defined as cervical intraepithelial neoplasia 2 or greater (CIN2+) or cervical cancer. Pressing down on the foot pedal again resumes the live feed from the HRME camera so other areas of the cervix can be imaged.
Clinical Exam
Participants who met the inclusion criteria and provided informed consent underwent a standard colposcopy examination. First, 5% acetic acid was applied to the surface of the cervix. The cervix was visibly inspected and then examined using a colposcope by a trained medical provider. Any visible lesions by visual inspection or during colposcopy were noted. While using the colposcope, the clinician provided an impression for each lesion (benign, low-grade precancer, high-grade precancer, or cancer). Next, HRME images were obtained of each abnormal lesion noted during the clinical exam along with one normal area. If no lesions were noted during the clinical exam, an HRME image was obtained of one normal area of the cervix at the transformation zone. Biopsies were then taken of each lesion. If no lesions were present, a biopsy was taken of the normal area imaged by HRME. An endocervical curettage (ECC) was performed if indicated by standard of care.
Histopathology & Follow-up
Histopathology was used as the gold standard to assess the diagnostic performance of traditional colposcopy versus colposcopy followed by HRME imaging to detect CIN2+. All pathology specimens were reviewed by the institutional pathologist at each clinical site. A central pathology review was not performed as all sites had pathologists with experience reviewing cervical dysplasia and cancer biopsy specimens. All diagnoses were categorized as being normal/benign (normal, inflammation, demonstrating HPV/reactive changes), CIN1, CIN2/3 (CIN2 and/or CIN3), adenocarcinoma in situ (AIS), cancer, or insufficient for diagnosis by the institutional pathologist. Patients were then treated or scheduled for follow-up based on the final histopathology results of cervical biopsies and ECC per standard of care.
Statistical Analysis
The sensitivity and specificity of traditional colposcopy and colposcopy followed by HRME imaging for the detection of CIN2+ were calculated on a per-patient and per-site basis. The worst grade colposcopy result and worst HRME result were compared to the worst grade histopathology biopsy result for each patient in the per-patient analysis, whereas the colposcopy result and HRME result of a particular cervical site were compared to the histopathology result of the biopsy taken at that site in the per-site analysis. A colposcopic impression of low-grade precancer and greater (Colpo LG+) was used as the threshold for positivity when evaluating the diagnostic performance of traditional colposcopy. Sensitivity and specificity calculations were calculated with 95% exact binomial confidence intervals. McNemar’s test was used to compare the sensitivity and specificity for each diagnostic method; differences were considered significant at p<0.05.
The quality of HRME images taken at each clinical site and by each medical provider was determined by calculating the average percentage of the field-of-view that could be analyzed by the automatic image analysis algorithm. One-way ANOVA was performed to assess whether differences between the clinical sites and medical providers were significant.
Results
Table 1 summarizes the providers, subjects, and final histopathology results at each of the clinical sites that were included in the final analysis. The study enrolled 50 women in Houston, TX and 132 women in the RGV. Of the 182 women enrolled, histopathology information was not recorded for six either because the clinical exam could not be completed or the biopsy samples taken were insufficient for diagnosis. Of the 176 women with histopathology results available, two did not have HRME images recorded due to an error in image collection. Overall, 174 women (227 cervical sites) had complete colposcopy, HRME, and histopathology results available and were included in the final analysis. Of the 174 women and 227 cervical sites evaluated, 21 women (12%) and 25 cervical sites (11%) were diagnosed with CIN2+ on histopathology. There were no diagnoses of invasive cervical cancer. The number of HRME+ results at each clinical site are also displayed in Table 1.
Table 1.
Summary of clinical data for enrolled patients included in the final analysis
| Houston, TX |
Rio Grande Valley, TX |
||||
|---|---|---|---|---|---|
| Clinical Sites | The University of Texas MD Anderson Cancer Center | Lyndon B. Johnson Hospital | UTMB Dysplasia and Cancer Stop Clinic | Su Clinica Brownsville | UTHealth Mobile Health Clinic |
| Medical Providers (No. Providers) | Gynecologic Oncologist (1) General Gynecologist (1) | Gynecologic Oncologist (1) General Gynecologist (1) | General Gynecologist (1) | General Gynecologist (1) Nurse Practitioner (1) | Physician Assistant (1) |
| Number of Patients | 18 | 28 | 71 | 48 | 9 |
| Final Pathology Diagnosis per Patient | |||||
| No. Patients (%) | |||||
| Normal/Benign | 11 (61%) | 20 (71%) | 49 (70%) | 23 (48%) | 3 (33%) |
| CIN1 | 6 (33%) | 3 (11%) | 11 (15%) | 21 (44%) | 6 (67%) |
| CIN2/3 | 1 (6%) | 5 (18%) | 11 (15%) | 3 (6%) | 0 (0%) |
| AIS | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Number of Cervical Sites | 19 | 36 | 85 | 74 | 13 |
| Final Pathology Diagnosis per Site | |||||
| No. Sites (%) | |||||
| Normal/Benign | 12 (63%) | 24 (66%) | 59 (70%) | 39 (53%) | 4 (31%) |
| CIN1 | 6 (32%) | 6 (17%) | 13 (15%) | 30 (40%) | 9 (69%) |
| CIN2/3 | 1 (5%) | 6 (17%) | 13 (15%) | 3 (4%) | 0 (0%) |
| AIS | 0 (0%) | 0 (0%) | 0 (0%) | 2 (3%) | 0 (0%) |
| Number of HRME+ Sites by Final Pathology Diagnosis (%) | |||||
| <CIN2 | 6 (33%) | 11 (37%) | 35 (49%) | 28 (41%) | 8 (62%) |
| CIN2+ | 1 (100%) | 2 (33%) | 12 (92%) | 4 (80%) | - |
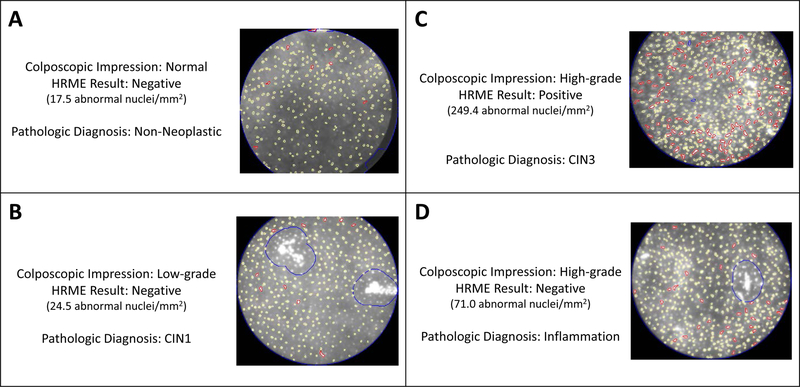
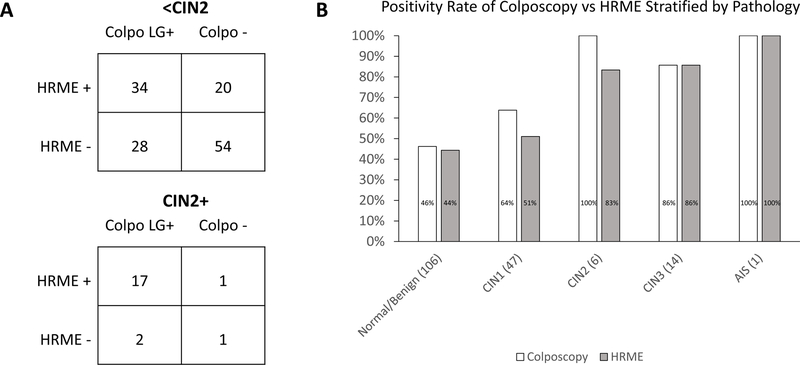
Figure 2 provides example HRME images along with the corresponding colposcopy impression and the final histopathology diagnosis of four different cervical sites for four different patients. Figures 2A–C show images for cases in which both the colposcopy impression and the HRME result corresponded with the final pathology result. Figure 2D shows an example in which the colposcopic impression differed from the HRME result (high-grade by colposcopy, negative by HRME), and HRME correctly identified that the area did not contain CIN2+. Figure 3 demonstrates the overall correlation of colposcopy and HRME results on a per-patient basis in comparison to the final histopathology diagnosis for each patient. The positivity rate for HRME was lower than the positivity rate for colposcopy (Colpo LG+) for patients who received final histopathology diagnoses of Normal/Benign, CIN1, or CIN2, but it was the same for patients who received a final histopathology diagnosis of CIN3 or AIS.
Figure 2:
HRME Images of Four Cervical Sites
HRME images of four cervical sites from four different patients. A-D) provides the colposcopic impression, the HRME result, and the final pathology diagnosis along with the corresponding HRME image for each cervical site. A-C) are cases in which both the colposcopy impression and the HRME result corresponded with the final pathology result, while D) is an example in which the colposcopic impression and HRME result differed and HRME correctly identified that the area did not contain CIN2+.
Figure 3:
Graphs Comparing the Positivity of Colposcopy vs HRME
Graphs showing the positivity rate for colposcopy and HRME in comparison to the final histopathology result on a per-patient basis. A) 2 x 2 tables showing the correlation of colposcopy and HRME results for patients diagnosed with <CIN2 (above) and CIN2+ (below). B) Bar graph showing positivity rate for colposcopy and HRME stratified by the final histopathology diagnosis for each patient.
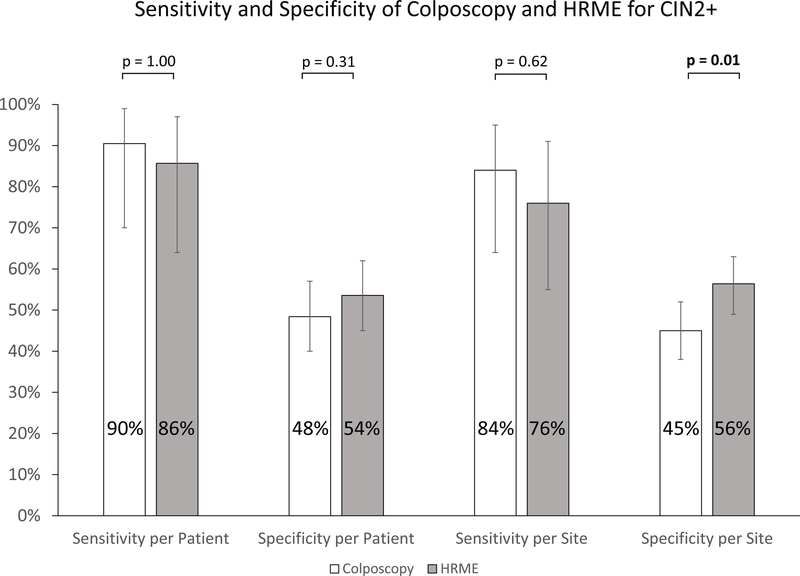
Figure 4 compares the calculated sensitivity and specificity for colposcopy and HRME (colposcopy followed by HRME imaging) for the detection of CIN2+. The performance of the HRME compared favorably to that of traditional colposcopy. There was no statistically significant difference in sensitivity of traditional colposcopy and HRME on a per-patient (91% and 86% respectively, p=1.00) or per-site basis (84% and 76% respectively, p=0.62). On a per-site basis, the specificity of HRME (56%) was significantly higher than that of traditional colposcopy (45%) (p=0.01), while the difference was not significant on a per-patient basis (54% and 48% respectively, p=0.31).
Figure 4:
Graph of Sensitivity and Specificity
Sensitivity and specificity of traditional colposcopy and HRME to detect CIN2+ on a per-patient and per-site basis. Significance was calculated using McNemar’s test. Error bars represent 95% exact binomial confidence intervals.
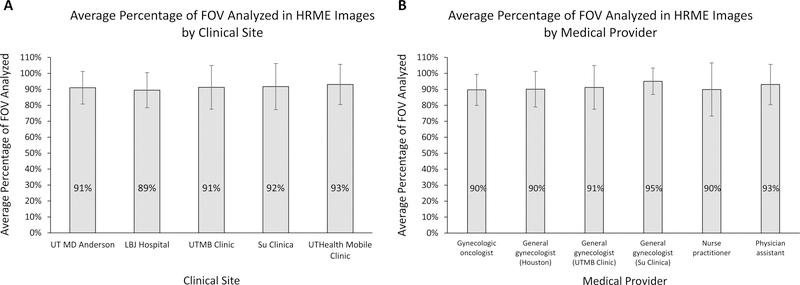
Image quality at each clinical site and between medical providers was assessed by calculating the average percentage of the field-of-view (FOV) that could be analyzed by the automatic image analysis algorithm (Figure 5). The average percentage of the FOV that could be analyzed ranged from 89% to 93% between clinical sites and from 90% to 95% among medical providers. There were no statistically significant differences in image quality between any of the clinical sites (p=0.77) or medical providers (p=0.33) by one-way ANOVA.
Figure 5:
Graphs of HRME Image Quality
The average percentage of the field-of-view (FOV) included in the automatic analysis of HRME images stratified by A) clinical site and B) medical provider. One-way ANOVA of the results revealed no statistically significant difference in image quality between clinical sites (p = 0.77) or medical providers (p = 0.33). Error bars represent one standard deviation
Discussion
In the U.S., women who have a positive cervical cancer screening test are referred for colposcopic evaluation. Medical providers, especially those in MUAs, do not always have access to the training required to perform colposcopy due to limited time, funding, or proximity to a training location. HRME imaging provides real time evaluation of the cervix that can be used in addition to colposcopy to support clinicians in detecting high-grade precancerous lesions at the point-of-care.
Moreover, the inclusion of HRME imaging with colposcopy can reduce the number of unnecessary biopsies, decreasing clinical costs and patient discomfort. The primary finding of our study is that quality HRME imaging of the cervix is possible in a variety of clinical settings, ranging from a high-resource cancer specialty clinic in Houston staffed by a gynecologic oncologist, to a mobile clinic located in one of the most underserved regions of Texas staffed by a physician assistant. There were no significant differences in HRME image quality between clinical sites or medical providers. This is most relevant for MUAs of the U.S. where expert clinicians might not always be available and the automatic and real time feedback of HRME imaging could offer assistance with cervical precancer detection.
In this study, the per-site specificity of colposcopy followed by HRME imaging was significantly higher than traditional colposcopy when using a colposcopic impression threshold of low-grade precancer or worse for detecting CIN2+. As part of standard of care, colposcopists take biopsies of any precancerous lesions found on exam to confirm any diagnosis of CIN2+ [21]. In this study, taking biopsies based on HRME would have resulted in 21% fewer unnecessary biopsies being taken (88 HRME false positive sites vs 111 false positive sites by traditional colposcopy) with no significant difference in the detection of CIN2+. Three patients (6 cervical sites) with CIN2+ were missed using HRME, in comparison to two patients (4 cervical sites) using traditional colposcopy, however the detection for CIN3+ was the same with both HRME and traditional colposcopy missing two patients (4 cervical sites) diagnosed with CIN3.
The HRME uses a 0.8 mm diameter probe to provide flexibility when imaging different parts of the cervix. A widefield imaging/visualization method, such as colposcopy, is needed to guide the placement of the probe so that HRME images are taken of the most suspicious areas of the cervix. However, expert colposcopists are not always available in MUAs, and even when colposcopists are available, image interpretation can still be inaccurate [22,23]. A recent study showed that using deep learning approaches to classify cervigrams (a widefield imaging technique used to screen for cervical cancer) could more accurately detect CIN2+ than the original cervigram interpretation provided by an expert physician colposcopist [24]. Following the publication of this study, MobileODT, a company that develops low-cost and portable visual assessment technology, is now exploring how to incorporate automated visual evaluation on their Enhanced Visual Assessment (EVA) system, a mobile smartphone-based colposcope, to provide reliable colposcopy results in MUAs [25]. Pairing automatic visual evaluation of the cervix with the automatic image interpretation of the HRME could make it possible to accurately detect CIN2+ without the need for biopsy.
The ultimate goal of the HRME is to omit biopsies altogether to provide the diagnosis and treatment of high-grade cervical precancer in a single patient visit. This is especially important in MUAs where patient follow-up is low or timely pathology services are unavailable. Future work is now focused on incorporating automatic visual evaluation methods of the cervix when using the HRME to increase the specificity of the HRME and further decrease the number of false positives associated with the current system.
The strengths of this study are the demonstration that HRME imaging can be used by medical providers of different levels of training at various clinical sites, including MUAs in Texas. Additionally, HRME imaging can help significantly decrease the number of false positives currently associated with traditional colposcopy. The main limitation of this study is that there were not as many cases of CIN2+ as there were <CIN2, resulting in larger confidence intervals when calculating sensitivity in comparison to specificity. Furthermore, not enough patients were recruited at each clinical site to provide a comparison of the sensitivity and specificity of HRME imaging between clinical sites or medical providers. Future work would expand the study to be able to provide such analysis.
HRME imaging offers a visual way to further interrogate abnormal lesions of the cervix during a traditional gynecologic exam that can help support clinicians to detect high-grade precancerous lesions at the point-of-care.
Acknowledgements:
The authors would like to thank Maria Daheri, Monica Gasca, and Jill Morales from the University of Texas Health Science Center at Houston and Jessica Gallegos from The University of Texas MD Anderson Cancer Center for their assistance in coordinating study efforts in the Rio Grande Valley. Funding for all authors and individuals named in the acknowledgements was provided by NIH R01 CA186132-01 and NIH R01 CA186132-Supplement, The University of Texas MD Anderson Cancer Center Support Grant (NCI Grant P30 CA016672), and The University of Texas MD Anderson Cancer Center R. Lee Clark Fellows Award. Parra S would also like to acknowledge the support of the Baylor College of Medicine Medical Scientist Training Program.
Footnotes
Conflict of Interest Statement: One author (Richards-Kortum R) is named as an inventor on patent applications for high resolution imaging owned by Rice University and The University of Texas. The authors report no other conflicts of interest.
References
- [1].American Cancer Society. Global cancer facts & figures. 3rd ed. Atlanta: American Cancer Society; 2015. [Google Scholar]
- [2].Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208 [doi]. [DOI] [PubMed] [Google Scholar]
- [3].National Institutes of Health. Fact Sheet: Cervical Cancer. https://www.report.nih.gov/nihfactsheets/viewfactsheet.aspx?csid=76. Updated October 2010. Accessed December 20, 2018.
- [4].Centers for Disease Control and Prevention. Cancer screening — united states, 2010. MMWR Morbidity and mortality weekly report. 2012;61(3):41. [PubMed] [Google Scholar]
- [5].Singh GK, Jemal A. Socioeconomic and Racial/Ethnic disparities in cancer mortality, incidence, and survival in the united states, 1950–2014: Over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi: 10.1155/2017/2819372 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Risser DR, Mokry B, Bowcock C, Miller EA, Williams MA, Magid R, Garcia R. Cervical cancer in Texas, 2010. Austin, TX: Texas Cancer Registry, Texas Department of State Health Services; Cancer Prevention Research Institute of Texas; 2010. [Google Scholar]
- [7].U.S. Cancer Statistics Working Group. U.S. cancer statistics data visualizations tool, based on november 2017 submission data (1999–2015). U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute Web site. https://gis.cdc.gov/Cancer/USCS/DataViz.html. Updated 2018. Accessed December 21, 2018. [Google Scholar]
- [8].U.S. Census Bureau. Small area income and poverty estimates (SAIPE). https://www.census.gov/data-tools/demo/saipe/saipe.html?map_geoSelector=aa_c&menu=map_proxy&s_state=. Updated 2017. Accessed December 21, 2018.
- [9].U.S. Census Bureau. Small area health insurance estimates (SAHIE). https://www.census.gov/data-tools/demo/sahie/#/?s_statefips=. Updated 2016. Accessed December 21, 2018.
- [10].Texas Department of State Health Services. Texas projections of supply and demand for primary care physicians and psychiatrists, 2017 – 2030. https://dshs.texas.gov/legislative/Reports-2018.aspx July 2018.
- [11].Saslow D, Solomon D, Lawson HW, et al. American cancer society, american society for colposcopy and cervical pathology, and american society for clinical pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542. doi: 10.1309/AJCPTGD94EVRSJCG [doi]. [DOI] [PubMed] [Google Scholar]
- [12].Shackelford DP, Griffin D, Hoffman MK, Jones DE. Influence of specialty on pathology resource use in evaluation of cervical dysplasia. Obstet Gynecol. 1999;94(5 Pt 1):709–712. doi: S0029784499004111 [pii]. [DOI] [PubMed] [Google Scholar]
- [13].Grant BD, Fregnani J, Resende JC, et al. High-resolution microendoscopy: A point-of-care diagnostic for cervical dysplasia in low-resource settings. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP). 2017;26(1):63–70. [DOI] [PubMed] [Google Scholar]
- [14].Pierce MC, Guan Y, Quinn MK, et al. A pilot study of low-cost, high-resolution microendoscopy as a tool for identifying women with cervical precancer. Cancer Prev Res (Phila). 2012;5(11):1273–1279. doi: 10.1158/1940-6207.CAPR-12-0221 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Quinn MK, Bubi TC, Pierce MC, Kayembe MK, Ramogola-Masire D, Richards-Kortum R. High-resolution microendoscopy for the detection of cervical neoplasia in low-resource settings. PLoS One. 2012;7(9):e44924. doi: 10.1371/journal.pone.0044924 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hunt B, Fregnani JHTG, Schwarz RA, et al. Diagnosing cervical neoplasia in rural brazil using a mobile van equipped with in vivo microscopy: A cluster-randomized community trial. Cancer Prev Res (Phila). 2018;11(6):359–370. doi: 10.1158/1940-6207.CAPR-17-0265 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pierce M, Yu D, Richards-Kortum R. High-resolution fiber-optic microendoscopy for in situ cellular imaging. J Vis Exp. 2011;(47). pii: 2306. doi(47): 10.3791/2306 . doi: 10.3791/230610.3791/2306. doi: 10.3791/2306 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pantano N, Hunt B, Schwarz RA, et al. Is proflavine exposure associated with disease progression in women with cervical dysplasia? A brief report. Photochem Photobiol. 2018;94(6):1308–1313. doi: 10.1111/php.12976 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Quang T, Schwarz RA, Dawsey SM, et al. A tablet-interfaced high-resolution microendoscope with automated image interpretation for real-time evaluation of esophageal squamous cell neoplasia. Gastrointest Endosc. 2016;84(5):834–841. doi: S0016-5107(16)01766-1 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shin D, Protano MA, Polydorides AD, et al. Quantitative analysis of high-resolution microendoscopic images for diagnosis of esophageal squamous cell carcinoma. Clin Gastroenterol Hepatol. 2015;13(2):272–279.e2. doi: 10.1016/j.cgh.2014.07.030 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wentzensen N, Massad LS, Mayeaux EJ Jr et al. Evidence-based consensus recommendations for colposcopy practice for cervical cancer prevention in the united states. J Low Genit Tract Dis. 2017;21(4):216–222. doi: 10.1097/LGT.0000000000000322 [doi]. [DOI] [PubMed] [Google Scholar]
- [22].Massad LS, Jeronimo J, Schiffman M, National Institutes of Health/American Society for Colposcopy and Cervical Pathology (NIH/ASCCP) Research Group. Interobserver agreement in the assessment of components of colposcopic grading. Obstet Gynecol. 2008;111(6):1279–1284. doi: 10.1097/AOG.0b013e31816baed1 [doi]. [DOI] [PubMed] [Google Scholar]
- [23].Massad LS, Jeronimo J, Katki HA, Schiffman M, National Institutes of Health/American Society for Colposcopy and Cervical Pathology Research Group. The accuracy of colposcopic grading for detection of high-grade cervical intraepithelial neoplasia. J Low Genit Tract Dis. 2009;13(3):137–144. doi: 10.1097/LGT.0b013e31819308d4 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019. doi: 10.1093/jnci/djy225 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Misrahi Y Automated visual evaluation (AVE) explained: Everything you need to know about the new AI for cervical cancer screening. January 14, 2019. Accessed June 10, 2019. [Google Scholar]