Abstract
Background
Chronically elevated interleukin-6 (IL-6) levels contribute to fatigue and functional decline via multiple pathways that often lead to frailty. Lesser known is the contribution of IL-6 to fatigue in relation to a standardized workload (fatigability), a precursor to functional decline. Therefore, the purpose of this study was to examine the longitudinal relationship between IL-6 and fatigability.
Methods
About 985 participants from the Baltimore Longitudinal Study of Aging (mean age: 70 ± 10 years) were evaluated every 1–4 years. IL-6 was measured in fasting serum samples at each visit and log-transformed for analyses. Perceived fatigability (PF) was defined as self-reported exertion (rate of perceived exertion; RPE) after a 5-min, 0.67 m/s, 0% grade treadmill walk. Continuous and categorical associations between IL-6 (baseline and repeated measures) and PF were assessed using generalized estimating equations, adjusting for demographics, behavioral factors, and comorbid conditions.
Results
In fully adjusted continuous models, twofold higher baseline IL-6 was associated with a 0.28 higher RPE (p = .03). This relationship tended to remain constant annually (baseline log IL-6 by time interaction p = .29). To provide clinical relevance, the sample median (3.7 pg/mL) was used to examine high versus low IL-6 levels. Over time, the high group reported an average 0.25 higher RPE (p = .03) than the low group. Annual change in logged IL-6 was not associated with annual change in PF (p = .48).
Conclusion
Findings suggest that elevated IL-6 is a biomarker of physiological dysregulation associated with greater fatigability, but there is no longitudinal association between IL-6 and fatigability. Future studies should evaluate whether interventions that aim to reduce inflammation also attenuate fatigability.
Keywords: Inflammaging, Chronic inflammation, Fatigue, Aging, IL-6, Older adults
Chronic inflammation marked by chronically elevated levels of pro-inflammatory biomarkers, such as interleukin-6 (IL-6) and C-reactive protein, frequently occurs in older persons and is often referred to as “inflammaging”. Numerous studies have reported that inflammaging adversely affects risk of developing chronic disease, disability and other phenotypic manifestations of aging (1). Over the past several decades, studies have shown that interleukin-6 (IL-6)—an important inflammatory marker—is strongly correlated with and predictive of poor physical function (2–8) and disability (9,10), even after adjusting for disease status and other potential confounders. Yet, little research has explored whether inflammation influences early preclinical markers of impending functional decline, or whether long-term exposure to inflammation is associated with greater fatigability, a precursor to accelerated functional decline (11).
Chronically elevated IL-6 has been linked to higher fatigue burden with respect to specific diseases (12–14), but its role in age-related fatigue is not well-understood. A recent study showed that changes in IL-6 over 6 months were negatively correlated with changes in self-reported vitality (the inverse of fatigue) (15). However, understanding whether IL-6 is associated with fatigue is intrinsically difficult because subjective measures of fatigue do not adequately reference symptoms to specified levels of physical effort or situational demands and may be masked by the normal decline of physical activity that occurs with aging (15). The construct of perceived fatigability (PF) has been associated with lower physical activity (16), poorer physical function (17), and heightened risk of functional decline (11), and has been found to provide a comprehensive, less subjective assessment of risk of adverse outcomes than traditional fatigue-like symptoms (eg, tiredness and low energy) (11,17). Further, a recent study demonstrated that PF was associated with clinically meaningful declines in usual gait speed, fast gait speed, physical performance, and self-described walking ability in mobility-intact older adults—highlighting PF’s role as an early precursor to impending functional decline (11). Yet, whether the age-related rise in IL-6 represents an underlying biological pathway that helps explain the association between PF and future physical decline remains unknown.
Serum levels of IL-6 may reflect tissue inflammation, such as skeletal muscle, which would be expected to negatively affect aerobic capacity or increase energy consumption (1). Moreover, circulating IL-6 can cross the brain–blood barrier and act on the prefrontal cortex and other brain areas to increase perception of tiredness or depressed mood (18,19), which are generally considered fatigue-like symptoms (20). If the association between IL-6 and fatigability is revealed at an early stage of a process that eventually leads to functional decline in well-functioning persons, steps to reverse chronic inflammation may curb deleterious health effects at a broader level. Examining IL-6’s association with PF may thus elucidate a means to identify adults who appear well-functioning but may be experiencing subclinical manifestations of accelerated aging, disability, and disease progression. Assessing the magnitude of this complex association using longitudinal data may deepen our understanding of the consequences of inflammaging.
The aim of this study was to understand whether IL-6, at baseline and throughout follow-up visits, was associated with annual changes in PF. The first aim examined whether baseline IL-6 was associated with longitudinal changes in PF. The second aim assessed whether changes in IL-6 over time contributed to changes in PF. We hypothesized that higher baseline levels of IL-6 would be associated with increased PF over time, and that increases in IL-6 between baseline and follow-up would correspond with higher PF.
Methods
Study Design and Population
This study utilized data from 1,280 men and women, aged 50–96 years old, whose inflammatory and functional measures were collected through the Baltimore Longitudinal Study of Aging (BLSA) between 2007 and 2016. The BLSA is an ongoing enrollment cohort study primarily focused on studying normative human aging, conducted by the National Institute on Aging’s (NIA) Intramural Research Program. Details about the BLSA enrollment criteria and sample have been previously published (21). In short, BLSA participants are healthy at enrollment, with no cognitive impairment, functional limitations, or major chronic diseases (except hypertension) or cancer within the past 10 years. Once enrolled, participants are followed for life, attending periodic comprehensive health, cognitive, and functional assessments during a 3-day stay at the NIA’s Clinical Research Unit located at Harbor Hospital in Baltimore, Maryland. These visits are regularly scheduled every 1–4 years depending on age. Specifically, those aged <60 years, 60–79 years, and 80+ years old attended a clinic visit every 4, 2, and 1 years, respectively. Participants were excluded if they had missing IL-6 values (n = 28) or missing PF data (n = 267). The final analytic sample used in this study consisted of 985 participants who had IL-6 and PF measured at each visit. Trained and certified study staff administer all assessments following standardized protocols. The study protocol has been approved by the National Institute for Environmental Health Sciences Institutional Review Board, and all participants gave written informed consent.
Serum IL-6 Measurement
Fasting (12 h or more) serum samples were collected at each BLSA visit. After the blood samples were obtained, they were centrifuged at 4°C, and serum immediately aliquoted and stored at −80°C. Serum IL-6 (pg/mL) concentrations were measured using an enzyme-linked immunosorbent assay (Quantikine ELISA; R&D System, Minneapolis, MN, USA. Intra-assay variation is 1.6–4.2%: inter-assay variation is 3.3–6.4%. The same antibody lot has been since 2005. Lower limit of detectability is 0.70 pg/mL). When ~80 samples had accumulated, the assay was performed. All assays were performed on a fresh frozen aliquot: no analysis was performed on a previously thawed and re-frozen aliquot. If a participant had multiple visits during this time period, previous IL-6 results were compared to current assay results to detect possible outliers. About 15% of the samples were assayed in duplicate to establish reliability. IL-6 values were log-transformed for analyses due to the skewed distributions of the raw values.
Perceived Fatigability
Perceived fatigability was measured at each BLSA visit using self-reported effort in response to a standardized, 5-min, slow-paced treadmill walk (0.67 m/s; 1.5 mph) at 0% grade. A speed of 0.67 m/s was selected to be low demand to minimize participant exclusion at the higher end of the age spectrum. Participants reported perceived effort using the Borg rating of perceived exertion (RPE) scale (22). This scale ranges from 6 to 20, where higher exertion levels represent higher PF.
Covariates
Participants reported age, sex, and race via an interview questionnaire administered every visit. Height and weight were measured objectively and body mass index (BMI) was derived as weight/height2 (kg/m2). Participants reported whether they were ever told by a doctor or other health professional that they had any of the following conditions: depression; cardiovascular disease including angina, myocardial infarction, congestive heart failure, peripheral arterial disease, and vascular-related procedures; hypertension or high blood pressure; cerebrovascular disease including stroke and transient ischemic attack; pulmonary disease including chronic bronchitis, emphysema, chronic obstructive pulmonary disease, or asthma; diabetes, glucose intolerance, or high blood sugar; cancer, a malignant growth, or malignant tumor; and arthritis or osteoarthritis.
Statistical Approach
Descriptive statistics for continuous variables (mean, standard deviation) and categorical variables (frequency, percentage of total) were tabulated for all covariates including age, sex, race, height, BMI, smoking history, and self-reported diagnosis of depression, cardiovascular disease, hypertension, cerebrovascular disease, pulmonary disease, diabetes, cancer, and osteoarthritis. Baseline IL-6 was categorized into two groups using the sample median of log baseline IL-6, a threshold determined a priori. Ad hoc sensitivity analyses were performed to explore a number of thresholds surrounding the median and their relationship to fatigability. Change in log IL-6 was calculated by subtracting baseline log IL-6 from all log IL-6 values for each participant. General estimating equations (GEE) were used to determine: (a) trajectories of log-transformed IL-6 and PF, (b) the association between log baseline IL-6 (continuous and categorical) with longitudinal change in PF, and (c) the association between longitudinal change in log IL-6 with longitudinal change in PF. The GEE analyses used an identity Gaussian distribution to characterize the variance and an exchangeable correlation matrix for estimation. All models were adjusted for the covariates noted above. Models testing the association between longitudinal change in log IL-6 and PF included baseline log IL-6 as a covariate. An alpha level of 0.05 was set to determine statistical significance using two-tailed hypothesis testing. All analyses were performed using STATA v14 (College Station, TX).
Results
Table 1 describes baseline characteristics of 985 participants who averaged 2 (SD = 2; range = 0–6) years between baseline and most recent follow-up visits. Overall, there were 1,010 longitudinal visits with 574 participants (58%) having at least one follow-up visit (mean = 2 visits, range = 1–6 visits). At baseline, the mean age was 70 (SD = 10) years, 53% were women, 24% were black, and the mean BMI was 27 (5) kg/m2. Prevalence of each disease condition in our analytic sample was under 20% except for hypertension (47%), cancer history/survivorship (32%), and osteoarthritis (53%). Those with high baseline IL-6 (n = 488) had a higher mean age, BMI, were more likely to be men and report a history of cardiovascular disease, hypertension, pulmonary disease, and/or cancer and had higher RPE scores (p < .038 for all).
Table 1.
Participant Characteristics at Baseline Stratified by Median Baseline Interleukin-6
| ≤3.7 pg/mL, n = 497 |
>3.7 pg/mL, n = 488 |
p-value | |
|---|---|---|---|
| Age (years), mean (SD) | 68.8 (9.7) | 72.5 (10.3) | <.001 |
| Female, n (%) | 294 (59.2) | 225 (46.1) | <.001 |
| Black, n (%) | 109 (21.9) | 126 (25.8) | .084 |
| Height (cm), mean (SD) | 167.4 (9.5) | 168.5 (9.0) | .070 |
| Body mass index (kg/m2), mean (SD) | 26.4 (4.2) | 27.7 (4.7) | <.001 |
| Smoked ever, n (%) | 163 (32.8) | 177 (36.3) | .252 |
| Depressiona, n (%) | 78 (15.7) | 74 (15.2) | .818 |
| Cardiovascular diseasea, n (%) | 41 (8.3) | 70 (14.3) | .002 |
| Hypertensiona, n (%) | 203 (40.9) | 261 (53.5) | <.001 |
| Cerebrovascular diseasea, n (%) | 15 (3.0) | 27 (5.5) | .051 |
| Pulmonary diseasea, n (%) | 55 (11.1) | 76 (15.6) | .037 |
| Diabetesa, n (%) | 77 (15.5) | 96 (19.7) | .085 |
| Cancera, n (%) | 136 (27.4) | 176 (36.1) | .003 |
| Osteoarthritisa, n (%) | 249 (50.1) | 268 (54.9) | .130 |
| Perceived fatigabilityb, mean (SD) | 8.3 (2.1) | 9.0 (2.4) | <.001 |
aSelf-reported history of being diagnosed by a doctor or other health professional.
bUnits are rate of perceived exertion (RPE) scores where higher scores represent higher perceived effort.
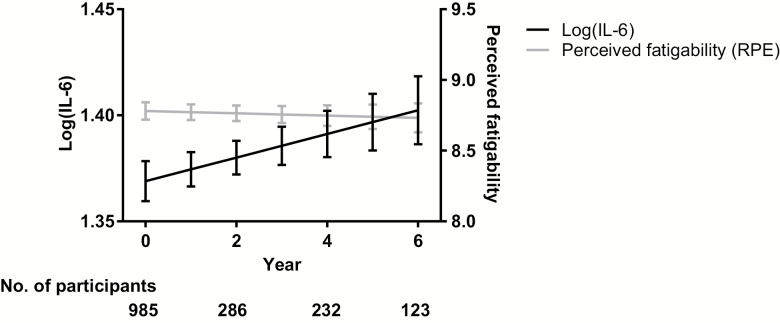
Figure 1 illustrates the trajectories of log IL-6 and PF after adjusting for demographic and behavioral factors and comorbidities. After full adjustment, mean baseline log IL-6 was 1.37 (SE = 0.01) log units. Additionally, IL-6 increased annually by +0.006 (SE = 0.003) log units, trending towards statistical significance (p = .082). Baseline PF averaged 8.78 (0.06) RPE, and remained relatively stable over time (−0.008 RPE, SE = 0.02, p = .676).
Figure 1.
Adjusted trajectories of log interleukin-6 (IL-6) and perceived fatigability over 6 years. Note: Figure is adjusted for baseline age, sex, race, height (cm), body mass index (kg/m2), smoking status, depression, cardiovascular disease, hypertension, stroke, pulmonary disease, diabetes, cancer, and osteoarthritis. IL-6 is in units of pg/mL; RPE = rate of perceived exertion.
Table 2 describes the association between baseline log IL-6 and longitudinal change in PF, with the effect of successive covariate adjustment. In the continuous analysis, for each one unit higher baseline log IL-6, there was a 0.4 (0.2) unit higher RPE score after full covariate adjustment (p = .034). For example, a twofold higher baseline IL-6 was associated with a 0.28 higher RPE (p = .03). This relationship remained constant over time, with no significant baseline log IL-6-by-time interaction (p = .291). In the categorical analysis, those with higher baseline IL-6 (≥ 3.7 pg/mL) reported a 0.3 (0.1) unit higher RPE at baseline when compared to those with lower IL-6 levels (p = .028). This relationship also remained stable over time (baseline IL-6 ≥3.7 pg/mL × time interaction p = .645).
Table 2.
Association Between Baseline Log IL-6 and Longitudinal Changes in Perceived Fatigability (RPE), n = 985
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Continuous | Beta coefficient (SE) | ||
| Baseline log(IL-6) | 1.34 (0.22)*** | 0.46 (0.19)* | 0.40 (0.19)* |
| Time (years) | 0.10 (0.09) | 0.002 (0.02) | −0.01 (0.02) |
| Baseline log(IL-6) × time | −0.07 (0.07) | NS† | NS† |
| Categorical | Beta coefficient (SE) (Ref: baseline IL-6 <3.7 pg/mL) | ||
| Baseline IL-6 ≥3.7 pg/mL | 0.70 (0.14)*** | 0.26 (0.11)* | 0.25 (0.11)* |
| Time (years) | 0.02 (0.03) | 0.002 (0.02) | −0.003 (0.02) |
| Baseline IL-6 ≥3.7 pg/mL × time | −0.02 (0.04) | NSa | NSa |
Notes: Model 1: Unadjusted. Model 2: Model 1 + baseline age, sex, race, height (cm), body mass index (kg/m2), and smoking. Model 3: Model 2 + depression, cardiovascular disease, hypertension, stroke, pulmonary disease, diabetes, cancer, and osteoarthritis. IL-6 = Interleukin-6 (pg/mL); RPE = Rate of perceived exertion; Ref. = Reference.
†Interaction term was removed from covariate-adjusted model when not significant (NS).
*p < .05; **p < .01; ***p < .001.
Ad hoc exploration of thresholds (ranging from 2.5 to 4.1 pg/mL) to categorize baseline IL-6 levels were explored; these thresholds were based on results from previous literature (9) and derived from tertiles of baseline log IL-6 from the analytic sample. However, these thresholds did not substantially alter the results when compared with the sample median of IL-6 of 3.7 pg/mL.
The association between longitudinal change in IL-6 and longitudinal change in PF is found in Table 3. No significant relationships were observed for change in logged IL-6 and change in PF (p > .05 for Models 1–3 [row A]). A significantly positive relationship between baseline IL-6 with PF was robust to covariate adjustment (p < .027 Models1-3 [row C]) and estimates were almost identical to those generated in the baseline models found in Table 2.
Table 3.
Association Between Longitudinal Change in IL-6 and Corresponding Change in Perceived Fatigability, n = 985
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| A. Change in log(IL-6) | 0.24 (0.17) | 0.11 (0.16) | 0.11 (0.16) |
| B. Time (years) | 0.01 (0.02) | 0.001 (0.02) | −0.01 (0.02) |
| C. Baseline log(IL-6) | 1.32 (0.21)*** | 0.49 (0.19)* | 0.43 (0.19)* |
Notes: Model 1: Unadjusted. Model 2: Model 1 + baseline age, sex, race, height (cm), body mass index (kg/m2), and smoking. Model 3: Model 2 + depression, cardiovascular disease, hypertension, stroke, pulmonary disease, diabetes, cancer, and osteoarthritis. IL-6 = Interleukin-6; RPE = Rate of perceived exertion.
*p < .05; **p < .01; ***p < .001.
Discussion
Chronic inflammation and high fatigability in older adults are serious health challenges that pose considerable threats to health and physical function. To our knowledge, this is the first study to evaluate the association between IL-6 and fatigability prospectively in a large cohort of middle-aged and older adults. Our results suggest that higher baseline levels of IL-6 are associated with greater PF, supporting our first hypothesis. Additionally, participants with higher baseline IL-6 (≥3.7 pg/mL) reported significantly higher PF than those with lower baseline IL-6 (<3.7 pg/mL). However, contrary to our second hypothesis, annual changes in IL-6 were not associated with longitudinal changes in PF. Despite the lack of a temporal relationship, these findings characterize an important longitudinal association between IL-6 and PF in a cohort of well-functioning older adults. Further investigation is warranted in studies with longer follow-ups and/or more clinically oriented populations to determine whether a dose–response relationship exists among those who live with the challenges of chronic inflammation and fatigability related to disease and disability.
Study results are consistent with previous cross-sectional evidence supporting an association between IL-6 and fatigue in 182 community-dwelling older adults (23). Similarly, a 2013 study in 7,509 participants aged 39–63 years old recruited for the Whitehall II prospective cohort study showed that baseline IL-6 was inversely correlated with self-reported vitality (the inverse of fatigue) measured with the 36-item Short Form Health Survey (SF-36) (24). Additionally, a 2016 study showed an inverse association between IL-6 and reported energy level in 167 overweight/obese middle-aged and older adults with osteoarthritis enrolled in the Intensive Diet and Exercise for Arthritis (IDEA) study (15). Our results strengthen and augment these findings by pairing IL-6 with the construct of fatigability, which has been shown to be a better predictor of functional decline than subjective reports of tiredness or low energy (11), in well-functioning older adults followed longitudinally.
Although the baseline association remained strong over time, there was no detectable longitudinal relationship between IL-6 and PF. This is contrary to the Whitehall II secondary analysis that showed participants with increasing IL-6 experienced decreases in vitality over 3 years of follow-up (24). Additionally, those categorized as having high IL-6 (>1.5 pg/mL) had a 45% higher odds of developing fatigue compared to those with low inflammation. Similarly, the IDEA sample experienced changes in the same vitality measure were inversely associated with changes in IL-6 among 167 adults aged ≥55 years old living with overweight/obesity (mean baseline IL-6 3.1 pg/mL) (15). However, this association was observed over 6 months, but not 18 months, of follow-up. Our sample’s baseline mean log IL-6 of 1.37 appeared to be lower than the mean log IL-6 level (1.92) of a large sample of well-functioning but even older adults participating in the Health, Aging and Body Composition study (n = 3,013; mean age of 74 ± 3 years) (25). Additionally, the sample median of 3.7 pg/mL appears to be higher than previous research in older women with disability (3.1 pg/mL) (26), but below that for frail older adults (4.2 pg/mL) (27). Differences in IL-6 levels across studies is likely due to varying assay kits used to measure inflammatory markers. Further, PF did not change materially over time in BLSA participants, which represent a healthier segment of the older adult population and exhibit higher functional status than the general population. Together with our findings, this suggests that the longitudinal origins between IL-6 and fatigue/fatigability may begin earlier in life or at a lower threshold of chronic inflammation. Although our results are adjusted for comorbidities, it is also possible that chronic IL-6 is simply a biomarker of a biological and detrimental condition that is associated with fatigability. Overall, IL-6 appears to be implicated in the development of fatigue and fatigability but only up to a certain threshold of IL-6.
Mechanisms underlying the positive relationship between IL-6 and PF may be explained through biological pathways. As IL-6 accumulates with age-related stressors, it stimulates signals across the blood-brain barrier via pathways such as the vagal nerve afference and humoral transmissions (28,29), leading to overexpression of IL-6 by neurons and microglia (eg, astrocytes) by the central nervous system and elevated levels of IL-6 in the brain (29,30). Experimental studies in both animals and humans implicate IL-6 in the modulation of neurotransmitter and neuroendocrine systems that can disrupt the blood–brain barrier and induce fatigue-like symptoms associated with sickness behavior (28,29,31,32). However, more research is needed confirm these biologic pathways and to fully elucidate acute versus chronic differences in whether IL-6’s pleiotropic nature is completely adverse to fatigue levels experienced by older adults (28).
There are limitations to acknowledge. First, we were not able to examine relationships among other inflammatory markers potentially related to PF such as tumor necrosis factor alpha and C-reactive protein. However, IL-6 is considered a primary inflammatory marker in aging and has been implicated in most research related to age-related physical function (1,5,9). Another limitation is that PF incorporates a subjective component into the measurement of activity-related fatigue, possibly introducing emotional and cognition biases. However, the subjective component (RPE) is a valid measure of exertion (22) and when coupled with a standardized treadmill activity, increases the sensitivity by isolating task-related fatigue and thus reducing the potential for misclassification. Another limitation is missing data loss to follow-up. Given the rigors of the 3-day clinic visit, BLSA participants who are sicker are less likely to return; therefore limiting our sample to robust older adults. Lastly, since BLSA is healthier than the general population, our results are likely to understate the true association between IL-6 and fatigability. Although this may be a limitation, it also reduces the potential for confounding by disease burden. Replication of this research is needed in clinical and research populations with greater chronic disease and functional burden.
In summary, we found that higher baseline IL-6 is associated with higher levels of PF and that annual changes in IL-6 are not associated with corresponding changes in PF among adults in mid-to-late life. Our findings point to IL-6 as a possible target for future interventions targeting fatigability and other precursors to functional limitation in older adults, and provide valuable insight into the degree to which IL-6 is related to PF both cross-sectionally and prospectively. Further research is needed to understand the biological mechanisms contributing to the association between IL-6 and fatigability and to further define the threshold at which IL-6 adversely affects perceptions of fatigue.
Funding
This research was supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health. Data used in the analyses were obtained from the Baltimore Longitudinal Study of Aging, a study performed by the National Institute on Aging Intramural Research Program. Fatigability analyses were supported by R21AG053198 and P30AG021334. A.W. is supported by T32AG000247 and P30AG021334. J.S. is supported by R21AG053198, P30AG021334, and U01AG057545. R.V. is partially supported by National Cancer Institute (P30CA006973).
Conflict of interest
E.S., S.S., L.F., and J.S. currently serve on the editorial board for the Journal of Gerontology: Medical Sciences. A.W. and R.V. have no conflicts of interest to disclose.
References
- 1. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2014;69(Suppl_1):S4–S9. doi:10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 2. Newman AB, Sanders JL, Kizer JR, et al. Trajectories of function and biomarkers with age: the CHS all stars study. Int J Epidemiol. 2016;45:1135–1145. doi:10.1093/ije/dyw092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: macarthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55:M709–M715. doi:10.1093/gerona/55.12.M709 [DOI] [PubMed] [Google Scholar]
- 4. Brinkley TE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–461. doi:10.1093/gerona/gln038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248. doi:10.1093/gerona/59.3.M242 [DOI] [PubMed] [Google Scholar]
- 6. Sousa AC, Zunzunegui M, Li A, Phillips SP, Guralnik JM, Guerra RO. Association between C-reactive protein and physical performance in older populations: results from the international mobility in aging study (IMIAS). Age Ageing. 2016;45:274–280. doi:10.1093/ageing/afv202 [DOI] [PubMed] [Google Scholar]
- 7. Verghese J, Holtzer R, Oh-Park M, Derby CA, Lipton RB, Wang C. Inflammatory markers and gait speed decline in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:1083–1089. doi:10.1093/gerona/glr099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Penninx BW, Kritchevsky SB, Newman AB, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–1113. doi:10.1111/j.1532-5415.2004.52308.x [DOI] [PubMed] [Google Scholar]
- 9. Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi:10.1111/j.1532-5415.1999.tb01583.x [DOI] [PubMed] [Google Scholar]
- 10. Verghese J, Holtzer R, Lipton RB, Wang C. High-sensitivity C-reactive protein and mobility disability in older adults. Age Ageing. 2012;41:541–545. doi:10.1093/ageing/afs038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simonsick EM, Glynn NW, Jerome GJ, Shardell M, Schrack JA, Ferrucci L. Fatigued, but not frail: perceived fatigability as a marker of impending decline in mobility-intact older adults. J Am Geriatr Soc. 2016;64:1287–1292. doi:10.1111/jgs.14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Appels A, Bär FW, Bär J, Bruggeman C, de Baets M. Inflammation, depressive symptomtology, and coronary artery disease. Psychosom Med. 2000;62:601–605. doi:10.1097/00006842-200009000-00001 [DOI] [PubMed] [Google Scholar]
- 13. Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi:10.1158/1078-0432.CCR-05-2398 [DOI] [PubMed] [Google Scholar]
- 14. Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–1158. doi:10.1210/jcem.85.3.6484 [DOI] [PubMed] [Google Scholar]
- 15. Nicklas BJ, Beavers DP, Mihalko SL, Miller GD, Loeser RF, Messier SP. Relationship of objectively-measured habitual physical activity to chronic inflammation and fatigue in middle-aged and older adults. J Gerontol A Biol Sci Med Sci. 2016;71:1437–1443. doi:10.1093/gerona/glw131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wanigatunga AA, Simonsick EM, Zipunnikov V, et al. Perceived fatigability and objective physical activity in mid-to late-life. J Gerontol A Biol Sci Med Sci. 2018;73:630–635. doi:10.1093/gerona/glx181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simonsick EM, Schrack JA, Glynn NW, Ferrucci L. Assessing fatigability in mobility-intact older adults. J Am Geriatr Soc. 2014;62:347–351. doi:10.1111/jgs.12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi:10.1016/j.pharmthera.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi:10.1016/ j.bbi.2006.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grygiel-Górniak B, Puszczewicz M. Fatigue and interleukin-6 - a multi-faceted relationship. Reumatologia. 2015;53:207–212. doi:10.5114/reum.2015.53998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stone JL, Norris AH. Activities and attitudes of participants in the Baltimore longitudinal study. J Gerontol. 1966;21:575–580. doi:10.1093/geronj/21.4.575 [DOI] [PubMed] [Google Scholar]
- 22. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. doi:10.1249/00005768-198205000-00012 [PubMed] [Google Scholar]
- 23. Valentine RJ, Woods JA, McAuley E, Dantzer R, Evans EM. The associations of adiposity, physical activity and inflammation with fatigue in older adults. Brain Behav Immun. 2011;25:1482–1490. doi:10.1016/ j.bbi.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 24. Cho HJ, Kivimäki M, Bower JE, Irwin MR. Association of C-reactive protein and interleukin-6 with new-onset fatigue in the Whitehall II prospective cohort study. Psychol Med. 2013;43:1773–1783. doi:10.1017/S0033291712002437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hall MH, Smagula SF, Boudreau RM, et al. Association between sleep duration and mortality is mediated by markers of inflammation and health in older adults: the health, aging and body composition study. Sleep. 2015;38:189–195. doi:10.5665/sleep.4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi:10.1046/j.1532-5415.2002.50605.x [DOI] [PubMed] [Google Scholar]
- 27. Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi:10.1111/j.1532-5415.2007.01186.x [DOI] [PubMed] [Google Scholar]
- 28. Jüttler E, Tarabin V, Schwaninger M. Interleukin-6 (IL-6): a possible neuromodulator induced by neuronal activity. Neuroscientist. 2002;8:268–275. doi:10.1177/1073858402008003012 [DOI] [PubMed] [Google Scholar]
- 29. Karshikoff B, Sundelin T, Lasselin J. Role of inflammation in human fatigue: relevance of multidimensional assessments and potential neuronal mechanisms. Front Immunol. 2017;8:21. doi:10.3389/fimmu.2017.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–653. doi:10.1016/S0889-1591(02)00021-1 [DOI] [PubMed] [Google Scholar]
- 31. Kelley KW, Bluthé RM, Dantzer R, et al. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;(17 Suppl 1):S112–S118. doi:10.1016/S0889-1591(02)00077-6 [DOI] [PubMed] [Google Scholar]
- 32. Rohleder N, Aringer M, Boentert M. Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci. 2012;1261:88–96. doi:10.1111/j.1749-6632.2012.06634.x [DOI] [PubMed] [Google Scholar]