Abstract
Therapeutic strategies for achieving sustained virologic remission are being explored in human immunodeficiency virus (HIV)–infected individuals who began antiretroviral therapy (ART) during the early phase of infection. In the evaluation of such therapies, clinical protocols should include analytical treatment interruption (ATI); however, the immunologic and virologic impact of ATI in individuals who initiated ART early has not been fully delineated. We demonstrate that ATI causes neither expansion of HIV reservoirs nor immunologic abnormalities following reinitiation of ART. Our findings support the use of ATI to determine whether sustained virologic remission has been achieved in clinical trials of individuals who initiated ART early during HIV infection.
Keywords: HIV reservoirs, acute/early HIV infection, analytical treatment interruption, antiretroviral therapy
We demonstrated that human immunodeficiency virus (HIV)–infected individuals who initiated antiretroviral therapy during the acute/early phase of infection do not experience irreversible expansion of the HIV reservoir or alterations in lymphocyte subsets following analytical treatment interruption and reinitiation of antiretroviral therapy.
Current antiretroviral therapy (ART) has proven to be unsuccessful at eradicating human immunodeficiency virus (HIV) from an infected individual because the vast majority of individuals experience plasma viral rebound upon treatment interruption [1]. Given that lifelong ART is currently required to maintain suppression of plasma viremia, novel therapeutic strategies aimed at achieving sustained virologic remission in the absence of ART are currently being explored [2]. HIV-infected individuals who began ART during the acute/early phase of infection are more likely to respond to therapeutic interventions aimed at inducing ART-free virologic remissions, possibly as a result of smaller HIV reservoirs, more-homogeneous viral quasispecies, and relatively intact immune systems, compared with individuals who initiated ART during the chronic phase of infection [3]. Therefore, these early treated individuals have been the focus of intense study. In this regard, the efficacy of any therapeutic strategy designed to achieve ART-free virologic remission can only be adequately assessed with analytical treatment interruption (ATI) [4]. Yet, there has been concern that plasma viral rebound resulting from ATI may have deleterious immunologic and virologic consequences. Previous studies have demonstrated that short-term ATI did not lead to permanent expansion of the HIV reservoir or irreversible damages to the immune system of infected individuals who initiated ART during the chronic phase of infection [5–8]. Furthermore, it has been shown that a brief course of ATI followed by immediate reinitiation of ART after the plasma viremia level rebounded to >1000 copies/mL did not lead to higher levels of HIV DNA in CD4+ T cells of individuals who initiated ART during the acute phase (Fiebig stage I) of infection [9]. Given the greater chance of achieving ART-free remission in early treated individuals, comprehensive assessment of curative interventions in such individuals may require extended periods of ATI despite high levels of plasma viral rebound [10]. Therefore, it is important to investigate the potential consequences of ATI in patients with significant levels of plasma viremia occur before reinitiation of ART. We conducted the present study to address the effect of ATI on immunologic and virologic parameters in early treated individuals.
MATERIALS AND METHODS
Study Participants
Twenty-two individuals with HIV infection who initiated ART and who had previously participated in a therapeutic vaccine trial [10] were included in this study (Table 1). Following a phase in which they received vaccine or placebo, all study subjects underwent ATI and resumed ART if they met any of the following criteria: a decrease of >30% in baseline CD4+ T-cell count or a decrease in the absolute CD4+ T-cell count to <350 cells/mm3, a sustained (for ≥ 4 weeks) plasma viremia level of >50 000 copies/mL [10]. Blood and leukapheresed products were collected in accordance with protocols approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. All subjects provided written informed consent.
Table 1.
Profiles of Human Immunodeficiency Virus (HIV)–Infected Study Subjects
| CD4+ T-Cell Parameter Before ATI | CD8+ T-Cell Parameter Before ATI | |||||||
|---|---|---|---|---|---|---|---|---|
| Subject Identifier | Viral Suppression Duration, y | Count, Cells/mm3 | Percentage | Count, Cells/mm3 | Percentage | Peak Plasma Viremia Level During ATI, Copies/mL | ATI Duration, d | ART Duration After ART Reinitiation, d |
| 1 | 4.4 | 855 | 47 | 309 | 17 | 164 792 | 62 | 890 |
| 2 | 5.8 | 511 | 39 | 301 | 23 | 105 918 | 126 | 1071 |
| 5 | 7.0 | 559 | 43 | 468 | 36 | 8 405 097 | 47 | 947 |
| 6 | 2.7 | 1443 | 51 | 679 | 24 | 21 997 | 127 | 910 |
| 7 | 3.2 | 495 | 34 | 466 | 32 | 86 049 | 121 | 894 |
| 10 | 7.2 | 582 | 27 | 798 | 37 | 1605 | 139 | 766 |
| 12 | 8.2 | 1628 | 59 | 441 | 16 | 118 209 | 140 | 850 |
| 13 | 12.1 | 1540 | 54 | 428 | 15 | 113 416 | 144 | 879 |
| 14 | 14.0 | 679 | 39 | 470 | 27 | 24 667 | 117 | 888 |
| 15 | 4.0 | 932 | 45 | 725 | 35 | 416 | 114 | 413 |
| 17 | 3.8 | 584 | 42 | 348 | 25 | 5248 | 319 | 640 |
| 19 | 3.5 | 545 | 34 | 400 | 25 | 25 358 | 207 | 697 |
| 20 | 1.9 | 508 | 33 | 523 | 34 | 401 130 | 139 | 690 |
| 21 | 1.8 | 524 | 33 | 715 | 45 | 3223 | 117 | 738 |
| 22 | 5.6 | 1025 | 50 | 492 | 24 | 162 388 | 120 | 706 |
| 23 | 2.8 | 603 | 37 | 505 | 31 | 3262 | 126 | 645 |
| 24 | 2.6 | 484 | 37 | 536 | 41 | 1935 | 217 | 557 |
| 25 | 11.7 | 514 | 34 | 454 | 30 | 72 026 | 86 | 632 |
| 26 | 2.7 | 647 | 45 | 475 | 33 | 15 247 | 112 | 596 |
| 28 | 2.4 | 649 | 34 | 458 | 24 | 362 467 | 56 | 580 |
| 29 | 2.4 | 721 | 34 | 912 | 43 | 32 046 | 115 | 511 |
| 31 | 4.3 | 465 | 26 | 1091 | 61 | 2180 | 242 | 360 |
| Median value | 3.9 | 594 | 38 | 473 | 31 | 28 702 | 124 | 702 |
Abbreviations: ATI, analytical treatment interruption; ART, antiretroviral therapy.
Longitudinal Measurements of HIV Reservoirs
The dynamics of HIV reservoirs were examined in all participants before ATI (referred to as the “pre-ATI” period), during ATI (the “ATI” period), and following reinitiation of ART (the “post-ATI” period). The frequency of cells carrying HIV DNA was determined by droplet digital polymerase chain reaction (PCR) analysis (Bio-Rad Laboratories), using restriction endonuclease (MscI; New England BioLabs)–digested genomic DNA isolated from CD4+ T cells as previously described [5]. The level of cell-associated HIV RNA was determined by reverse transcription PCR (RT-PCR). Total RNA was isolated (by the RNeasy Mini kit; Qiagen) from CD4+ T cells, followed by synthesis of complementary DNA (qScript XLT cDNA Master Mix, Quanta Biosciences) and droplet digital PCR analysis (Bio-Rad Laboratories) as previously described [5]. HIV RNA copy numbers were normalized per 1 × 106 copies of the housekeeping gene TBP, which encodes TATA-box binding protein. The level of inducible HIV was determined by stimulating 106 CD4+ T cells with a phorbol ester (50 nM of prostratin analog 11c) [11] in triplicate for 48 hours, followed by quantitation of cell-free HIV RNA, using the Cobas Ampliprep/Cobas Taqman HIV-1 Test, version 2.0 (Roche Diagnostics). The frequency of cells carrying replication-competent HIV was determined by quantitative coculture assays, using serially diluted (1 × 106, 200 000, 40 000, 8000, 1600, and 320) and replicates of 5 × 106 CD4+ T cells as described elsewhere [5].
Longitudinal Measurements of Immune Parameters
The levels of lymphocyte subsets were determined by staining peripheral blood mononuclear cells with the following fluorophore-conjugated antibodies: CD3 (clone SK7), CD4 (clone SK3), CD8 (clone SK1), CD19 (clone SJ25C1), CD16 (clone B73.1), CD56 (clone NCAM16.2), CD38 (clone HB7), and HLA-DR (clone L243; BD Biosciences). Flow cytometry data were acquired on a BD FACS Canto II flow cytometer with FACSDiva software and analyzed using FlowJo.
Statistical Analysis
Three-way comparisons were performed using the Friedman test, followed by pair-wise comparisons with the Wilcoxon signed rank test if findings were statistically significant.
RESULTS
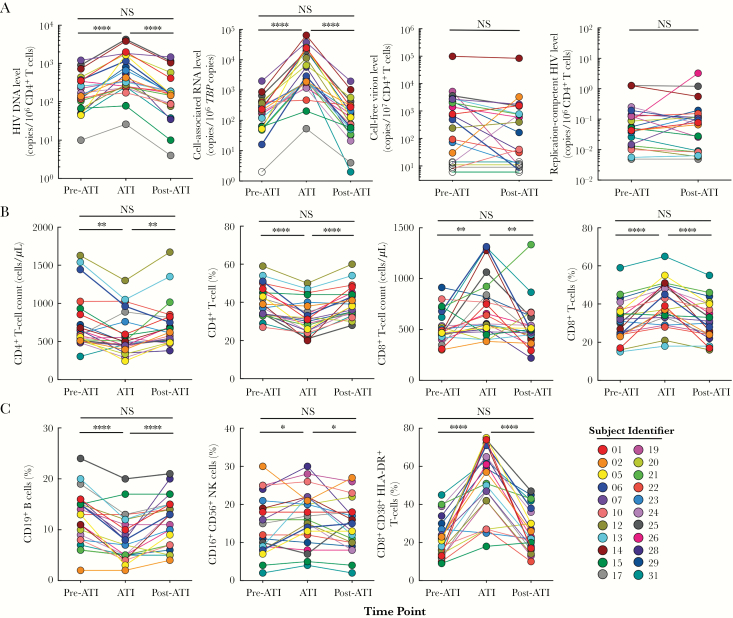
The study cohort comprised 22 HIV-infected individuals who initiated ART during the acute/early phase of infection and subsequently participated in a therapeutic vaccine trial [10]. The median duration of the ATI phase was 124 days (range, 56–242 days), and the plasma viremia level rebounded in all participants, with a median peak level of 28 702 copies/mL (range, 416–8 405 097 copies/mL). All study subjects resumed ART, per the predefined criteria [10], and received antiretroviral drugs for a median of 702 days (range, 360–1071 days) at the time of analysis. During ATI, the vast majority of study participants experienced significant increases in the frequency of CD4+ T cells carrying HIV DNA and cell-associated HIV RNA (Figure 1A). However, following reinitiation of ART, the frequency of CD4+ T cells carrying HIV DNA and cell-associated HIV RNA returned to baseline levels, and there were no significant differences for either virologic marker between the pre-ATI and post-ATI time points (Figure 1A). Given that a large proportion of HIV present in infected CD4+ T cells is replication defective, we conducted 2 additional assays designed to address the level of infectious HIV in CD4+ T cells. First, the frequency of cells carrying inducible HIV was assessed before ATI and following reinitiation of ART, by stimulating CD4+ T cells for 48 hours with a phorbol ester (prostratin analog). Second, we conducted quantitative coculture assays at the pre-ATI and post-ATI time points to assess the change in the size of the HIV reservoir carrying replication-competent virus. As shown in Figure 1A, there were no significant differences in frequencies of CD4+ T cells carrying either inducible or replication-competent HIV between the pre-ATI and post-ATI periods. Of note, when the data were analyzed after excluding the study subjects who had received the therapeutic vaccine regimens [10], there was no impact on the changes in virologic markers shown in Figure 1A. Collectively, these data indicate that ATI and subsequent reinitiation of ART does not alter the size of HIV reservoirs in early treated individuals.
Figure 1.
Kinetics of human immunodeficiency virus (HIV) reservoirs and immunologic parameters before and following analytical treatment interruption (ATI) and reinitiation of antiretroviral therapy (ART). A, The frequency of CD4+ T cells carrying HIV DNA and cell-associated viral RNA was measured before ATI (the “pre-ATI” period), following ATI (the “ATI” period), and upon reinitiation of ART (the “post-ATI” period). The level of inducible cell-free virions and replication-competent HIV was measured before ATI and following reinitiation of ART. Open symbols indicate values under limits of detection. B and C, The frequency and cell count of CD4+ and CD8+ T cells (B) and the frequency of B (CD19+), natural killer (CD16+CD56+), and CD8+CD38+HLA-DR+ T cells (C) are shown at the pre-ATI, ATI, and post-ATI time points. NK, natural killer; NS, not significant. *P < .05, **P < .01, and ****P < .0001, by the Wilcoxon signed rank test.
Having established that ATI leading to plasma viral rebound followed by reinitiation of ART had no measurable impact on the size of the persistent HIV reservoir, we examined several immune parameters longitudinally. During ATI, all subjects experienced a transient decrease in the numbers and frequencies of CD4+ T and B (CD19+) cells, as well as increases in CD8+ T, natural killer (CD16+CD56+), and CD8+CD38+HLA-DR+ T cells that coincided with plasma viral rebound (Figure 1B and 1C). Despite these changes, levels of all immune parameters examined returned to baseline, with no significant difference between pre-ATI and post-ATI time points (Figure 1B and 1C). Taken together, these data demonstrate that active HIV replication/rebounding plasma viremia following ATI contributes to transient perturbations in the frequency of circulating lymphocyte populations that return to pre-ATI frequencies following reinitiation of ART.
DISCUSSION
The development of therapeutic strategies aimed at achieving sustained virologic remission following discontinuation of ART has received considerable attention in light of overwhelming evidence that HIV reservoirs persist during clinically effective ART [12, 13] and that rapid plasma viral rebound occurs upon cessation of therapy in the vast majority of infected individuals [2]. Efforts to determine the efficacy of therapeutic interventions in clinical trials in which ATI was not used have included a variety of qualitative and quantitative laboratory-based assays designed to measure the size of persistent viral reservoirs [14], as well as immune responses against HIV [15]. However, most of these assays are unable to predict the likelihood of achieving durable control of HIV in infected individuals following ATI and as such are of limited clinical relevance [2]. Given that the plasma viremia level remains the only clinically relevant virologic marker available, clinical trials that evaluate therapeutic agents aimed at achieving ART-free virologic remission should include treatment interruption with intensive and extended monitoring of relevant immunologic and virologic parameters. In this regard, several studies have addressed the impact of ATI and reinitiation of ART on the dynamics of HIV reservoirs and immune markers in cohorts of infected individuals who initiated ART during the chronic phase of infection [5–8]. These studies have shown that a short course of ATI does not lead to irreversible expansion of the HIV reservoir or damage to the immune system [5–8]. However, there is a growing interest in exploring novel therapeutic interventions in HIV-infected individuals who have not thus far been extensively studied for the effects of ATI and reinitiation of ART, namely those who began ART during the acute/early phase of infection. Previous studies have shown that, compared with initiation of ART during the chronic phase of infection, early intervention is associated with smaller viral reservoir size, homogeneous viral quasispecies, and preservation of immune function, factors that might predict a better likelihood of controlling HIV replication following discontinuation of ART [3]. Given the reported differences in immunologic and virologic parameters associated with early versus delayed initiation of ART [3], the evaluation of such parameters following ATI and subsequent reinitiation of ART in early treated individuals is warranted. In this regard, a recent study involving a small number of HIV-infected individuals who initiated ART during the earliest possible stage of infection (ie, Fiebig stage I) revealed that the plasma viremia level rebounded with a median of 26 days following treatment interruption [9], similar to the time to rebound reported in cohorts of individuals whose therapy was initiated during the chronic stage of infection [1, 6]. Although the ATI study involving early treated patients did not find evidence of changes in the frequency of CD4+ T cells carrying HIV DNA between the pre-ATI period and 6 months after reinitiation of ART, the findings were confounded by a prompt restart of ART, defined per protocol, as soon as the plasma viremia level reached 1000 copies/mL. It is possible that a longer period of treatment interruption may have provided more valuable information without compromising the clinical status of the patients. In this regard, we previously demonstrated that a substantial proportion of HIV-infected individuals who initiated ART early in the course of infection spontaneously controlled their plasma viremia for extended periods following high levels of initial plasma viral rebound [10]. Had we reinitiated ART immediately when the patients reached 1000 copies/mL, the subsequent prolonged control of plasma viremia observed in the absence of ART would have been missed. Based on these observations, we feel that it is justified and safe for clinical trials of potentially curative interventions in early treated HIV patients to include a period of ATI that could potentially lead to transiently high levels of plasma viral rebound. The study subjects in the present study underwent ATI that lasted a median of 124 days, with a median peak plasma viremia of 28 702 copies/mL followed by a median ART duration of 702 days after ATI. Despite the relatively high peak plasma viremia level and longer duration of ATI as compared to the previous study [9], we did not find evidence of irreversible expansion of the measurable HIV reservoir or alterations in subsets of immune cells in the peripheral blood. Future experiments involving phylogenetic analyses of rebounding virus and persistent HIV reservoirs, as well as in-depth examination of immune responses to the virus, would be necessary to support our findings. One caveat of our study was the inclusion of study subjects who received therapeutic vaccines before the ATI phase [10]. However, it is unlikely that the vaccine regimen had an impact on the parameters examined in this study, given the lack of effect of the vaccine regimens on the size of HIV reservoirs and HIV-specific immune responses [10]. Indeed, exclusion of the vaccine group from our current analyses and inclusion of only the placebo group had no impact on the overall conclusions. However, further investigations are warranted, including analyses of tissue compartments where a higher viral burden than that in the peripheral blood is expected. Collectively, our data indicate that ATI with close monitoring of plasma viremia and inclusion of strict ART restart guidelines is safe. Furthermore, we propose that the importance of including ATI in clinical trials designed to examine the efficacy of therapeutic interventions aimed at achieving ART-free HIV remissions in infected individuals who initiated ART early in the course of infection outweighs the potential risks of ATI.
Notes
Acknowledgments. We thank the study volunteers, for their participation in this study; and the National Institute of Allergy and Infectious Diseases HIV Outpatient Clinic staff, for their assistance in the execution of this study.
Financial support. This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) and by the NIH (grants AI124743 and CA31845 to P. A. W.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Davey RT Jr, Bhat N, Yoder C, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A 1999; 96:15109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chun TW, Moir S, Fauci AS. HIV reservoirs as obstacles and opportunities for an HIV cure. Nat Immunol 2015; 16:584–9. [DOI] [PubMed] [Google Scholar]
- 3. Robb ML, Ananworanich J. Lessons from acute HIV infection. Curr Opin HIV AIDS 2016; 11:555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wen Y, Bar KJ, Li JZ. Lessons learned from HIV antiretroviral treatment interruption trials. Curr Opin HIV AIDS 2018; 13:416–21. [DOI] [PubMed] [Google Scholar]
- 5. Clarridge KE, Blazkova J, Einkauf K, et al. Effect of analytical treatment interruption and reinitiation of antiretroviral therapy on HIV reservoirs and immunologic parameters in infected individuals. PLoS Pathog 2018; 14:e1006792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Papasavvas E, Lada SM, Joseph J, et al. Analytical antiretroviral therapy interruption does not irreversibly change preinterruption levels of cellular HIV. AIDS 2018; 32:1763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salantes DB, Zheng Y, Mampe F, et al. HIV-1 latent reservoir size and diversity are stable following brief treatment interruption. J Clin Invest 2018; 128:3102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strongin Z, Sharaf R, VanBelzen DJ, et al. Effect of short-term antiretroviral therapy interruption on levels of integrated HIV DNA. J Virol 2018; 92:e00285–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colby DJ, Trautmann L, Pinyakorn S, et al. ; RV411 study group Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med 2018; 24:923–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sneller MC, Justement JS, Gittens KR, et al. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med 2017; 9:eaan8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beans EJ, Fournogerakis D, Gauntlett C, et al. Highly potent, synthetically accessible prostratin analogs induce latent HIV expression in vitro and ex vivo. Proc Natl Acad Sci U S A 2013; 110:11698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997; 278:1295–300. [DOI] [PubMed] [Google Scholar]
- 13. Wong JK, Hezareh M, Günthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 1997; 278:1291–5. [DOI] [PubMed] [Google Scholar]
- 14. Henrich TJ, Deeks SG, Pillai SK. Measuring the size of the latent human immunodeficiency virus reservoir: the present and future of evaluating eradication strategies. J Infect Dis 2017; 215:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rouzioux C, Hocqueloux L, Sáez-Cirión A. Posttreatment controllers: what do they tell us? Curr Opin HIV AIDS 2015; 10:29–34. [DOI] [PubMed] [Google Scholar]