Abstract
Identifying the neural changes that support recovery of cognitive functions after a brain lesion is important to advance our understanding of human neuroplasticity, which, in turn, forms the basis for the development of effective treatments. To date, the preponderance of neuroimaging studies has focused on localizing changes in average brain activity associated with functional recovery. Here, we took a novel approach by evaluating whether cognitive recovery in chronic stroke is related to increases in the differentiation of local neural response patterns. This approach is supported by research indicating that, in the intact brain, local neural representations become more differentiated (dissimilar) with learning (Glezer et al., 2015). We acquired fMRI data before and after 21 individuals received approximately 12 weeks of behavioral treatment for written language impairment due to a left-hemisphere stroke. We used Local-Heterogeneity Regression Analysis (Purcell and Rapp, 2018) to measure local neural response differentiation associated with written language processing, assuming that greater heterogeneity in the pattern of activity across adjacent neural areas indicates more well-differentiated neural representations. First, we observed pre to post-treatment increases in local neural differentiation (Local-Hreg) in the ventral occipital-temporal cortex of the left hemisphere. Second, we found that, in this region, higher local neural response differentiation prior to treatment was associated with less severe written language impairment, and that it also predicted greater future responsiveness to treatment. Third, we observed that changes in neural differentiation were systematically related to performance changes for trained and untrained items. Fourth, we did not observe these brain-behavior relationships for mean BOLD responses, only for Local-Hreg. Thus, this is the first investigation to quantify changes in local neural differentiation in the recovery of a cognitive function and the first to demonstrate the clear behavioral relevance of these changes. We conclude that the findings provide strong support for the novel hypothesis that the local re-differentiation of neural representations can play a significant role in functional recovery after brain lesion.
Keywords: Spelling, dysgraphia, differentiation, orthography, stroke
1. INTRODUCTION
Given the loss of neural tissue subsequent to stroke, recovery of cognitive abilities necessarily involves functional reorganization of the remaining intact substrates. A precise understanding of recovery-related functional neural changes is vital both for understanding the neuroplastic capacities of the human brain and for developing targeted neural interventions. In this investigation, we specifically examined the hypothesis that an increase in the local differentiation of neural representations supports recovery of function.
1.1. Acquired dysgraphia
Recovery of written communication abilities is especially important for individuals with severe impairments in spoken communication. In this work, we specifically examined the recovery/re-learning of written spelling in the context of stroke-induced acquired dysgraphia, focusing on the central (core) processes of spelling rather than those involved in writing and motor execution. Spelling involves multiple cognitive functions, key among which are the cognitively and neurally distinct functions of orthographic long-term memory (OLTM) and orthographic working memory (OWM) (Buchwald and Rapp, 2009; Rapp et al., 2015). Orthographic LTM processes involve the storage and retrieval of learned knowledge of the spellings of words (constituent letters and their order), while orthographic WM maintains this information active while peripheral processes produce each letter serially in a specific format (e.g., writing, oral spelling or typing). Disruption to orthographic LTM and WM processes, as may occur subsequent to a stroke, results in characteristic behavioral patterns. Specifically, disruption to OLTM results in frequency-sensitive spelling (i.e., higher frequency words are spelled more accurately than lower frequency words). In contrast, disruption to OWM is characterized by sensitivity to the number of letters in a word, such that the probability of producing an incorrect letter increases with word length. Neurally, impairments to OLTM tend to be associated with left hemisphere inferior frontal and ventral occipitotemporal regions, while impairments to OWM tend to be associated with left parietal damage (Rapp et al., 2015). Another core component of the spelling system, which we do not focus on in this work, is the sublexical system (also referred to as phoneme-grapheme conversion). This system applies learned knowledge of the systematic relations between sounds and letters to generate plausible spellings for phonological strings and has been found to be associated with left hemisphere perisylvian regions (e.g., DeMarco et al., 2017; Henry et al., 2007).
A number of studies have reported improvement in spelling in individuals with acquired dysgraphia, in many cases despite persistent issues in spoken language (Beeson et al., 2010, 2003; Orjada and Beeson, 2005; Rapp and Wiley, 2019; Tsapkini and Hillis, 2013). However, few studies have examined the underlying neural mechanisms associated with post-stroke recovery in acquired dysgraphia. Here, we examine the novel hypothesis that the re-differentiation of neural representations supports recovery of function; in other words, that “re-learning to be different” may be an important part of the neural basis of functional recovery.
1.2. Recovery of function in the brain: Prior research and current hypotheses
Generally, post-stroke recovery is due either to neurophysiological changes (spontaneous or clinically induced) such as edema reduction, revascularization and reperfusion (Hillis et al., 2002; Kelly-Hayes et al., 1989) or to functional reorganization. In terms of the recovery of language functions, changes in the chronic post-lesion phase are thought to be largely due to functional reorganization driven by experience and/or therapy (Mohr, 2017).
Previous studies have overwhelmingly used mean task-based BOLD to evaluate functional reorganization, providing mixed findings in terms of the neurotopography of recovery-based functional changes. These studies have attributed positive recovery outcomes to: ipsilesional-perilesional reorganization (Fridriksson, 2010; Postman-Caucheteux et al., 2010; Winhuisen et al., 2007), activity modulation of contralesional areas homologous to the stroke (Thulborn et al., 1999; Gold and Kertesz, 2000; Blasi et al., 2002; Turkeltaub et al., 2012), or reorganization of both left perilesional and homologous contralesional areas (Crosson et al., 2005; Fridriksson et al., 2006; Kuest and Karbe, 2002). In addition to a range of findings regarding the neurotopography of neuroplastic changes, there have also been mixed results in terms of the directionality of the relationship between neural and behavioral changes. Some studies examining pre to post-treatment changes in mean BOLD have found activation increases (up-regulation) to be associated with recovery (Cardebat et al., 2003; Fridriksson et al., 2006; Johansen-Berg et al., 2002; Lindberg et al., 2007; Meinzer et al., 2008), while others have found recovery to be associated down-regulation (Blasi et al., 2002; Ward et al., 2003), and still others have reported both (Cramer et al., 1997; Liu et al., 2014; Saur et al., 2006). Similarly, functional connectivity studies have reported that connectivity increases (Fan et al., 2015), both increases/decreases (Zhang et al., 2016), or neither (Nijboer et al., 2017) are associated with positive recovery outcomes.
This range of neural patterns is also clearly illustrated in research comparing learning in older and younger healthy adults. For example, Bråthen (2018) reported on a study of episodic memory training in which they reported, among other things, on the relationship between learning and a measure of local resting state activity (fALFF) in the hippocampus. They found that while fALFF levels were generally positively related to learning in young adults, they were negatively correlated in older adults, even if they considered only those older adults with positive learning outcomes. Along similar lines, Lange et al. (2019) examined the relationship between white-matter changes and word-list learning over 10 weeks in older and younger healthy adults. They found that while the younger group exhibited significantly more learning than the older group, the magnitude of learning was related to microstructural white matter changes only in the older group. This occurred despite the fact that the learning task was dynamically adjusted to be comparably challenging across participants. De Lange (2017) interpreted these seemingly counterintuitive findings within a “supply and demand” framework proposed by Lövdén et al., (2010) according to which the magnitude of neuroplastic changes is a complex function of a number of factors including the demands placed on the brain and the capacity of existing systems (supply) to respond to them flexibly with/without engaging neuroplastic processes. Such findings highlight the complex and often non-linear nature of the relationship between the neural and cognitive changes associated with neuroplasticity and learning. These relationships are likely to be particularly complex when learning/re-learning takes place in the context of aging and/or brain lesions.
1.3. Neural representations in fMRI
More recently, beyond characterizing the neurotopography of mean changes in activation levels, research has begun to focus on understanding the content and nature of the neural representations that specifically underlie behavioral recovery (Fischer-Baum et al., 2017; Lee et al., 2017). The work reported here is in that vein, specifically, we apply a novel analytic approach (Purcell and Rapp, 2018) to measure treatment-induced changes in the local properties of neural representations in intact tissue.
Based on evidence that representational information is spatially distributed across adjacent voxels (Cox and Savoy, 2003; Haxby et al., 2001; Kriegeskorte et al., 2008), multi-voxel pattern analyses (MVPA) examine patterns of BOLD activation across adjacent voxels to explore local representations in a manner not possible in the analysis of mean activity alone (e.g., Mahmoudi et al., 2012). These analytic approaches have only very recently been used to investigate the nature and content of post-stroke neural representations. For example, recent work (Fischer-Baum et al., 2017) used MVPA to compare the neurotopography of visual and orthographic representations between healthy controls and an alexic individual with a left ventral occipitotemporal cortex (vOTC) lesion, finding a contralesional shift specifically for orthographic representations. Another recent study used fMRI MVPA classification in an individual with post-stroke anomia to discriminate between neural patterns for correctly vs. incorrectly named pictures in regions of the contralesional hemisphere (Lee et al., 2017). Although both studies demonstrate the utility of multi-voxel approaches for understanding changes in neural representations after brain lesion, as single-case studies they represent important proofs of concept that require further validation with larger participant numbers. Furthermore, to support strong inferences about the neural mechanisms underlying recovery-based neuroplastic changes, it is important to link neural representational effects to behavioral recovery.
1.4. Local representational differentiation
Here we present work which also involves multi-voxel analysis and is theoretically grounded in sparse coding theories which posit that, with learning, neural representations become sparser and more highly tuned (Olshausen and Field, 1996; Rolls and Tovee, 1995). This theoretical work is supported by electrophysiological evidence reporting that responses to well-learned stimuli involve stronger activation of smaller numbers of neurons compared to responses to less well-learned stimuli (Freedman et al., 2006; Kobatake et al., 1998). Furthermore, the finding that with learning, local populations of neurons have less correlated (i.e., more heterogeneous) firing patterns (Bair et al., 2001; Jermakowicz et al., 2009) has facilitated the application of sparse coding theories to the “coarser grain-size” of fMRI. On this basis, it has been proposed that well-learned neural representations involve smaller numbers of sharply tuned neurons distributed across adjacent voxels, and that this can be quantified by measuring the relative heterogeneity of BOLD responses across adjacent voxels. Accordingly, it is claimed that the relative heterogeneity of the BOLD response of adjacent voxels can index the degree of learning/differentiation of local neural representations (Jiang et al., 2013). This hypothesis was supported by the Jiang et al. (2013) finding of greater local, cross-voxel heterogeneity of BOLD responses to face stimuli in face-sensitive cortex in autistic individuals with better face perceptual discrimination performance, and more recently, by the finding that higher local heterogeneity is related to stronger cognitive abilities in healthy older adults (Jiang et al.,2017).
Recently, we built on previous approaches to measuring local response heterogeneity (Deshpande et al., 2009; Jiang et al., 2013; Zang et al., 2004) to develop Local Heterogeneity Regression Analysis (Local-Hreg; Purcell and Rapp, 2018). To quantify the relationship between the time-course of task-related activation across adjacent voxels, Local-Hreg adapts gPPI (general psychophysiological interaction) analysis (McLaren et al., 2012) that is traditionally used to examine the functional connectivity of non-adjacent brain regions. Furthermore, unlike previous approaches (Deshpande et al., 2009; Jiang et al., 2013; Zang et al., 2004), Local-Hreg uses a regression approach instead of correlation to analyze condition-specific variability across adjacent voxels, providing the following benefits: 1) it leaves the time series intact, identifying the task-relevant aspects of the signal through model interaction terms rather than signal segmentation; 2) it provides a direct method of quantifying local neural heterogeneity independent of the mean activity by incorporating the mean task-related response into the model; 3) it accounts for shared noise (e.g., due to motion) across adjacent voxels via motion-specific time-series interaction regressors; 4) it is a flexible method that is easily implemented in various fMRI designs and also as a spatial searchlight, allowing for analyses not limited to predefined regions. Confirming the validity of Local-Hreg for indexing increased representational differentiation that results from learning, in Purcell & Rapp (2018) we reported that for neurologically healthy adults who read words while undergoing fMRI, the local response heterogeneity of BOLD was greater for well-learned (high frequency) words relative to less well-learned or unknown letter strings (low frequency words and pseudowords); these patterns were observed in left ventral temporal brain areas typically associated with orthographic representation in reading (Cohen & Dehaene, 2004; Cohen et al., 2000).
1.5. Current study
The investigation reported on here involved 21 individuals with chronic dysgraphia (an impairment affecting central orthographic spelling processes; (Purcell et al., 2011)) following a single left-hemisphere stroke. For each participant, individualized lists of Training words were selected based on low spelling accuracy and, thus, were indexed to each participant’s specific skill level. As a control to evaluate the specificity of any neural changes, we also selected individualized lists of Known words that each participant could accurately spell. Participants underwent approximately 12 weeks of spelling rehabilitation and, before and after treatment, they were administered a comprehensive battery of language and cognitive tasks and underwent fMRI scanning. Brain activity data from an fMRI spelling task (Rapp and Lipka, 2011) were used in a series of analyses directed at evaluating the local differentiation of neural representations involved in spelling at both pre- and post-treatment time-points.
Given that local response heterogeneity indexes the integrity of neural representations by quantifying neural differentiation, we hypothesized that a brain region that participates in both the storage and “re-storing” of orthographic representations of word spellings should exhibit the following neural differentiation properties: (1) the degree of differentiation for a set of words should be related to spelling performance on those words; (2) assuming that the degree of differentiation for a subset of orthographic representations indexes the general integrity of the orthographic representational system, we would expect that the degree of differentiation for a word set to predict the magnitude of future behavioral improvements was due to training; (3) orthographic (re)-learning should be associated with an increase in neural differentiation; and (4) the amount of (re)-learning should be related to the degree of change in neural differentiation. Any region exhibiting these properties likely plays a pivotal role in the re-learning/recovery of orthographic representations. Our expectation was that area/s exhibiting these properties are most likely to be situated in brain regions known to be involved in the representation and processing of orthographic long-term memory representations. A key candidate region is ventral occipitotemporal cortex – a region (which includes the Visual Word Form Area) that has been previously identified as playing a key role in orthographic representation and processing in both reading (Cohen et al., 2000; Dehaene and Cohen, 2011; Gaillard et al., 2006; Martin et al., 2015) and spelling (Purcell et al., 2017, 2011; Rapcsak and Beeson, 2004; Rapp and Lipka, 2011). In particular, this region has been specifically associated with the processing and representation of orthographic long-term memory representations of word spellings (Glezer et al., 2015, 2009; Rapp et al., 2015; Rapp and Dufor, 2011; Szwed et al., 2011) and was shown, as indicated above, in Purcell & Rapp (2018) to exhibit greater differentiation as measured by Local-Hreg for well-learned compared to less well-learned orthographic representations in reading.
In order to investigate these issues, we used the Local-Hreg analysis to quantify the local response heterogeneity for Training words targeted in rehabilitation as well as for Known words. Local-Hreg was used to track pre- to post-treatment changes in neural representations and relate these to behavioral changes and characteristics. Specifically, we carried out four sets of Local-Hreg analyses. First, we identified areas throughout the whole brain in which local neural differentiation (Local-Hreg) significantly changed from pre to post-treatment. Second, we examined these areas of neural change for the relationship between Local-Hreg and various behavioral measures: pre-treatment Local-Hreg and severity of spelling impairment on the to-be-trained items; pre-treatment Local-Hreg and future response to treatment; pre-treatment Local-Hreg and generalization of behavioral treatment effects to Untrained items; and, importantly, the relationship between changes in neural differentiation and behavioral improvement (both for Training items and for generalization to Untrained spelling items). Third, we examined the relationship between Local-Hreg and local mean BOLD response in order to understand the extent to which these two “perspectives” on the BOLD signal reveal similar or different properties. Applying these analyses, we identified only one region - left vOTC - that demonstrated the properties that were expected to be associated with an area involved in recovery-based neural differentiation. These findings provide strong evidence that representational changes specifically within ipsilesional left vOTC play a key role in supporting recovery in dysgraphia subsequent to brain lesion. Further, they more provide strong support for the conclusion that cross-voxel response heterogeneity indexes the local differentiation of neural representations and that increases in neural representational differentiation may be important for the recovery of cognitive functions.
2. METHODS
2.1. Participants
The primary inclusion criteria for this study were that each participant have a left hemisphere stroke, be in the chronic phase of recovery (i.e. > 1 year post-stroke), have an acquired impairment in spelling, no contraindication for fMRI, and no other neurological disease or history of developmental dyslexia/dysgraphia. Twenty-five individuals with chronic spelling impairments subsequent to a single left-hemisphere stroke were enrolled. Each individual participated in the following: pre-treatment fMRI data acquisition that was distributed across two days (within a 1-week period), a behavioral treatment program of approximately 3 months, and post-treatment fMRI data acquisition that was distributed across two days (within a 1-week period). In order to track retention of behavioral improvements due to treatment, behavioral data was also obtained at follow-up after a 3-month period during which no treatment was administered. One participant was excluded due to data acquisition error and four participants withdrew due to health reasons, but there was sufficient data for one (participant AES; see Table 1) to be included in Analyses 1 and 2. See Table 1 for demographic, lesion and other information; three were left handed while two were ambidextrous. Consent was obtained using procedures consistent with the Declaration of Helsinki and the Johns Hopkins University Institutional Review Board.
Table 1:
Acquired Dysgraphia Participant Characteristics
| Participant Characteristics | Spelling Performance | |||||||
|---|---|---|---|---|---|---|---|---|
| ID | Gender | Age (yrs) | Education (yrs) | Time post stroke (months) | Lesion volume (cc) | Number of treatment sessions | Untrained Items (% correct) | Type of spelling deficit |
| DTE | F | 80 | 18 | 14 | 64.7 | 40 | 76% | OWM |
| PQS | M | 54 | 18 | 17 | 92.8 | 17 | 86% | OWM |
| THD* | M | 67 | 18 | 76 | 215.0 | 21 | 84% | OWM |
| TTR* | F | 46 | 16 | 21 | 113.9 | 21 | 51% | OWM |
| CCN* | M | 40 | 18 | 38 | 100.7 | 23 | 91% | OLTM |
| CIE | F | 62 | 14 | 85 | 45.9 | 17 | 61% | OLTM |
| DSK | M | 67 | 16 | 59 | 135.5 | 12 | 74% | OLTM |
| JGL | F | 72 | 16 | 32 | 36.3 | 48 | 86% | OLTM |
| RFZ | M | 60 | 18 | 46 | 55.3 | 16 | 94% | OLTM |
| RHH | M | 45 | 16 | 82 | 108.8 | 18 | 87% | OLTM |
| RHN | F | 75 | 19 | 27 | 7.7 | 16 | 90% | OLTM |
| ABS | M | 58 | 18 | 97 | 138.9 | 19 | 84% | OWM+OLTM |
| AEF | F | 55 | 16 | 101 | 214.6 | 25 | 82% | OWM+OLTM |
| ESG | M | 62 | 16 | 38 | 102.8 | 27 | 54% | OWM+OLTM |
| FCE | M | 64 | 12 | 119 | 42.2 | 19 | 77% | OWM+OLTM |
| KMN | M | 55 | 15 | 28 | 52.0 | 48 | 77% | OWM+OLTM |
| KST | M | 61 | 14 | 46 | 18.2 | 29 | 78% | OWM+OLTM |
| MSO | M | 45 | 18 | 103 | 166.9 | 30 | 56% | OWM+OLTM |
| TCI | F | 69 | 12 | 45 | 67.0 | 22 | 66% | OWM+OLTM |
| TCK | M | 69 | 16 | 68 | 21.1 | 26 | 85% | OWM+OLTM |
| AES† | F | 59 | 16 | 208.9 | 182.1 | 24 | 82% | OLTM |
ID - Participant Identification; yrs - years; cc- cubic centimeters;
Auditory Comprehension deficit (see Supplemental Materials Table S1);
OWM - Orthographic Working Memory; OLTM - Orthographic Long Term Memory. Untrained spelling performance on a composite spelling battery described further in Supplemental Materials Section S1.
Only included in the behavioral and fMRI Analyses 1 and 2 given that post-treatment behavioral data, but not post-treatment fMRI is available.
2.2. Behavioral Methods
2.2.1. Cognitive/Language Assessments
At both pre and post-treatment time-points, a battery of language and cognitive tests was administered to determine if treatment affected only the spelling of trained words or if it also generalized to untrained words and/or to cognitive functions more generally (see Supplemental Materials Section S1). Letter accuracy, rather than whole-word accuracy, was used throughout, as it is a more precise measure of spelling performance (e.g., the misspelling of BOAT as BOET has a 75% letter accuracy while a misspelling such as BUAD has a 25% letter accuracy). Although some participants (e.g., RFZ) had high accuracy on the spelling assessments, their post-stroke spelling abilities were known to have decreased relative to their very high pre-stroke levels. Importantly, each training regimen was individualized (discussed further below) ensuring low performance (even for these participants) on the individualized Training word lists (< 81% letter accuracy).
Spelling deficit type and accuracy were quantified with multiple spelling tests. Spelling deficit type was characterized as affecting orthographic long-term memory, orthographic working memory or both. The former was defined by sensitivity to word frequency (Brysbaert and New, 2009) but not length and the latter by the reverse pattern (Rapp et al., 2015); see Supplemental Materials Table S1 for detailed behavioral results. To examine generalization of spelling treatment, sets of Untrained words were used and assessed at both time-points. The sets of Untrained words varied across participants (mean n = 195 words, range 40-454) and served as a measure of general spelling severity (see Supplemental Materials Section S1); see Table 1 for pre-treatment accuracy on Untrained words. Although the Untrained word lists did vary across participants, 16/21 participants spelled a shared subset of 129 words, providing some common basis for evaluating general severity1. For three participants with additional auditory comprehension impairments (< 90% accuracy on spoken word-picture matching (Thompson et al., 2012); see Supplemental Materials Table S1), spelling evaluations and scanner tasks were the same as for the other participants with the sole exception that visual pictures were used rather than auditory stimuli.
2.2.2. Treatment Methods
Prior to pre-treatment scanning, an individualized Training word set was developed for each participant, consisting of words with 25 - 80% letter accuracy on two baseline tests (n = 40); see Supplemental Materials Section S1 for further information on individualized word list selection. A Spell-Study-Spell treatment (Beeson, 1999) was administered targeting the Training items during 60-80 minute sessions during which multiple spelling items were trained. Each training trial proceeded as follows: (1) the individual heard a target word, repeated it, and attempted to write the spelling, (2) regardless of accuracy, the individual was shown the correct spelling while the experimenter said aloud the word’s letters; then the individual copied the word once and was instructed to study the word. If the word had been spelled correctly at Step 1, then the training trial ended and the experimenter continued to the next item. Otherwise, the word was removed from view and Steps 1 and 2 were repeated until the word was spelled correctly, or for a maximum of three times before moving to the next item. Sessions were administered twice per week, for an average of 12.4 weeks (standard deviation = 5 weeks; min = 6 and max = 24). Training ended with greater than 90% accuracy on Training items for two consecutive sessions or failure to improve after six sessions. To measure training-based improvement, assessments of spelling accuracy for Training items were administered at pre and post-treatment time-points. In order to measure retention, spelling accuracy for Training items was also assessed after a 12-week follow-up period during which no treatment was carried out (Note: no neuroimaging data were acquired at the follow-up time-point).
2.3. Neuroimaging Design, Acquisition and Pre-processing
2.3.1. Stimuli
Three individualized word sets were identified for each participant: Training words (randomly selected from the Training items; n=30), Known words (100% accuracy on two baseline tests; n=30); Case Verification words (matched on frequency and length to Training and Known words; n=30).
2.3.2. FMRI: Spelling Task
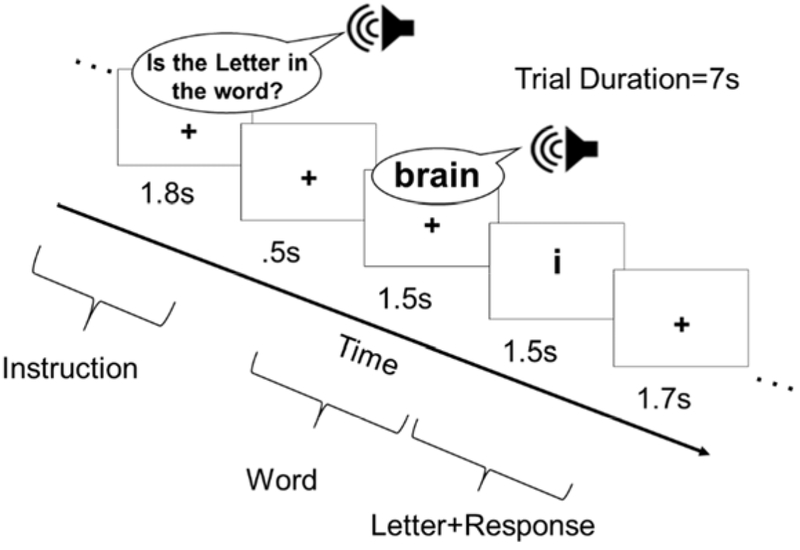
An event-related design included two tasks: Spelling Probe (SPELL) and Case Verification control task (CASE) (Rapp and Lipka, 2011). Each SPELL trial began with an instruction: “Is the letter in the word” (1800 ms), followed by 500 ms of silence, and an auditorily presented target word (1500 ms); this was followed by a visually presented lower-case letter (1500 ms) and, finally, 1700 ms of silence during which participants responded with a yes/no button press if the visually presented letter was in the spelling of the word (Figure 1). Stimuli corresponded to either Training or Known words. The control Case Verification trials were identical (i.e., a cue, followed by an auditory word, followed by a letter were presented), except that Case Verification words were used with the instruction: “Is the letter Upper Case?” and participants responded yes/no if the visual letter was uppercase or not (in this condition,, target letters were never in the spelling of the word).
Figure 1:
Sample Spelling Probe trial with timing parameters. For the Case Verification control task, the instructions were “Is the Letter Upper Case?”.
This spelling task - with a left-handed button-press response rather than written response - was designed to facilitate ease of response across all of the stroke participants, despite sensory or motor difficulties or paresis. The task was not, however, designed for precisely quantifying behavioral changes associated with spelling performance (i.e., there is a 50/50 chance of a correct response on any given trial), but did require each participant to retrieve and query the spelling of each word within an event-related trial scheme.2
To minimize the amount of time participants were in the scanner within one session, data acquisition at pre and post-treatment time-points took place over two scan-sessions across two days (acquired within one week of each other). Each of the two scan sessions was 1.5 hours in duration and had two Spelling probe runs each; additional functional data were acquired for: resting state, reading, a basic visual-motor task, visual object recognition, Diffusion Tensor Imaging (DTI), and Arterial Spin Labeling (ASL), but are not discussed in this report. For each run there were 15 trials per condition; therefore, across all four runs at each pre or post-treatment time-point there were 60 trials per condition (stimuli were repeated twice). Trial sequences and jittered, between-trial fixation periods were pseudorandomized (range of 2 to 5 secs duration) using the MATLAB based tool easy-optimize.X (http://www.bobspunt.com/easy-optimize-x/). Each run was 7 min, 42 sec., with 32% of the time dedicated to fixation periods.
2.3.3. Structural MRI: Data acquisition and lesion tracing
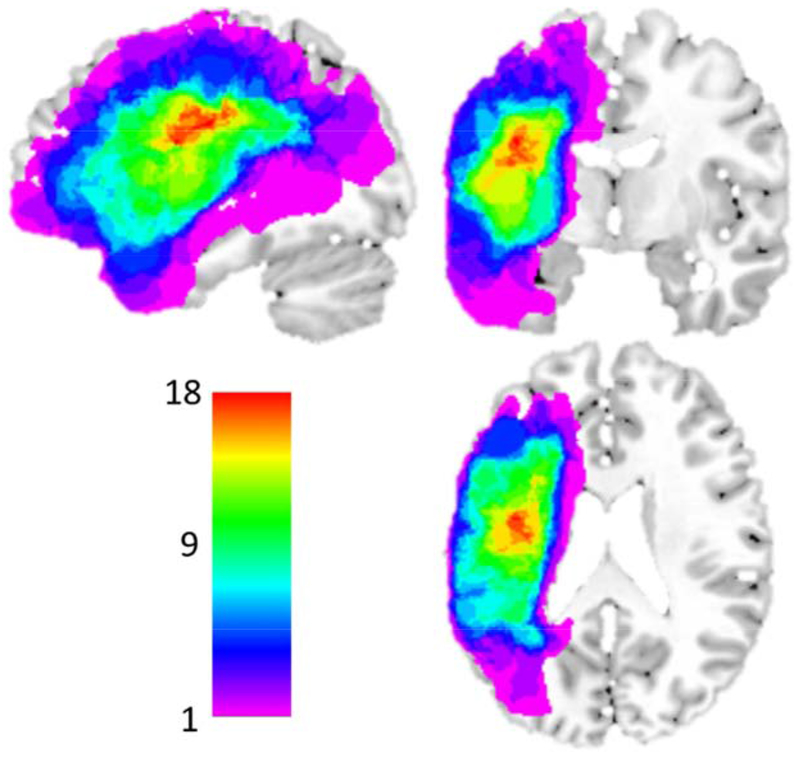
Structural MPRAGE scan: 176 sagittal slices, multishot, turbo field echo pulse sequence, slice thickness = 1mm, and in-plane resolution of 1x1mm2. Each structural scan was aligned to the AC-PC plane and lesion masks were drawn using MRIcron (Rorden and Brett, 2000) within voxels with T1 hypointensity. Given that our participants were primarily older, images were normalized to a standard older T1 weighted template (Rorden et al., 2012). In order to account for brain lesion abnormalities, enantiomorphic normalization approach was used (see Nachev, Coulthard, Jäger, Kennard, & Husain, 2008) using SPM12. Normalization parameters were applied to the structural and functional data for normalization to MNI space. Analyses were constrained to a gray matter mask (Harvard-Oxford atlas cortical and sub-cortical regions; (Desikan et al., 2006); see Figure 2 for a lesion distribution map.
Figure 2: Distribution of MNI normalized lesions in the 21 acquired dysgraphia participants.
Only participants with left hemisphere stroke were included in this study; inclusion based on lesion location was not constrained in any other way. Color scale indicates the number of overlapping lesions at each voxel. The areas of greatest lesion overlap include left posterior frontal, insula, anterior parietal and superior temporal regions. Note that the left ventral occipitotemporal cortex was mostly intact in this sample.
2.3.4. Functional MRI data acquisition and preprocessing
We acquired 27 axial slices of fMRI Echo Planar Imaging scans: FOV = 240 x 240mm, TR/TE = 1500/30 ms, 27 axial slices, 3x3 mm in plane resolution, and 3 mm slice thickness with 1.5 mm gap. This field of view was sufficient to cover the entire cerebrum in every individual, but not the cerebellum. Three dummy scan volumes were run and discarded by scanner. Paradigms were developed and presented in Eprime 3.0 (Schneider et al., 2012). Each participant’s vision was normal or corrected to normal.
Functional and anatomical data were analyzed using a processing pipeline integrated in MATLAB that incorporated BrainVoyager QX 2.4 (BV) and SPM12. BV pre-processing steps included slice-time correction with the first slice as reference with sinc interpolation, rigid body correction for motion to the first volume using trilinear interpolation for detection, sinc for actual correction, and re-sampling to 3mm3. Then, in SPM12, pre-processed functional data were co-registered to the un-normalized T1 weighted structural scan, and then normalization parameters were applied to the data. This was followed by high-pass temporal filtering (90 Hz) applied via MATLAB. For the Standard mean BOLD GLM analysis (but not the Local-Hreg Analysis) described below, data were smoothed by 6mm FWHM via SPM12 using a fixed kernel.
2.4. FMRI Analysis Approaches
Although the primary goal of the investigation was to quantify local representational heterogeneity using Local Heterogeneity Regression (Local-Hreg), for comparison purposes, we also carried out standard mean BOLD GLM analysis.
2.4.1. Standard mean BOLD GLM
A random-effects GLM was evaluated at each voxel, including all participants, pre and post-treatment data and a minimum of fourteen regressors: 6 regressors corresponded to the trial portions of the time series (see Figure 1): the instruction, the word, and 4 for the visual letter+response (one each for Training, Known, Case, and no- response trials (on which participants failed to respond)); to account for participant-specific item variability: 2 item-specific regressors for word frequency and length (mean-centered and convolved with the canonical HRF), 6 motion parameters (roll, pitch, yaw, x, y, z), and from 0 to 7 principle component analysis (PCA) regressors that estimate physiologically plausible noise from high variable voxels (highest 2% standard deviation across the time series) and a cerebral spinal fluid mask (optimized compcor method; Soltysik et al., 2015). Fixations were left un-modeled and constituted the implicit baseline. In-scanner performance was recorded and in-scanner accuracy and reaction time are reported in Supplemental Materials Section S2. (See Section S3 for a report on the overall “dysgraphic” spelling network obtained based on a contrast between Spelling Probe and Case Verification tasks combined across both Pre- and Post-Treatment time-points).
2.4.2. Local-Hreg
Local-Hreg (Purcell and Rapp, 2018) uses a general psychophysiological interaction approach (gPPI; McLaren et al., 2012) to quantify the similarity of condition-specific BOLD signal across adjacent voxels. Local-Hreg is a searchlight analysis performed on un-smoothed, pre-processed, MNI normalized data. Here, each searchlight contained 7 voxels; a central voxel and the 6 shared-face surrounding voxels3. For each searchlight, a general psychophysiological interaction model (gPPI) was used to characterize the center voxel using the following equation (adapted from McLaren et al., 2012):
In the equation, Yk is the time-series obtained from the center voxel of the searchlight, and is used to develop a gPPI model that predicts the time series of each adjacent voxel sharing a face with the center voxel. In a searchlight of seven voxels, there are six adjacent voxels, denoted by yi. The equation corresponds to the linear combination of the mean BOLD fit estimates, the interaction BOLD fit estimates, and the error term. In the mean BOLD fit estimates portion of the model, H(gp) corresponds to the condition regressors (e.g. Training items) gp convolved with the canonical hemodynamic function H. N corresponds to a matrix of nuisance regressors (e.g., motion parameters). The H(gp) and N regressors are exactly the same as those used in used in the mean BOLD GLM analysis described just above. βG are the parameter estimates for Yk, the condition regressors and the nuisance regressors; these terms estim ate the condition specific mean response in Yi. Including Yk accounts for physiologically based variance in Yi. The mean BOLD fit estimates portion of the model essentially accounts for the task-based (i.e. psychological) and physiological shared variance of the center voxel relative to each adjacent voxel.
The interaction BOLD fit estimate portion of this equation takes each term used in the mean fit estimates portion and multiplies it by the center voxel time series Yk, thereby generating interaction terms (βi) for both regressors of interest and non-interest. These interaction estimates quantify the amount of shared variance across adjacent voxels during the portion of a time series corresponding to a given condition (e.g. Training items). This model derived from the center voxel time series is then used via regression to predict the adjacent voxel time series; from this we obtain β estimates that reflect shared physiological variance (i.e., time series), the conditions (i.e., original condition regressors), and the critical shared condition-specific variance (i.e., the interaction terms). For each searchlight, the Local-Hreg values for a given condition of interest correspond to the inverse of the median of these six βi estimates (i.e., higher Local-Hreg values correspond to higher heterogeneity and lower Local-Hreg values correspond to lower heterogeneity). These values are assigned to the center voxel of each searchlight.
For the analyses described below, we extracted the following for each participant (at each voxel and each pre and post-treatment time-point): Local-Hreg values for Training and Known conditions. No additional smoothing was applied for subsequent Local-Hreg analyses with the exception of those presented in Supplemental Materials Section S4.
2.5. Behavioral treatment statistical analyses
First, to assess spelling improvement associated with the training from pre- to post-treatment, two linear mixed effects (LME) models (Baayen et al., 2008) per participant were evaluated (R. lme4; Bates et al., 2015), for Training items and Untrained items. The dependent variable was percent letters correct per word; fixed effects: time-point (pre, post, and follow-up), word frequency, and word length; random effects: a random intercept and random slope for time-point by item. Improvement betas corresponded to the effect of time-point (pre-treatment coded as −1 and post-treatment as +1). These models tested the significance of improvement for each individual participant (p-values obtained via Satterthwaite approximation; Kuznetsova et al., 2017).
Second, to assess retention of spelling improvement beyond the training period, two additional linear mixed effects models for each individual participant (in the same manner as above) evaluated the change between the pre-treatment time-point and the 3-month follow-up time-point. This was done for both for Training items and Untrained items.
Third, to obtain robust effect size estimates for each participant for subsequent neural-behavioral analyses, we evaluated two LME models (for Training and Untrained items) for the group. These were identical to the participant models, with the addition of a maximal random effects structure by participants (Barr et al., 2013)4. These models provided the participant-specific Pre-Treatment, Post-Treatment, and Change (i.e., post minus pre-treatment) β estimates, for both Training and Untrained words, that were used in subsequent analyses.
2.6. Analysis 1: Identifying brain areas in which Local-Hreg changed from before to after treatment
If increases in representational differentiation support recovery of function, then we should be able to identify brain areas in which Local-Hreg changed from before to after treatment. For each participant and at each voxel within a whole brain gray matter mask including cortical and sub-cortical gray matter (Harvard Oxford atlas; Desikan et al., 2006)), we evaluated the difference between the pre and post-treatment Local-Hreg values for the Training items using two-way t-tests (i.e., both positive and negative change was evaluated). A voxel-wise threshold = p < 0.05 was used and a cluster-size threshold was identified using a non-parametric permutation test (Nichols and Holmes, 2002) involving label-scrambling (e.g., whether a condition was pre or post-treatment) for each of 10,000 iterations (extent threshold = 30 voxels). Furthermore, to evaluate the specificity of pre to post Local-Hreg changes, the same analysis was carried out using pre and post-treatment Local-Hreg values for the Known items.
2.7. Analysis 2: Examining the relationship between Local-Hreg and spelling performance
For any significant Local-Hreg “change cluster” identified in Analysis 1, we carried out two sets of region of interest (ROI) analyses (Sections 2.7.1 & 2). Analysis 2A evaluated the relationship between pre-treatment Local-Hreg values and pre-treatment spelling performance. Analysis 2B evaluated the relationship between pre- to post-treatment changes in Local-Hreg and pre- to post-treatment changes in spelling accuracy.5 Note that in this, and all other subsequent analysis examining the relationship between a neural measure and spelling performance, we considered spelling performance as measured on assessments administered outside the scanner (e.g., at pre and post-treatment time-points) rather than spelling behavior measured during scanning. We believe that this approach provides a much more stringent test of whether the neural characteristics (e.g., Local-Hreg) are related to spelling competence since these measures are independent from the activation signal used to obtain the Local-Hreg values.
2.7.1. Analysis 2A: Is the magnitude of pre-treatment Local Hreg associated with contemporaneous spelling accuracy and/or future responsiveness to treatment?
First, for any ROI identified in Analysis 1, we evaluated an LME model in which pre-treatment Local-Hreg values for every voxel were used as the dependent variable and both Pre-Treatment Accuracy βs and Accuracy Change β’s for the Training items served as key independent variables of interest. Because the analysis included both of these regressors, it addressed the potential confound between pre-treatment accuracy on the Training items and the maximum amount of improvement that would have been possible given an individual’s starting accuracy.
Furthermore, for this and subsequent ROI analyses, we also included the additional z-normalized fixed-effects which accounted for participant characteristics (age, lesion volume), general deficit severity (as indexed by pre-treatment accuracy on Untrained items), and mean condition specific in-scanner reaction time. An age regressor was included based on work indicating that aging can be associated with poorly differentiated representations (e.g., Park et al., 2004), a lesion volume regressor was included to account for any modulation in the BOLD signal or behavior that might be due to lesion size, and a general deficit severity regressor was included to account for individual differences at the start of the study in terms of the general integrity of the spelling system. An in-scanner reaction time regressor was included as it is reported to be associated with BOLD modulation across a wide range of tasks (Yarkoni et al., 2009) and, therefore, its inclusion should help account for BOLD response variability related to response-time differences stemming from cognitive functions unrelated to spelling (e.g. attentional, sensorimotor). Additionally, a fixed effect regressor of the mean Local-Hreg value of each voxel’s surrounding voxels (i.e., the 26 surrounding voxels) was included to account for cross-voxel spatial autocorrelation (Hanke and Wichern, 2005). In order to account for variability across participants and voxels within the ROI, there were random intercepts and slopes for mean-neighboring value by-participants and by-voxels. All analyses were carried out in MATLAB, with degrees of freedom estimated with the Satterthwaite method (Kuznetsova et al., 2017).
Second, we evaluated whether pre-treatment Local-Hreg predicted future changes in spelling accuracy for Untrained words. To examine this, we used the same LME model analysis as described just above, except that behavioral improvement for the Untrained words (instead of for Training words) was the primary independent variable of interest (i.e., Untrained Accuracy Change β’s; see Section 2.5).
2.7.2. Analysis 2B: Do changes in pre to post-treatment Local-Hreg correlate with pre to post-treatment changes in spelling accuracy?
For any ROI identified in Analysis 1, we first tested whether the magnitude of the Local-Hreg increases from pre to post-treatment were related to the magnitude of improvement in spelling accuracy for Training items. To do this we evaluated an LME model in which both the pre-treatment and post-treatment Local-Hreg values for every voxel were used as the dependent variable. In this model the fixed effects of interest were Accuracy Change βs for Training items (see Section 2.5), time-point (pre-treatment coded as −1 and post-treatment as +1), and the interaction between Accuracy Change βs for Training items and time-point. The interaction quantified whether the change in Local-Hreg for Training items was related to the change in their spelling accuracy.
Second, we tested whether the magnitude of the Local-Hreg increases were related to the magnitude of improvement in spelling accuracy for Untrained words. Local-Hreg is based on the BOLD signal during the in-scanner Spelling task specifically for the Training words. Because these words were selected to target a moderate difficulty level for each person, they differ across individuals and, thus, may index only the degree of representational differentiation associated with the Training items and not with the differentiation of the orthographic system more generally. However, there is the possibility that the Local-Hreg analysis may also be sensitive to the differentiation of orthographic system if we consider that the Training word representations may be functionally integrated in an interdependent manner within of a broader orthographic representational network. In that case, Local-Hreg values at pre-treatment may also index the general integrity/health of the orthographic system and, accordingly, may predict response to treatment even for Untrained words. To examine this possibility, we evaluated another LME model like the one described in the previous paragraph except that the Accuracy Change βs for Untrained words were included as the fixed effect of interest instead of the Accuracy Change βs for Training words. The interaction between time-point and Accuracy Change βs for Untrained words quantified whether the change in Local-Hreg for Training words also supported improvement in spelling accuracy for Untrained words.
2.8. Analysis 3: Evaluating the relationship between Local-Hreg and mean local BOLD
To date, changes in mean BOLD activations have constituted the primary measure evaluated in investigations of the neural changes that support recovery. Since both mean BOLD and Local-Hreg measures involve analysis of the BOLD signal, it is important to not only also examine changes in the mean BOLD response, but to also understand the relationship between these two measures. To this end, we carried out three sets of analyses (2.8.1-3)
2.8.1. Analysis 3A: Are there brain areas in which the mean BOLD response changes from pre to post-treatment?
In order to determine if areas involved in pre to post-treatment Local-Hreg changes also exhibited mean BOLD changes, we calculated a whole-brain mean BOLD difference map between pre and post-treatment values for the Training items. A cluster-wise extent threshold was obtained by running a Monte Carlo simulation (Forman et al., 1995) via the AlphaSim program in the NeuroElf toolbox estimating the smoothness of the data and maximal cluster due to chance at an alpha level < 0.05 after 10,000 iterations of simulated random voxel responses that are non-isometric in the x, y, and z dimensions, yielding a cluster threshold of 49 voxels.
2.8.2. Analysis 3B: Do pre to post-treatment changes in mean BOLD correlate with behavioral changes for Trained items?
To specifically evaluate the relationship between mean BOLD change and behavioral treatment changes, for each cluster identified in the previous step, we extracted the mean BOLD responses from each voxel and used them as dependent variables in two LME models (per cluster), as described in Section 2.7.2, except that the neural measure was mean BOLD, rather than Local-Hreg.
2.8.3. Analysis 3C: Do mean BOLD changes correlate with Local-Hreg changes?
To directly examine the relationship between changes in mean BOLD and Local-Hreg, we performed an LME model analysis on any Local-Hreg change clusters identified in Analysis 1. The dependent measure was Local-Hreg for Training items, and the fixed effect of interest was the mean BOLD for Training items, using both pre and post-treatment data and a fixed effect for time-point. Additional z-normalized fixed effects: age, lesion volume, spelling deficit type, deficit severity, and in-scanner reaction time. Finally, to quantify the amount of variance in Local-Hreg explained by the mean BOLD response in a cluster, we calculated the proportion of total variance explained (R2) for the fixed effects with and without the mean BOLD regressor (Nakagawa and Schielzeth, 2013); this identifies the percentage of Local-Hreg variance explained that is accounted for by the mean BOLD response.
3. RESULTS
3.1. Evaluation of behavioral treatment effects
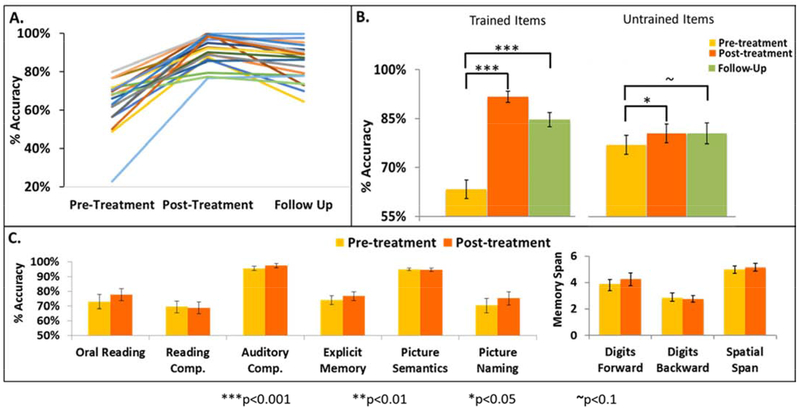
First, in order to quantify the effectiveness of spelling treatment we examined changes in spelling for Training items accuracy from Pre-Treatment, Post-Treatment, and at Follow-Up (Figure 3A). As summarized in Figure 3B (left), a post-hoc t-test from a repeated measures ANOVA (F(2,38) = 66.7, corrected p < 0.001) indicates not only a significant increase from pre- to post-Treatment (Bonferroni corrected p < 0.001) but also a large effect size (Cohen’s d = 2.3) which demonstrates the clear effectiveness of the spelling treatment. To confirm that these behavioral gains were maintained for at least 3 months after the treatment was completed, another post-hoc t-test confirmed that there was also a significant increase when comparing spelling accuracy on Training items from Pre-Treatment to Follow-Up (Bonferroni corrected p < 0.001, Cohen’s d = 1.6). These group results were confirmed with individual LME models that included performance on every spelling trial. We found that 20 out of the 21 participants demonstrated significant (p<0.05) increases in performance, while one demonstrated a p <0.1 increase. Furthermore, from Pre-Treatment to Follow-Up, 16 out of 20 participants demonstrated significant (p<0.05) increases, while 2 demonstrated p <0.1 increases, and only 2 did not maintain significant performance gains at Follow-Up. These results demonstrate that nearly every participant improved in spelling accuracy as a result of treatment and that the gains were robust as they were largely maintained for 3 months after treatment was completed.
Figure 3: Scores on spelling and other language and cognitive tests.
A. Raw score percent Accuracy on Training Items at Pre-Treatment, Post-Treatment, and Follow-Up. Each color depicts an individual participant. B. Average percent spelling accuracy at Pre-Treatment, Post-Treatment, and Follow-Up for the Training Items on the left and Untrained Items on the right. C. Performance at Pre- and Post-Treatment for tests not associated with spelling; see Supplemental Materials Sections S1 for task details. For B and C, the error bars are standard error of means; corrected thresholds *** p< 0.001; *p< 0.05; ~ p <= 0.1
Second, we tested whether these treatment effects generalized to spelling items that were Untrained. As summarized in Figure 3B (right), a post-hoc t-test from a repeated measures ANOVA (F(2,38) = 4.995, corrected p = 0.012) indicates a significant increase from pre- to post-treatment (Bonferroni corrected p = 0.031, Cohen’s d = 0.63), demonstrating the generalization of the spelling treatment. To confirm that these generalization gains were maintained for at least 3 months after the treatment was completed, another post-hoc t-test confirmed that there was also a marginally significant increase when comparing spelling accuracy on Untrained items from Pre-Treatment to Follow-Up (Bonferroni corrected p = 0.1, Cohen’s d = 0.49). These group results were confirmed with individual LME models that included performance on every spelling trial. We found that 12 out of the 20 participants exhibited significant (p<0.05) increases in performance on Untrained items while 1 demonstrated a p <0.1 increase. Furthermore, from Pre-Treatment to Follow-Up, 13 out of 20 participants exhibited significant (p<0.05) increases. These results indicate that over half of the participants demonstrated spelling improvements that generalized to Untrained items and that these gains were maintained for 3 months after treatment was completed.
Finally, we evaluated the selectivity of the treatment effects in order to allow for the attribution of any observed neural changes to treatment-based spelling recovery. Here, we considered if treatment effects generalized to other language and cognitive functions (see Supplemental Materials Section S2 for test details). To do so, we examined pre to post-treatment accuracy changes on a battery of language and cognitive tests (Bonferroni adjusted for multiple comparisons). The analysis indicated that no other language/cognitive tasks showed significant improvement, indicating that the spelling treatment selectively targeted only spelling (Figure 3C), increasing confidence that the neuroimaging results reported below were primarily due to treatment-related recovery and not to other cognitive changes that could have taken place between pre and post-treatment time-points.
3.2. Analysis 1 Results: Identifying brain areas in which Local-Hreg values changed from before to after treatment
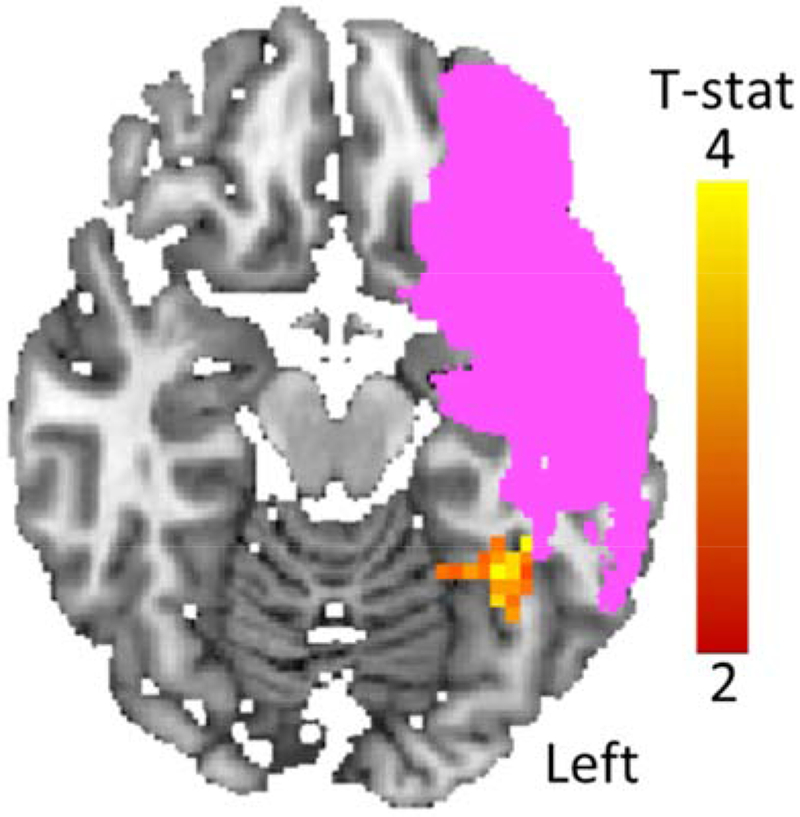
This whole brain analysis sought to identify areas(s) that exhibited a significant change in local heterogeneity from pre to post-treatment. For Training items, the analysis identified a single significant cluster - in the left vOTC - in which Local-Hreg values for Training items significantly increased from pre to post-treatment (n = 20, Peak MNI x, y, z location = −35 −47 −21, Max t = 5.03, cluster size = 35, corrected p-value = 0.028); see Figure 4. All participants, except for one, exhibited pre to post-treatment increases in Training item Local-Hreg beta values, with increased values ranging from −0.09 to 0.34 (SD = 0.1). The same analysis carried out with Known words identified no clusters in which Local-Hreg significantly changed from pre to post-treatment, strengthening the conclusion that the treatment resulted in targeted neural changes.
Figure 4: Pre to post-treatment Local-Hreg changes (Analysis 1: whole-brain analysis).
The only cluster in which there was significant change in Local-Hreg from pre to post-treatment was in the left vOTC: Peak MNI location = −35 −47 −21 Voxel-wise p <0.05; corrected p <0.05. Axial slice = −17. Pink indicates voxels lesioned in at least one participant.
3.3. Analysis 2 Results: Examining the relationship between Local-Hreg and spelling performance
3.3.1. Analysis 2A: Is the magnitude of pre-treatment Local Hreg associated with contemporaneous spelling accuracy and/or future responsiveness to treatment?
These analyses were carried out using the single Local-Hreg left vOTC change cluster identified in Analysis 1 (see Section 3.2 and Figure 4). In examining the individual variability in Local-Hreg values at pre-treatment within this cluster, we observed the following three main findings.
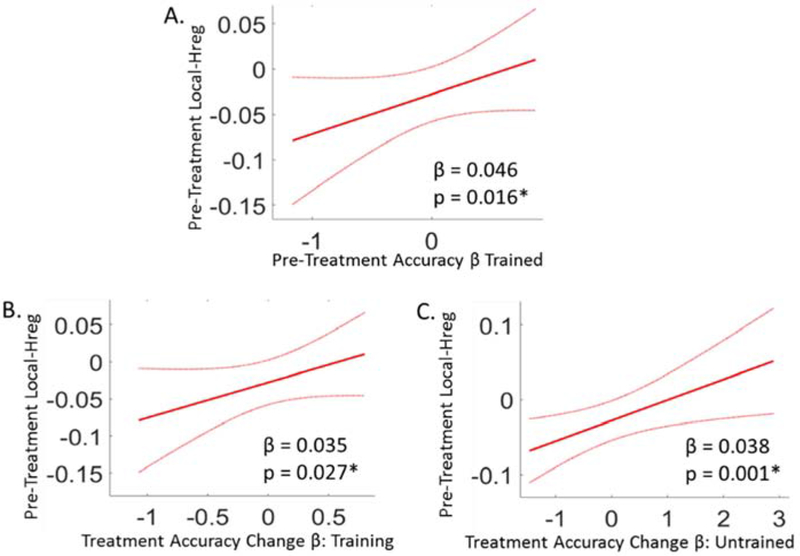
First, there was a significant positive relationship between pre-treatment Local-Hreg and pre-treatment spelling accuracy (i.e. Pre-Treatment Accuracy βs) for the Training items (n = 21, β = 0.046, p = 0.016): depicted in Figure 5A (see Supplemental Materials Table S3 for full model results). Recall that in all of these analyses, spelling accuracy was measured outside the scanner so the Local-Hreg levels did not simply reflect scanner task accuracy. To understand whether the relationship between pre-treatment Hreg and spelling accuracy was strictly limited to the ROI that was identified in Analysis 1 (i.e., based on changes in pre to post-treatment Local-Hreg ), we also directly evaluated the relationship between pre-treatment Local-Hreg and spelling accuracy with a voxel-based evaluation of the entire bilateral vOTC region. This analysis not only revealed only one significant cluster (see Supplemental Materials, Section S4.1 and Figure S5), but this cluster overlapped with the ROI cluster identified in Analysis 1/Figure 4 (5 voxels).
Figure 5: Analysis of the relationship between pre-treatment Local-Hreg and spelling accuracy, within the left vOTC cluster identified in Analysis 1.
Overall, these results reveal that for the left vOTC cluster that showed significant change in pre to post Local-Hreg, pre-treatment Local-Hreg values were tightly coupled to spelling severity and responsiveness to treatment. (Note: All Local-Hreg values were based on the neural response to Training items and all behavioral effects were based on spelling accuracy for these items data collected outside the scanner). (A) The relationship between pre-treatment Local-Hreg values (y-axis) and pre-treatment accuracy β’s for the Training items (x-axis). (B) The relationship between pre-treatment Local-Hreg (y-axis) and pre to post-treatment change in spelling accuracy β’s for Training items (x-axis). (C) The relationship between pre-treatment Local-Hreg (y-axis) and pre to post-treatment changes in β accuracies for Untrained items (x-axis). Figure Details: Red lines depict the β-estimate regression lines; red dotted lines depict the 95% confidence intervals. β estimates and p-values of these relationships are reported at the bottom of each plot, and obtained from LME models, see Supplementary materials Table S3 for the full model results. *Significant relationship at an alpha level of 0.05.
Second, we found that pre-treatment Local-Hreg within the ROI was also positively related to future responsiveness to spelling treatment (Accuracy Change β’s) for the Training items (n = 21, β = 0.035, p = 0.027) (see Figure 5B and Table 2 for a summary and also Supplemental Materials Table S3 for full model results). This finding was also examined further using a voxel-wise analysis within bilateral vOTC, although in this case the analysis did not reveal any significant clusters (see Supplemental Materials Section S.4.1).
Table 2:
The relationship between Pre-Treatment BOLD (Local-HREG and mean BOLD) and the magnitude of future pre to post treatment behavioral changes, within the left vOTC ROI (Figure 4).
| BOLD Measure | Behavioral Improvement (Betas) | |||
|---|---|---|---|---|
| Training Items | Untrained Items | |||
| Beta | p-value | Beta | p-value | |
| Local-HREG | 0.035 | 0.027* | 0.038 | 0.001* |
| Mean BOLD | 0.050 | 0.588 | −0.020 | 0.819 |
significant relationship at an alpha level of 0.05
Third, we evaluated whether Local-Hreg might index the integrity of the orthographic representational system beyond that of the Training items themselves. If so, higher pre-treatment Local-Hreg for Training items would predict improvement not only on the Training items themselves (Figure 5B), but would also support generalization of treatment benefits to the Untrained items. Consistent with this possibility, we found, as depicted in Figure 5C, that pre-treatment Local-Hreg was significantly and positively related to future Accuracy Change β’s for Untrained items (n = 20, β = 0.038, p = 0.001).
3.3.2. Analysis 2B: Do changes in pre to post-treatment Local-Hreg correlate with pre to post-treatment changes in spelling accuracy?
As was the case for the analyses reported in the previous section, these analyses examined the left vOTC Local-Hreg change cluster identified in Analysis 1 (Figure 4), focusing on the pre to post-treatment changes in Local-Hreg, rather than the pre-treatment Local-Hreg values that were examined in Analysis 2A. There were three main findings.
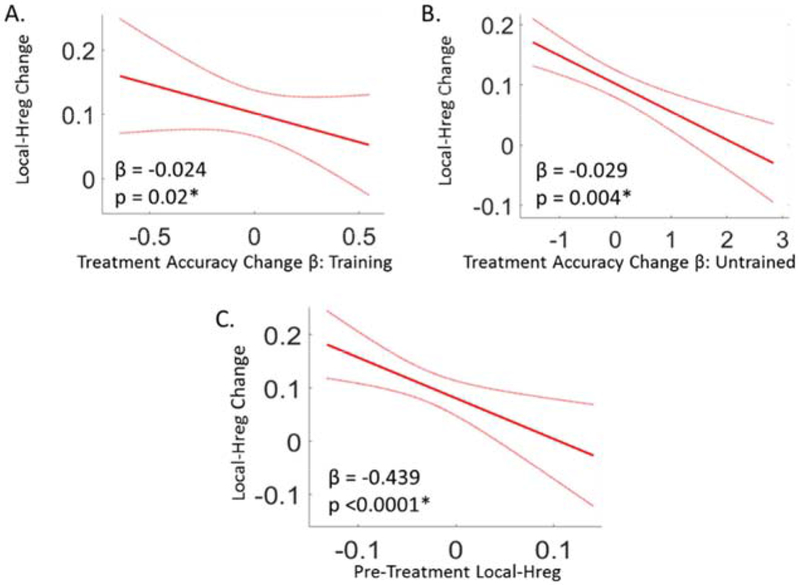
First, we examined the relationship between individual participant Hreg changes and their behavioral improvements from pre- to post-treatment for Training items. This revealed a significant negative relationship between the magnitude of the Local-Hreg change from pre to post-treatment and the magnitude of improvement in spelling accuracy for Training words (n = 20, β = −0.024, p = 0.02) (Figure 6A). Second, a significant and also negative relationship was observed in a separate analysis of the Untrained words (n = 20, β = −0.029, p = 0.004) (Figure 6B). See Table 3 for a summary, and Supplemental Materials Table S4 for the full LME model fixed effect estimates.
Figure 6: Analysis of the relationship between Local-Hreg changes and changes in spelling accuracy, within the left vOTC cluster identified in Analysis 1.
Overall, these results reveal that changes in Local-Hreg within the left vOTC region were tightly coupled to treatment-related spelling improvements. (Note: All Local-Hreg values are based on the Training items only). (A) The relationship between the pre to post-treatment changes in Local-Hreg for Training items (y-axis) and the pre to post-treatment Accuracy change β’s for Training items (x-axis). (B) The relationship between the pre to post-treatment change in Local-Hreg (y-axis) and the Accuracy Change βs (x-axis) for Untrained items. (C) The relationship between pre to post-treatment changes in Local-Hreg (y-axis) and pre-treatment Local-Hreg (x-axis). Figure Details: Red lines depict the β-estimate regression lines and red dotted lines depict the 95% confidence intervals. β estimates and p-values of these relationships are reported at the bottom of each plot, and obtained from an LME model. *Significant relationship at an alpha level of 0.05.
Table 3:
The relationship between the magnitude of neural changes (Local-Hreg and mean BOLD) and the magnitude of pre to post-treatment behavioral changes, within the left vOTC (see Figure 4).
| BOLD Measure | Behavioral Improvement (betas) | |||
|---|---|---|---|---|
| Training Items | Untrained Items | |||
| Beta | p-value | Beta | p-value | |
| Local-HREG | −0.024 | 0.02* | −0.029 | 0.004* |
| Mean Bold | −0.042 | 0.298 | −0.054 | 0.151 |
significant relationship at an alpha level of 0.05
Third, a voxel-wise analysis over the entire bilateral vOTC directly evaluating the relationship between Local-Hreg changes and behavioral changes was also carried out. No clusters survived correction for multiple comparisons (see Supplemental Materials Section S4.2).
While the previous analyses found that all participants improved their spelling accuracy and, further, that all but one had higher Local-Hreg values after treatment in the critical left vOTC region, the negative correlation reported in Analysis 2B indicated that individuals with smaller increases in Local-Hreg had larger behavioral improvements than did individuals with larger increases in Local-Hreg (Figure 6A/B). We considered two possible explanations: (1) a maladaptivity account according to which the observed changes in Local-Hreg in the left vOTC were maladaptive, or (2) a recovery efficiency account according to which healthier systems require less neural change to achieve greater behavioral benefits. Although it is not obvious how to definitively distinguish between these two possibilities, we found that the preponderance of evidence favored the recovery efficiency hypothesis. We discuss this in more detail in the General Discussion but here we report two relevant results. First, the hallmark of maladaptivity is that higher levels of some neural factor (in this case Local-Hreg) after treatment should be associated with lower post-treatment behavioral accuracy. However, we found no significant relationship between these two variables at the post-treatment time-point (n = 20, β = −0.007, p = 0.475; see Supplemental Materials Table S5 for full model results). Second, the recovery efficiency account specifically assumes that individuals with more intact pre-treatment orthographic representations (higher levels of differentiation prior to treatment) would demonstrate more recovery efficiency, and thus would require less additional re-differentiation (smaller Local-Hreg changes) in order to achieve larger behavioral gains. While Analysis 2A already established that higher pre-treatment Local-Hreg values were associated with larger future improvements, this account also predicts that pre-treatment Local-Hreg values should be negatively correlated with Local-Hreg change values. This prediction was confirmed in an LME model analysis in which pre- to post-treatment Local-Hreg change was the dependent variable and pre-treatment Local-Hreg was a fixed effect (n = 20, β = −0.439, p<0.0001; see Figure 6C and Supplemental Materials Table S6 for full model results).
3.3.3. Are the relationships between Local-Hreg and spelling accuracy present when also accounting for the different types of spelling impairments?
It is possible the type of spelling deficit (OWM or OLTM) would affect the pattern of spelling recovery. For example, given previous work associating left vOTC with O-LTM but not O-WM (see, Rapp et al., 2015), we might not find neural evidence of recovery in this region in individuals with O-WM deficits. Although we did not have a sufficient number of participants to perform a comparison of recovery in those with pure O-WM or O-LTM deficits (n = 4 and 8 respectively), we did re-run Analyses 2A and B (Sections 3.3.1 and 2) including additional categorical regressors indicating the presence of O-WM or O-LTM deficits, or both. This allowed us to determine if the effects reported for Analyses 2A and B were significant regardless of deficit type. In these re-analyses we also included a categorical regressor for an auditory comprehension deficit to account for any unique variability due to the use of the picture based paradigm with those participants (n = 3; see Section 2.2.1).
The results were not substantively different from those reported for Analysis 2A (Section 3.3.1) which did not include these regressors: there was a significant positive relationship between pre-treatment Local-Hreg and the pre-treatment Training item accuracy (n = 21, β = 0.043, p = 0.022), the amount of future Training item improvement (n = 21, β = 0.04, p = 0.02), and the amount of future Untrained item improvement (n = 21, β = 0.047, p = 0.001). The re-run results from Analysis 2B (Section 3.3.2) did not substantively change either: there was a significant negative relationship between the magnitude of the Local-Hreg change from pre to post-treatment and the magnitude of improvement in spelling accuracy for Training words (n = 20, β = −0.026, p = 0.013) and for Untrained words, (n = 20, β = −0.029, p = 0.004). The full results are presented in Supplemental Materials Table S7–8 where it can be seen that the effects reported for Analyses 2A and B are still statistically significant even when deficit type is accounted for, and that the deficit type regressors themselves were not significant.
3.3.4. Summary of analyses examining the relationship between Local-Hreg and spelling accuracy.
The results of Analyses 2A and B (Sections 3.3.1 and 2) provide a critical link between, on the one hand, the observed neural changes in local neural differentiation and, on the other hand, the observed behavioral changes in spelling accuracy. Importantly, this relationship was robust even though performance improvements in spelling were measured outside the scanner and, therefore, were not directly influencing the fMRI signal analyzed. Further, this relationship is present even while accounting for individual variability in lesion volume, age, or type of spelling deficit.
3.4. Analysis 3 Results: Evaluating the relationship between Local-Hreg and mean local BOLD
Since both mean BOLD change and Local-Hreg approaches involve analysis of the BOLD signal we thought it important to understand the relationship between the two analysis approaches and, specifically, to understand the extent to which changes in Local-Hreg could be driven by changes in mean BOLD or if, alternatively, the approaches evaluate different aspects of the neural changes that support recovery.
3.4.1. Are there brain areas in which the mean BOLD response changes from pre to post-treatment?
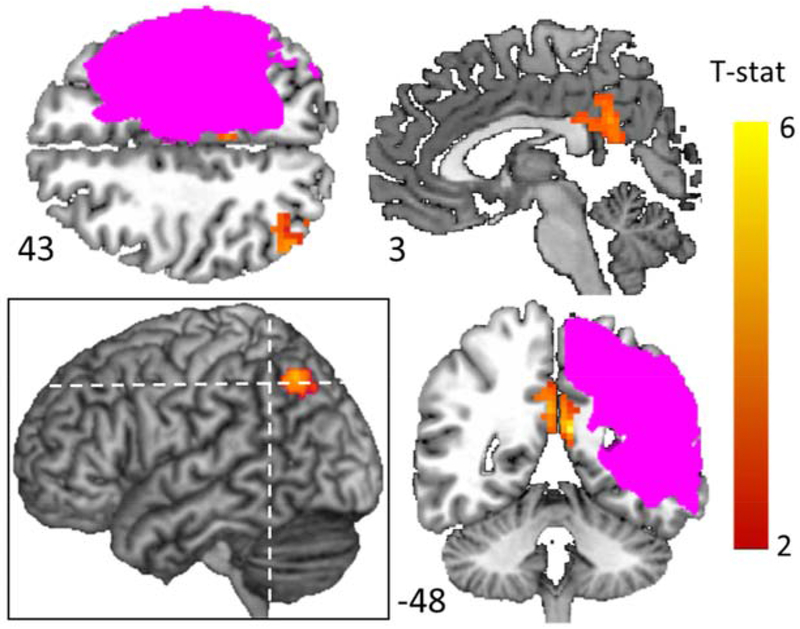
First, in contrast to the left vOTC region associated with pre to post-treatment Local-Hreg changes, mean BOLD changes were identified both at the intersection of the superior lateral occipital cortex, angular gyrus and the intraparietal sulcus (S-LOC/AG/IPS) and bilateral posterior cingulate cortex (Figure 7/Table 4). Furthermore, analyses revealed no significant relationships between mean BOLD changes in each of these clusters and treatment-based behavioral changes for Training items (right superior LOC/AG/IPS: n = 20, β = 0.004, p = 0.941) (right PCC : n = 20, β = 0.028, p = 0.571) (left PCC: n = 20, β = 0.068, p = 0.202) or Untrained items (right superior LOC/IPS: n = 20, β = −0.017, p = 0.707) (right PCC: n = 20, β = −0.059, p = 0.214) (left PCC: n = 20, β = −0.075, p = 0.12). Finally, in order to determine if Local-Hreg was related to treatment-based change within these ROIs, we also examined these relationships and observed no significant relationships in these clusters between Local-Hreg changes and behavioral change for Training items (right superior LOC/AG/IPS: n = 20, β = 0.002, p = 0.836) (right PCC: n = 20, β = 0.002, p = 0.802) (left PCC: n = 20, β = 0.005, p = 0.545) or Untrained items (right superior LOC/IPS: n = 20, β = 0.007, p = 0.45) (right PCC: n = 20, β = 0.008, p = 0.329) (left PCC: n = 20, β = 0.005, p = 0.507). For full model results, see Supplemental Materials Tables S9–11.
Figure 7: Significant pre to post-treatment changes in mean BOLD.
Voxel-wise p <0.01; corrected p <0.05. Pink indicates voxels lesioned in at least one participant. Dotted lines depict locations of axial slice 43 and horizontal slice −48. The analysis is described in Materials and Methods, Analysis 3, and for a detailed description of the clusters see Table 4.
Table 4.
List of peak coordinates with significant changes in pre to post-treatment mean BOLD response for Training items (Voxel-wise p< 0.01; Cluster level corrected at p< 0.05)
| Region (Estimated from Harvard/Oxford Atlas) | voxels | Tmax | MNI Coordinates |
|||
|---|---|---|---|---|---|---|
| X | Y | z | ||||
| Parietal | L Posterior Cingulate | 102 | 5.40 | −8 | −52 | 17 |
| R Posterior Cingulate | 57 | 4.30 | 1 | −49 | 26 | |
| R S-LOC/AG/IPS | 61 | 4.40 | 44 | −64 | 44 | |
MNI-Montreal Neurological Institute; L - Left; R - Right; S-LOC – superior lateral occipital gyrus, AG – angular gyrus, and IP –intraparietal sulcus.
3.4.2. Do pre to post treatment changes in mean BOLD correlate with improvements in spelling accuracy for Training items?
We evaluated whether mean BOLD changes and behavioral changes were related within the left vOTC cluster identified in Analysis 1. To do so, we performed an LME model analysis to determine if the magnitude of pre to post-treatment change in spelling accuracy was related to the change in mean BOLD response values within this cluster. As reported in Table 2 there was no relationship between the pre-treatment mean BOLD response and Accuracy Change βs for Training (β = −0.05, p = 0.588) or Untrained items (β = −0.02, p = 0.819). See Supplemental Materials Table S3 for full report of the LME model output. Furthermore, this analysis did not reveal a significant relationship between pre to post-treatment changes in mean BOLD and the magnitude of the accuracy changes for either the Training items (β = −0.042, p = 0.298) or the Untrained items (β = −0.054, p = 0.151). See Table 3 and Supplemental Materials Table S4 for a full report of the LME model output.
3.4.3. Do mean BOLD changes correlate with Local-Hreg changes?
To directly evaluate the relationship between mean BOLD and Local-Hreg, we performed an LME model analysis within the left vOTC Local-Hreg change cluster identified in Analysis 1. This analysis revealed that although, in terms of individual differences, the magnitude of changes in Local-Hreg and mean BOLD changes were not related (β = −0.009, p = 0.25), there was a significant main effect (β = −0.02, p = 0.015) such that lower overall mean BOLD was significantly associated with greater overall local heterogeneity. However, despite this general relationship, we determined that mean BOLD only explained an additional 1.3% of the total variance when it was included in the regression model predicting Local-Hreg (see Section 2.8.3. Analysis 3C) relative to when it was excluded from the model. This leaves 98.7% of the explained variance in Local-Hreg that is not accounted for by the mean BOLD response.
This set of analyses clearly reveal that changes in mean BOLD and Local-Hreg cannot be reduced to one another and have a strikingly different neurotopography. These findings support the important conclusion that these two analysis approaches reveal different aspects of the neural changes that support recovery, with Local-Hreg apparently more tightly coupled to individual differences in behavior and behavioral changes.
4. DISCUSSION
In order to test the hypothesis that re-learning due to treatment in post stroke recovery is associated with increased neural differentiation of local representations, we deployed a novel analytic approach - Local Heterogeneity Regression (Local-Hreg; described in Purcell and Rapp, 2018). Local-Hreg builds both on multi-voxel pattern analysis approaches that assume neural representations are spatially distributed across adjacent voxels (Haxby, 2012; Kriegeskorte et al., 2008; Mahmoudi et al., 2012) and also on theories that posit that, with learning, neural representations become sparser and more sharply tuned (e.g., Olshausen and Field, 1996; Rolls and Tovee, 1995) and are less locally correlated (i.e., better differentiated) (e.g., Bair et al., 2001; Jermakowicz et al., 2009). Accordingly, Local-Hreg quantifies the relative heterogeneity of the BOLD time-courses across adjacent voxels to index the local differentiation of neural representations. This approach was specifically used to examine recovery in individuals with post-stroke written language impairments who were scanned before and after a 3-month period of behavioral treatment. We found that: 1) Local-Hreg values prior to treatment indexed pre-treatment spelling accuracy and also predicted the magnitude of subsequent behavioral response to treatment; 2) Local-Hreg values showed treatment-specific increases from pre- to post-treatment; 3) Local-Hreg provided different information about the neural underpinnings of recovery than that provided by mean BOLD; 4) A convergence of the findings from this study revealed that neural differentiation within the left ventral occipital-temporal cortex (vOTC) may play a key role in the recovery of spelling in acquired dysgraphia.
4.1. Representational differentiation before treatment
Before training, individuals with higher Local-Hreg values within the left vOTC for the to-be-trained items also more accurately spelled those items outside the scanner. This finding supports the claim that Local-Hreg indexes the local differentiation of orthographic representations in this area, such that, even before treatment, individuals with more differentiated neural representations had higher spelling accuracy. A plausible interpretation is that the lesion caused de-differentiation of local orthographic representations. In other words, the spelling deficits could be due (at least in part) to stroke-induced de-differentiation of representations within the left vOTC. This interpretation is also generally consistent with reports that in normal aging there is de-differentiation in multiple neural systems (e.g., Park et al., 2004; Rieckmann et al., 2018; St-Laurent et al., 2014).
Additionally, we found that pre-treatment Local-Hreg values in the left vOTC predicted the responsiveness to treatment for Training items and generalization of treatment benefits to Untrained items. These findings support the interpretation that individuals with higher-integrity (i.e., well differentiated) orthographic representations are better positioned to benefit from treatment. These findings are promising with regard to eventual clinical applications, allowing local heterogeneity measures to serve as a potential biomarker to inform decisions about prognosis and allocation of intervention resources and providing an index of responsiveness to specific treatment approaches.
4.2. The role of increased neural differentiation in recovery of function
In this section, we specifically discuss the evidence that increased neural representational differentiation (re-differentiation) supports recovery of function. In this regard, it is important to first note that the specificity of the observed behavioral treatment effects (significant improvement in the spelling of Training and Untrained items but not on any other task) increases confidence in attributing pre to post-treatment neural changes to treatment-targeted recovery. Confidence is further increased by the specificity of the neural changes that were associated only with responses within the scanner to Training but not Known items. Against this backdrop, the key findings regarding pre to post-treatment changes are as follows: 1) all participants experienced significant improvement in their spelling of Trained items, 2) whole-brain analyses revealed significant pre- to post-treatment changes in Local-Hreg (within the left vOTC), 3) the magnitude of pre to post-treatment behavioral improvement for Training items and the generalization of treatment benefits to Untrained words were both predicted by pre-treatment Local-Hreg levels within left vOTC, and 4) pre to post-treatment changes in the magnitude of Local-Hreg in this region were negatively correlated with behavioral improvement such that individuals with larger behavioral improvements exhibited smaller changes in Local-Hreg.
The association of behavioral improvement with increased local neural differentiation (Findings 1 and 2) provides a strong argument for the role of neural re-differentiation in the recovery of spelling. The fact that the behavioral improvements were strongly linked to pre-treatment Local-Hreg levels (Finding 3) further strengthens the plausibility of the claim that Local-Hreg plays a role in recovery. The only “unexpected” result is Finding 4 - that larger changes in Local-Hreg were associated with smaller behavioral changes. Certainly, the most intuitive prediction is that larger increases in neural differentiation should result in larger behavioral changes. However, as briefly reviewed in the Introduction, there are many findings in the neuroimaging literature of non-linear relationships between neural and behavioral measures. On this basis, in the specific context of this study, we need to consider possible interpretations of the observed negative relationship between changes in Local-Hreg and changes in spelling accuracy.
First it is worth remembering that these results cannot be explained by differences in general pre-treatment spelling severity, lesion volume, age, or accuracy on the words targeted in treatment as these possibilities were dealt with analytically by including these factors in the statistical models used to quantify treatment-based behavioral change (see Analysis Section 2). Second, we have been able to generate only two possible accounts of this negative relationship which we have referred to as the “maladaptivity” and the “recovery efficiency” accounts. According to the maladaptivity account, larger Local-Hreg increases produce smaller behavioral gains because increased Local-Hreg negatively impacts the spelling system. The most obvious prediction of this account is that at the post-treatment time-point individuals with larger Local-Hreg values should exhibit worse spelling accuracy. However, this relationship was not observed (see Section 3.3.2). While this may not definitively rule out a maladaptivity account, there is no independent support for this account. Instead, the findings are all consistent with the “recovery efficiency” account, which falls within the general “supply and demand” proposal of Lövdén et al. (2010). According to the recovery efficiency account, higher post-treatment neural differentiation is indeed beneficial and the negative relationship was observed because individuals with higher pre-treatment Local-Hreg (i.e., more highly differentiated orthographic representations) could benefit more from treatment with smaller adjustments to the underlying local orthographic neural representational system due to a greater efficiency of the system. With regard to neural changes, this account predicts that individuals with high pre-treatment Local-Hreg values should experience smaller changes in Local-Hreg from pre to post-treatment – which was found to be case (see Section 3.3.2). Thus, the “recovery efficiency” account is consistent with all of the results we have reported. While we appreciate that the evidence may not be definitive, in the absence of a better alternative, this is the preferred hypothesis. Post-stroke recovery under this account could be seen as analogous to the effects of a hurricane such that less devastated areas may make a full recovery of services with relatively small amounts of reconstruction, while more devastated areas may undergo a greater amount of reconstruction to achieve less recovery; i.e., reconstruction is more efficient in the first case than the second. To summarize, these results support the conclusion that greater representational differentiation within the left vOTC corresponds to healthier orthographic representations, and that the representational system experiences greater “recovery efficiency” when re-differentiating representations such that larger behavioral responses to treatment require less neural reorganization for both Trained and Untrained words. The complexity of the findings of this investigation highlight the need for empirical and theoretical work to better understand the relationship between neural and behavioral changes, especially in the complex environment of a lesioned system.
4.3. Left vOTC
The left mid-vOTC has been shown to play an important role in orthographic processing, both in spelling (Purcell et al., 2011) and reading (Martin et al., 2015), where it is often referred to as the visual word form area (VWFA; Cohen et al., 2002). One specific claim about its function is that it is key for the long-term memory storage of word spellings in both reading and spelling (Glezer et al., 2009; Kronbichler et al., 2004; Purcell et al., 2017; Rapp and Dufor, 2011; Rapp and Lipka, 2011; Szwed et al., 2011).
On this basis, we assume that the representations quantified by Local-Hreg in this region correspond to long-term memory orthographic representations of word spellings. We propose that treatment serves to “tune” these representations by increasing their relative dissimilarity which is reflected in the increasing differentiation of BOLD response patterns (see also, Glezer et al., 2015). This interpretation supports the predictions - confirmed in this investigation - that neural representational differentiation in this region should be related to spelling performance prior to training, predict response to training, and that neural differentiation changes should be associated with recovery of function. In fact, this investigation finds that the left vOTC is the only region to demonstrate these multiple properties. Thus, these findings provide a deeper understanding of the role of left vOTC in orthographic processing. However, one limitation of the study is that we were not able to compare the Local-Hreg changes associated with recovery for individuals with OLTM and OWM deficits. It would be important to understand if the Local-Hreg changes in left vOTC supported recovery for both deficit types. One might predict that the two deficit types might differ in this regard. However, this is not a strong prediction as it is certainly possible that the re-differentiation of orthographic representations could have benefits throughout the spelling system. Importantly, as we have reported, the obtained results were still significant even when a deficit-type regressor was included in the analysis (Section 3.3.3), indicating that findings were unlikely to have been carried by a single deficit type. Nonetheless, future work with larger number of individuals with each deficit type would allow for a more direct evaluation of these issues.
4.4. Local-Hreg and other analytic approaches
In this investigation, by comparing findings from mean BOLD and Local-Hreg analyses, we found that these two approaches characterize distinct aspects of the neuroplastic changes supporting recovery. First, the two approaches identified different recovery-sensitive regions, with Local-Hreg results converging on left vOTC and mean BOLD identifying right IPS/AG/LOC and bilateral PCC. Second, unlike for the left vOTC, no significant relationships were found in any of these clusters between pre- to post-treatment mean BOLD change and behavioral improvement for Training or Untrained items. This may indicate that the mean BOLD response is not as tightly coupled to performance outside of the scanner as is Local-Hreg. Third, within the left vOTC cluster, within which there were significant pre to post-treatment changes in Local-Hreg, the magnitude of changes in Local-Hreg was not predicted by the magnitude of changes in mean BOLD. Finally, although there was a significant relationship between the magnitude of overall mean BOLD and Local-Hreg in the left vOTC, this relationship explained less than 2% of Local-Hreg variance. Generally, this suggests that these two analytic approaches – even when used to analyze the same BOLD signal in the same voxels - capture distinct aspects of the changes that support recovery. We hypothesize that Local-Hreg captures experience-dependent aspects of the BOLD signal that reflect the relative differentiation of neural representations, whereas the mean BOLD response is more associated with the amount of cognitive load/demand required for task performance (Gould et al., 2003).
4.5. Conclusions: Neural substrates supporting recovery of function
In this work, we report strong evidence that increased local neural differentiation can play a key role in the recovery of cognitive functions after brain lesion. With regard to the specific deficit – acquired dysgraphia – convergent findings from this study consistently implicate an area of left ventral occipitotemporal cortex that forms part of the neural network normally recruited for orthographic processing. The results indicate that recovery is supported by re-learning to differentiate neural representations from one another. The findings support the more general claim that when the network of areas supporting a function is damaged, other (ipsilesional) regions within that network can contribute to the neuroplastic changes that support functional recovery. The Local-Hreg approach can be applied to other language and cognitive disorders to understand the degree to which changes in representational differentiation are more generally found to support recovery and re-learning.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge NIH support for the multi-site project examining the neurobiology of language recovery in aphasia of which that this work forms a part (DC012283). We are grateful for the many valuable contributions to this research from Jennifer Shea and Donna Gotsch.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Another approach would have been to measure severity based on a common list of words. This would have been possible using a relatively short list with a small range of stimulus properties. However, we decided that a much larger word sets which overlapped across many, if not all, of the participants would provide a more representative estimate of spelling deficit severity.
It is worth noting that we have previously established with neurotypical individuals that there is a similar distribution of spelling-related brain responses for the spelling probe task and a spelling-to-dictation task requiring written responses during scanning (Rapp & Lipka, 2011 and Rapp & Dufor, 2011)
This is smallest possible searchlight that samples surrounding voxels along the x, y, and z axes.
Specifically, the random intercept and slope for time-point by-items were crossed with a random intercept and slopes for time-point, word frequency, and word length by-participants.
Note that, importantly, these analyses were based on functionally defined ROI analyses, such that the identification of an ROI was based on pre vs post-treatment Local-Hreg differences (Section 2.6) which would be independent from the comparisons of interest (e.g., the magnitude of behavioral improvement from pre to post-treatment).
REFERENCES
- Baayen RH, Davidson DJ, Bates DM, 2008. Mixed-effects modeling with crossed random effects for subjects and items. J. Mem. Lang., Special Issue: Emerging Data Analysis 59, 390–412. 10.1016/j.jml.2007.12.005 [DOI] [Google Scholar]
- Bair W, Zohary E, Newsome WT, 2001. Correlated firing in macaque visual area MT: time scales and relationship to behavior. J. Neurosci. Off. J. Soc. Neurosci 21, 1676–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DJ, Levy R, Scheepers C, Tily HJ, 2013. Random effects structure for confirmatory hypothesis testing: Keep it maximal. J. Mem. Lang 68 10.1016/j.jml.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S, 2015. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw 67 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Beeson P, M. Hirsch F., A. Rewega M, 2010. Successful single-word writing treatment: Experimental analyses of four cases. Aphasiology April 01, 473–491. 10.1080/02687030244000167 [DOI] [Google Scholar]
- Beeson PM, 1999. Treating acquired writing impairment: strengthening graphemic representations. Aphasiology 13 10.1080/026870399401867 [DOI] [Google Scholar]
- Beeson PM, Rising Kindle, Volk Jennifer, 2003. Writing Treatment for Severe Aphasia. J. Speech Lang. Hear. Res 46, 1038–1060. 10.1044/1092-4388(2003/083) [DOI] [PubMed] [Google Scholar]
- Blasi V, Young AC, Tansy AP, Petersen SE, Snyder AZ, Corbetta M, 2002. Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron 36, 159–170. 10.1016/S0896-6273(02)00936-4 [DOI] [PubMed] [Google Scholar]
- Bråthen ACS, de Lange A-MG, Rohani DA, Sneve MH, Fjell AM, Walhovd KB, 2018. Multimodal cortical and hippocampal prediction of episodic-memory plasticity in young and older adults. Hum. Brain Mapp 39, 4480–4492. 10.1002/hbm.24287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brysbaert M, New B, 2009. Moving beyond Kucera and Francis: A critical evaluation of current word frequency norms and the introduction of a new and improved word frequency measure for American English. Behav. Res. Methods 41, 977–990. 10.3758/BRM.41.4.977 [DOI] [PubMed] [Google Scholar]
- Buchwald A, Rapp B, 2009. Distinctions between orthographic long-term memory and working memory. Cogn. Neuropsychol 26, 724–751. 10.1080/02643291003707332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardebat D, Démonet JF, de Boissezon X, Marie N, Marié RM, Lambert J, Baron JC, Puel M, 2003. Behavioral and Neurofunctional Changes Over Time in Healthy and Aphasic Subjects A PET Language Activation Study. Stroke 34, 2900–2906. 10.1161/01.STR.0000099965.99393.83 [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, 2004. Specialization within the ventral stream: the case for the visual word form area. Neuroimage 22, 466–476. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F, 2000. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain 123, 291–307. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S, 2002. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain 125, 1054–1069. [DOI] [PubMed] [Google Scholar]
- Cox DD, Savoy RL, 2003. Functional magnetic resonance imaging (fMRI) “brain reading”: detecting and classifying distributed patterns of fMRI activity in human visual cortex. NeuroImage 19, 261–270. 10.1016/S1053-8119(03)00049-1 [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, Kennedy DN, Finklestein SP, Rosen BR, 1997. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke 28, 2518–2527. [DOI] [PubMed] [Google Scholar]
- Crosson B, Moore AB, Gopinath K, White KD, Wierenga CE, Gaiefsky ME, Fabrizio KS, Peck KK, Soltysik D, Milsted C, Briggs RW, Conway TW, Gonzalez Rothi LJ, 2005. Role of the right and left hemispheres in recovery of function during treatment of intention in aphasia. J. Cogn. Neurosci 17, 392–406. 10.1162/0898929053279487 [DOI] [PubMed] [Google Scholar]
- de Lange A-MG, Bråthen ACS, Rohani DA, Grydeland H, Fjell AM, Walhovd KB, 2017. The effects of memory training on behavioral and microstructural plasticity in young and older adults. Hum. Brain Mapp 38, 5666–5680. 10.1002/hbm.23756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, 2011. The unique role of the visual word form area in reading. Trends Cogn. Sci 15, 254–262. 10.1016/j.tics.2011.04.003 [DOI] [PubMed] [Google Scholar]
- DeMarco AT, Wilson SM, Rising K, Rapcsak SZ, Beeson PM, 2017. Neural substrates of sublexical processing for spelling. Brain Lang. 164, 118–128. 10.1016/j.bandl.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, LaConte S, Peltier S, Hu X, 2009. Integrated local correlation: a new measure of local coherence in fMRI data. Hum. Brain Mapp 30, 13–23. 10.1002/hbm.20482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Fan Y, Wu C, Liu H, Lin K, Wai Y, Chen Y, 2015. Neuroplastic changes in resting-state functional connectivity after stroke rehabilitation. Front. Hum. Neurosci 9 10.3389/fnhum.2015.00546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Baum S, Bruggemann D, Gallego IF, Li DSP, Tamez ER, 2017. Decoding levels of representation in reading: A representational similarity approach. Cortex 90, 88–102. 10.1016/j.cortex.2017.02.017 [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC, 1995. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. Off. J. Soc. Magn. Reson. Med. Soc. Magn. Reson. Med 33, 636–647. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK, 2006. Experience-Dependent Sharpening of Visual Shape Selectivity in Inferior Temporal Cortex. Cereb. Cortex 16, 1631–1644. 10.1093/cercor/bhj100 [DOI] [PubMed] [Google Scholar]
- Fridriksson J, 2010. Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. J. Neurosci. Off. J. Soc. Neurosci 30, 11558–11564. 10.1523/JNEUROSCI.2227-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Morrow-Odom L, Moser D, Fridriksson A, Baylis G, 2006. Neural recruitment associated with anomia treatment in aphasia. NeuroImage 32, 1403–1412. 10.1016/j.neuroimage.2006.04.194 [DOI] [PubMed] [Google Scholar]
- Gaillard R, Naccache L, Pinel P, Clemenceau S, Volle E, Hasboun D, Dupont S, Baulac M, Dehaene S, Adam C, Cohen L, 2006. Direct intracranial, FMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron 50, 191–204. [DOI] [PubMed] [Google Scholar]
- Glezer LS, Jiang X, Riesenhuber M, 2009. Evidence for highly selective neuronal tuning to whole words in the “visual word form area.” Neuron 62, 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer LS, Kim J, Rule J, Jiang X, Riesenhuber M, 2015. Adding Words to the Brain’s Visual Dictionary: Novel Word Learning Selectively Sharpens Orthographic Representations in the VWFA. J. Neurosci 35, 4965–4972. 10.1523/JNEUROSCI.4031-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Kertesz A, 2000. Right Hemisphere Semantic Processing of Visual Words in an Aphasic Patient: An fMRI Study. Brain Lang. 73, 456–465. 10.1006/brln.2000.2317 [DOI] [PubMed] [Google Scholar]
- Gould RL, Brown RG, Owen AM, Ffytche DH, Howard RJ, 2003. fMRI BOLD response to increasing task difficulty during successful paired associates learning. NeuroImage 20, 1006–1019. 10.1016/S1053-8119(03)00365-3 [DOI] [PubMed] [Google Scholar]
- Hanke JE, Wichern DW, 2005. Business Forecasting, 8th ed Pearson, Prentice Hall, New Jersey. [Google Scholar]
- Haxby James.V., 2012. Multivariate pattern analysis of fMRI: The early beginnings. Neuroimage 62, 852–855. 10.1016/j.neuroimage.2012.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P, 2001. Distributed and Overlapping Representations of Faces and Objects in Ventral Temporal Cortex. Science 293, 2425–2430. 10.1126/science.1063736 [DOI] [PubMed] [Google Scholar]
- Henry ML, Beeson PM, Stark AJ, Rapcsak SZ, 2007. The role of left perisylvian cortical regions in spelling. Brain Lang 100, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Barker PB, Beauchamp NJ, Gailloud P, Murphy K, Cooper O, Metter EJ, 2002. Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain J. Neurol 125, 1094–1104. [DOI] [PubMed] [Google Scholar]
- Jermakowicz WJ, Chen X, Khaytin I, Bonds AB, Casagrande VA, 2009. Relationship between spontaneous and evoked spike-time correlations in primate visual cortex. J. Neurophysiol 101, 2279–2289. 10.1152/jn.91207.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Bollich A, Cox P, Hyder E, James J, Gowani SA, Hadjikhani N, Blanz V, Manoach DS, Barton JJS, Gaillard WD, Riesenhuber M, 2013. A quantitative link between face discrimination deficits and neuronal selectivity for faces in autism. NeuroImage Clin. 2, 320–331. 10.1016/j.nicl.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Petok JR, Howard DV, Howard JH, 2017. Individual Differences in Cognitive Function in Older Adults Predicted by Neuronal Selectivity at Corresponding Brain Regions. Front. Aging Neurosci 9 10.3389/fnagi.2017.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM, 2002. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain J. Neurol 125, 2731–2742. 10.1093/brain/awf282 [DOI] [PubMed] [Google Scholar]
- Kelly-Hayes M, Wolf PA, Kase CS, Gresham GE, Kannel WB, D’Agostino RB, 1989. Time Course of Functional Recovery After Stroke: The Framingham Study. J. Neurol. Rehabil 3, 65–70. 10.1177/136140968900300202 [DOI] [Google Scholar]
- Kobatake E, Wang G, Tanaka K, 1998. Effects of shape-discrimination training on the selectivity of inferotemporal cells in adult monkeys. J. Neurophysiol 80, 324–330. 10.1152/jn.1998.80.1.324 [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Mur M, Bandettini P, 2008. Representational Similarity Analysis – Connecting the Branches of Systems Neuroscience. Front. Syst. Neurosci 2 10.3389/neuro.06.004.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G, 2004. The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. Neuroimage 21, 946–953. [DOI] [PubMed] [Google Scholar]
- Kuest J, Karbe H, 2002. Cortical activation studies in aphasia. Curr. Neurol. Neurosci. Rep 2, 511–515. 10.1007/s11910-002-0038-x [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB, 2017. ImerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw 82 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Lee YS, Zreik JT, Hamilton RH, 2017. Patterns of neural activity predict picture-naming performance of a patient with chronic aphasia. Neuropsychologia 94, 52–60. 10.1016/j.neuropsychologia.2016.11.010 [DOI] [PubMed] [Google Scholar]
- Lindberg PG, Schmitz C, Engardt M, Forssberg H, Borg J, 2007. Use-dependent up- and down-regulation of sensorimotor brain circuits in stroke patients. Neurorehabil. Neural Repair 21, 315–326. 10.1177/1545968306296965 [DOI] [PubMed] [Google Scholar]
- Liu H, Song L, Zhang T, 2014. Changes in brain activation in stroke patients after mental practice and physical exercise: a functional MRI study. Neural Regen. Res 9, 1474–1484. 10.4103/1673-5374.139465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövdén M, Bodammer NC, Kühn S, Kaufmann J, Schütze H, Tempelmann C, Heinze H-J, Düzel E, Schmiedek F, Lindenberger U, 2010. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia 48, 3878–3883. 10.1016/j.neuropsychologia.2010.08.026 [DOI] [PubMed] [Google Scholar]
- Mahmoudi A, Takerkart S, Regragui F, Boussaoud D, Brovelli A, 2012. Multivoxel pattern analysis for FMRI data: a review. Comput. Math. Methods Med 2012, 961257 10.1155/2012/961257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Schurz M, Kronbichler M, Richlan F, 2015. Reading in the brain of children and adults: A meta-analysis of 40 functional magnetic resonance imaging studies. Hum. Brain Mapp 36, 1963–1981. 10.1002/hbm.22749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC, 2012. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage 61, 1277–1286. 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Breitenstein C, Wienbruch C, Elbert T, Rockstroh B, 2008. Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. NeuroImage 39, 2038–2046. 10.1016/j.neuroimage.2007.10.008 [DOI] [PubMed] [Google Scholar]
- Mohr B, 2017. Neuroplasticity and Functional Recovery after Intensive Language Therapy in Chronic Post Stroke Aphasia: Which Factors Are Relevant? Front. Hum. Neurosci 11 10.3389/fnhum.2017.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Coulthard E, Jäger HR, Kennard C, Husain M, 2008. Enantiomorphic normalization of focally lesioned brains. Neuroimage 39, 1215–1226. 10.1016/j.neuroimage.2007.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Schielzeth H, 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol 4, 133–142. 10.1111/j.2041-210x.2012.00261.x [DOI] [Google Scholar]
- Nichols TE, Holmes AP, 2002. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp 15, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijboer TCW, Buma FE, Winters C, Vansteensel MJ, Kwakkel G, Ramsey NF, Raemaekers M, 2017. No changes in functional connectivity during motor recovery beyond 5 weeks after stroke; A longitudinal resting-state fMRI study. PloS One 12, e0178017 10.1371/journal.pone.0178017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ, 1996. Emergence of simple-cell receptive field properties by learning a sparse code for natural images. Nature 381, 607–609. 10.1038/381607a0 [DOI] [PubMed] [Google Scholar]
- Orjada SA, Beeson P, 2005. Concurrent treatment for reading and spelling in aphasia.
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR, 2004. Aging reduces neural specialization in ventral visual cortex. Proc. Natl. Acad. Sci. U. S. A 101, 13091–13095. 10.1073/pnas.0405148101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postman-Caucheteux WA, Birn RM, Pursley RH, Butman J. a, Solomon JM, Picchioni D, McArdle J, Braun AR, 2010. Single-trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasic patients. J. Cogn. Neurosci 22, 1299–1318. 10.1162/jocn.2009.21261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell JJ, Jiang X, Eden GF, 2017. Shared orthographic neuronal representations for spelling and reading. NeuroImage 147, 554–567. 10.1016/j.neuroimage.2016.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell JJ, Rapp B, 2018. Local response heterogeneity indexes experience-based neural differentiation in reading. NeuroImage 183, 200–211. 10.1016/j.neuroimage.2018.07.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell JJ, Turkeltaub PE, Eden GF, Rapp B, 2011. Examining the central and peripheral processes of written word production through meta-analysis. Front. Psychol 2, 239 10.3389/fpsyg.2011.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapcsak SZ, Beeson PM, 2004. The role of left posterior inferior temporal cortex in spelling. Neurology 62, 2221–2229. [DOI] [PubMed] [Google Scholar]
- Rapp B, Dufor O, 2011. The neurotopography of written word production: an FMRI investigation of the distribution of sensitivity to length and frequency. J. Cogn. Neurosci 23, 4067–81. 10.1162/jocn_a_00109 [DOI] [PubMed] [Google Scholar]
- Rapp B, Lipka K, 2011. The literate brain: the relationship between spelling and reading. J. Cogn. Neurosci 23, 1180–1197. 10.1162/jocn.2010.21507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B, Purcell JJ, Hillis AE, Capasso R, Miceli M, 2015. Neural bases of orthographic long-term memory and working memory in dysgraphia. Brain awv348 10.1093/brain/awv348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B, Wiley RW, 2019. Re-learning and remembering in the lesioned brain. Neuropsychologia 132, 107126 10.1016/j.neuropsychologia.2019.107126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann A, Johnson KA, Sperling RA, Buckner RL, Hedden T, 2018. Dedifferentiation of caudate functional connectivity and striatal dopamine transporter density predict memory change in normal aging. Proc. Natl. Acad. Sci. U. S. A 10.1073/pnas.1804641115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Tovee MJ, 1995. Sparseness of the neuronal representation of stimuli in the primate temporal visual cortex. J. Neurophysiol 73, 713–726. [DOI] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath H-O, 2012. Age-specific CT and MRI templates for spatial normalization. Neuroimage 61, 957–965. 10.1016/j.neuroimage.2012.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Brett M, 2000. Stereotaxic display of brain lesions. Behav Neurol. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C, 2006. Dynamics of language reorganization after stroke. Brain 129, 1371–1384. 10.1093/brain/awl090 [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A, 2012. E-Prime User’s Guide Psychology Software Tools, Inc., Pittsburgh. [Google Scholar]
- Soltysik DA, Thomasson D, Rajan S, Biassou N, 2015. Improving the use of principal component analysis to reduce physiological noise and motion artifacts to increase the sensitivity of task-based fMRI. J. Neurosci. Methods 241, 18–29. 10.1016/j.jneumeth.2014.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Laurent M, Abdi H, Bondad A, Buchsbaum BR, 2014. Memory reactivation in healthy aging: evidence of stimulus-specific dedifferentiation. J. Neurosci. Off. J. Soc. Neurosci 34, 4175–4186. 10.1523/JNEUROSCI.3054-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwed M, Dehaene S, Kleinschmidt A, Eger E, Valabrègue R, Amadon A, Cohen L, 2011. Specialization for written words over objects in the visual cortex. NeuroImage 56, 330–344. 10.1016/j.neuroimage.2011.01.073 [DOI] [PubMed] [Google Scholar]
- Thompson CK, Lukic S, King MC, Mesulam MM, Weintraub S, 2012. Verb and noun deficits in stroke-induced and primary progressive aphasia: The Northwestern Naming Battery(). Aphasiology 26, 632–655. 10.1080/02687038.2012.676852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulborn KR, Carpenter PA, Just MA, 1999. Plasticity of language-related brain function during recovery from stroke. Stroke J. Cereb. Circ 30, 749–754. [DOI] [PubMed] [Google Scholar]
- Tsapkini K, Hillis AE, 2013. Spelling intervention in post-stroke aphasia and primary progressive aphasia. Behav. Neurol 26, 55–66. 10.3233/BEN-2012-110240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Coslett HB, Thomas AL, Faseyitan O, Benson J, Norise C, Hamilton RH, 2012. The right hemisphere is not unitary in its role in aphasia recovery. Cortex J. Devoted Study Nerv. Syst. Behav 48, 1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ, 2003. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain J. Neurol 126, 2476–2496. 10.1093/brain/awg245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, Heiss WD, 2007. The Right Inferior Frontal Gyrus and Poststroke Aphasia: A Follow-Up Investigation. Stroke 38, 1286–1292. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Barch DM, Gray JR, Conturo TE, Braver TS, 2009. BOLD Correlates of Trial-by-Trial Reaction Time Variability in Gray and White Matter: A Multi-Study fMRI Analysis. PLOS ONE 4, e4257 10.1371/journal.pone.0004257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y, Jiang T, Lu Y, He Y, Tian L, 2004. Regional homogeneity approach to fMRI data analysis. NeuroImage 22, 394–400. 10.1016/j.neuroimage.2003.12.030 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu H, Wang L, Yang J, Yan R, Zhang J, Sang L, Li P, Wang J, Qiu M, 2016. Relationship between functional connectivity and motor function assessment in stroke patients with hemiplegia: a resting-state functional MRI study. Neuroradiology 58, 503–511. 10.1007/s00234-016-1646-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.